Summary
The field of small-diameter vascular grafts remains a challenge for biomaterials scientists. While decades of research have brought us much closer to developing biomimetic materials for regenerating tissues and organs, the physiological challenges involved in manufacturing small conduits that can transport blood while not inducing an immune response or promoting blood clots continue to limit progress in this area. In this short review, we present some of the most recent methods and advancements made by researchers working in the field of small-diameter vascular grafts. We also discuss some of the most critical aspects biomaterials scientists should consider when developing lab-made small-diameter vascular grafts.
Subject areas: Cardiovascular medicine, Bioengineering, Tissue engineering, Biomaterials
Graphical abstract
Cardiovascular medicine; Bioengineering; Tissue engineering; Biomaterials
Introduction
The body’s vasculature performs multiple functions in the body, including delivering oxygen and nutrients to the cells, removing waste (like carbon dioxide and degradation metabolites), regulating the coagulation process, modulating the infiltration of immune cells into other tissues, and regulating blood pressure, to mention a few.1 This system is so critically important that a failure to maintain homeostasis of the vasculature is the leading cause of cardiovascular disease and the number one cause of death worldwide.2
Cardiovascular diseases (CVDs) represent a group of disorders of the heart and the blood vessels which include coronary heart disease, cerebrovascular disease, rheumatic, and congenital heart diseases, among others. The most important behavioral risk factors for developing CVD include an unhealthy diet, physical inactivity, smoking, and alcohol consumption. Commonly, patients with an underlying vascular condition may experience myocardial infarction, known as a heart attack, typically characterized by pain in the center of the chest and pain or discomfort in the arms, left shoulder, elbows, jaw, or back.2 Different pharmacotherapies have been developed over the past decades to treat the vascular system when it fails to regulate tonicity, coagulation, or lipid accumulation.3 These approaches include the use of antiplatelets, beta-blockers, angiotensin-converting enzyme inhibitors, statins, and calcium channel blockers, which can effectively restore vascular function and improve the quality of life of patients with vascular disease. Despite this progress in non-invasive therapies, the prevalence of CVDs has continued to rise.2,4
If there is a localized problem of the vasculature system that does not resolve with medication, more invasive interventional approaches can be used depending on the location. As an example, during a myocardial infarction due to blockage(s) in the coronary arteries of the heart, specialized catheters can be used to perform an angioplasty or to insert a stent to expand the conduit and allow for reperfusion of the infarcted area.5 Alternatively, surgery can be performed to insert a blood vessel replacement, called a vascular graft (VG).6 These VGs are classified based on the source and include autografts (from the patient), allografts (from a donor of the same species), xenografts (from a different species),7 or tissue-engineered vascular grafts (e.g., electrospun scaffolds, 3D-printed grafts, fibrous capsule).7,8 Also, for human applications, they can be classified as large vascular grafts (LVGs) when the internal diameter (ID) is greater than 6 mm, or small vascular grafts (SVGs) when the ID is below 6 mm.9 Examples of surgical VG replacements are aortic aneurysm graft replacement, coronary artery bypass graft (CABG) surgery, or peripheral vascular bypass (PVB).10 Another important candidate for VG replacements are arteriovenous fistula as they are the main conduit used for permanent hemodialysis access for patients living with kidney failure.11
A VG must meet several criteria, the most important ones being low coagulation induction (patency), pressure resistance, and no immune rejection.12 Several xenograft transplantation approaches have been tested in humans, with none yet approved for widespread clinical use due to the immune response to these types of grafts and the need for a long-term regimen of immunosuppressants.13 For this reason, in LVG replacement, polymeric materials are the gold standard, with Dacron and polytetrafluorethylene (ePTFE) grafts being the most widely used materials. The large diameter of these LVGs and the fast flow of blood through them minimize coagulation events, such as seen in aortic dissection graft replacement.14 Nevertheless, blood in contact with these materials can still promote coagulation, and so heparinized surfaces have been developed to improve their patency.15 For SVG, the blood flow is considerably slower compared to the large conduits, so polymeric graft materials that are used as LVG do not behave ideally in terms of coagulation and are not used in the clinic. Therefore, autografts are the gold standard in clinical use for SVG. As an example, for PVB in the femoral vessels, the patient’s own saphenous vein (SV) is the ideal graft for bypass surgery. Likewise, in CABG surgery, the internal thoracic (mammary) artery (IMA), the radial artery, the gastroepiploic artery, or the SV are ideal candidates as the SVG source.16 However, the use of autografts can be limited by a lack of suitable graft availability due to previous harvesting, anatomical variability, or poor quality of the available tissue, which makes engineered vascular grafts an attractive alternative approach for creating SVG.17
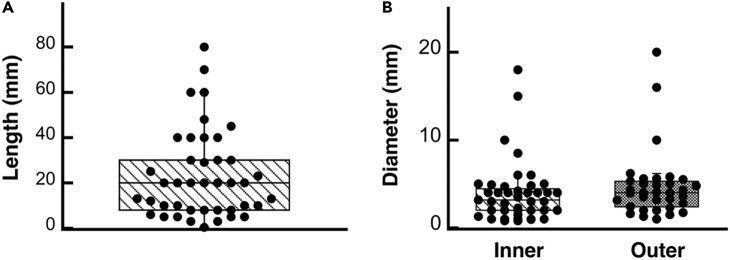
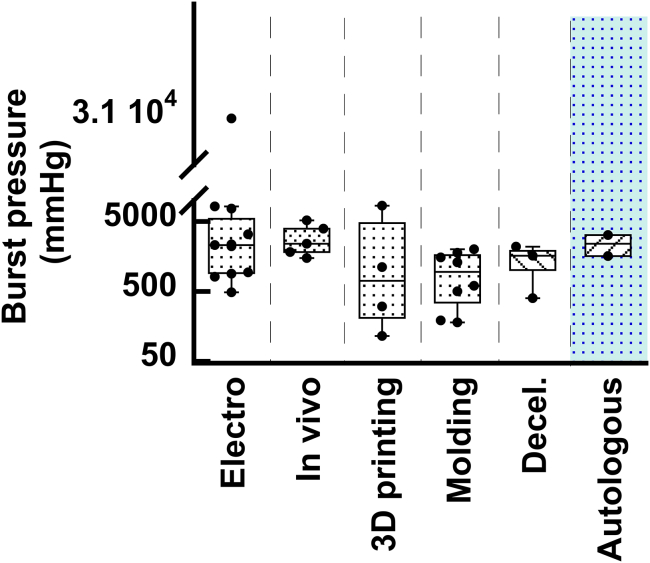
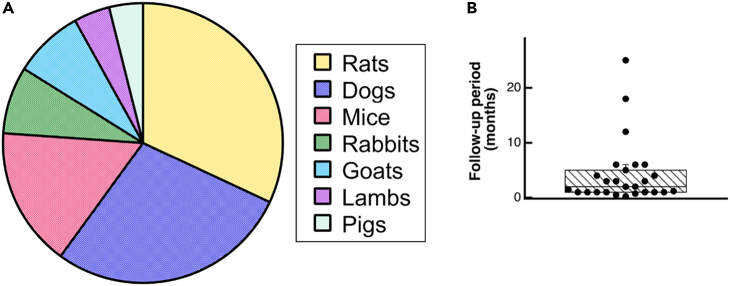
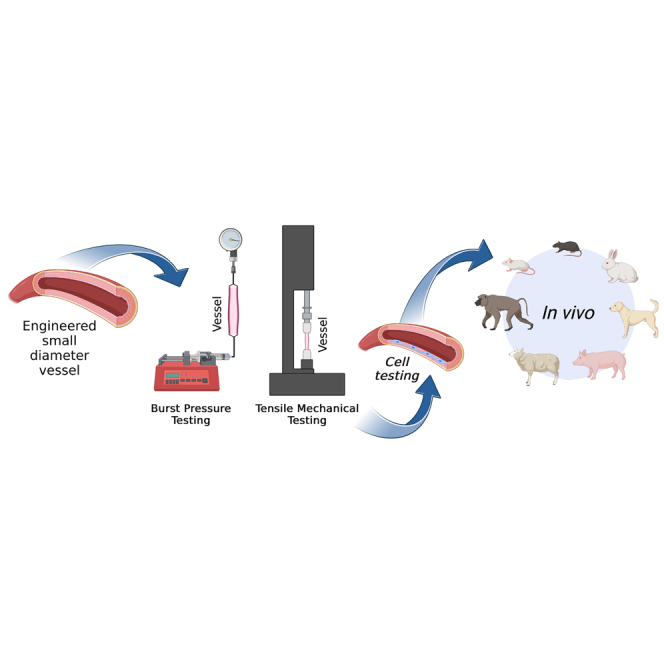
Despite decades of research in engineered VGs, none of them have yet translated to the clinic, due mainly to coagulation issues in the long term.18 New approaches in 3D bioprinting, advanced biomaterials, and tissue engineering have been seen as possible alternative solutions to resolve all the unmet needs for SVG. In the following pages, we review some of the most used techniques in vascular tissue engineering for the fabrication of small-diameter vascular grafts. Articles considered for inclusion in this review were retrieved through Google Scholar, spanning from 2007 to the present. The chosen studies were specifically centered on the development of SVGs, with a particular focus on their manufacturing method, mechanical characterization with an emphasis on burst pressure assessment, and in vitro/in vivo testing assessments. The keywords used in the search included the following: “electrospinning small vascular grafts”, “mechanical properties of electrospinning small vascular grafts”, “bioengineered 3D printed vascular grafts”, “manufacturing methods for vascular grafts”, “decellularized vascular graft”, and “3D bioprinted vascular graft”. Roughly 300 studies were initially identified, and a subset of 48 studies was ultimately selected for inclusion in the mini review. In arriving at the 48 selected studies, the main exclusion criteria were studies that did not include both in vivo testing and burst pressure assessment.
Vascular tissue engineering
Tissue engineering is a multidisciplinary field encompassing principles from bioengineering, medicine, and biology to facilitate tissue and organ repair or regeneration, utilizing the fundamental components of native tissue, such as cells and scaffolds. Vascular tissue engineering, in particular, has gained prominence due to the prevalence of blood vessel-related diseases and the critical role of angiogenesis in tissue regeneration. This branch of tissue engineering is divided into three categories for constructing vascular grafts: in vitro, in vivo, and in situ. In vitro vascular tissue engineering involves crafting functional vascular grafts outside the body through the integration of cells, scaffolds, and bioreactors.9 In contrast, in vivo vascular tissue engineering creates autologous vascular grafts within the body’s natural environment as a bioreactor, allowing the development of living tissue around a template, which can later be harvested as a graft. Further, in situ vascular tissue engineering aims to avoid prolonged in vitro culture periods by creating cellular or acellular vascular grafts that leverage the host’s inherent regenerative capacities, promoting blood vessel regeneration directly at the implantation site. A growing trend is the development of bioactive vascular grafts that promote in situ blood vessel regeneration. Small-diameter vascular grafts (<6 mm) are of particular interest to tissue engineers due to their relevance in addressing occlusions caused by conditions like atherosclerosis and embolism, which often lead to end-organ ischemia.19
Currently, the demand for small blood vessels continues to rise, and the available options are highly limited due to all the challenges faced by this field, including rejection, thrombosis, and calcification, among others. For instance, synthetic grafts employing biologically inert polymers exhibit limited resistance to thrombus formation on their surfaces, potentially leading to graft obstruction and diminished patency rates. Consequently, there is a pressing need to develop non-thrombogenic endothelial linings. Despite being the thinnest layer, the endothelium plays an essential role in vessel function, primarily by forming a tight, non-thrombogenic barrier.20,21 Hence, promoting rapid endothelialization emerges as a promising approach to address this challenge.19 Additionally, over the past decade, heparin-coated grafts have shown promise by enhancing patency rates. Furthermore, several studies have highlighted heparin’s beneficial impact on inhibiting the proliferation of smooth muscle cells (SMCs), a major contributor to intimal hyperplasia and the narrowing of small-diameter artificial blood vessels, in addition to its remarkable anticoagulant properties.14 Therefore, surface heparinization serves as a potent strategy to improve anticoagulation properties.19,20,21,22 Besides, calcification remains a major challenge in SVGs and is linked to vascular failure due to mechanical dysfunction. During the calcification process, vascular SMCs (VSMCs) undergo osteo-chondrogenic transdifferentiation, resulting in ectopic mineralization in the vascular walls of the graft.23 Current strategies to address vascular graft calcification include preventing passive calcium deposition through polymer coating and heparinization,19 promoting matrix remodeling to reduce exposure to foreign materials and prevent calcification, producing small blood vessels with immunomodulatory capabilities by incorporating bioactive materials and molecules to reduce inflammation and hinder calcification, and creating grafts with antioxidant compounds capable of scavenging free radicals and maintaining intracellular reactive oxygen species balance.23
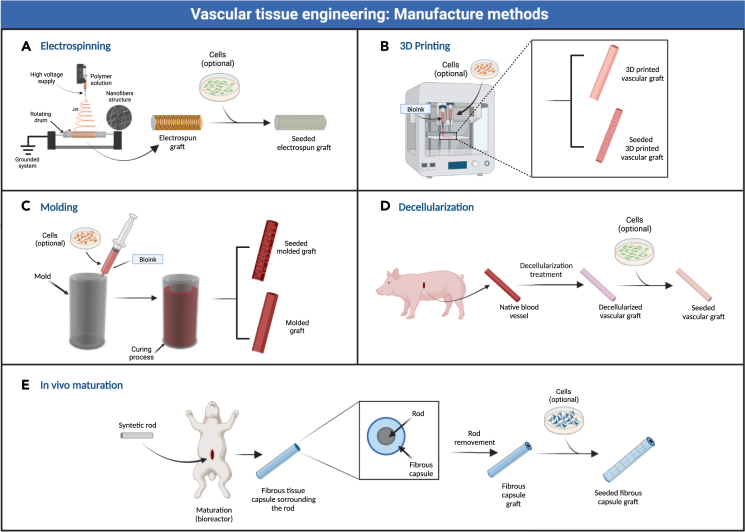
In the following pages, we start by presenting some of the most used manufacturing methods for the creation of SVGs as well as representative examples of materials used in such approaches. Subsequently, we will explore the utilization of animal models in validating SVGs, highlighting their contributions to evaluating key parameters like graft patency, endothelialization, thrombosis, inflammation, and immune response. To facilitate the reading of this mini review, the manufacturing methods have been classified electrospinning, 3D printing, molding, decellularization, and in vivo maturation. Figure 1 summarizes all these methods.
