Abstract
Protocadherin19 (PCDH19)-related epilepsy syndrome is a rare disorder characterized by early-onset epilepsy, intellectual disability, and autistic behaviors. PCDH19 is located on the X chromosome and encodes a calcium-dependent single-pass transmembrane protein, which regulates cell-to-cell adhesion through homophilic binding. In human, 90% of heterozygous females, containing PCDH19 wild-type and mutant cells due to random X inactivation, are affected, whereas mutant males, containing only mutant cells, are typically not. The current view, the cellular interference, is that the altered interactions between wild-type and mutant cells during development, rather than loss of function itself, are responsible. However, studies using Pcdh19 knockout mice showed that the complete loss of function also causes autism-like behaviors both in males and females, suggesting that other functions of PCDH19 may also contribute to pathogenesis. To address whether mosaicism is required for PCDH19-related epilepsy, we generated Xenopus tropicalis tadpoles with complete or mosaic loss of function by injecting antisense morpholino oligonucleotides into the blastomeres of neural lineage at different stages of development. We found that either mosaic or complete knockdown results in seizure-like behaviors, which could be rescued by antiseizure medication, and repetitive behaviors. Our results suggest that the loss of PCDH19 function itself, in addition to cellular interference, may also contribute to PCDH19-related epilepsy.
Keywords: epilepsy in females with mental retardation, Dravet syndrome, mosaicism, autism spectrum disorder, seizure
PCDH19 is composed of six consecutive extracellular cadherin (EC) domains, a transmembrane region, and a cytoplasmic domain with a C-terminal tail. PCDH19 is highly expressed in the developing neuroepithelium, during which homophilic interaction between the EC domains regulates cell-to-cell adhesion (1). Indeed, most pathogenic mutations in human are located in the EC domain (2), particularly in the adhesive interface (3). Mutations in the PCDH19 gene have been mostly found in female heterozygous patients with epilepsy, who often inherited the mutant gene-containing X chromosome from the asymptomatic father (2). This unusual pattern of inheritance is explained by the cellular interference theory, which posits that the coexistence of wild-type and mutant neuroepithelial cells in the developing female brain (due to random X inactivation) or mosaic males (4–6) interfere with normal homophilic adhesion causing clustering of like cells, abnormal brain development, and the disease.
