Abstract
Purpose of Review.
Over the last decade, evidence suggests that a combination of behavioral and neuroimaging findings can help illuminate changes in functional dysconnectivity in schizophrenia. We review the recent connectivity literature considering several vital models, considering connectivity findings and relationships with clinical symptoms.
Methods.
We reviewed resting state fMRI studies from 2017–2023. We summarized the role of two sets of brain networks (cerebello-thalamo-cortical (CTCC) and the triple network set) across three hypothesized models of schizophrenia etiology (neurodevelopmental, vulnerability-stress, and neurotransmitter hypotheses).
Recent Findings.
The neurotransmitter and neurodevelopmental models best explained CTCC-subcortical dysfunction, which was consistently connected to symptom severity and motor symptoms. Triple network dysconnectivity was linked to deficits in executive functioning, and the salience network (SN) – Default Mode Network dysconnectivity was tied to disordered thought and attentional deficits.
Summary.
This paper links behavioral symptoms of schizophrenia (symptom severity, motor, executive functioning, and attentional deficits) to various hypothesized mechanisms.
Keywords: schizophrenia, disconnectivity, functional connectivity, dysconnectivity, brain networks
1. Introduction
Dysconnectivity is defined as “abnormal functional integration across brain circuits” [1]. “The dysconnection hypothesis” [2]; [3] posits that schizophrenia arises from deficits in functional brain integration. These disconnections have been explained by multiple biological, genetic, and computational models [4]; [5]; [6]; [7]. Dysconnectivity stemming from genetic, neural, and cellular levels is most likely responsible for positive and negative schizophrenia symptoms [8]; [9]. In addition to disruptions at these levels, the origins of schizophrenia as a disrupted neurodevelopmental process were reviewed by Andreasen et al. [10]. These neurodevelopmental delays are hypothesized to cascade to more specific neural circuitry deficits. Consequences of disrupted circuitry in schizophrenia include reduced modularity and disrupted networks, e.g., frontoparietal-cingulo opercular (FP-CO) [11]. Satterthwaite and colleagues also noted in their comprehensive review that the origins of psychosis may stem from a developmental delay, affecting the development and refinement of cortical association systems. Additional explanations regarding a neurodevelopmental model include impaired metabolic activity due to oxidative stress [12]. Thus, it is essential to contextualize widespread patterns of hypo/hyper-connectivity related to dysregulated behavior concerning biological and neurodevelopmental models. No review paper has identified the most salient models highlighting dysconnectivity trends in the last five years.
Given these gaps in the literature, the goals of this review paper are to 1) review the functional connectivity literature in schizophrenia related to various hypotheses of causation, 2) delve into related hypo vs. hyperconnectivity patterns in specific networks, and 3) identify which models best explain these connectivity findings to behavioral symptoms (e.g., executive dysfunction, positive and negative symptoms).
2. Literature Search Process
We performed initial keyword searches focused on fMRI and resting state studies in schizophrenia, yielding over 20,000 articles (keywords: “schizophrenia,” “dysconnectivity,” “resting state,” “fMRI”). To narrow the scope of the review, we limited papers to 2017–2023, with occasional citations outside of this time range. We restricted the searches to be more succinct, based on keywords falling into six domains: 1) serotonin, 2) dopamine, 3) connectivity & neuromediators, 4) dysconnectivity, 5) salience, and 6) effective connectivity. For each specific brain network (e.g., cerebello-thalamo-cortical, triple networks), additional keywords such as cerebello-thalamo-cortical, thalamo-cortical, cerebellar, cortical-subcortical-cerebellar, white matter, default mode network, salience network, glutamate, neurodevelopmental, neurotransmitter, dopamine, vulnerability, stress, and serotonin were added to reference specific large scale brain networks and the models. Functional connectivity was defined as correlated blood oxygenation-level dependent (BOLD) functional magnetic resonance imaging (fMRI) signals between brain regions. Searches were conducted on both PubMed and Google Scholar. Studies unrelated to schizophrenia and fMRI and studies falling outside of these six domains were subsequently excluded (see Figure 1). The few animal studies that were included serve as translational templates for future genetic research that could aid in understanding patterns of dysconnectivity. 63 papers were included in this review, listed in the references. These were selected based on A.) topic fit, B.) mentioned at least one of the three models of interest, or C.) included one or more of the two large-scale functional connectivity brain networks. See Figure 1 for a flowchart of the process.
Figure 1.