Figure 1.
Graphical summary of the manufacturing methods for small vascular grafts
(A) Electrospinning.
(B) 3D printing.
(C) Molding.
(D) Decellularization.
(E) In vivo maturation.
Electrospinning for production of biomimetic structures
Electrospinning was first patented in 1934,24 and, since then, it has had broad applications in multiple fields. It has received significant growing interest in the field of tissue engineering due to its potential for fabricating scaffolds with biomimetic structures similar to the scale and morphology of the native extracellular matrix (ECM).25
This technique involves the application of a high electric field to produce ultra-fine polymeric fibers with nano/micrometer diameters. The electrospinning apparatus includes a high-voltage power supply, a syringe pump to control the polymer flow rate, an injection needle, and a conductive collector to catch the electrospun fibers. Basically, it can be considered as a process in which a high direct current voltage is applied to the solution causing a repulsive force within like charges in the liquid, which causes the droplets to elongate, generating a conical shape (Taylor cone). When the applied voltage is high enough to overcome the surface forces acting on the Taylor cone, a jet of liquid travels toward the collector. As the liquid jet travels to the collector, the solvent from the jet evaporates and a solid nanofiber is obtained (see Figure 1A). These nanofibers have a great potential for mimicking the microenvironment of natural ECM and thus have garnered increasing interest for the fabrication of fibrous membranes to create tissue-engineered vascular grafts.17,25,26
Synthetic polymer-based electrospun scaffolds
Many groups have attempted to fabricate VGs using electrospun synthetic polymers, which have the capability to be modified or functionalized (high tunability) and have excellent mechanical and physical features.27 For example, a poly-ε-caprolactone (PCL)-based scaffold SVG with an inner diameter of 2 mm was produced by electrospinning. The mechanical assessment showed a burst pressure (BP) of ∼3,280 mmHg,28 which is comparable to the values observed in native SV (BP ∼1,599 mmHg) and native IMA (BP ∼3,196 mmHg), considered as the gold standard for CABG procedures.29,30 The scaffolds were implanted in the abdominal aorta of Sprague-Dawley rats for up to 18 months. While tissue formed by collagen, myofibroblasts, and macrophages was observed in the SVG at early time points, this did not persist, and the grafts showed insufficient cellularization of the vascular wall in the long term. Between 12 and 18 months, endochondral ossification occurred at the sites of chondroid metaplasia and the calcifications spread transmurally.
Another group, also with PCL, used smaller diameter electrospun fibers to produce an SVG also with an inner diameter of 2 mm.31 The grafts were implanted into the abdominal aortas of Sprague-Dawley rats and were followed for up to 12 weeks. After 3 weeks, the endothelial coverage was limited to the endoluminal graft surface near the distal and proximal anastomoses. After 6 weeks, graft endothelialization was almost complete except for a small area in the middle. Full endothelialization with some intima hyperplasia formation was observed after 12 weeks. At this time, the vessels showed good patency, no anastomotic stenosis, and no aneurysmatic dilatation. However, in vivo healing and degradation characteristics of the electrospun PCL grafts need to be studied more extensively and over a longer time period in comparison to standard vascular prostheses before clinical application is considered.
Among the synthetic polymers, polyurethane (PU) is also attractive because of its good antithrombogenicity and excellent compliance, beneficial physical/mechanical properties, and biocompatibility. As an example, tubular PU grafts with an inner diameter of 4 mm were developed using the electrospinning technique.32 The PU grafts showed good mechanical properties.33 In another study, these PU grafts were assessed by replacing a 2 cm segment of the abdominal aorta with the vascular graft in beagle dogs for up to 24 weeks. The assessment showed a patency of 83% with complete endothelialization and a bilayer structure formation, like seen in host vessels. It was also found that a limited amount of tissue grew on the walls of PU grafts and the neointimal thickness remained constant, contributing to improved graft patency with relatively limited neointimal hyperplasia, but it remains to be elucidated whether this lack of tissue ingrowth onto the PU graft wall may cause other complications (e.g., inadequate mechanical strength in the long term, formation of aneurysm/dissection, initiation of rupture). It is important to find an optimal balance between wall tissue ingrowth and long-term patency.
In another study, PU and polylactid acid (PLLA) were used to create SVGs. By controlled steering of the electrospinning jet, a biostable PU and a degradable PLLA were electrospun in different spinning modes to guide the jet and generate SVGs with 2 mm of inner diameter.34 The evaluation of mechanical properties for the PU grafts showed a burst pressure of ∼894 and 606 mmHg for circumferential and axial oriented fibers, respectively. For PLLA SVGs, much higher burst pressure values of 7,641 and 1,587 mmHg were observed for circumferential and axial oriented fibers, respectively. Compared to IMA and SV native vessels, the BP values of PU grafts are significantly lower for both fiber orientations, while the BP value is also lower for the PLLA grafts with axial fiber orientation. In contrast, the PLLA SVG with circumferential fiber orientation not only meets, but also exceeds the BP values of gold-standard vessels. However, none of the fiber orientations of both materials could mimic the mechanical behavior of the rat aorta. To obtain an optimal non-linear stress-strain behavior, a synthetic vessel would probably have to be constructed from several layers with different mechanical properties.
Homopolymers often lack one or more of the desired characteristics such as mechanical properties, degradability, biocompatibility, and bio-functionality. Hence, incorporating desired characteristics from different materials into polymer blends may overcome the limitations of using a single polymer in cardiovascular applications. Polymer blending enables the creation of scaffolds with improved hydrophilicity for better cell interaction and enhanced biodegradability.35 As an example of synthetic polymer blends, a group using PU and PCL and polyethylene terephthalate (PET) developed a triad-hybrid PU/PCL/PET scaffold with an inner diameter of 4 mm.36 The mechanical properties assessment showed a burst pressure of ∼2,200 mmHg. The triad-hybrid electrospun scaffolds were tested in vivo by implanting them subcutaneously in rats and were assessed for local tissue responses for 45 days. The results showed an increase in cell growth from day 0 to day 7.
Another group fabricated thermoplastic polyurethane (TPU)/graphene oxide (GO) scaffolds via electrospinning with an inner diameter of 3.18 mm. The electrospun scaffolds were treated with oxygen plasma using a plasma etcher to activate the scaffold surface to improve the scaffold’s hydrophilicity. The mechanical properties of the hybrid TPU/GO scaffolds were assessed and showed a burst pressure of 31,000 mmHg. To study their biocompatibility and potential to be used as vascular grafts, mouse fibroblasts and human endothelial cells were cultured on the scaffolds. The plasma-treated scaffolds supported cell viability as high as 99%, indicating good biocompatibility.37
Polyglycerol sebacate as a core along with a PCL electrospun sheath has also been developed as a hybrid synthetic graft.38 The graft was designed with different internal diameters for use based on the application. The graft was tested for patency in vivo using rats for a period of 90 days and a survival rate of 87.5% was achieved, with most failure occurring in grafts with an internal diameter under 3.0 mm due to acute thrombosis. Mechanical testing of the graft showed a burst pressure of 2,350 mmHg, similar to the gold-standard vessel values.
Natural polymer-based electrospun scaffolds
Natural polymers have been widely used by many research groups to fabricate VGs due to their biocompatibility properties and hydrophilic nature, which can simulate the ECM of native blood conduits.39 As an example, silk fibroin from the B. mori silkworm has been successfully used to fabricate SVG using electrospinning due to its unique combination of impressive mechanical properties, slow degradability, and biocompatibility. One group generated silk SVGs with an inner diameter of 5 mm and the mechanical properties assessment showed a burst strength of 811 mmHg (average),40 which was lower than IMA and SV values. Cell studies were carried out to assess the ability of the electrospun silk scaffolds to support the growth of human endothelial cells and SMCs. The results showed that cells attached, spread, and grew on silk mats. The scaffolds have promising features to be used as vascular grafts.
Gelatin is one of the most commonly used natural polymers since it is a low-cost derivative of collagen, the main fibrous protein of native vessel ECM.41 Gelatin significantly improves infiltration, adhesion, spreading, and proliferation of cells on gelatin-based scaffolds and has shown no denaturation phenomena due to the interaction with the applied electric field of electrospinning.42 As an example of its applications, gelatin SVGs with an inner diameter of 5 mm were developed. After the electrospinning process, the grafts were crosslinked using glutaraldehyde, so that the scaffold would not dissolve in an aqueous medium. The evaluation of mechanical properties of gelatin-based scaffolds showed a strain to failure of 11.7%, lower than the strain to failure of arteries (35%) and a high Young’s modulus of 33.8 MPa in the axial direction compared to natural collagen Young’s modulus of 5–10 MPa.41
Elastin is the principal structural component responsible for energy storage and recovery in elastic arteries and contributes to their unique mechanical properties. Hence, a group of researchers have attempted to fabricate electrospun SVG containing recombinant human tropoelastin (rTE), which, when crosslinked, mimics native elastin fibers.43 The SVG containing rTE had an inner diameter of 4.0 mm and burst pressure values of ∼485 mmHg which are insufficient for implantation compared to those of gold-standard autograft vessels. To assess the growth of vascular cells on the lumenal surface of electrospun polymeric rTE vascular grafts, porcine bone-marrow-derived endothelial outgrowth cells were used. The cells attached, spread, and grew on the electrospun fiber matrix and formed a confluent monolayer with typical endothelial cell cobblestone morphology after 48 h in culture. For potential clinical use, higher failure pressures of at least 1,500 mmHg would need to be achieved to provide sufficient strength to avoid rupture.
Synthetic/natural electrospun scaffolds
Polymeric blend scaffolds can also be designed to simultaneously take advantage of the desirable biological attributes found in natural polymers/materials, alongside the outstanding mechanical and physical characteristics of synthetic polymers.27 As an example of this advantageous combination, a research group used natural silk fibroin and TPU to produce SVGs with an inner diameter of 5.0 mm.44 The TPU/fibroin grafts were in vitro tested using human endothelial cells and after 14 days of culture, the cells attached to the tubular grafts exhibited high viability and it was found that cells grew on both the inner and outer surfaces of the TPU/fibroin graft.
Type I collagen is a predominant component of blood vessels and finds extensive use in biomedical applications owing to its excellent biocompatibility, minimal antigenicity, and ability to facilitate cell adhesion and growth. However, collagen scaffolds tend to possess inadequate mechanical properties on their own. To address this limitation, a common approach is to blend collagen with synthetic polymers.45 As an example of this natural/synthetic polymeric combination, electrospun gelatin type A/PCL and type I collagen/poly(lactide-co-ε-caprolactone) (PLCL) scaffolds were produced for SVG applications with inner diameters of 4 mm. The gelatin/PCL scaffolds showed Young’s modulus of ∼1.5 MPa and the collagen/PLCL scaffolds showed Young’s modulus of ∼1.8 MPa. The grafts were assessed in vivo using a subcutaneous implant mouse model. The scaffolds were implanted under the skin of the dorsum and 6 weeks following implantation, the Young’s modulus of gelatin/PCL decreased to ∼0.75 MPa and the Young’s modulus of the collagen/PLCL scaffolds increased to ∼6 MPa. Considering that Young’s modulus of healthy coronary arteries is 1.48 MPa,46 both grafts have similar values before implantation; however, after implantation, mechanical changes can be observed. Gross observation of the transplanted scaffolds revealed that the engineered collagen/PLCL scaffolds were smoother and brighter than the gelatin/PCL scaffolds and hematoxylin and eosin staining showed that these materials formed relatively homogenous vessel-like tissues.47
Another SVG with an inner diameter of 3 mm was designed with a thin inner layer composed of longitudinally aligned PCL/collagen nanofibers and a relatively thick outer layer composed of PCL/silica nanofibers.48 Biocompatibility of the bilayer SVGs was evaluated by cell attachment assays by seeding the inner layer with human endothelial cells and the outer layer with mouse fibroblasts. The results indicated that the aligned PCL/collagen nanofiber structure (inner layer) allowed endothelial cells to adhere, proliferate, and rapidly migrate and reached a 100% cell migration ratio after 24 h. On the other hand, the PCL/silica outer layer showed a favorable fibroblast adhesion, with a large number of cells attached to its surface and stimulated proliferation.