However, there have been reports of autism-related behaviors (7, 8) and seizure activity (9) in complete knockout mice, suggesting that additional mechanisms may contribute to pathogenesis. Xenopus tadpoles are a unique model to investigate this possibility as the embryo develops by holoblastic divisions, during which the fate of each cell is well described, allowing complete or mosaic delivery of gene expression–altering reagents. In this study, we knocked down pcdh19 gene expression in all or half of the cells of the central nervous system (CNS) and compared their phenotypes relevant to PCDH19-related epilepsy, using the previously established methods to model epilepsy and autism (10, 11).
Results
Experimental Strategy.
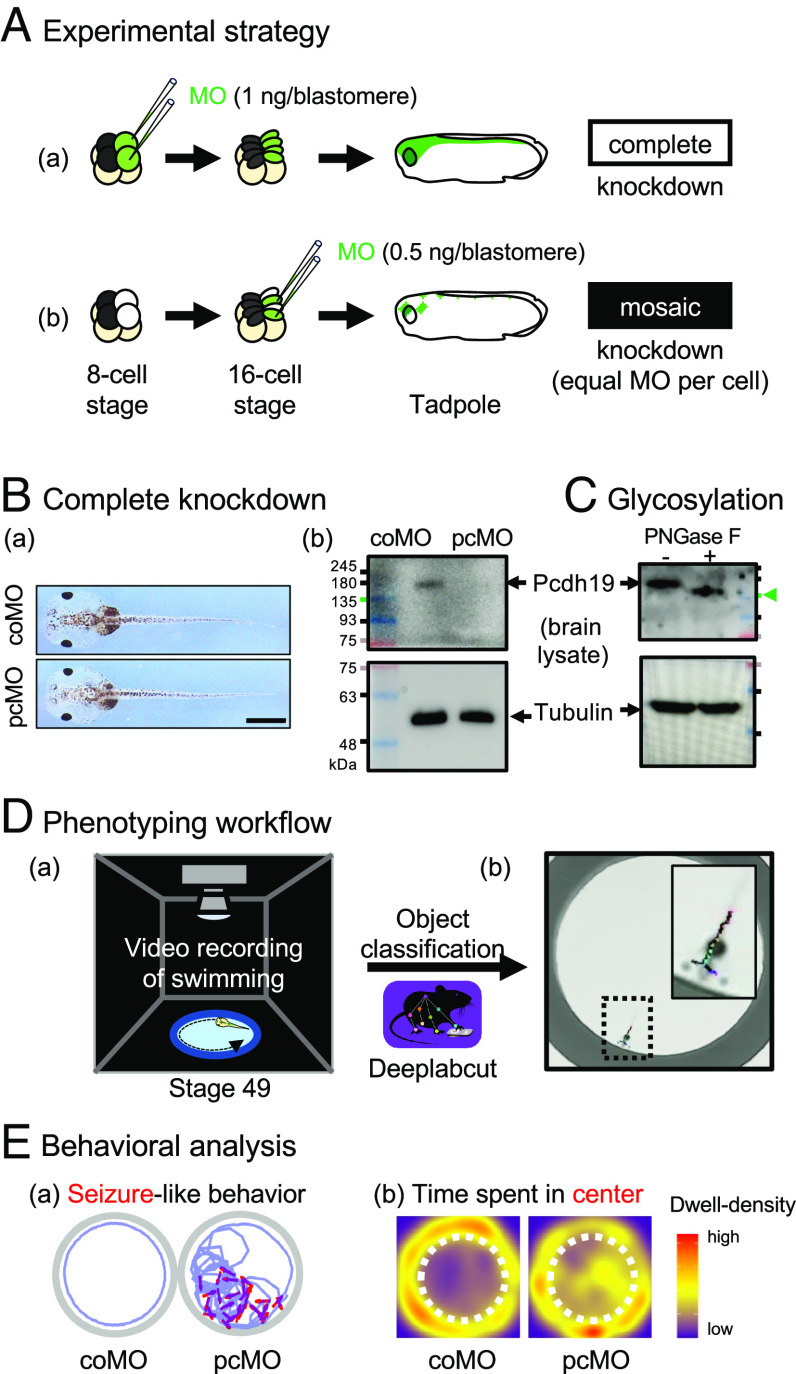
At the eight-cell stage following three rounds of cleavage, the two dorsal animal blastomeres contribute to most of the CNS in the tadpole (12). Therefore, injecting morpholino (MO) oligonucleotides into these two blastomeres at this stage leads to knockdown of gene expression in most cells in the CNS of the tadpole (Fig. 1 A, a). On the other hand, injecting a half dose of the same reagent into one of their two daughter cells following an additional round of cleavage would result in the same degree of gene knockdown in half of the CNS cells in the tadpole since MO activities are restricted to the daughter cells (Fig. 1 A, b) (12). We used a pcdh19 translation-blocking MO (pcMO) or control MO (coMO) and found that complete knockdown resulted in the normal-looking tadpole (Fig. 1 B, a) with a level of Pcdh19 in the brain undetectable by western blot (Fig. 1 B, b). The Pcdh19 band migrated more slowly than expected for its calculated mass (~130 kDa) due to glycosylation (Fig. 1C). We analyzed the swimming behaviors of complete or mosaic knockdown tadpoles as previously described (10, 11) in a 2.5 cm arena for 10 min, extracted their poses using DeepLabCut trained with our dataset, and analyzed the swimming trajectory, swimming activity, and abrupt turning behaviors (Fig. 1D). One phenotype that we quantified is seizure-like behaviors (Fig. 1E, a, red arrow), characterized by intermittent bouts of rapid swimming, repetitive circling, lateral movement of the head with tremors, and/or C-shaped contractions (10, 11) (Movie S1). Another phenotype that we quantified is the time spent in the center of the arena (Fig. 1 E, b). As previously described (10), tadpoles tend to swim around the edge, whereas pcMO-injected tadpoles display short-ranged, repetitive circling behaviors in the center of the arena (dashed circle).
Fig. 1.
Experimental strategy. (A) Microinjection strategy. (a) Complete knockdown. (b) Mosaic knockdown. (B) Efficiency of translation-blocking MO. (a) Normal gross morphology of stage 49 tadpoles injected with control (coMO) or pcdh19 morpholino (pcMO). (Scale bar, 100 µm.) (b) Pcdh19 western blot from the brain. (C) Deglycosylation of Pcdh19 by PNGase F (arrowhead). (D) Trajectory analysis. (a) Video recording. (b) Pixel classification. (E) Phenotyping. (a) Seizure-like behaviors (red) in swimming trajectory (purple). (b) The center (dashed circle)-dwelling time visualized as a density plot.