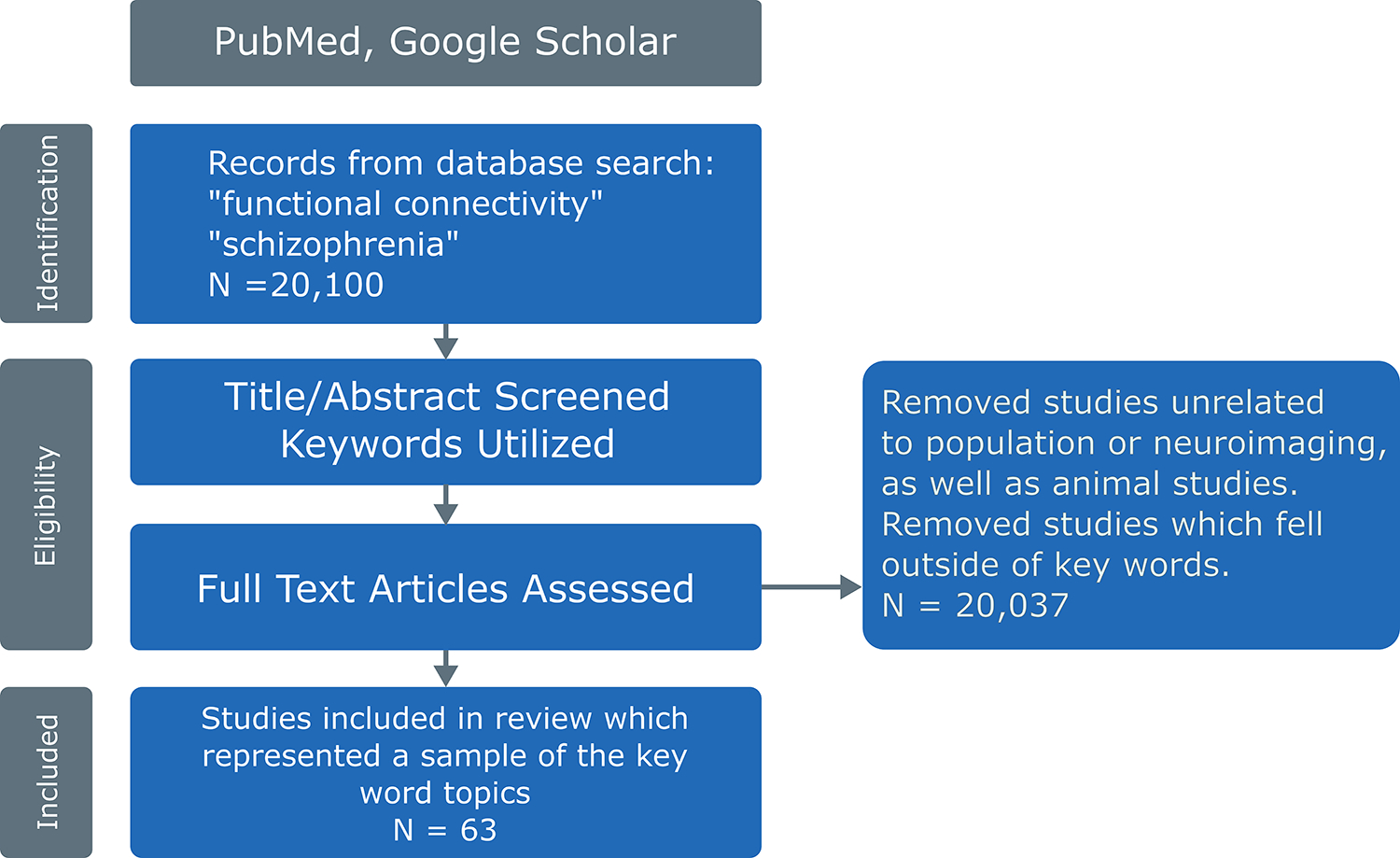
A flowchart explaining the literature review process. The initial search performed on PubMed and Google Scholar yielded over 20,000 papers. After removing studies outside our keyword search, 63 articles were included in this review.
3. Models of Interest
Ľupták and colleagues [13••] recently outlined several models of schizophrenia, which guided the focus of this paper. We concentrate on the genetic, neurodevelopmental, vulnerability-stress-inflammation, and neurotransmitter (dopamine, glutamate, and serotonin) dysfunction models. Given that models focusing on neurodevelopmental delays and neurotransmitter dysfunctions have featured currently and historically in the neuroimaging literature, we decided to take these models from Ľupták’s paper to examine the literature through this lens critically. We focus mainly on those models and the evidence from dysconnectivity studies among two large-scale sets of brain networks: A) Cerebello-Thalamo-cortical / Subcortical, and B) the Triple Network (comprised of the Salience, Default Mode Network, Central Executive Networks). Finally, we discuss the model that best explains schizophrenia behavioral symptoms based on aberrant connectivity patterns. The hope is that this paper will aid schizophrenia researchers in picking the best models to help guide future neuroimaging research.
3.1. Measuring Sources of Dysconnectivity in Schizophrenia
While this review paper has focused exclusively on understanding networks in the context of various models and theories, it is essential to touch on multiple techniques utilized to measure dysconnectivity patterns. Functional MRI has been around for over 20 years, with the primary goal of resting state fMRI being to measure the changes in blood oxygen level dependent (BOLD) signal at rest [14]. While diverse imaging methods have been employed to study schizophrenia (see Keshavan et al. [15] for a comprehensive review), questions remain concerning accurately classifying and predicting symptoms systematically. Here, dimension reduction techniques such as independent components analysis, or ICA, have played an essential role in helping identify and characterize schizophrenia related patterns of functional connectivity. Group ICA is a widely used technique in which components can be extracted from brain networks [16, 17]. Additional ICA techniques (e.g., multiscale ICA, which investigates functional sources at multiple spatial scales) have found sex-specific differences in schizophrenia [18] as well as increased predictive ability in identifying FNC between spatial scales in schizophrenia and healthy controls [19]. The use of ICA has demonstrated overall classification accuracy using large-scale FNC data in schizophrenia [20] and successfully distinguished between schizophrenia, bipolar disorder, and schizoaffective disorder using resting state fMRI networks alone [21]. Additional techniques such as graph theory, decomposition (see [22] for a review of different imaging methods), as well as seed-based techniques [23] have been used to understand disrupted resting state networks.
4. Dysconnectivity Across Large-Scale Brain Networks: Cerebello-Thalamo-cortical Connectivity (CTCC) and Subcortical Dysconnectivity
4.1. CTCC Overview
We broadly investigated Friston’s dysconnectivity hypothesis [3] of schizophrenia across large-scale brain networks, the first being the cerebello-thalamo-cortical and subcortical networks. Consistent with Friston’s conjecture, exploration of thalamo-cortical hypoconnectivity revealed a network of prefrontal and thalamic regions linked to motor control and executive functioning deficits. We summarize the recent literature relating these networks to various symptoms and models in schizophrenia.
4.2. CTCC Network: Motor Deficits, Symptom Severity, and Cortical Connectivity Trends
Dysconnectivity between the motor cortices and cerebellar areas has been noted as a typical pattern in schizophrenia. However, schizophrenia has been characterized as a series of hyperconnectivity links from the motor cortices -> thalamus ->; motor cortices -> cerebellum ->, as well as links to the prefrontal cortex -> subthalamic nucleus [24]. In their study, Walther and colleagues found that connectivity between the motor cortex and cerebellum was significantly correlated to spontaneous motor activity [24]. The main conclusions to note were increased connectivity in these three linkages, and a significant correlation was found between thalamo-cortical hyperconnectivity and motor dysfunction in individuals with schizophrenia.