One more SVG using natural/synthetic blends was designed by functionalizing electrospun poly(ester-urethane) urea (PEUU) grafts with poly (ethylene glycol) (PEG) and heparin (Hep) via covalent grafting to increase their anticoagulant and anti-adhesive capabilities.49 The grafts had an inner diameter of 2 mm and the mechanical properties assessment for the PEUU-PEG-Hep grafts showed a burst pressure of ∼8,200 mmHg, which exceeded that of IMA and SV vessels. For the in vitro tests, endothelial cells were seeded onto the surface of electrospun grafts to evaluate the capability of grafts to rapidly regenerate the endothelial layer. Endothelial cells adhered and proliferated on the surface of grafts, indicating their cytocompatibility. Also, a quantitative assessment of cell coverage on the electrospun grafts demonstrated that the covalent grafting of PEG and heparin greatly elevated cell coverage (up to ∼77%) compared to either component alone, demonstrating the complementary effects of heparin and PEG on quick endothelialization. For the in vivo assessment, PEUU-PEG-Hep grafts were implanted into the left carotid artery of New Zealand rabbits. When the grafts were removed from the body 10 and 30 days after implantation, an examination of the inner graft surface revealed a smooth cell monolayer resembling the natural vascular endothelium, suggesting in situ remodeling. Also, no platelet adhesion or thrombus matrix was found in the lumen of the grafts, suggesting that they possess antithrombotic properties similar to the native blood vessels. Although the results are promising, the length of the implanted grafts tested might be insufficient to assess clinical relevance, and the short implant time assessed did not allow for observation of the graft’s degradation and stability. Future studies are needed to focus on the long-term outcomes of the PEUU-PEG-Hep grafts in large animal models.
Cellular electrospun scaffolds
As previously mentioned, electrospinning is a promising approach in the field of vascular engineering; however, its biocompatible properties could be enhanced by seeding cells into the scaffolds to prevent from acute thrombosis and to generate an anti-thrombogenic layer.50 As an example, an SVG was created by seeding a PLCL electrospun scaffold (coated by type I collagen) with SMCs on both sides. The graft had an inner diameter of 4 mm and reached a burst pressure of ∼933 mmHg and tensile strength of ∼3.23 MPa at 4 weeks of assessment.51 These small-diameter vascular grafts had tensile properties similar to that of a rabbit’s aorta. However, the BP was significantly lower compared to those achieved by the IMA and SV native vessels.
As another approach, a thermally induced phase separation (TIPS) process and an electrospinning method were applied sequentially to obtain bilayered tubular scaffolds made of PEUU.52,53 The SVGs were produced with an inner diameter of 4.7 mm and achieved a burst pressure of ∼2,300 mmHg. Also, the scaffolds were bulk seeded with muscle-derived stem cells to assess cell loading within a short seeding time and showed cell seeding efficiencies of greater than 90%.52 In other work, these PEUU SVGs were assessed in vivo using Lewis rats for up to 8 weeks52,53 A segment of the rats’ infrarenal aorta was replaced with seeded PEUU grafts. The results showed patency rates of 65%, with no signs of stricture and no aneurysms detected. However, a potential limitation of this study is the amount of polymer degradation that was observed at 8 weeks; although the TIPS layer appeared to be heavily remodeled and infiltrated by cells, the electrospun layer remained intact, making it difficult to evaluate when the newly formed tissue would replace all the polymer. The degradation rates need to be optimized for future studies.
Researchers have also studied the effect of incorporating human adipose-derived stromal vascular fraction cells into the luminal surface of a synthetic graft made of ePTFE or a PLCL electrospun scaffold.54 To promote cell adhesion, the internal surfaces of the grafts were first modified by placing the scaffold in a bioreactor and flowing conditioned medium through them. Subsequently, the cells were added, and the grafts were cultured for 14 days. Histology data showed minimal infiltration cells within the wall of the ePTFE graft compared to the electrospun scaffold and no smooth muscle actin (SMA) markers were detected in either scaffold.54
Takeaway – Electrospinning
Based on the findings of the analyzed studies, it can be concluded that electrospun synthetic polymer blood vessel grafts exhibit favorable mechanical characteristics. However, many fell short in terms of endothelialization, adhesion, and/or biodegradability, which are crucial for meeting the performance standards required for small blood vessels. In contrast, SVGs crafted from natural polymers, while displaying good biocompatibility and endothelialization properties, lagged in mechanical performance. It is worth emphasizing that for a blood vessel graft to transition to the clinic, it must excel in both its mechanical and biological characteristics.55
To this end, the utilization of polymer blends as well as the addition of cells could emerge as a highly promising approach. Notable examples include PEUU/PEG/Hep and PEUU SVGs. These grafts exhibited superior mechanical properties, facilitated rapid endothelialization, supported cellular viability, and actively participated in the remodeling processes. This suggests that combining the good mechanical attributes of synthetic polymers with the excellent biocompatibility of natural components and cells holds great promise for addressing small blood vessel creation.18 Additionally, the maintenance of a native blood vessel’s perfusion characteristics is crucial,56 and according to the reviewed studies, electrospun grafts can withstand perfusion flow rates similar to native vessels, with many of them undergoing perfusion tests showing beneficial outcomes. Nonetheless, it is imperative to recognize that several challenges persist, including the assessment of these grafts in biologically human-like environments and extending the evaluation time for more comprehensive results.
3D printing of vascular graft
3D printing is a type of additive manufacturing where a specific scaffold or complex object is constructed through the sequential deposition of layers of material with specific shapes or pattern.57 In the most common form of 3D printing, fused deposition modeling, plastic filament is fed through a heated nozzle tip which melts the filament and deposit strands of melted plastic on a platform in an arranged pattern to build the desired object layer by layer.58 The affordability and simplicity of this manufacturing method made it widely adopted in both industry and academia for the rapid prototyping of complex 3D design. One key advantage of 3D-printed grafts over other manufacturing method is the ability to create conduits with complex shapes and angles directly from a 3D design file. Specifically for vascular graft, dimensions of the native artery can be imported into computer-aided design software using magnetic resonance imaging or computed tomography (CT) data in order to accurately recreate the artery.57
Over the last decade, 3D bioprinting emerged as a branch of 3D printing where plastic filament is replaced with a bioink that can contain a mix of different natural or synthetic polymer and cells, and the layers of material are deposited either on a build platform (see Figure 1B) or in a suspended support material for softer materials.59
3D printing using natural polymers
Stereolithography 3D printing is a technology that employs the uses of a photosensitive material in a tank and, using a laser, the desired shape is printed by polymerizing particles in each layer and the process is repeated layer-by-layer until the desired scaffold is obtained.60 Using this technique, a research group developed a tubular scaffold using methacrylate gelatin (GelMA) with irgacure-2959 as a photoinitiator and embedded it with mouse fibroblasts.61 The tube was manufactured using a bioprinting setup fitted with a 365 nm light source to crosslink the polymer. The bed of the printer was filled with the GelMA solution, and the cells were suspended in the mix before printing. Imaging post-printing showed a porous structure with pore sizes increasing by 5% after a 24-h incubation period and staying constant past this time point. Cell viability within the construct was 75%, while the presence of cells within the matrix was shown to decrease the maximum strength of the graft to 1.7 kPa compared to 2.4 kPa for the acellular graft, and the strain decreased to 14.6% compared to 18.7%.61
Coaxial 3D bioprinting is a manufacturing technique where a coaxial needle is used to produce a tubular structure with 2 concentric materials: the core (inner layer) and sheath (outer layer).62 Using this approach, a 0.65 mm thick vascular graft was bioprinted on a coaxial nozzle where alginate mixed with human umbilical vein SMCs cells was flowed through the sheath of the nozzle as the graft material and calcium chloride was flowed through the core for crosslinking. With this approach, tubes of up to 80 cm in length could be printed, and no leakage or occlusion occurred when perfused. Burst pressure tests showed that the graft could withstand pressure of 303 mmHg. Finally, long-term static culture of the graft for 6 weeks showed cell sheets and smooth muscle deposition on both surfaces.56
Researchers have also tested a catechol-functionalized GelMA (GelMA/C) scaffold with embedded human coronary artery SMCs (HCASMCs).63 The graft was manufactured using an extrusion-based 3D printer where the printer head contained multiple needles connected to a syringe pump filled with the different bioinks. For this configuration, different inks were used for printing the internal and external layers of the graft, with a GelMA/C and HCASMC mixture for the external layer and a pluronic F127, sodium periodate (NaIO4), and endothelial cell mixture for the internal layer, with gelation occurring through the diffusion of NaIO4. After printing, the grafts were matured in cell media for 24 h and then subcutaneously implanted in mice. After 16 weeks, they found that the GelMA/C scaffold experienced significantly less degradation than the GelMA scaffold. While the presence of inflammatory cells increased for up to week 4 with a fibrosis layer forming on the gel, this inflammation was significantly reduced thereafter and by week 16, almost all inflammatory cells were gone, and the scaffold was completely encapsulated in fibrosis tissue. Furthermore, fluorescence imaging showed the presence of vasculature within the tissue by 6 weeks post-implantation indicated by the presence of SMCs and CD31+ endothelial cells.63
Similarly, another group from Soochow University developed a bilayer bioprinted vascular construct using GelMA supplemented with hyaluronic acid. The bilayer design was achieved by synthesizing bioinks with unique concentration (6% GelMA for internal layer and 4% GelMA for the external layer) and loading each of the bioinks with a specific cell line. Human umbilical vein endothelial cells (HUVECs) were embedded in the hydrogel for the internal layer while SMCs were embedded in the hydrogel for the external layer. The grafts were vertically printed on a multi-syringe extrusion-based bioprinter64 with an internal diameter of 4 mm, length of 20 mm, and wall thickness of 0.8 mm, with the internal layer representing one-third of the total wall thickness. After printing, the strength of the grafts was characterized through tensile strength and suture testing while the cell proliferation of embedded cells was studied for 7 days. The bilayer graft achieved an ultimate tensile strength of 12 kPa, significantly higher than a graft with a 4% GelMA bioink while being lower than the graft with a 6% bioink. Fluorescent imaging showed a high proliferation of cells in both layers at 7 days post-printing, while staining showed specific markers in the distinct layers.65
Lastly, as another example of natural polymers used for cardiovascular applications, a scaffold made from a gelatin-fibrinogen bioink embedded with fibroblasts was manufactured using a rotary three axis 3D bioprinter. During printing, the liquid bioink was deposited on a polystyrene rotating mandrel pre-treated with pluronic F-127 and, as the vessel is printed, the printer head slowly moves axially to deposit material on the length of the construct and radially to slowly increase the thickness of the scaffold. After printing, the grafts were submerged in thrombin to crosslink the fibrinogen and were then cultured in cell media for up to 45 days. After 7 days, the reduction in the thickness of the graft was significantly higher in samples with higher cell concentrations. In terms of mechanical strength, while the compliance significantly decreased over the course of 2 months, both the elastic modulus and burst pressure increased with a maximum of 1,110 mmHg obtained. Fluorescence imaging over the 2-month period showed a realignment of the fibroblasts such that they elongated along the circumference of the graft.66
3D printing using synthetic polymers
Digital light processing (DLP) 3D printing is another technology similar to stereolithography 3D printing but instead of using a single laser to polymerize individual points on each layers of a print, DLP printer uses a projector to polymerize the entire layer at once.60 Using that technique, a polypropylene fumarate synthetic graft was manufactured with DLP. After printing, the grafts were post-process using UV light curing, were characterized through mechanical testing, and the grafts were implanted in female mice for 6 months. Mechanical testing results revealed a maximum tensile strength of 2.06 ± 1.28 MPa and a Young’s modulus of 11.32 ± 2.82 MPa. As for the patency test, no thrombosis or stenosis was observed in any of the grafts, and they remained patent after 6 months. Ultrasound imaging showed no significant change in diameter, wall thickness, and length while scanning electron microscopy imaging demonstrated the endothelialization of the grafts.67
The use of a lithium phenyl-2,4,6-trimethylbenzoylphosphinate photoinitiated peptide-based material composed of PEG-co-depsipeptide and acryl-PEG-RGDS has been also reported.68 Since this peptide is crosslinked using visible light, the scaffold was manufactured using stereolithography 3D printing, and endothelial cells were encapsulated within the graft by suspending the cells in the peptide solution during printing. Fluorescence images were taken to assess the survivability of the cells within the construct while its elastic modulus was evaluated before and after a 7-day incubation in PBS. While the hydrogel reached an elastic modulus ranging from 3 to 38 kPa, it was significantly decreased to 0.5–1.5 kPa after swelling. Even with this decrease in elasticity, the structural integrity of the scaffold was maintained. One advantage of this decrease in elastic modulus is that cell proliferation increases as the stiffness of the scaffold is reduced, meaning that after the incubation period, the cells could slowly remodel the graft composition as the hydrogel content decreases.68
3D printing using polymer blends
Using a coaxial bioprinting process, Zhou et al. developed a GelMA vascular graft where the core was made of Pluronic F127 while the sheath was made of the GelMA solution mixed with VSMCs. After printing, the tube was cultured in growth media, and a vascular endothelial cell and gelatin solution were continuously injected in the lumen of the graft such that the endothelial cell slowly attaches to the arterial wall while the core is gradually degraded by the alginate lyase, ultimately forming a tubular structure with 2 cell layers, wall thickness of 0.3 mm, and an internal diameter of 1 mm. Mechanical testing showed a non-linear stress-strain behavior with a maximum tensile strength of 1 MPa. Fluorescent imaging showed the proliferation of the 2 distinct cell layers 6 days post-printing while immunocytochemical staining shows the presence of α-SMA and CD31 in the graft.69
Using the same bioprinting technology, a GelMA/gelatin/sodium alginate composite was used to bioprint a graft. In this particular setup, the coaxial nozzle employs 3 concentric channels such that during the printing process, the hydrogel was flowed in the middle channel while calcium chloride was flowed in the inner and outer channel for crosslinking. After printing, the graft was further crosslinked with UV light and the resulting graft had a length of 1 cm, wall thickness of 0.52 mm, and inner diameter of 2 mm. To test the in vivo degradation and cytocompatibility, the graft was implanted subcutaneously for 2 weeks and harvested for further analysis. H&E staining showed degradation at the interface between the graft and tissue as well as cell infiltration. Immunofluorescent staining showed an increase of CD31-positive markers, indicating that the composite graft could promote vascularization. Burst pressure test of the graft showed a maximum pressure of 1 MPa, significantly higher than the graft printed with only sodium alginate (0.4 MPa) and GelMA/sodium alginate (0.7 MPa) while having significantly higher compliance.70
Lastly, a 3-layer vascular graft was bioprinted with PCL in a cross-striping pattern on the inner layer, a sodium alginate/mesenchymal stem cells solution in the middle layer, and helically oriented PCL in the outer layer. The final graft had a length of 40 mm, inner diameter of 4 mm, and wall thickness of 0.5 mm. For patency testing, grafts with and without cells were implanted in the carotid and femoral arteries of 8 dogs and continuously monitored for 2 weeks. After experiment, the grafts were extracted, histology was performed, and the animals were sacrificed. Grafts with cells had a higher patency than the ones without cells while having a higher level of endothelial cells on the surface. Furthermore, a higher level of inflammation was observed in the graft without cells.71
Takeaway – 3D-printed vascular graft
Based on the findings of the analyzed studies, 3D printing is a versatile manufacturing method for the construction of vascular grafts due to its ability to recreate complex geometry at a wide range of sizes directly from a 3D-designed drawing. A large portion of the grafts studied in this section had cells embedded in the final scaffold which promoted the presence of protein markers observed in native arteries and accelerated surface endothelialization of the graft.68 However, one issue observed with bioprinted grafts is the dependence of the final mechanical properties on the printing orientation, as grafts with longitudinally aligned polymers, such as the ones obtained from coaxial bioprinting, exhibited values as low as 303 mmHg69
From all the approaches studied in this article, grafts trying to replicate the multi-layer structures of native arteries such as the three-layer grafts with PCL and MSCs71 showed promising results as the orientations of the material through the layers were optimized to provide the best mechanical properties.