Histological Analysis of Complete and Mosaic pcdh19 Knockdown in the Developing Brain.
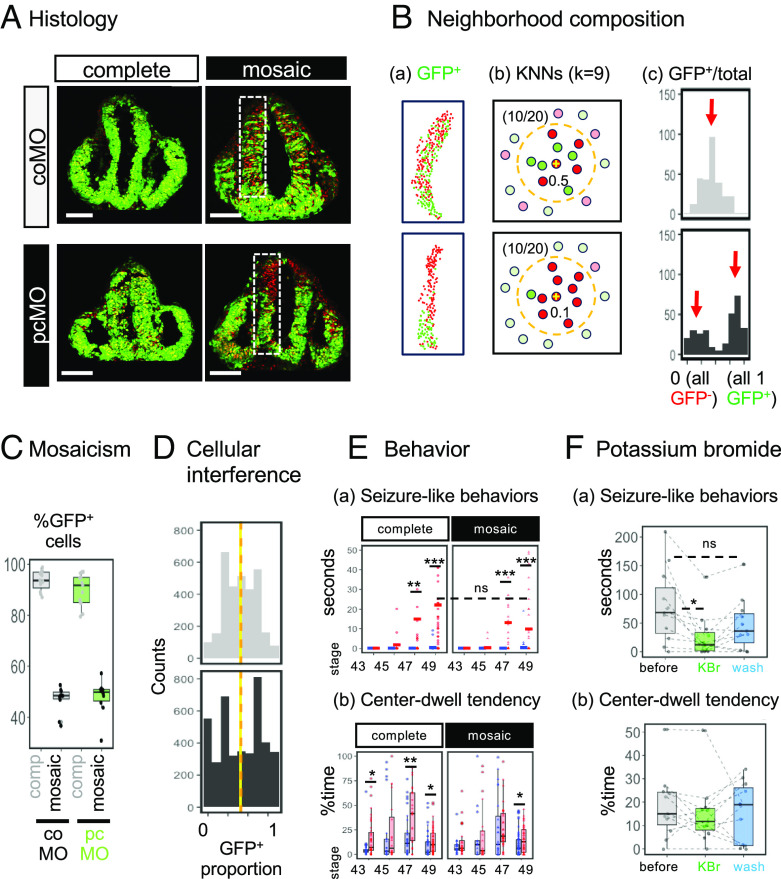
First, we asked whether cellular interference is observed in Xenopus. Complete and mosaic knockdown strategies delivered MO to approximately 96% and 50% of cells in the neuroepithelium, respectively (Fig. 2A). The distribution of GFP-positive cells showed clear difference depending on which MO was injected: pcMO-containing cells were segregated from noncontaining cells, in line with previous observations in mouse. We quantified this distribution by the k-nearest neighbors method: For every cell in the image, nine-nearest neighbors were selected forming a neighborhood composed of 10 cells, and the proportion of GFP+ cells in the neighborhood was calculated (Fig. 2B). These values in the coMO group showed a bell-shaped distribution centered around 0.5. In contrast, those in the pcMO group showed two peaks around 0 and 1 (red arrows), indicating that GFP+ and GFP− cells segregate. This result also indicates that pcMO knocks down Pcdh19 proteins to a functionally significant degree. Both tissues were composed of similar proportions of GFP+ cells (Fig. 2C), and the distribution, but not mean value, of GFP+ cell proportions in neighborhoods was different (Fig. 2D). Therefore, cellular interference was observed in this Xenopus model.
Fig. 2.
Histological and behavioral outcomes of mosaic versus complete knockdown. (A) Representative images of GFP+ (MO-containing) cells in the developing neuroepithelium (stage 27). Nuclei are counterstained in red. (Scale bars, 100 µm.) (B) Assessing the distribution of GFP+ cells. (a) Cell counting. (b) K-nearest neighbors (KNNs). For each cell, a 10-cell neighborhood (k = 9, orange dashed circle) is defined and the GFP+ proportion is calculated. In this example, 50% are GFP+ in both conditions (i.e., 10/20), but the coMO and pcMO cells receive 0.5 and 0.1, respectively. (c) The distribution of neighborhood compositions. (C) Proportion of GFP+ cells per brain. (D) Distribution of GFP+ cells in all analyzed animals. The y axis represents the number of counted cells. P < 2.2 × 10−16 (D = 0.1408) in the asymptotic Kolmogorov–Smirnov test. Vertical lines represent means: coMO = 0.486 (yellow) and pcMO = 0.494 (orange), P = 0.29 in the unpaired t test. (E, a) Seizure-like behaviors (solid lines represent means) and (b) center-dwelling time of coMO (blue) and pcMO (red) tadpoles over time. Each point represents an observation (see SI Appendix, Extended Materials and Methods for the numbers of analyzed animals). *P < 0.05, **P < 0.01, ***P < 0.001, and ns, not significant; post hoc Tukey test following two-way ANOVA. (F) Effect of potassium bromide at stage 49. (a) Seizure-like behaviors. (b) Center-dwell tendency. *P < 0.05 and ns, not significant; repeated-measure ANOVA followed by post hoc Bonferroni tests (n = 11).