Increased resting state connectivity between the cerebellum and other brain areas has been noted after analyzing inter-regional correlations and functional integration of widespread brain regions [25]. This review also noted decreased connectivity among the insula, temporal lobe, prefrontal cortex, and basal ganglia. Clark and colleagues reported that when comparing schizophrenia patients to healthy controls (HCs), HCs showed more robust connectivity than patients between several cerebellar lobules and cortical regions; specifically, these relationships were strong between motor-related cerebellar lobules (V and VIIIa/b) as well as the temporal and parietal cortices [26•]. Notably, Clark et al. found that disrupted cerebellocortical connectivity was chiefly associated with reduced processing speed in individuals with schizophrenia. In addition to reduced processing speed, Anticevic et al. [27] noted thalamic hyperconnectivity noted with bilateral sensory-motor cortices; however, thalamic hypoconnectivity was linked to prefrontal-striatal-cerebellar regions compared to healthy controls. These were reported to be influenced by sensory gating and other factors.
Additional findings noted that thalamic hypoconnectivity was coupled with prefrontal–striatal–cerebellar regions relative to controls [28]. In addition to this hypoconnectivity, hyperconnections were identified in sensory regions (i.e., motor, somatosensory, temporal, occipital, and insular cortical regions). Patients had greater thalamic connectivity with multiple sensory-motor areas, including bilateral pre- and post-central gyri, middle/inferior occipital gyrus, and middle/superior temporal gyrus [29]. Thalamus-to-middle temporal gyrus connectivity correlated positively with hallucinations and delusions, while thalamus-to-cerebellar connectivity negatively correlated with delusions and bizarre behavior [29]. The strong negative relationship between these thalamo-cortical deflections suggests that they share a common mechanism and may account for some psychosis symptoms. Taken together, these results suggest patterns of both hypo and hyperconnectivity through the thalamus and its connections across regions in the cortex, and cerebellum have a relationship with both positive and negative psychotic symptoms.
4.4. CTCC: Neurodevelopmental Model
The neurodevelopmental model is particularly salient to thalamo-cortical dysconnectivity, as multiple aberrant connectivity patterns emerge when adolescent brains develop toward adulthood. Giraldo-Chica et al. [5] systematically reviewed thalamo-cortical dysconnectivity in schizophrenia, noting that thalamo-cortical network dysconnectivity might arise from atypical neurodevelopment between childhood and adolescence [5]. Specifically, studies have shown that abnormal brain maturation combined with thalamo-cortical network dysfunction (e.g., reduced PFC-thalamic connectivity increased somatomotor connectivity) contributed to atypical development in childhood and adolescence [11]. Benoit et al. [30•] concluded that disrupted thalamo-cortical connectivity development observed in youth coincided with early psychotic symptom onset and increases in symptom severity, which also has implications for disrupted prefrontal cortex (PFC) functioning. In addition to these critical linkages between CTCC regions, Bernard et al. [31] noted that cerebello-thalamo-cortical network connectivity was linked to positive symptoms in schizophrenia. Specifically, the team pointed out that the neurodevelopmental model was the best model to explain links between disrupted cerebello-thalamo-cortical networks chiefly associated with positive symptoms. In addition to positive symptoms, Bernard et al. noted relationships between motor-striatal connectivity in addition to increased connectivity between the primary motor cortex and basal ganglia were chiefly associated with negative symptoms [32]. In summary, the neurodevelopmental model seems to be a predominant model seen in the current literature to explain disrupted thalamo-cortical dysfunction in schizophrenia. Both positive and negative symptoms are strongly tied to dysconnectivity present in different CTCC regions.
5. Triple Network Dysconnectivity
5.1. Triple Network Overview
The triple network includes the Central Executive Network (CEN), the Default Mode Network (DMN), and the Salience Network (SN), with their interactions proposed to be deficient in schizophrenia [33, 34]. The CEN is the specifically lateral frontoparietal network (L-FPN), primarily composed of the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex. It is involved in sustained attention, complex problem-solving and working memory. The bilateral frontoparietal network (FPN) more broadly serves as an important functional module specific to executive control, e.g., [35]. The DMN is composed primarily of medial prefrontal cortex, posterior cingulate cortex/precuneus and angular gyrus, as well as the inferior and mesial temporal lobe [36]. The SN is primarily anchored in anterior insula (AI) and dorsal anterior cingulate cortex (dACC), but also includes rostral prefrontal cortex (PFC), supramarginal gyrus (SMG) and subcortical limbic areas (striatum, thalamus and amygdala); it facilitates switching between the FPN and DMN [37].
The triple network has been defined as these three essential networks that are considered brain “hubs” for perceptual, emotional, and behavioral processing [33] as well as introspection, theory of mind (TOM), and self-awareness. Studies suggest that SN, FPN, and DMN dysconnectivity plays an important role in the cognitive impairment seen in schizophrenia [38] with some studies indicating the SN, the CEN, and the DMN as “the holy trinity” as outlined in [33].
Triple network aberrations have been notably found in schizophrenia (see comprehensive review by Menon et al.) [33]. Jiang et al. [39] compared individuals with depression and schizophrenia, showing that dysconnectivity patterns differed between the two groups; DMN-CEN connectivity was increased in schizophrenia while CEN-SN connectivity was decreased in both groups. This differing pattern was thought to be linked to self-referential processing in both the depression and schizophrenia patient groups. Neuroimaging studies comparing fractional amplitude of low frequency fluctuations (fALFF), regional homogeneity (ReHo) and FC techniques have notably found that overall, fALFF in the central executive network (CEN) was increased in drug naïve first episode schizophrenia (dn-FES) patients and ultra-high risk groups of psychosis compared to healthy controls [40]. However, a limitation of this finding was difficulty distinguishing the dn-FES group from the ultra-high-risk group based on fMRI data, which needs to be explored in greater detail.