Molding of vascular graft
Molding, also known as casting, is one of the oldest method for the manufacturing of parts made from a wide variety of materials ranging from polymers to metal and ceramic. In the simplest form of molding, known as gravity casting, the liquid material is poured into the mold cavity until the entire mold is filled and, after curing, the mold is disassembled and the desired scaffold can be extracted (see Figure 1C). In order to mold structures with cavities such as vascular graft, a removable core can be inserted in the mold before pouring the liquid material and can be removed after solidification.72
One of the key challenges with mold cast is the manufacturing of the mold such that the mold cavity precisely mimics the structure of the desired scaffold.73 Ideally, the mold should be made from a material that won’t melt or deform when in contact with the liquid polymer and is inert.
Molded natural polymer vascular graft
Researchers have developed a bilayer scaffold using porcine skin gelatin crosslinked with a microbial transglutaminase enzyme for the outer layer and fibrin derived from fibrinogen and thrombin for the inner layer (Singh et al.). For manufacturing, the graft was built in half-lumen sections which were then seeded with endothelial cells. After an incubation period of 2 h, the two half-sections were joined using fibrin, and the tubular scaffold was then incubated for another 4 days. Mechanical characterization of the scaffold showed an average burst pressure of 2,000 mmHg. Staining showed endothelialization on the fibrin layer, making it suitable to undergo remodeling and maturation when implanted in vivo.74
Centrifugal mold-casting is a type of manufacturing technique where a polymer is injected into a mold and the mold is rotated at high speed. This results in the polymer being slowly compacted against the wall of the mold cavity, generating a thinner but denser structure.75 Using this technique, a three-layer fibrinogen graft embedded with cells was developed. More specifically, the middle layer was composed of a high concentration fibrinogen mixture with SMCs, the outer layer was composed of a low concentration fibrinogen mixture with HUVEC, and adipose-derived stem cells (ADSCs), and the inner layer was generated by seeding HUVEC cells on the internal surface using a flow bioreactor. Performance of the graft was evaluated using a pulsatile perfusion system at physiological pressure, flow, and temperature for 72 h. Histology on the dynamically stimulated grafts showed longitudinally aligned endothelial cells on the lumen while cells in the middle layer were circumferentially aligned and expressed α-SMA. Finally, the graft itself had an internal diameter of 1.5 mm with wall thickness of 0.4 mm after pulsatile perfusion and could withstand burst pressures of ≈192 mmHg76
In another study, a group developed a fibrinogen graft with embedded human fibroblasts.77 To manufacture the graft, a solution of fibrinogen and fibroblasts were injected into a tubular mold with a cylindrical glass mandrel in the center and left in the incubator until gelation occurred. Once obtained, the grafts were placed in static culture for 2 weeks before being dynamically cultured for 9 weeks. During the dynamic culture, one group was cultured under a constant flow rate while the other group was under a pulsatile flow rate mimicking physiological conditions. Grafts cultured with a cyclic flow achieved a burst pressure of 600 mmHg compared to 350 mmHg for the grafts cultured with constant flow. Furthermore, the maximal burst of the graft was shown to significantly increase after a 4-week culture, showing that remodeling occurred during the stimulation process. Finally, stained sections of the graft showed the presence of collagen along with muscle fibers in the medial layer.77
Following on their previous work, a multi-layer mold casting approach was used to manufacture a bilayer fibrinogen vascular graft with embedded dermal fibroblasts. To prepare the graft, a mixture of fibrinogen solution and dermal fibroblasts were poured into a tubular mold with a mandrel in the center until gelation. After 18 days of static culture while still mounted on the mandrel, bi-layer scaffolds were prepared by wrapping one construct around another one reinserting the mandrel into the inner construct such that both constructs were compressed together. Finally, the bi-layer (DL) construct was statically cultured for 3 weeks alongside a single-layer version (SL) of the construct and a bi-layer version where both layers were separated every week to prevent fusion (DL-U). After culture, burst pressure and morphology were assessed. Histological analysis showed remodeling between the 2 layers of the DL scaffold, which was not observed on the DU-L graft, while the cells in the DL graft remained segregated to their distinct layer. Finally, the DL graft resisted a burst pressure of 530 mmHg, double that of the SL and DL-U grafts, showing that the fusion between the layers significantly contributed to the strength of the graft.78
In another study, a collagen vascular graft embedded with either human bone-marrow-derived mesenchymal stem cells or human neonatal dermal fibroblasts was designed, which was seeded with endothelial cells on the inner surface through continuous perfusion. The graft itself was initially casted in a 3-cc syringe as a mold in which a 0.8 mm rod was inserted to form the lumen of the graft. After casting, the rod with the scaffold was removed and wrapped in kimwipes in order to reduce the water content and increase the collagen density. The grafts were then injected with endothelial cells in the lumen and were centrifuged to evenly seed the cells. Using a perfusion system, the grafts were dynamically stimulated for a period of 5 weeks during which the change in size and strength of the graft was measured. Grafts perfused for 1 week had a burst pressure of 1,777 mmHg compared to 1,104.5 mmHg for the graft cultured under static condition while the inner and outer diameter went from 0.8 to 1.6 mm down to 0.5 and 0.6 mm. Finally, grafts stimulated under dynamic conditions showed significantly greater expression of α-SMA and calponin markers compared to statically matured grafts.79
One interesting approach in vascular tissue engineering is the ability to develop in vitro disease models to better understand the physiology and progression of diseases. Using the manufacturing technique of the previous study,79 collagen-based vascular grafts were produced using induced pluripotent stem cell-derived SMCs and endothelial cells (ECs) from Hutchinson-Gilford progeria syndrome (HGPS) patients, which were then compared against grafts containing cells from healthy donors. For context, HGPS is a genetic condition shown to cause atherosclerosis in young patients, which can lead to stroke or heart attacks at a young age due to the excessive accumulation of the progerin protein. Progerin is present in the vascular SMCs, fibroblasts, and ECs of patients with HGPS.80 After the grafts were casted and seeded with ECs, they were dynamically stimulated in a perfusion system for 5 weeks. As expected, compared to healthy grafts, the grafts cultured with HGPS-derived cells showed reduced vasodilation and vasoconstriction as well as increased expression of endothelial markers associated with cardiovascular disease. Finally, the graft with HGPS-derived cells exhibited similar maximal burst pressure (1,575 mmHg) compared to the graft with healthy cells (1,500 mmHg).81
Another example of collagen for engineered vascular grafts is the work by Li et al., whereby a high-concentration collagen mixture was cast in a cylindrical polydimethylsiloxane mold of 8 mm diameter with a stainless-steel rod of 1 mm in the center to form the lumen. After solidification, the scaffolds were dried at 37°C overnight, after which they were rehydrated and the mandrel was removed. Finally, the rehydrated grafts were crosslinked with genipin. Mechanical characterization of the crosslinked graft resulted in a maximum burst pressure of 1,313 mmHg, significantly higher than the non-crosslinked graft (63 mmHg). Furthermore, a suture retention test when attached to a native rat femoral artery showed that the crosslinked graft could withstand higher anastomotic strength compared to the non-crosslinked vessel. Finally, the crosslinked tube was implanted into a rat femoral artery and was anastomosed on both ends with 16 sutures; the authors then observed that blood flow was maintained for 20 min without leaks.82
Molded synthetic polymer vascular graft
Polyglycolic acid (PGA) is one of the potential candidates for synthetic vascular graft. This particular study employs the use of a biodegradable PGA scaffold coated with poly-4-hydroxybutyrate as a potential graft candidate. After molding, myofibroblasts were seeded on the scaffold and cultured in a bioreactor for 14 days. Once matured, the grafts were surgically implanted into lambs’ pulmonary arteries and studied for 100 weeks, during which CT and echocardiography were regularly performed. Imaging showed no presence of thrombosis or aneurysm while a 45% increase in graft length was observed over the duration of the study and a smooth luminal layer was present inside the grafts. As for the mechanical properties, a significant increase in tensile strength was observed post-implantation (2 MPa) compared to the pre-implantation graft (1.25 MPa).83
Thermoplastic polyurethane (TPU) is another potential candidate for synthetic graft due to their desirable mechanical properties. As such, a group from the Korea University Ansan Hospital developed a TPU vascular graft which was molded around a sacrificial 3D-printed polyvinyl alcohol core. After molding, the core was removed through sonication and the grafts were obtained. Patency test was performed in a rat model where the graft was implanted as a vascular patch on a 1.5 cm incision across the aorta and kept for 30 days. Finally, the animals were sacrificed, and the aorta was collected for histology and staining. While the TPU graft showed a higher endothelial activation compared to the commercial graft at day 7, it was not present in either graft by day 30. Histological analysis showed that the TPU molded graft had a significantly lower level of thrombogenesis, slightly lower calcification, and slightly higher neovascularization compared to the control ePTFE graft. Mechanical testing show that, while the tensile strength was 42% lower than a standard ePTFE commercial synthetic graft (23 MPa compared to 15 MPa), the molded graft was significantly more flexible than the commercial vessel.84
Molded vascular graft from blends
Freeze casting is a manufacturing process where a solid structure is generated by subjecting a liquid polymer through a large temperature gradient with one end on a cold source such as liquid nitrogen. As the polymer cools down, ice crystals start to nucleate on the cold side and slowly grow directional to the temperature gradient. Once solidified, the scaffold is freeze-fried to sublimate the ice crystal, generating a porous structure and the size of the pores can be reduced by sintering.85 Using that technique, hybrid gelatin and silk fibrinogen were manufactured and as a control, grafts using the same composition were generated by freeze drying. Finally, the grafts were coated with a layer of PCL on the outer surface. Scanning electron microscope images of the freeze-cast grafts shows regular lamellar structure oriented in the longitudinal direction compared to the random structure of the freeze-dried graft. Based on preliminary computational fluid dynamic simulation, the lamellar structure of the freeze-cast graft should result in a more laminar flow and less turbulence than the freeze-dried graft and should result in lower shear stress on the blood. These observations were confirmed in the in vivo model where the graft was implanted for 3 months on the carotid arteries of rabbits where the graft with the lamellar structure had excellent patency and did not experience any occlusion compared to the freeze-dried graft.86
Takeaway – Molded vascular grafts
Based on the findings of this section, it can be concluded that mold casting remains a viable approach for the manufacturing of vascular grafts. The simplicity of the manufacturing setup makes this approach accessible for most materials, engineers, and scientists as it only requires a simple cylindrical shape mold. Most of the grafts showed a great level of endothelialization and protein marker expression similar to that of native arteries. However, one of the remaining challenges with mold casting is the relatively low burst pressure of some of the scaffold directly after molding. Thus, some form of post-processing, such as long-term maturation in dynamic conditions,76 the addition of another synthetic material through electrospinning,86 or adding a crosslinker,82 is necessary to increase the strength of the graft. One of the most promising approaches is the freeze-casted graft reinforced with electrospun PCL fibers as it has a lamellar anisotropic structure that promotes laminar flow and prevents high shear stress, which can lead to thrombosis, while offering high burst pressures due to the addition of the fibers.