Behavioral Phenotypes of Complete and Mosaic pcdh19 Gene Knockdown.
We compared seizure-like behaviors in tadpoles with complete or mosaic knockdown with their matching controls. Animals in both conditions showed a pcMO-dependent increase in seizure-like behaviors and the repetitive swimming activity in the center of the arena (Fig. 2E). These behaviors were rarely observed in coMO-injected tadpoles, and there was no difference between mosaic and complete pcdh19 knockdown groups, suggesting that additional mechanisms other than the cellular interference contribute to seizure-like behaviors in Xenopus. Seizure-like behaviors of pcMO-injected tadpoles began to appear at premetamorphic stages with “adult-like” circuits (stages 47 to 49) but not in late embryonic stages (stages 43 to 45), as previously reported (10, 11) (Fig. 2E).
Relevance of Behaviors in Understanding PCDH19-related Epilepsy.
If the seizure-like behavior induced by pcdh19 knockdown in Xenopus represents epilepsy in human, antiseizure medication that can control seizures in patients with PCDH19-related epilepsy, such as potassium bromide (13), is expected to ameliorate it. To test this possibility, we selected the complete pcdh19 knockdown tadpoles that displayed a high incidence of seizure-like behaviors and compared these behaviors before and after the treatment. We found that seizure-like behaviors, but not the center-dwelling tendency, were reduced in over 90% of tadpoles tested (Fig. 2F). These results indicate that pcdh19 gene knockdown (complete or mosaic) in Xenopus produces two distinct behavioral phenotypes: seizure-like behaviors that can be prevented by antiseizure medications and repetitive behaviors that cannot be prevented.
Discussion
This is a direct and comprehensive comparison of littermate animals, in which pcdh19 gene expression was knocked down in all or half of the cells in the brain. We found that both complete and mosaic knockdown induce similar degrees of behavioral abnormality: seizure-like and repetitive swimming behaviors. These behaviors occurred with or without cellular interference, suggesting that mechanisms other than the cellular interference contributed, in line with a recent finding of network hyperexcitability in pcdh19 knockout zebrafish (14).
It is noteworthy that our findings contrast with cases in human, in which almost all nonmosaic male mutants are asymptomatic, with very few potential exceptions. We found that pcMO-dependent seizure-like behaviors appeared at premetamorphic stages, which suggests that they result from defects in Pcdh19-dependent synaptic refinements that occur during the preceding late embryonic stages. We speculate that synaptic refinements in human, which occur over a much longer duration than in model animals, are more resilient to perturbation. Therefore, cellular interference, which may influence global wiring patterns, is a main driver of pathogenesis in human, whereas both cellular interference and defective synaptic refinements contribute to pathogenesis in model animals.
Abnormal synaptogenesis underlies neurodevelopmental diseases such as autism-spectrum disorders, in which apparent anatomical and cellular abnormalities are not seen in the brain. In this sense, it is intriguing that PCDH19 regulates the excitability of neurons (15). As the excitation–inhibition balance is established during development and its alteration is associated with autism spectrum disorders, we speculate that the loss of function in the PCDH19 gene itself increases the vulnerability in developing a hyperactive neural network, the result of which may manifest as seizure-like and autism-like behaviors in Xenopus (this study), zebrafish (14), and mouse (8), but not often in human.