In conjunction with trends towards hypoconnectivity, hyperconnectivity trends have also been reported, for example right ITG and MTG, SN and right precentral gyrus [41], SN and cortico-cerebellar sub-circuit and prefrontal cortex [42], within the lateral part of the left middle occipital gyrus [43], and within the frontoparietal modular region [44]. Furthermore, distinctive features of schizophrenia patients include the presence of amygdala (AMY)-SPL (superior parietal lobule) and AI-AMY connections as detailed in effective connectivity studies [45]. [45] also noted that dysfunctions in self-regulation of the salience network may underlie some of the features of dysconnectivity seen in schizophrenia. The most pronounced changes are observed in both intra-network and inter-network connections of the SN. Huang and colleagues showed that first-episode schizophrenia (FES) was associated with reduced functional connectivity within cortico-striatal-thalamic-cortical (CSTC) sub-circuit of the SN [41]. In general, schizophrenia patients tend to show reduced functional connectivity between distinct networks but not within-networks [46]. Additional psychosis related alterations have been examined in first episode groups compared to healthy controls with Sarpal and colleagues [47] indicating lower background connectivity (i.e. removal of task effects) between the DLPFC and the bilateral superior parietal lobule (SPL) and left inferior parietal lobule [47]. Results of this study showed intact frontoparietal connectivity, but impaired cognitive functioning when participants had to perform a cognitive task. Thus, it is clear that patients with schizophrenia demonstrate impaired cognitive functioning (i.e. executive functioning deficits) that correlate with deficits broadly in various triple network regions. Taken together, these studies demonstrate the importance of further examining salience network and DLPFC dysconnectivity in conjunction with social cognition, self-referential processing, and executive functioning deficits.
5.2. Triple Network, Symptom Severity and Cognitive Associations
Triple network dysconnectivity has been hypothesized to play an essential role in symptoms including executive functioning deficits, negative symptoms, and abnormal salience and mood states [33]. One effective connectivity measuring approach is dynamic causal modeling (DCM), which shows how coupling among brain regions changes over time [48]. Evidence from DCM dysregulation across the three networks suggested that dysconnectivity between the DMN-CEN was a critical potential biomarker [49]. Specifically, Rodriguez et al. [50] teased apart cognitive profiles of first episode schizophrenia based on a cluster analysis correlated with clinical variables, and resting state fMRI data. [50]. They found that for Cluster 1, hyperconnectivity between the cerebellum-precentral gyrus, SN-insula cortex, and reduced connectivity between the DMN-SN were noted. For Cluster 2, hyperconnectivity between the DMN- cerebellum, SN-precentral gyrus, and FPN-IFG were found; finally, hypoconnectivity between the DMN-insula and SN-pallidum were noted for Cluster 3. Taken together, distinct cognitive profiles were noted with Cluster 1 being linked to decline in attention, working memory/flexibility, and verbal memory deficits. Cluster 2 was linked to decline in verbal memory and increased performance in the attention domain. Finally, Cluster 3 was linked to severe deficits in all cognitive domains. Additional studies by Xu et al. [51] also noted relationships between disrupted intrinsic connectivity in midbrain dopaminergic regions, specifically finding a link between social amotivation and a negative relationship with functional connectivity in key regions. These regions included the substantia niagra, ventral tegmental area, medial-and lateral prefrontal cortex, temporoparietal junction, and dorsal and ventral striatum. In summary, decreased connectivity of dopaminergic areas was chiefly associated with social amotivation, and these brain regions were linked with reward, social and executive functioning [51]. Thus, a constellation of positive and negative symptoms are associated with dysconnectivity in a variety of brain regions spanning the triple network and other areas.
Wylie and colleagues [52] measured working memory using the MATRICS Consensus Cognitive Battery (MCCB), to assess the dysfunction of various components of working memory in relationship to intrinsic connectivity networks measured using ICA. Overall, the study found a strong relationship between working memory task performance and functional connectivity, including both voxel-wise global connectivity measures across frontal, temporal, and parietal regions, and the connectivity of the ICA networks identified with the CEN to these inferior frontal and parietal regions.
In addition to disruptions in cognitive and attentional functioning, additional triple network studies have also noted links to hallucinatory symptoms with triple network dysfunction. For example, compared to healthy controls, patients with visual hallucinations had lower functional connectivity in all vision-related networks, both intra-network and inter-network. This decrease was most prominent for intra-network functional connectivity in the Ventral Attention Network (VAN; that has also been equated with the SN) and the Dorsal Attention Network [43]. Hallucinations have been suggested to stem from a selection mechanism, where sensory representations become chosen in an atypical fashion (compared to healthy controls) rather than being filtered out [53]. Indeed, the SN-DMN connectivity pattern has also been demonstrated as a critical network linked to positive and negative symptoms [54]. Specifically, Hare and colleagues showed that SN-DMN FC was associated with key symptoms such as disordered thought and attentional deficits in schizophrenia, even after participants were controlled for confounding effects of medication. Kandilarova et al. [45] showed that measures of overall schizophrenia symptom severity correlated with reduced connectivity between the anterior insula and the inferior frontal gyrus. Specific network alterations, particularly related to self-inhibitory connection of the anterior insula was noted as a dysconnectivity pattern [45].
5.3. Triple Network: Neurotransmitter, Vulnerability/Stress, and Glutamate Hypothesis Theories
The triple network has been associated with multiple theories, including the neurotransmitter, vulnerability/stress and glutamate hypotheses. McCutcheon et al. [9] noted in a relatively recent review that environmental and genetic risk factors such as disrupted neurodevelopment led to increased risk of prodromal symptomatology; and cortical excitatory-inhibitory imbalance poses individuals to be at risk for developing both cognitive and negative symptoms of schizophrenia [9]. Finally, a combination of psychosocial, psychological stressors in combination with dopamine dysfunction in subcortical regions may play a significant role in the onset of positive symptoms of schizophrenia [9]. Taken together, these findings demonstrate a close relationship between the functions of the salience network and mesolimbic dopamine system [9]. In the serotonergic pathway, 5-HT signaling through the raphe nuclei related pathways was associated with decreased sensorimotor network activity and increased DMN activity [55]. Han et al. [56] confirmed decreased functional connectivity of bilateral striatum, pallidum, and thalamus with the salience network and raphe nucleus [56] which also correlated with total negative scores on the Positive and Negative Syndrome Scale (PANSS). Decreased functional connectivity was also shown between SN and left caudate in schizophrenia patients [42]. Moreover, the FPN–SN connection exhibited aberrant age-associated alteration in adolescent-onset schizophrenia (AOS) [57]. Maximo et al. [58•] investigated the role of glutamatergic metabolism related to SN dysconnectivity in first-episode psychosis (FEP) subjects, reporting complex neurotransmitters related between group connectivity differences [58]. Specifically, they found that when measuring glutamate and glutamine (Glx) in the dorsal anterior cingulate cortex (dACC) that positive FC in the SN was found to be disrupted in medication naïve first episode patients compared to healthy controls. Shukla and colleagues also speculated that GABA and glutamate relationships may modulate the reduced FC of key DMN regions in schizophrenia (right posterior cingulate cortex, precuneus, bilateral anterior cingulate, parahippocampal gyrus, and medial frontal gyrus) [59]. In summary, a complex cocktail of environmental, biological, and neurotransmitter dysfunctions cascade into dysconnectivity disruptions that adversely affect triple network function. These cascading effects seem to have a critical role in the development of both positive and negative symptoms.