Decellularization
Decellularization is a widely used technique for the development of SVGs. All of the scaffold’s cell content is removed using a mixture of freeze-thawing, osmotic gradients, solvents, detergents, enzymes, chelating agents, and physical techniques on tissues derived from allografts or xenografts. Following the removal of cells, the ECM, which is mainly made of collagen, is maintained (Figure 1D).87,88,89,90 This scaffold possesses abundant cell signaling molecules that can facilitate cell adhesion, migration, and proliferation.87 As an example of this approach, Gu et al. developed a decellularized porcine carotid artery where the scaffolds were subsequently crosslinked with genipin.91 Histological analysis of the decellularized scaffold showed that the structure was not damaged by the decellularization process. Mechanical testing of the modified scaffold resulted in a BP of 2,182 mmHg, significantly higher than the decellularized scaffold (1,747 mmHg) and almost on par with the harvested graft (2,270 mmHg). The grafts were implanted subcutaneously in beagle dogs for 4 weeks and showed that the crosslinked decellularized graft had the lowest inflammatory response while experiencing the least amount of degradation among all three types of graft.91
An interesting approach for the development of SVGs has been made by Humacyte, a company that is actively working in the field and created the human acellular vessel (HAV).92 In this innovative method, the company begins isolating human VSMCs, expands them in culture, and seeds them onto a biodegradable PGA mesh scaffold, which is maintained within a bioreactor and subjected to pulsatile, cyclic distension. Subsequently, the scaffold degrades, and the newly formed matrix is decellularized to remove immunogenic material. The inner diameter of this HAV is 6 mm; however, it can be created in different sizes. The HAV is currently in various stages of clinical trials targeting multiple applications and in the long term, the HAV is planned to be applied to coronary circulation.93
As another approach, porcine aortas were peeled to produce thin sheets, which then underwent a high-hydrostatic pressure decellularization treatment. Following the decellularization treatment, the sheets were carefully wrapped around a 1 mm rod, forming tubes that were then glued at the ends. Subsequent mechanical assessment showed a BP of 400 mmHg, which occurred at the bonded sites of the graft. These decellularized grafts were then surgically implanted into the carotid arteries of rats, and a 3-week follow-up was conducted. At the end of the observation period, no signs of acute rejection or occlusion were detected. Notably, a layer of endothelial-like cells was found in the inner surface of the grafts, although limited host cell infiltration was observed.94
In one study, enzymatic digestion was used to decellularize fetal pig aortas with an inner diameter of 4 mm. Vascular ECs were subsequently isolated from seven dogs, cultivated to the fourth passage, and seeded onto decellularized scaffolds. The grafts were placed in a bioreactor for 10 days (7 days for a dynamic culture and 3 days for a static culture), and then were implanted into the common carotid artery of the seven cell donor dogs for 6 months. The results revealed a consistent inner cell layer and a dense tunica media, with no signs of stenosis. Additionally, all grafts demonstrated good patency and in situ remodeling. These results were compared to those of grafts without vascular EC seeding, which showed significantly lower patency rates of about 60%. The study indicates that one potential limitation may be the acquisition of ECs from recipients. Additionally, it is mentioned that extending the follow-up period after implantation could provide valuable insights.95
As another example, the culture of allogeneic SMCs from cadaveric donors on a degradable PGA scaffold led to the formation of a collagenous matrix that was deposited as the PGA degraded.96 Subsequently, the obtained tissue was decellularized to remove antigenic and allogeneic cells in order to produce a non-immunogenic VG. The scaffold was then seeded with autologous ECs from recipient canines. Three grafts were implanted into the left anterior descending coronary artery of dogs with a follow-up of 1 month and another four were implanted as carotid artery bypass grafts with a follow-up of 12 months. The results showed a patency of 83% of the implanted VGs. After implantation into canines, the VGs showed considerable in situ remodeling. The grafts were well integrated with the native tissue at the anastomotic sites and cells with EC-positive markers were identified in the lumen of the grafts, close to the anastomotic sites and midgraft. Additionally, αSMA-positive cells were also found. Intimal hyperplasia aneurysmal dilatation or calcification in the long term was not observed. In terms of mechanical properties, a BP of 1,618 mmHg was achieved for the 3 mm grafts. A limitation of this study is the small number of implanted grafts and the short follow-up for those implanted into the LAD coronary artery.96
As a similar approach, rats’ aortas were collected and underwent a decellularization treatment. Then, the decellularized scaffolds were seeded with dermal fibroblasts from thrombospondin-2 knockout (TSP2 KO) mice to create a platelet-adhesion-resistant ECM. After culture, the seeded scaffolds underwent a secondary decellularization. The obtained grafts were implanted into abdominal interpositions and a follow-up of 4 weeks was made. The TSP2 KO ECM-modified grafts showed fewer adherent platelets in comparison with an unmodified control and a patency of 100%. Additionally, SMC-like cells were found in the media along with an increased luminal endothelial cell presence. This study mentions that additional testing in a bigger model is required to validate the pro-migratory effect of the modified scaffolds on ECs and further analysis is also necessary to determine whether the modified ECM could disturb the equilibrium between ECM production and degradation by repopulating cells.97
Takeaway – Decellularization
As previously described, decellularization remains one of the most widely used and promising approaches for creating SVGs. However, some limitations persist, such as residual cellular structures and modifications to the ECM during the decellularization treatment,89,98 which can elicit immune reactions and potentially lead to immune rejection of the decellularized matrix.17,31,87 To address these challenges, some of the reviewed studies explored the incorporation of recipient’s cells to enhance the biocompatibility of the graft and promote in situ regeneration and integration with the native tissue. The results demonstrated improved attachment and proliferation of cells, as well as higher patency rates compared to non-cellular decellularized matrices.
In vivo maturation
As opposed to most lab-made grafts which are manufactured outside the body, capsule autografts are produced by using the patient’s own body as a bioreactor in an in vivo maturation process. Essentially, a fibrous capsule composed of cells and collagen is formed on the surface of a tubular support and, after maturation, the graft is collected and reimplanted into the patient (Figure 1E).99 The phenomenon of capsule formation occurs due to an immune response from the body when a foreign object is present in the body.100 One key advantage of this method is the fact that the resulting graft is composed of the patient’s own cells and, in theory, should not produce any adverse immune response and would have a great level of biocompatibility.100 As an example of this manufacture method, plastic polyethylene tubes (8 mm long, 0.96 mm diameter) were implanted in the peritoneal cavities of mice, and tissue capsules were collected after 8 weeks and stained for the presence of cells and collagen material.101 Antibody staining showed that the capsules were mostly composed of a collagenous material, but the absence of both SMC and endothelial cell markers suggested a lack of vascular cells within the capsule. To test their patency as a vascular graft, the capsules were surgically implanted into the abdominal aorta of the same animals with the control consisting of arterial and venous iso-grafts. After 16 weeks, they found a survival rate of 61.9% for mice receiving the capsule graft versus 90.9% for those with arterial grafts and 76.9% for those who received venous grafts. As for the graft’s functional performance, micro ultrasound results showed minimal deformation in both diameter and length while laser Doppler imaging showed no difference in flow between all 3 graft types.101
In a more recent study, two cylindrical rods were implanted into the neck of adult female Dutch milk goats, and tissue capsules were collected for a period of 1 month.102 After maturation, the resulting capsular grafts were harvested. One capsular graft was reimplanted between the carotid artery and jugular vein of the same goat for 2 months and the other capsule was used for mechanical testing and histology. Mechanical testing of the capsule showed an average burst pressure of ∼2,400 mmHg (within the range of gold-standard vessel values). Staining showed that the capsule is mainly composed of ECM components, fibroblasts, myofibroblasts, and collagen. Two months after grafting, it was found that the graft went through significant in situ remodeling with a high level of elastin and SMCs present.
As another approach, caged molds were designed for the creation of biotubes. The caged molds were embedded into the dorsal subcutaneous pouches of beagle dogs for 4 weeks. After harvesting, biotubes were obtained by removing the extra peripheral tissues and the caged molds. The obtained biotubes were formed from collagen-based tissue, with a collagen layer oriented circumferentially. Mechanical assessment indicated a burst pressure of around 1,825 mmHg. Subsequently, the biotubes were implanted into the femoral arteries of the beagle dogs, and a follow-up of 7 days was conducted. Angiography results showed 100% patency, with no signs of stenosis or aneurysms. The short follow-up period is mentioned as a limitation that should be addressed in further studies.103
Based on the promising results of their initial in vivo study with the biotube,103 the same research group expanded the scope of the biotube concept by significantly increasing the length of the graft obtained in vivo up to 50 cm of length and expanding the length of the in vivo patency test to 3 months. Burst pressure of the 50 cm biotubes reached 1,500 mmHg and was consistent at every section of the graft, showing that manufacturing longer biotubes did not negatively affect the mechanical properties. Angiography of the graft 3 months post-implantation showed normal flow with no sign of thrombosis and no leak or failure was observed at the anastomosis point between the artery and graft. Finally, histology of the grafts implanted for 3 months showed presence of endothelial cell layer on the internal surface as well as presence of SMA inside the arterial wall.104
In another study, polyethylene oxide terephthalate/polybutylene terephthalate rods with an inner diameter of 4.2 mm and length of 80 mm were implanted subcutaneously in the abdominal area of pigs as an in vivo bioreactor for 4 weeks, and then they were harvested. The rods were removed from the tissue capsule, and subsequently, 4 cm segments of tissue capsules were bilaterally implanted as autologous carotid artery interpositions. After 4 weeks of grafting, the fibrous capsule was composed of circumferentially aligned collagen fibers and vascular SMC-like cells along the 4 cm graft. Additionally, an endothelial lining was observed after 4 weeks of implantation, suggesting in situ remodeling. The mechanical assessment showed a BP of around 3,950 mmHg before grafting and around 5,200 mmHg after grafting. No acute thrombosis was observed, and the patency rates after 4 weeks were of 87.5%, attributed to peri-anastomotic intimal hyperplasia of one of the grafts. Some limitations of the study were the short follow-up period and the use of healthy pigs with high re-endothelialization capacity.105
As a different approach, silicon rods with inner diameter of 2 mm and length of 30 mm were embedded into the dorsal subcutaneous pouches of rabbits (four rods per rabbit). After 1 month, the rods surrounded by connective tissue were harvested and the rods were removed. Subsequently, the obtained biotubes were seeded with ADSCs from the rabbits and after culture, they were implanted into the rabbit’s carotid artery with a follow-up of 5 months. The cell-seeded biotubes demonstrated 100% patency rates after 5 months of implantation, which was higher than a control group of biotubes that were not seeded with ADSCs (60% after 3 months). It was additionally observed that ADSCs began differentiating into not only ECs but also SMCs.106
Takeaway – In vivo maturation
Based on the reviewed studies for this section, it can be concluded that fibrous tissue capsules formed by in vivo maturation process remain as a promising approach in the field of vascular engineering. These fibrous capsules, composed of autologous cells and ECM components, demonstrate high biocompatibility levels reducing the risk of immune rejection.100 However, some challenges persist, such as incomplete cell infiltration and long culture periods.107,108 After reviewing these studies, it was also observed that some of these grafts underwent a remodeling process, thus integrating with the native tissue and improving their patency. Additionally, some studies included the addition of cells which significantly improved the patency rates as well as induced differentiation into ECs and SMCs. These findings highlight the potential of this manufacturing method.
Discussion
Several research groups have worked on the fabrication of small-diameter vascular grafts that meet the requirements for microvascular replacement by focusing on improvements in biocompatibility, gradual degradation, reducing cytotoxicity, the promotion of cell adhesion, spreading and proliferation, and suitable mechanical properties.48,109 In the following sections, we will analyze the data of the articles discussed in this review to illustrate the manufacture methods, range of properties, and tests carried out by the different research groups. This analysis is intended to provide the reader with a more in-depth understanding of the most recent state-of-the-art technologies and materials in the field of small-diameter vascular grafts.
Manufacturing methods
Different manufacture techniques have been widely applied for the creation of SVGs. This review focuses on commonly used approaches for SVG creation, including electrospinning, 3D printing, molding, decellularization, and in vivo maturation. Additionally, some examples of each method were provided. Table 1 outlines the different vascular graft manufacturing methods, as well as their associated pros and limitations.
Table 1.