Materials and Methods
All the analyzed data were obtained from three or more independent experiments. Xenopus experiments were performed using the following morpholinos (OR, USA): pcMO, 5′-CCCTGCTCAGCCACAACCACATAGT-3′; coMO, 5′-CCTCTT ACCTCAGTTACAATTTATA-3′. Western blot analysis was performed using anti-PCDH19 (ab191198) or anti-Tubulin antibodies (ab6160) (Abcam) and PNGase F (P7367, Sigma-Aldrich). Data processing, visualization, and statistical analyses were performed using R (v4.3.0). Methods details are provided in SI Appendix, Extended Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Related to Figure 1E. Examples of seizure-like and normal swimming behaviors in tadpoles. (A) Repetitive circling. (B) Lateral movement of the head with tremors. (C) C-shaped contractions. (D) Intermittent bouts of rapid swimming in Pcdh19 knockdown tadpoles. (E) Typical swimming behavior of a healthy tadpole. Timestamp, seconds:centiseconds.
Acknowledgments
J.P. performed all experiments. J.O. and H.J. designed the experiments, analyzed the data, and wrote the paper with the help of all authors. E.L. and C.H.K. gave inputs to the design of the experiments, the data, and the manuscript. We thank Irene Cho and Nirali Anand for technical assistance. This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (2017R1A2B4002683, 2018R1A5A2025079, and 2022M3E5E8018388 to H.J.; 2019R1A2C3002354 to C.H.K.) and the Ministry of Education (RS-2023-00247585 to J.O.).
Author contributions
J.O. and H.J. designed research; J.P. performed research; J.O. and H.J. analyzed data; E.L. and C.H.K. gave inputs to the design of the experiments, the data, and manuscript; and J.O. and H.J. wrote the paper.
Competing interests
The authors declare no competing interest.
Contributor Information
Jiyeon Ohk, Email: jyeon1703@yonsei.ac.kr.
Hosung Jung, Email: hosungjung@yonsei.ac.kr.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Pederick D. T., et al. , Abnormal cell sorting underlies the unique X-linked inheritance of PCDH19 epilepsy. Neuron 97, 59–66.e55 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Depienne C., et al. , Mutations and deletions in PCDH19 account for various familial or isolated epilepsies in females. Hum. Mutat. 32, E1959–E1975 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper S. R., Jontes J. D., Sotomayor M., Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy. Elife 5, e18529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lange I. M., et al. , Male patients affected by mosaic PCDH19 mutations: Five new cases. Neurogenetics 18, 147–153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Depienne C., et al. , Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 5, e1000381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romasko E. J., et al. , PCDH19-related epilepsy in a male with Klinefelter syndrome: Additional evidence supporting PCDH19 cellular interference disease mechanism. Epilepsy Res. 145, 89–92 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Hayashi S., et al. , Loss of X-linked Protocadherin-19 differentially affects the behavior of heterozygous female and hemizygous male mice. Sci. Rep. 7, 5801 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim J., Ryu J., Kang S., Noh H. J., Kim C. H., Autism-like behaviors in male mice with a Pcdh19 deletion. Mol. Brain 12, 95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakotomamonjy J., Sabetfakhri N. P., McDermott S. L., Guemez-Gamboa A., Characterization of seizure susceptibility in Pcdh19 mice. Epilepsia 61, 2313–2320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt K. G., Khakhalin A. S., Modeling human neurodevelopmental disorders in the Xenopus tadpole: From mechanisms to therapeutic targets. Dis. Model Mech. 6, 1057–1065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo Y., et al. , GABBR2 mutations determine phenotype in rett syndrome and epileptic encephalopathy. Ann. Neurol. 82, 466–478 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Nutt S. L., Bronchain O. J., Hartley K. O., Amaya E., Comparison of morpholino based translational inhibition during the development of Xenopus laevis and Xenopus tropicalis. Genesis 30, 110–113 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Lotte J., et al. , Effectiveness of antiepileptic therapy in patients with PCDH19 mutations. Seizure 35, 106–110 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Robens B. K., et al. , Mosaic and non-mosaic protocadherin 19 mutation leads to neuronal hyperexcitability in zebrafish. Neurobiol. Dis. 169, 105738 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serratto G. M., et al. , The epilepsy-related protein PCDH19 regulates tonic inhibition, GABA(A)R kinetics, and the intrinsic excitability of Hippocampal neurons. Mol. Neurobiol. 57, 5336–5351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Related to Figure 1E. Examples of seizure-like and normal swimming behaviors in tadpoles. (A) Repetitive circling. (B) Lateral movement of the head with tremors. (C) C-shaped contractions. (D) Intermittent bouts of rapid swimming in Pcdh19 knockdown tadpoles. (E) Typical swimming behavior of a healthy tadpole. Timestamp, seconds:centiseconds.
Data Availability Statement
All study data are included in the article and/or supporting information.