6. Summary and Conclusions
We summarize the overall findings across these networks in Figure 2. We noted that the CTCC dysconnectivity was chiefly associated with the neurodevelopmental model (e.g. atypical development that occurred during childhood and adolescence) supporting patterns of abnormal connectivity in the CTCC network. Although the CTCC network includes all cortical regions and the cerebellum, we chose to focus on organization from the cerebellum to the thalamus and on thalamo-cortical connectivity. The neurotransmitter model was associated as well with CTCC-subcortical dysfunction. Behavioral deficits associated with this network included motor symptoms and the positive and negative symptoms of schizophrenia.
Figure 2.
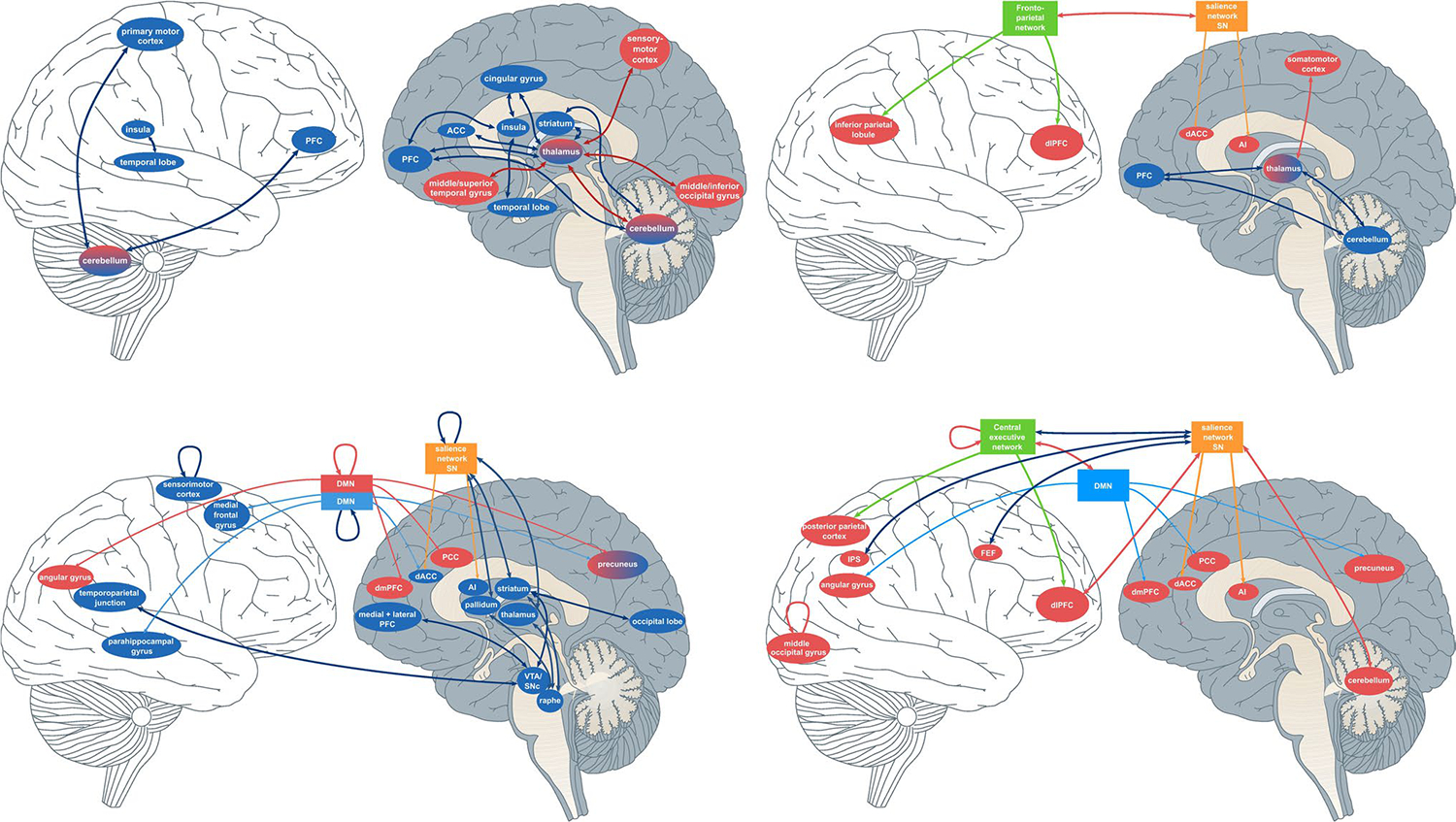
A graphical summary demonstrating the interplay of the different brain regions and networks associated with FC connections in patients with schizophrenia. Blue arrows indicate decreased functional relationships in schizophrenia vs. healthy controls; red arrows indicate functional relationships that increase in patients vs. healthy controls. In summary, a complex “highway” of brain regions such as various sensorimotor, thalamic, and prefrontal areas that moderate behavioral and cognitive symptoms seen in schizophrenia. From L-R: 1.) Left top: a graphical summary of the cerebello-thalamo-cortical and subcortical dysconnectivity. 2.) Right top: Neurodevelopmental model 3.) Bottom left: Neurotransmitter model. Bottom right: 4.) Triple Network dysconnectivity represented graphically.
Next, we investigated the triple network for various links between dysconnectivity, behavioral deficits and symptoms. SN-DMN dysconnectivity was tied specifically to hallucinations and symptom severity in schizophrenia. Links between disrupted cognitive control, executive functioning, and attention were noted with disruptions in the triple network. Consequently, a mix of environmental, biological, and neurotransmitter related dysfunctions were noted to contribute to the development of positive and negative symptoms in schizophrenia.