Manufacturing methods, pros, and limitations
| Vascular graft manufacturing methods | Pros | Limitations |
|---|---|---|
| Electrospinning | Electrospinning can create nanofibrous scaffolds that mimic the microenvironment of natural ECM, promoting cell interaction.26 This method provides control over fiber diameter and scaffold porosity.88 Additionally, the nanofibers’ structure could control cell orientation110 | Practical challenges with electrospinning include restricted cellular ingrowth and penetration due to a small pore size which limits cell migration into the scaffold.111,112 Additional toxicity from chemical debris within the fibers or from post-processing are a potential risk112 |
| 3D printing | 3D printing offers the ability to directly manufacture a graft based on a 3D CAD design which can be imported from CT or MRI data57 Large variety of 3D printing techniques offer different possible arrangements of the polymers and the ability to use different polymers in a single scaffold71 Cells can be directly mixed within the liquid polymer and precisely spatially arranged within the final scaffold which was shown to improve the endothelialization and vascularization of the graft after in vivo implantation65 |
Mechanical properties of the graft are dependent on the direction of the printing113 Viscosity of the bioink needs to be optimized when used with an extrusion bioprinter to avoid applying excessive shear stress to cells114 Softer biomaterial/hydrogel requires the use of a support bath to maintain the integrity of the material during printing59 |
| Molding | Accurate and consistent way of producing vascular graft with a simple setup79 Compatible with a wide variety of materials from natural to synthetic polymers, blends, and metals115 Freeze casting produces a scaffold with a determined anisotropic lamellar structure85 | Depending on the type of mold and desired level of accuracy, the cost associated with the manufacturing and machining of the core and mold can be significant73 Injection process of the polymer inside the mold makes it hard to control the final microstructure of the scaffold82 Presence of cavity in the final scaffold requires the use of core, limiting the complexity of the final geometry72 |
| Decellularization | After cells are removed, the ECM preserves its inherent properties, offering a surface abundant in cell signaling molecules that facilitate cell adhesion, migration, proliferation, and differentiation87,88 | After decellularization treatment, debris of cellular structures may persist, and the physical and chemical stresses involved in this process may result in modifications to the ECM components, which may affect the vessel’s biomechanical properties89,90 Additionally, strong immunological responses following the implantation of decellularized scaffolds could cause thrombosis, immune rejection, and infection17,31,87 |
| In vivo maturation | The grafts are composed of the patient’s own cells, ensuring biocompatibility, and reducing the risk of adverse immune responses. Additionally, they are associated with low risk of thrombus formation100,107,108 | Long maturation times and elevated costs remain as limitations to their wide use in clinical applications107,108 |
In summary, while electrospinning provides precise control and tunability over the scaffold properties, challenges such as limited cellular infiltration need to be addressed. Similarly, 3D printing method offers customization properties but further optimization remains to be done. Molding methods offer simplicity; however, mold fabrication may involve elevated costs. On the other hand, decellularization and in vivo maturation approaches offer biocompatibility but face challenges related with immunological responses and long maturation times.
Additionally, Table 2 offers valuable information about the categories of the materials used for the manufacturing methods including synthetic, natural, and blends of natural/synthetic, and their advantages, limitations, and polymer examples as well as their properties. Similarly, in Table 3, the materials used for each manufacturing method are listed.
Table 2.
List of polymers with material categories, pros, limitations, and properties
| Material category | Pros | Limitations | Materials | Properties |
|---|---|---|---|---|
| Synthetic | They can be readily modified and functionalized, providing a high degree of tunability to meet specific requirements. Furthermore, synthetic polymers frequently exhibit excellent mechanical properties, enhancing their suitability for use in vascular grafts88,109 | Vascular grafts made of synthetic polymers face some limitations as thrombogenicity, incomplete endothelialization, and stenosis. Additionally, they could trigger immune inflammatory responses88 | PCL | Biocompatible and biodegradable88 |
| PLCL | Copolymer of PCL and PLA. Slow degradation, biocompatibility, and elasticity88 | |||
| PU | High elasticity, optimum strength and compliance, and good biocompatibility32,36 | |||
| PEUU | Cytocompatibility, with non-toxic degradation products and high tunability52 | |||
| PLLA | Biocompatible and biodegradable with tunable mechanical properties. It has a hydrophobic character88 | |||
| PET | Biocompatible, resilient, stiff, and durable polymer with high tensile strength36 | |||
| TPU | It is a class of PU with high elongation and tear resistance properties. It also has good biocompatibility37 | |||
| GO | Contains a large number of hydrophilic groups. In high concentrations can be cytotoxic37 | |||
| PGS | Fast degrading elastomer with good biocompatibility and tunable mechanical properties38 | |||
| PEG | Antifouling hydrophilic polymer. Shows great resistance against protein adsorption49 | |||
| PPF | Biodegradable polymer with great mechanical properties often used in SLA 3D printing67 | |||
| PEG-co-PDP | Photocrosslinkable biodegradable polymer used for stereolithography 3D printing68 | |||
| PGA | Thermoplastic polymer with great biocompatibility83 | |||
| PVA | Water soluble polymer used to form a sacrificial core for hollowed features in 3D printing and molding84 | |||
| PE | Durable polymers. Commonly used to create rods for the in vivo maturation method101,106 | |||
| Silicon | ||||
| Natural | They have biocompatibility properties, and closely mimic the ECM of native tissues, promote cellular attachment, and are biodegradable116 | Natural polymers often lack the mechanical properties required for vascular engineering applications. This can limit their suitability for use in vascular grafts88 | Silk fibroin | Excellent biocompatibility and non-cytotoxicity88 |
| Fibrinogen | Glycoprotein present in fibrin and responsible for the clotting ability of native artery117 | |||
| Collagen | Low antigenicity, high biocompatibility, biodegradability, and support for cell adhesion88 | |||
| Gelatin | Low-cost derivative of collagen. Improves cell infiltration, adhesion, spreading, and proliferation42 | |||
| Methacrylated gelatin (GelMA) | Gelatin functionalized with methacrylic groups. Can be crosslinked into a hydrogel with good biocompatibility and stable at physiological temperature61 | |||
| rTE | Monomer unit of elastin. When cross-linked, it mimics native elastin (responsible of energy storage and recovery)43 | |||
| Sodium alginate | Biocompatible material that forms a gel in the presence of sodium chloride70 | |||
| Fibrous tissue capsule | Fibrous material made from cells and collagen formed due to the body’s immune response in the presence of a foreign object101,102,103,104,105,106 | |||
| Decellularized vessels | ECM scaffolds mainly composed of collagen. They underwent a decellularization process87,88,89,90 | |||
| TPU + silk fibroin | Combines the non-immunogenicity properties of silk fibroin and the resistance and elongation properties of TPU44 | |||
| Blends (natural + synthetic) | These blends incorporate the biological characteristics of natural and synthetic polymers to produce scaffolds with good mechanical properties and that promote cellular adhesion and biodegradability88 | One challenge of polymer blend is to ensure uniform distribution and integration between the difference class of polymers within a composite material35 | Gelatin+ PCL |
Combines the biocompatible and cell adhesion properties of gelatin and collagen with the mechanical strength and high tunability of PCL and PLCL47 |
| Collagen+PLCL | Combines the biocompatible and cell adhesion properties of gelatin and collagen with the mechanical strength and high tunability of PCL and PLCL47 Pluronic F127 is used as a temporary support material for GelMA during printing due to its thermal sensitivity69 |
|||
| Collagen + PCL | ||||
| GelMA + Pluronic F127 | ||||
| PCL + sodium alginate | Combine the strength of PCL with the biocompatibility and easy gelation of sodium alginate71 |
Table 3.
List of materials for each manufacturing method
| Category | Material | ES | 3D printing | Mold casting | Decel. | In vivo mat. |
|---|---|---|---|---|---|---|
| Synthetic | PCL | ✓ | ||||
| PLCL | ✓ | |||||
| PU | ✓ | |||||
| PEUU | ✓ | |||||
| PLLA | ✓ | |||||
| PET | ✓ | |||||
| TPU | ✓ | ✓ | ||||
| GO | ✓ | |||||
| PGS | ✓ | |||||
| PEG | ✓ | |||||
| PPF | ✓ | |||||
| PEG-co-PDP | ✓ | |||||
| PGA | ✓ | ✓ | ||||
| PVA | ✓ | |||||
| Silicon | ✓ | |||||
| PE | ✓ | |||||
| Natural | Silk fibroin | ✓ | ||||
| Fibrinogen | ✓ | ✓ | ||||
| Collagen | ✓ | ✓ | ||||
| Gelatin | ✓ | ✓ | ||||
| rTE | ✓ | |||||
| GelMA | ✓ | |||||
| GelMA/C | ✓ | |||||
| Gelatin-Fibrinogen | ✓ | ✓ | ||||
| Sodium alginate | ✓ | ✓ | ||||
| Blends (natural + synthetic) | TPU/silk fibroin | ✓ | ||||
| Gelatin/PCL | ✓ | ✓ | ||||
| Collagen/PLCL | ✓ | |||||
| Collagen/PCL | ✓ | |||||
| GelMA + Pluronic F127 | ✓ | |||||
| PCL + Sodium Alginate | ✓ |
Physical dimensions and mechanical properties
Most of the vascular grafts considered in this review have an inner diameter between 2 and 4.8 mm, an outer diameter of around 2.5.3–5.5 mm, and a length between 8 and 30 mm (Figure 2). The inner diameter dimensions of these engineered grafts are within the range of sizes of autologous coronary arteries (∼4.0 mm),118 suggesting that they are promising for use as suitable conduits in coronary artery bypass surgery.
Figure 2.
Physical dimensions of the engineered small vascular grafts discussed in this review
(A) Inside and outside diameter (mm).
(B) Length (mm). Values in plots are depicted in boxplots, where the box encloses 50% of the data and the upper and lower quartiles, with the median value of the variable displayed as a line inside the box.
In this section, we have focused on further analyzing the burst pressure as opposed to the Young’s modulus or ultimate tensile strength (UTS) due to the anisotropy of native vascular tissue. Native arteries and veins are composed of 3 main layers (tunica intima, tunica media, and tunica externa) where the SMCs and elastin/collagen fibers are differently oriented depending on the layers.119 As such, uniaxial mechanical characterization such as a tensile test will result in a different UTS or Young’s modulus depending on the direction in which the test was performed.120 On the contrary, direct burst pressure measurement is a form of multi-axial testing which considers the anisotropic behavior of the tissue as the graft is deformed both circumferentially and longitudinally.30 The burst pressure can be used to predict the behavior of the graft in vivo as the burst pressure of native arteries is known from literature and blood pressure is continuously monitored in patients. A minimum burst pressure is required for engineered grafts in order to prevent rupture upon implantation.
Lastly, most biomaterials exhibit a non-linear stress-strain relationship, meaning that the Young’s modulus varies depending on the applied load, making this parameter non-ideal for predicting the behavior of the graft in physiological conditions.121 Ideally, a more practical value for measuring the elasticity of a graft is vascular compliance as it gives a percentage change in diameter for a specific change in pressure.121 However, most of the articles reviewed here omitted this test as it requires a more complicated testing setup and methodology.
Since autologous veins and arteries are the gold standards for coronary revascularization,29 it is imperative for engineered vascular grafts to achieve mechanical properties that enable them to withstand burst pressure levels that are at least as high as those observed in autologous vascular grafts such as native SV (1,599 ± 877 mmHg) and native IMA (3,196 ± 1,264 mmHg).29 The engineered vascular grafts analyzed in this review were divided into five categories by manufacturing method: electrospinning, 3D printing, molding, decellularization, and in vivo maturation.
While the highest burst pressures were achieved in grafts manufactured through electrospinning, there was no significant difference in burst pressure between grafts made by electrospinning, in vivo maturation, and 3D printing methods (Figure 3). However, this might be due to the small sample size in the 3D printing category as most of the grafts were only tested in uniaxial tensile tests instead of burst pressure. Furthermore, the variability in the electrospun vessel burst pressure is likely attributable to the large variety of polymers used, which all have different stiffness and rigidity based on their chemical compositions.122 Molded and decellularized grafts performed similarly but had lower burst pressure than electrospinning, in vivo maturation, 3D printing, and autologous grafts. Based on the available data, electrospinning and in vivo manufactured grafts hold potential as a viable solution in developing engineered vascular grafts with adequate burst pressure values to mimic those of autologous vessels, but more examples with the other manufacturing methods would be needed to properly assess their viability.
Figure 3.
Burst pressure plot of the different categories of vascular grafts
Electrospun (Electro), in vivo maturation (in vivo), 3D printing, molding, decellularization (Decel.), and autologous vessels (Autologous). Values in plots are depicted in boxplots, where the box encloses 50% of the data and the upper and lower quartiles, with the median value of the variable displayed as a line inside the box.
Going forward, a critical aspect to consider in the development of engineered vascular grafts is to evaluate them based on international standards recognized by federal agencies, such as ISO 7198:2016 Cardiovascular implants and extracorporeal systems – vascular prostheses-tubular vascular grafts and vascular patches. These standards include the requirements for the evaluation of tubular vascular substitutes implanted by surgical techniques with the aim of replacing, bypassing, or creating shunts between different segments of the human vascular system. The methods proposed by ISO 7198:2016 to evaluate the mechanical properties of the vascular grafts are dynamic radial compliance test, circumferential tensile strength, diaphragm pressurized burst strength, longitudinal tensile strength, pressurized burst strength, and probe burst strength.30,123 Nonetheless, most VGs included in this review did not undergo all these assessments and the testing equipment employed may differ, which is why ISO provides specific testing equipment specifications for each mechanical property that needs to be evaluated. Whenever feasible, the testing protocols should adhere to the ISO’s recommendations and include testing in both the longitudinal and circumferential directions,30,123 as this will help standardize the field, improve the interpretation and comparison of results, and advance translation to the clinic.
Animal models for testing engineered vascular graft in vivo
Preclinical testing of engineered vascular grafts in appropriate animal models is crucial in order to evaluate their safety and effectiveness. See Figure 4 for a summary of the animal models used in the studies reported in this review. This helps to anticipate any potential harm to patients and predict the likelihood of successful long-term clinical application. Therefore, selecting the most suitable animal model is essential. While there is no perfect animal model that meets all the requirements for testing vascular grafts, there are several animal species that can be used in different test settings. Consequently, it is advisable to implant the grafts in a reasonable number of animals for an appropriate observation period, and all animals involved in the study, including those that are excluded from the final analysis, should be documented and reported.124
Figure 4.
In vivo assessment of the discussed grafts
(A) Animal models used for the in vivo assessment of the reviewed engineered small vascular grafts.
(B) Follow-up periods of the in vivo experiments for the articles reviewed in this mini review. Values in B are depicted in boxplots, where the box encloses 50% of the data and the upper and lower quartiles, with the median value of the variable displayed as a line inside the box.