7. Limitations
As demonstrated in Figure 1, many thousands of papers on neuroimaging and schizophrenia have been published in recent years. The scope of this review was on recent resting state fMRI connectivity studies and their findings considering two primary networks. The CTCC focuses on cerebello-thalamic and thalamo-cortical networks and their relationship; the triple network framework focuses on a subset of cortico-cortico connections. Many papers which focused on individual regions or analyses which did not consider connectivity measures were outside the scope of this review.
Similarly, there are many other considerations in the neural deficits in schizophrenia. We touched on environmental stressors [9], and there are many considerations of genetic risk both from individual genetic loci or combined genetic effects that can lead to neural circuitry deficits with disruptions that occur in early brain development [7]. Recent approaches that combine neuroimaging connectivity measures with post-mortem genetic expression atlases have shown that expression profiles of SZ risk genes within the cortex significantly correlate with known dysconnectivity patterns in SZ, for example [60], and the potential for these approaches to examine trajectories of disease progression in dysconnectivity patterns is becoming clear. Thus, a single model of dysconnection is less explanatory than one integrating genetic (specifically Genome-Wide Association Studies (GWAS)), neurotransmitter, and neurodevelopmental models. Additionally, no papers in the literature search yielded links between the neurotransmitter model and the CTCC network. Thus, more research should be conducted to broadly investigate potential links in this area.
It is also important to note that medication effects are commonly confounded with disease effects in schizophrenia studies, including those examined here. As shown in the Supplementary Table 1, 16 out of 42 papers included only medicated subjects. However, in these papers, the clinical high risk and first episode studies complement the studies of medicated individuals, without identifying fundamentally different patterns of deficits. Additionally, while the CTCC and triple networks were examined for this paper, several other networks disrupted in schizophrenia (i.e., Theory of Mind (TOM) could be further examined to understand future clinical interventions and treatment. [61, 62]. Consequently, a comprehensive review of Positron Emission Tomography (PET) studies to understand neurotransmitter deficits about dysconnectivity would also be helpful. While a few studies understanding neurotransmitter imbalances were noted in the literature [58, 59, 63], a more comprehensive review of PET studies tied to patterns of dysconnectivity across various brain regions in schizophrenia is needed.
In conclusion, the findings of this review demonstrate the need to incorporate a translational, multidisciplinary approach to schizophrenia research. Many gaps remain when attempting to understand the origins of dysconnectivity. Understanding these gaps will be particularly useful as research expands into investigating global patterns of dysconnectivity both in schizophrenia, and with other linked, comorbid disorders.
Supplementary Material
Table 1.
Summarizing key findings for the CTCC network. See supplementary table for a full list of papers
| Paper Citations | Summary of Findings | Model or Theory |
|---|---|---|
|
| ||
| Walther et al.; Calhoun 2009; Clark et al., 2020; Anticevic et al. 2014; Ramsay, 2019; Ferri et al., 2018 | Overall, these papers found various results related to resting state functional connectivity within CTCC nework in schizophrenia. They correlate hyper/hypoconnectivity and less robust connections with motor dysfunctions and schizophrenia symptoms. | N/A |
| Benoit et al., 2022; Bernard et al., 2017a, 2017b | The development and connectivity of the cerebello-thalamo-cortical network at baseline are linked to a positive symptoms in schizophrenia, indicating that these cerebellar networks could serve as a biomarker for the progression of the disease. | Neurodevelopmental |
Table 2.
Summarizing key findings for the Triple network. See supplementary table for a full list of papers
| Paper Citations | Summary of Findings | Model or Theory |
|---|---|---|
|
| ||
| Menon, 2019; Jiang et al., 2017; Ma et al., 2023; Huang et la., 2020; Huang et al., 2022; van Ommen et al., 2022; Xiang et al., 2019; Kandilarova et al., 2021; Hummer et al., 2020; Sarpal et al., 2022 | Disconnectivity between Salience Network, Central Executive Network and Default Mode Network is related to cognitive impairments in schizophrenia. Connectivity within SN, CEN and DMN networks is usually increased, however the connections between them are decreased. | N/A |
| van Ommen et al., 2022; Kandilarova et al., 2021; Xi et al., 2021; Rodriguez et al., 2019; Xu et al., 2019; Hare, 2021; Hare et al., 2019 | Triple network disconnectivity and aberrant patterns between and within networks are associated with schizophrenia negative and positive symptoms. | N/A |
| McCutcheon et al, 2020; Huang et al., 2022; Conio et al., 2020; Han et al., 2020; Fan et al., 2021; Maximo et al., 2021 | Mesolimbic dopamine system, serotonergic pathways, glutamatergic metabolism and GABA may modulate functional disconnectivities and that are associated with particular schizophrenia symptoms. | Neurotransmitter Theory |
| McCutcheon et al, 2020 | The interplay of psychosocial and psychological stressors, coupled with dopamine dysfunction in subcortical regions, may significantly contribute to the emergence of positive symptoms in schizophrenia. | Vulnerability/Stress Model |
Funding Sources
This work was supported by the National Institute of Health (NIH) grants R01MH129047 and partly by NSF 2112455 and NIH 2R01EB006841. Amritha Harikumar is currently supported by the Georgia State University Brains and Behavior Fellowship.
Footnotes
Statements and Declarations
The authors do not have competing disclosures or conflicts of interest to report.
Human/Animal Rights and Informed Consent Statement
This paper is a review paper and reviews studies that obtained informed consent.
References
- 1.Stephan KE, Friston KJ, Frith CD (2009) Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-monitoring. Schizophr Bull 35:509–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friston KJ (1998) The disconnection hypothesis. Schizophr Res 30:115–125 [DOI] [PubMed] [Google Scholar]
- 3.Friston K, Brown HR, Siemerkus J, Stephan KE (2016) The dysconnection hypothesis (2016). Schizophr Res 176:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limongi R, Jeon P, Mackinley M, Das T, Dempster K, Théberge J, Bartha R, Wong D, Palaniyappan L (2020) Glutamate and Dysconnection in the Salience Network: Neurochemical, Effective Connectivity, and Computational Evidence in Schizophrenia. Biol Psychiatry 88:273–281 [DOI] [PubMed] [Google Scholar]
- 5.Giraldo-Chica M, Woodward ND (2017) Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res 180:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraguljac NV, Frölich MA, Tran S, White DM, Nichols N, Barton-McArdle A, Reid MA, Bolding MS, Lahti AC (2017) Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry 22:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnbaum R, Weinberger DR (2017) Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci 18:727–740 [DOI] [PubMed] [Google Scholar]
- 8.Correll CU, Schooler NR (2020) Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr Dis Treat Volume 16:519–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCutcheon RA, Reis Marques T, Howes OD (2020) Schizophrenia—An Overview. JAMA Psychiatry 77:201. [DOI] [PubMed] [Google Scholar]
- 10.Andreasen NC (2010) The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues Clin Neurosci 12:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satterthwaite TD, Baker JT (2015) How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol 30:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier M, Ferrari C, Baumann PS, et al. (2014) Impaired Metabolic Reactivity to Oxidative Stress in Early Psychosis Patients. Schizophr Bull 40:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ľupták M, Michaličková D, Fišar Z, Kitzlerová E, Hroudová J (2021) Novel approaches in schizophrenia-from risk factors and hypotheses to novel drug targets. World J Psychiatry 11:277–296 **This is a seminal paper from which our review adopted the models. Various models including biomarkers of schizophrenia are discussed here.
- 14.Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med 34:537–41 [DOI] [PubMed] [Google Scholar]
- 15.Keshavan MS, Collin G, Guimond S, Kelly S, Prasad KM, Lizano P (2020) Neuroimaging in Schizophrenia. Neuroimaging Clin N Am 30:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salman MS, Du Y, Lin D, et al. (2019) Group ICA for identifying biomarkers in schizophrenia: ‘Adaptive’ networks via spatially constrained ICA show more sensitivity to group differences than spatio-temporal regression. NeuroImage Clin 22:101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calhoun VD, Liu J, Adalı T (2009) A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage 45:S163–S172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iraji A, Faghiri A, Fu Z, et al. (2022) Multi-spatial-scale dynamic interactions between functional sources reveal sex-specific changes in schizophrenia. Netw Neurosci 6:357–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X, Iraji A, Fu Z, et al. (2022) Multi-model order spatially constrained ICA reveals highly replicable group differences and consistent predictive results from fMRI data. 10.1101/2022.11.02.514809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassanzadeh R, Abrol A, Calhoun V (2022) Classification of Schizophrenia and Alzheimer’s Disease using Resting-State Functional Network Connectivity. In: 2022 IEEE-EMBS Int. Conf. Biomed. Health Inform. BHI. IEEE, Ioannina, Greece, pp 01–04 [Google Scholar]
- 21.Du Y, Pearlson GD, Liu J, Sui J, Yu Q, He H, Castro E, Calhoun VD (2015) A group ICA based framework for evaluating resting fMRI markers when disease categories are unclear: application to schizophrenia, bipolar, and schizoaffective disorders. NeuroImage 122:272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahhosseini Y, Miranda MF (2022) Functional Connectivity Methods and Their Applications in fMRI Data. Entropy 24:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Q, Allen EA, Sui J, Arbabshirani MR, Calhoun VD (2015) Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV (2017) Aberrant Hyperconnectivity in the Motor System at Rest Is Linked to Motor Abnormalities in Schizophrenia Spectrum Disorders. Schizophr Bull 43:982–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calhoun VD (2009) Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 10.3389/neuro.09.017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark SV, Tannahill A, Calhoun VD, Bernard JA, Bustillo J, Turner JA (2020) Weaker Cerebellocortical Connectivity Within Sensorimotor and Executive Networks in Schizophrenia Compared to Healthy Controls: Relationships with Processing Speed. Brain Connect 10:490–503 *This paper discusses the link between reduced processing speed and cerebello-cortical connectivity in schizophrenia. We deemed it important given it is a timely paper with salient findings tying together cognitive deficits and cerebello-cortical connectivity.
- 27.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC (2014) Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cereb Cortex 24:3116–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsay IS (2019) An Activation Likelihood Estimate Meta-analysis of Thalamocortical Dysconnectivity in Psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging 4:859–869 [DOI] [PubMed] [Google Scholar]
- 29.Ferri J, Ford JM, Roach BJ, et al. (2018) Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med 48:2492–2499 [DOI] [PubMed] [Google Scholar]
- 30. Benoit LJ, Canetta S, Kellendonk C (2022) Thalamocortical Development: A Neurodevelopmental Framework for Schizophrenia. Biol Psychiatry 92:491–500 * This paper is an important and timely paper that discusses in great detail how the neurodevelopmental framework/model best explains thalamocortical development. We found this to be a crucial paper in explaining the neurodevelopmental deficits in frontal and thalamic dysfunction.