Non-human animal model organisms are utilized to mimic the human response, with specific characteristics that should resemble the human target of interest. Disease animal models can be categorized as either spontaneous or induced and may be relevant for testing and developing engineered vascular grafts. Ultimately, the ideal animal model should exhibit all stages of the disease.124
Small animal models
Small animal models are commonly employed to screen potential biomaterial candidates for developing vascular substitutes and to perform early functional tests. This is due to their easy availability, low maintenance and handling requirements, and reasonable experimental costs even with large cohorts. In addition, a variety of genetically modified strains are available. Small animal models have certain limitations due to their smaller anatomical size, shorter life expectancy, and physiological differences in parameters such as cardiovascular physiology and hemostasis mechanisms when compared to humans.125 Nonetheless, if the results of preclinical rodent models are to be cautiously interpreted, and limitations carefully considered, these models can be an essential resource in the development of vascular grafts.124 Among the available small animal models, rats and rabbits emerged as the most appropriate candidates for initial-stage preclinical trials. Rats can be employed to evaluate the conduit immune response, biodegradation, biocompatibility, and performance. On the other hand, rabbits, due to their size, are better suited for surgical interventions. Moreover, rabbits exhibit hemostasis and endothelialization mechanisms like those of humans, which are necessary for the evaluation of remodeling and thrombogenic properties of vascular grafts. These characteristics make rabbits the ideal candidates for preliminary preclinical trials before transitioning to assessments in larger animal models.124 Table 4 describes the advantages and disadvantages of the most used small animal models in SVG testing.
Table 4.
Small animal models commonly used in testing SVG
| Species | Pros | Limitations |
|---|---|---|
| Mouse | Grafts have been successfully implanted into the carotid or femoral artery of mice. Mouse models can provide advantages in the study of pathophysiological mechanisms as there are numerous genetically modified strains with targeted mutations in the area of cardiovascular disease readily available124 | Due to their limited vessel size, mice are rarely used in the development of tissue-engineered vascular prostheses. Even in vessels with larger diameters, such as the aorta with an inner diameter of 1 mm, implant insertion is extremely challenging124 |
| Rat | Preferred over mice due to their larger body weight and vessel size. They are commonly used in preclinical studies to assess the immune response, biodegradation, biocompatibility, and the performance of tissue-engineered vascular grafts (TEVGs), prior to conducting large animal studies. The adult Sprague-Dawley rat has an internal diameter of up to 1.5–2 mm in the abdominal aorta, making it the most used animal model for this purpose124 | Rats are more susceptible to ischemia/reperfusion phenomena, as evidenced by irreversible hindlimb ischemia124 |
| Rabbit | Commonly employed as a preliminary model to test various tissue-engineered conduits. They are easy to breed and have a high number of offspring, have lower costs for purchase and maintenance compared to larger animals, and are easy to handle for routine controls and graft assessment. The size of the rabbit’s blood vessels (diameter and length) is more suitable for surgical interventions than in small rodents. Additionally, their hemostasis and endothelialization mechanisms are more similar to humans than in small rodents124 |
Coagulation and fibrinolysis in rabbits is more active compared to humans124 |
For a more comprehensive and in-depth exploration of the different animal models suitable for preclinical trials, encompassing a thorough examination of the strengths and limitations associated with each type, the reader can consult reviews and research articles related to this subject matter.125,126,127,128 Additionally, it is highly recommended to refer to the regulatory guidelines and recommendations offered by authorities such as the Food and Drug Administration and other relevant regulatory agencies. These resources provide invaluable insights into the selection considerations surrounding animal models in preclinical research, ensuring the highest standards of safety and efficacy in medical and scientific advancements.
Large animal models
To ensure successful clinical translation, it is imperative to validate findings from small animal model studies in large animal models. Large animal models exhibit greater similarity to humans in terms of vascular anatomy and physiology.129 Within this group, pigs and sheep remain as the more suitable large animal models for preclinical testing. Sheep have anatomic and hemodynamic characteristics that closely resemble those of humans and have shown the highest degree of similarity in terms of their coagulation activity and fibrinolysis system. Moreover, blood thrombogenic and calcification mechanisms are very similar which is necessary to evaluate the performance of SVGs under these conditions. Additionally, pigs are widely used in translational research due to their physiological and anatomical similarities to humans, for example, the size of the pig heart and large arteries, such as the aorta and pulmonary artery, closely approximates that of humans. These characteristics make pigs also ideal candidates for evaluating tissue-engineered vascular grafts for preclinical trials.124 Table 5 highlights the pros and cons of various large animal models used for the preclinical evaluation of SVGs.
Table 5.
Large animal models commonly used in small vascular graft testing
| SPECIES | PROS | Limitations |
|---|---|---|
| Sheep and goat | Appropriate animal models for cardiovascular research due to their anatomic and hemodynamic characteristics that closely resemble those of humans. Moreover, blood thrombogenic mechanisms are very similar. Recent literature has demonstrated that, among various laboratory animals, sheep exhibit the highest degree of similarity to humans in terms of the activity of their coagulation and fibrinolysis system. As a result, sheep have been employed to investigate the in vivo performance of small-diameter vascular substitutes, with particular emphasis on implant thrombogenicity and calcification tendency124 | It has been stated that the increased calcium metabolism of sheep predestines implanted tissues to calcify and is therefore a challenging parameter in the evaluation of graft performance Domesticated sheep are currently not available as genetically modified lines or inbred strains124 |
| Pigs | Widely used in translational research due to their physiological and anatomical similarities to humans. Due to the resemblance of the cardiovascular system of pigs to that of humans, cardiovascular devices and TEVGs are frequently used in pig cardiovascular models. Besides, the size of the pig heart and large arteries, such as the aorta and pulmonary artery, closely approximates that of humans124 | Pigs can be difficult to manage and do not tolerate manipulations very well Pig arteries are delicate and exhibit a high propensity for muscular spasms Pigs of special strains are susceptible to malignant hyperthermia during anesthesia124 |
| Dogs | Numerous TEVGs have been applied in dogs in short- and long- term studies Canines are easy to handle and show tolerance to prolonged anesthesia Provide a range of implantation sites with the possibility of paired prostheses evaluation and have appropriate vessels for large and small-diameter applications124 |
Trans-anastomotic ingrowth of endothelial cells in dogs like in other animal species is much faster than in humans They have an active immune system which restricts study lengths124 |
| Nonhuman primates | Their anatomy and cardiovascular physiology is very similar to humans which make them an excellent model for cardiovascular graft assessment They can develop atherosclerotic lesions after high-cholesterol, high-fat diet feeding Nonhuman primate’s hemostatic mechanisms are very similar to humans124 |
High purchase costs and ethical concerns limit their use for biocompatibility assessment and functionality studies of TEVGs124 |
Among the vascular grafts discussed in this review, only a few underwent preclinical testing using animal models, with rats and dogs being the most commonly used (32% and 28%, respectively), followed by mice (16%), goats, and rabbits (8% each) and finally lambs and pigs (4% each) (Figure 4). From the reviewed studies, it can be observed that several in vivo validations were performed by implanting the SVGs subcutaneously. While this outside-of-the-circulation approach helps to evaluate the initial immune and healing response of the host animal, as well as the biodegradability of the material,126,130 it does not fully replicate the complex mechanical and physiological conditions that vascular grafts experience. Additionally, while small animal models are a good starting point for in vivo studies, it is essential to evaluate vascular grafts in large animal models to ensure their safety and efficacy in long-term applications and to minimize potential harm to patients. Furthermore, most vascular grafts were tested in healthy animal models, emphasizing the need to use disease animal models to simulate the pathological conditions in which the grafts will be used.
It can be also observed that most implantation times were in the range of 2–5 months, with only a few exceeding this period. The follow-up period in testing engineered grafts is crucial for in vivo validation.130 Its duration is typically determined by the study’s objectives; however, for safety and efficacy considerations, a follow-up of 6 months is often conducted. When assessing graft performance, it is advisable to harvest samples at 1, 3, and 6 weeks, and at 3 and 6 months post-implantation. For studies focused on graft patency evaluation, follow-up is recommended 4–6 weeks post-implantation.130
Outlooks and future directions
In the field of vascular grafting, autologous grafts remain the gold standard for SVG applications. However, there are patients that have low availability of suitable vessels because of previous harvesting or lack of good-quality vessels.12 In recent years, several techniques have gained attention and have emerged as promising approaches for the creation of SVGs, including electrospinning, 3D printing, molding, decellularization, and in vivo maturation. Nevertheless, despite the intense efforts, none of the grafts has been successfully translated to the clinic because of their inadequate mechanical properties, long maturation periods, and post-implantation complications such as calcification, thrombosis, immunological rejection, and/or poor patency.31,89,107 Insufficient endothelialization of the inner layer of the graft is frequently the cause of thrombogenicity, while increased bacterial susceptibility of the graft material might cause infection and subsequent inflammatory reactions.31,89 Consequently, it is crucial to develop grafts that encourage quick endothelialization, facilitate nutrient exchange, and have outstanding mechanical and biocompatible properties.
To address these challenges, several groups have tested surface modifications of their scaffold, which adds molecules that regulate cellular responses. This strategy seeks to maximize the biological activity of vascular grafts by precisely regulating cellular responses and accelerating in situ tissue remodeling.7
The vascular tissue engineering field is moving toward the creation of off-the-shelf vascular grafts, which, ideally, would be available for immediate use in case of emergency. Additionally, the grafts would have suitable mechanical properties and facilitate cell attachment, ingrowth, and remodeling to integrate with the native tissue.97
Patient-specific engineered vascular grafts containing cells derived from the patient (either seeded or embedded) would potentially offer the best level of biocompatibility and provide endothelialized grafts with protein markers found in native arteries. However, this approach would not be viable in the clinical setting due to the lengthy time needed for proper maturation of the graft prior to implantation. Indeed, studies have shown that increasing the waiting time between the initial diagnostic and surgical procedure from 1 week to 1 month significantly increases the risk of mortality of patients with acute myocardial infarction.131 Most of the scaffolds with embedded cells covered in this article require long maturation time in a bioreactor to achieve adequate burst pressure and endothelialization,76,77 making it non-viable for emergency patients. While universal donor cells lacking immunogenicity could be a viable alternative to patient-derived cells, more research needs to be done to fully understand them and how they could be used in bioengineered vascular grafts.132
Conclusion
For a variety of reasons, healthy autologous vessels for use in bypass surgery in coronary artery disease are not always available. Although there are already engineered large-diameter grafts being used in the clinic as substitutes to the autograft, small-diameter vascular grafts for this purpose are still not available. While much progress has been made in recent years, as illustrated by the breadth of work reviewed herein (Table 6), significant challenges remain and there is still a long way to go before we see engineered vascular grafts being used in the clinic. Critical for clinical translation will be to develop vascular grafts that meet standards such as ISO 7198:2016, which specifies the types of tests and evaluation criteria that vascular grafts must undergo for more conclusive results on their safety and effectiveness. Additionally, more preclinical studies using small animal models followed by large animal model testing are needed to increase the number of products that make it to clinical trials.
Table 6.