- 31.Bernard JA, Orr JM, Mittal VA (2017) Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. NeuroImage Clin 14:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard JA, Goen JRM, Maldonado T (2017) A case for motor network contributions to schizophrenia symptoms: Evidence from resting-state connectivity: Motor Networks and Schizophrenia. Hum Brain Mapp 38:4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon B (2019) Towards a new model of understanding – The triple network, psychopathology and the structure of the mind. Med Hypotheses 133:109385. [DOI] [PubMed] [Google Scholar]
- 34.Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506 [DOI] [PubMed] [Google Scholar]
- 35.Tu P-C, Lee Y-C, Chen Y-S, Li C-T, Su T-P (2013) Schizophrenia and the brain’s control network: Aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res 147:339–347 [DOI] [PubMed] [Google Scholar]
- 36.Hu M-L, Zong X-F, Mann JJ, Zheng J-J, Liao Y-H, Li Z-C, He Y, Chen X-G, Tang J-S (2017) A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci Bull 33:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sridharan D, Levitin DJ, Menon V (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci 105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neill A, Mechelli A, Bhattacharyya S (2019) Dysconnectivity of Large-Scale Functional Networks in Early Psychosis: A Meta-analysis. Schizophr Bull 45:579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y, Duan M, Chen X, Chang X, He H, Li Y, Luo C, Yao D (2017) Common and distinct dysfunctional patterns contribute to triple network model in schizophrenia and depression: A preliminary study. Prog Neuropsychopharmacol Biol Psychiatry 79:302–310 [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Yang WFZ, Zheng W, et al. (2023) Neuronal dysfunction in individuals at early stage of schizophrenia, A resting-state fMRI study. Psychiatry Res 322:115123. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Botao Z, Jiang Y, et al. (2020) Aberrant resting-state functional connectivity of salience network in first-episode schizophrenia. Brain Imaging Behav 14:1350–1360 [DOI] [PubMed] [Google Scholar]
- 42.Huang H, Chen C, Rong B, Wan Q, Chen J, Liu Z, Zhou Y, Wang G, Wang H (2022) Resting-state functional connectivity of salience network in schizophrenia and depression. Sci Rep 12:11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Ommen M, Invernizzi A, Renken RJ, Bruggeman R, Cornelissen FW, van Laar T (2022) Impaired functional connectivity in patients with psychosis and visual hallucinations. 10.1101/2022.05.06.22274666 [DOI] [Google Scholar]
- 44.Xiang Q, Xu J, Wang Y, et al. (2019) Modular Functional-Metabolic Coupling Alterations of Frontoparietal Network in Schizophrenia Patients. Front Neurosci 13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandilarova S, Stoyanov DSt, Paunova R, Todeva-Radneva A, Aryutova K, Maes M (2021) Effective Connectivity between Major Nodes of the Limbic System, Salience and Frontoparietal Networks Differentiates Schizophrenia and Mood Disorders from Healthy Controls. J Pers Med 11:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hummer TA, Yung MG, Goñi J, Conroy SK, Francis MM, Mehdiyoun NF, Breier A (2020) Functional network connectivity in early-stage schizophrenia. Schizophr Res 218:107–115 [DOI] [PubMed] [Google Scholar]
- 47.Sarpal DK, Tarcijonas G, Calabro FJ, Foran W, Haas GL, Luna B, Murty VP (2022) Context-specific abnormalities of the central executive network in first-episode psychosis: relationship with cognition. Psychol Med 52:2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friston KJ, Harrison L, Penny W (2003) Dynamic causal modelling. NeuroImage 19:1273–1302 [DOI] [PubMed] [Google Scholar]
- 49.Xi Y-B, Guo F, Liu W-M, et al. (2021) Triple network hypothesis-related disrupted connections in schizophrenia: A spectral dynamic causal modeling analysis with functional magnetic resonance imaging. Schizophr Res 233:89–96 [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez M, Zaytseva Y, Cvrčková A, et al. (2019) Cognitive Profiles and Functional Connectivity in First-Episode Schizophrenia Spectrum Disorders – Linking Behavioral and Neuronal Data. Front Psychol 10:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu P, Klaasen NG, Opmeer EM, Pijnenborg GHM, van Tol M-J, Liemburg EJ, Aleman A (2019) Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophr Res 208:353–359 [DOI] [PubMed] [Google Scholar]
- 52.Wylie KP, Harris JG, Ghosh D, Olincy A, Tregellas JR (2019) Association of Working Memory With Distributed Executive Control Networks in Schizophrenia. J Neuropsychiatry Clin Neurosci 31:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hare SM (2021) Hallucinations: A Functional Network Model of How Sensory Representations Become Selected for Conscious Awareness in Schizophrenia. Front Neurosci 15:733038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hare SM, Ford JM, Mathalon DH, et al. (2019) Salience–Default Mode Functional Network Connectivity Linked to Positive and Negative Symptoms of Schizophrenia. Schizophr Bull 45:892–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conio B, Martino M, Magioncalda P, Escelsior A, Inglese M, Amore M, Northoff G (2020) Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders. Mol Psychiatry 25:82–93 [DOI] [PubMed] [Google Scholar]
- 56.Han S, Cui Q, Guo X, Fan Y-S, Guo J, Zong X, Hu M, Lu F, Chen X, Chen H (2020) Disconnectivity between the raphe nucleus and subcortical dopamine-related regions contributes altered salience network in schizophrenia. Schizophr Res 216:382–388 [DOI] [PubMed] [Google Scholar]
- 57.Fan Y, Li L, Peng Y, et al. (2021) Individual-specific functional connectome biomarkers predict schizophrenia positive symptoms during adolescent brain maturation. Hum Brain Mapp 42:1475–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maximo JO, Briend F, Armstrong WP, Kraguljac NV, Lahti AC (2021) Salience network glutamate and brain connectivity in medication-naïve first episode patients – A multimodal magnetic resonance spectroscopy and resting state functional connectivity MRI study. NeuroImage Clin 32:102845. *This was an interesting study that linked neurotransmitter functioning to functional connectivity; we found this study to be particular interesting given that dysconnectivity was noted in medication naive patients, and few studies examined neurotrasmitter links + functional connectivity.
- 59.Shukla DK, Wijtenburg SA, Chen H, Chiappelli JJ, Kochunov P, Hong LE, Rowland LM (2019) Anterior Cingulate Glutamate and GABA Associations on Functional Connectivity in Schizophrenia. Schizophr Bull 45:647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romme IAC, De Reus MA, Ophoff RA, Kahn RS, Van Den Heuvel MP (2017) Connectome Disconnectivity and Cortical Gene Expression in Patients With Schizophrenia. Biol Psychiatry 81:495–502 [DOI] [PubMed] [Google Scholar]
- 61.Vass E, Fekete Z, Simon V, Simon L (2018) Interventions for the treatment of theory of mind deficits in schizophrenia: Systematic literature review. Psychiatry Res 267:37–47 [DOI] [PubMed] [Google Scholar]
- 62.Doody GA, Götz M, Johnstone EC, Frith CD, Cunningham Owens DG (1998) Theory of mind and psychoses. Psychol Med 28:397–405 [DOI] [PubMed] [Google Scholar]
- 63.Shin S, Jung WH, McCutcheon R, Veronese M, Beck K, Lee JS, Lee Y-S, Howes OD, Kim E, Kwon JS (2022) The Relationship Between Frontostriatal Connectivity and Striatal Dopamine Function in Schizophrenia: An 18F-DOPA PET and Diffusion Tensor Imaging Study in Treatment Responsive and Resistant Patients. Psychiatry Investig 19:570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