Summary table of the grafts scoped in this article along with their characteristics
| Graft material | Cell type | Type | Manufacturing process | I.D (mm) | Burst pressure (mmHg) | Animal model | Source |
|---|---|---|---|---|---|---|---|
| PCL | Synthetic | Electrospinning | 2 | 3,280 | Rat | de Valence et al.28 | |
| PCL | 2 | Rat | Nottelet et al.31 | ||||
| PU | 4 | Dog | Hu et al.,32 He et al.33 | ||||
| PU + PLLA | N/A | 894 | N/A | Grasl et al.34 | |||
| PU + PCL + PET | 4 | 2,200 | Rat | Jirofti et al.36 | |||
| TPU + GO | 3.18 | 31,000 | N/A | Jing et al.37 | |||
| PCL + PGS | 1 | 2,350 | Rat | Wu et al.38 | |||
| PEUU | MDSCs | 4.7 | 2,300 | Rat | Soletti et al.,52 Nieponice et al.53 | ||
| Nieponice et al.53 | |||||||
| PLCL | SVF | N/A | N/A | Touroo et al.54 | |||
| PLCL | SMCs | 4 | 933 | N/A | Mun et al.51 | ||
| Silk fibroin | Natural | 5 | 811 | N/A | Soffer et al.40 | ||
| Gelatin | N/A | N/A | Salifu et al.41 | ||||
| Elastin | 4 | 485 | N/A | McKenna et al.43 | |||
| Silk fibroin + TPU | Blend | 5 | N/A | Yu et al.44 | |||
| Gelatin + PCL or Collagen + PLCL | 4 | Mouse | Fu et al.47 | ||||
| PCL + Collagen + Silica | 3 | N/A | Park et al.48 | ||||
| PEUU + PEG + Heparin | 2 | 8,200 | Rabbit | Zhu et al.49 | |||
| GelMA | Fibroblasts | Natural | 3D Printing | 2 | N/A | Wadnap et al.61 | |
| Sodium alginate | HUVSMCs | 0.99 | 303 | N/A | Zhang et al.56 | ||
| GelMA/C | HCASMC | 10 | 115 | Mice | Cui et al.63 | ||
| GelMA | HUVECs | 3 | N/A | Xu et al.65 | |||
| Gelatin + Fibrinogen | Fibroblasts | 4.9 | 1,110 | N/A | Freeman et al.66 | ||
| PPF | Synthetic | 1 | Mice | Melchiorri et al.67 | |||
| PEG-co-PDP and acryl-PEG-RGDS | EC | 8.5 | N/A | Elomaa et al.68 | |||
| GelMA + Pluronic F127 | Blend | 1 | N/A | Zhou et al.69 | |||
| GelMA + Gelatin + Sodium alginate | 2 | 8,400 | N/A | Zhou et al.70 | |||
| PCL + Sodium alginate | MSCs | 4 | Dog | Jang et al.71 | |||
| Gelatin + Fibrinogen + Thrombin | Natural | Molding | 6 | 2,000 | N/A | Singh et al.74 | |
| Fibrinogen | SMC + HUVEC + ASC | 1.5 | 191.78 | N/A | Helms et al.76 | ||
| Fibrinogen | Fibroblast | 3.5 | 600 | N/A | Syedain et al.77 | ||
| Fibrinogen | Dermal fibroblast | 4.8 | 500 | N/A | Huynh et al.78 | ||
| Collagen | hMSCs or hNDF | 0.8 | 1,776 | N/A | Fernandez et al.79 | ||
| Collagen | SMC and EC | 0.8 | 1,525 | N/A | Atchison et al.81 | ||
| Collagen + Genipin | 1 | 1,300 | Rat | Li et al.82 | |||
| PGA | Myofibroblasts | Synthetic | 18 | Lamb | Hoerstrup et al.83 | ||
| TPU + PVA | 15 | Rat | Sohn et al.84 | ||||
| Gelatin + Silk Fibrinogen + PCL | Blend | 2.25 | 180 | Rabbit | Wang et al.86 | ||
| Decellularized Porcine Carotid Artery + Genipin | Decellularization | N/A | 2,182 | Dog | Gu et al.91 | ||
| PGA | hVSMCs | 6 | N/A | Lauria et al.93 | |||
| Decellularized porcine aorta | 1 | 400 | Rat | Negishi et al.94 | |||
| Decellularized fetal pig aorta | ECs | 3 | Dog | Ma et al.95 | |||
| PGA | hSMCs | 3 | 1,618 | Canine | Dahl et al.96 | ||
| Decellularized rat aorta | Dermal fibroblasts | 1.3 | Rat | Kristofik et al.97 | |||
| Fibrous tissue capsule | In vivo maturation | 0.96 | Mice | Song et al.101 | |||
| Fibrous tissue capsule | 2 | 1,825 | Dog | Furukoshi et al.103 | |||
| Fibrous tissue capsule | 4 | 1,500 | Dog | Nakayama et al.104 | |||
| Fibrous tissue capsule | 2 | 3,950 | Pig | Rothuizen et al.105 | |||
| Fibrous tissue capsule | 2 | Rabbit | Tseng et al.106 | ||||
| Fibrous tissue capsule | 2,400 | Goat | Geelhoed et al.102 |
Limitations of the study
The limitations of this mini review include the following: (1) None of the SVGs (<6 mm) explored in this article was tested in human which could potentially results in a different response in terms of long-term patency and immunogenic response in the clinical setting compared to animal models,125 and (2) there is variability in the methodologies used for the mechanical characterization of their VG which could result in different behaviors when further tested in either animal models or clinical settings due to the anisotropic behaviors of biological tissues.133 For instance, some groups directly measured the burst pressure using a pressurized fluid and a gauge, which is the current clinical standard for testing VG, while others used indirect methods such as the circumferential stress or tensile stress which are not as accurate.134
Acknowledgments
The authors thank the support of INTBIOTECH-CREATE funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) of Canada. D. H.-S. was supported by MITACS Graduate Fellowship award. M.C.-B. was supported by a Graduate Scholarship from the University of Ottawa Heart Institute Foundation. This work was made possible by funding from the University of Ottawa Heart Institute, Heart & Stroke Foundation Canada, NSERC (RGPIN-2023-03813), and an Early Career Researcher Award from the Province of Ontario to E.I.A. M.M. thanks the University of Ottawa Heart Institute, the Strategic Research Endowed Funds for a Strategic Research Postdoctoral Fellowship, and the CIHR Fellowship MFE-186357.
Author contributions
D.H.S. and M.C.-B. conducted the literature review and structured the main body of the manuscript including the different techniques and the discussion with input from E.I.A., E.J.S., M.M., and M.R. M.M. wrote the introduction. E.I.A. and E.J.S. supervised the work and revised the content of the review.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Erik J. Suuronen, Email: esuuronen@ottawaheart.ca.
Emilio I. Alarcon, Email: ealarcon@uottawa.ca.
References
- 1.Tanishita K., Yamamoto K. Introduction. Vascular Engineering. 2016:1–11. doi: 10.1007/978-4-431-54801-0_1. [DOI] [Google Scholar]
- 2.Organization, W.H. 2021. Cardiovascular Diseases (CVDs)https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- 3.Kennedy S., Touyz R.M. In: Textbook of Vascular Medicine. Touyz R.M., Delles C., editors. Springer, Cham; 2019. Anatomy and Pharmacology of Vessels; pp. 3–11. [DOI] [Google Scholar]
- 4.Mohebi R., Chen C., Ibrahim N.E., McCarthy C.P., Gaggin H.K., Singer D.E., Hyle E.P., Wasfy J.H., Januzzi J.L., Jr. Cardiovascular Disease Projections in the United States Based on the 2020 Census Estimates. J. Am. Coll. Cardiol. 2022;80:565–578. doi: 10.1016/j.jacc.2022.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J.L., Morrow D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 6.Diodato M., Chedrawy E.G. Coronary artery bypass graft surgery: the past, present, and future of myocardial revascularisation. Surg. Res. Pract. 2014;2014 doi: 10.1155/2014/726158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obiweluozor F.O., Emechebe G.A., Kim D.-W., Cho H.-J., Park C.H., Kim C.S., Jeong I.S. Considerations in the development of small-diameter vascular graft as an alternative for bypass and reconstructive surgeries: a review. Cardiovasc. Eng. Technol. 2020;11:495–521. doi: 10.1007/s13239-020-00482-y. [DOI] [PubMed] [Google Scholar]
- 8.Benrashid E., McCoy C.C., Youngwirth L.M., Kim J., Manson R.J., Otto J.C., Lawson J.H. Tissue engineered vascular grafts: Origins, development, and current strategies for clinical application. Methods. 2016;99:13–19. doi: 10.1016/j.ymeth.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Song H.H.G., Rumma R.T., Ozaki C.K., Edelman E.R., Chen C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell. 2018;22:340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennel T., Zilla P. In: Tissue-Engineered Vascular Grafts. Walpoth B.H., Bergmeister H., Bowlin G.L., Kong D., Rotmans J.I., Zilla P., editors. Springer, Cham; 2020. Clinical Applications and Limitations of Vascular Grafts; pp. 3–34. [DOI] [Google Scholar]
- 11.Arasu R., Jegatheesan D., Sivakumaran Y. Overview of hemodialysis access and assessment. Can. Fam. Physician. 2022;68:577–582. doi: 10.46747/cfp.6808577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiob M.A., She S., Muiznieks L.D., Weiss A.S. Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts. ACS Biomater. Sci. Eng. 2017;3:712–723. doi: 10.1021/acsbiomaterials.6b00220. [DOI] [PubMed] [Google Scholar]
- 13.Naegeli K.M., Kural M.H., Li Y., Wang J., Hugentobler E.A., Niklason L.E. Bioengineering Human Tissues and the Future of Vascular Replacement. Circ. Res. 2022;131:109–126. doi: 10.1161/CIRCRESAHA.121.319984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshi R.A., Van Lith R., Jen M.C., Allen J.B., Lapidos K.A., Ameer G. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials. 2013;34:30–41. doi: 10.1016/j.biomaterials.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslani S., Kabiri M., HosseinZadeh S., Hanaee-Ahvaz H., Taherzadeh E.S., Soleimani M. The applications of heparin in vascular tissue engineering. Microvasc. Res. 2020;131 doi: 10.1016/j.mvr.2020.104027. [DOI] [PubMed] [Google Scholar]
- 16.Gaudino M., Bakaeen F.G., Benedetto U., Di Franco A., Fremes S., Glineur D., Girardi L.N., Grau J., Puskas J.D., Ruel M., et al. Arterial Grafts for Coronary Bypass: A Critical Review After the Publication of ART and RADIAL. Circulation. 2019;140:1273–1284. doi: 10.1161/CIRCULATIONAHA.119.041096. [DOI] [PubMed] [Google Scholar]
- 17.Hasan A., Memic A., Annabi N., Hossain M., Paul A., Dokmeci M.R., Dehghani F., Khademhosseini A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang S., Ellman D.G., Andersen D.C. Review: Tissue Engineering of Small-Diameter Vascular Grafts and Their In Vivo Evaluation in Large Animals and Humans. Cells. 2021;10 doi: 10.3390/cells10030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S., Sengupta D., Chien S. Vascular tissue engineering: from in vitro to in situ. WIREs Mechanisms. of. Disease. 2014;6:61–76. doi: 10.1002/wsbm.1246. [DOI] [PubMed] [Google Scholar]
- 20.Ng H.Y., Lee K.-X.A., Kuo C.-N., Shen Y.-F. Bioprinting of artificial blood vessels. Int. J. Bioprint. 2018;4 doi: 10.18063/IJB.v4i2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devillard C.D., Marquette C.A. Vascular tissue engineering: challenges and requirements for an ideal large scale blood vessel. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.721843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Z., Xing Y., Wang H., Geng X., Ye L., Zhang A.-y., Gu Y., Feng Z.-g. Remodeling of structurally reinforced (TPU+ PCL/PCL)-Hep electrospun small-diameter bilayer vascular grafts interposed in rat abdominal aortas. Biomater. Sci. 2022;10:4257–4270. doi: 10.1039/d1bm01653a. [DOI] [PubMed] [Google Scholar]
- 23.Brown T.K., Alharbi S., Ho K.J., Jiang B. Prosthetic vascular grafts engineered to combat calcification: Progress and future directions. Biotechnol. Bioeng. 2023;120:953–969. doi: 10.1002/bit.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anton, F. (1934) Process and apparatus for preparing artificial threads. US patent US1975504A, patent application US500283A.
- 25.Xie X., Chen Y., Wang X., Xu X., Shen Y., Khan A.u.R., Aldalbahi A., Fetz A.E., Bowlin G.L., El-Newehy M., Mo X. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020;59:243–261. doi: 10.1016/j.jmst.2020.04.037. [DOI] [Google Scholar]
- 26.Rahmati M., Mills D.K., Urbanska A.M., Saeb M.R., Venugopal J.R., Ramakrishna S., Mozafari M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021;117 [Google Scholar]
- 27.Mohammadalizadeh Z., Bahremandi-Toloue E., Karbasi S. Synthetic-based blended electrospun scaffolds in tissue engineering applications. J. Mater. Sci. 2022;57:4020–4079. [Google Scholar]
- 28.de Valence S., Tille J.C., Mugnai D., Mrowczynski W., Gurny R., Möller M., Walpoth B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 2012;33:38–47. doi: 10.1016/j.biomaterials.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Konig G., McAllister T.N., Dusserre N., Garrido S.A., Iyican C., Marini A., Fiorillo A., Avila H., Wystrychowski W., Zagalski K., et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542–1550. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camasao D.B., Mantovani D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater Today Bio. 2021;10 doi: 10.1016/j.mtbio.2021.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nottelet B., Pektok E., Mandracchia D., Tille J.C., Walpoth B., Gurny R., Möller M. Factorial design optimization and in vivo feasibility of poly(epsilon-caprolactone)-micro- and nanofiber-based small diameter vascular grafts. J. Biomed. Mater. Res. 2009;89:865–875. doi: 10.1002/jbm.a.32023. [DOI] [PubMed] [Google Scholar]
- 32.Hu Z.J., Li Z.L., Hu L.Y., He W., Liu R.M., Qin Y.S., Wang S.M. The in vivo performance of small-caliber nanofibrous polyurethane vascular grafts. BMC Cardiovasc. Disord. 2012;12:115. doi: 10.1186/1471-2261-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He W., Hu Z., Xu A., Liu R., Yin H., Wang J., Wang S. The preparation and performance of a new polyurethane vascular prosthesis. Cell Biochem. Biophys. 2013;66:855–866. doi: 10.1007/s12013-013-9528-5. [DOI] [PubMed] [Google Scholar]
- 34.Grasl C., Stoiber M., Röhrich M., Moscato F., Bergmeister H., Schima H. Electrospinning of small diameter vascular grafts with preferential fiber directions and comparison of their mechanical behavior with native rat aortas. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;124 doi: 10.1016/j.msec.2021.112085. [DOI] [PubMed] [Google Scholar]
- 35.Toh H.W., Toong D.W.Y., Ng J.C.K., Ow V., Lu S., Tan L.P., Wong P.E.H., Venkatraman S., Huang Y., Ang H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021;146 [Google Scholar]
- 36.Jirofti N., Mohebbi-Kalhori D., Samimi A., Hadjizadeh A., Kazemzadeh G.H. Fabrication and characterization of a novel compliant small-diameter PET/PU/PCL triad-hybrid vascular graft. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/ab8743. [DOI] [PubMed] [Google Scholar]
- 37.Jing X., Mi H.Y., Salick M.R., Cordie T.M., Peng X.F., Turng L.S. Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;49:40–50. doi: 10.1016/j.msec.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 38.Wu W., Allen R.A., Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012;18:1148–1153. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazemzadeh G., Jirofti N., Kazemi Mehrjerdi H., Rajabioun M., Alamdaran S.A., Mohebbi-Kalhori D., Mirbagheri M.S., Taheri R. A review on developments of in-vitro and in-vivo evaluation of hybrid PCL-based natural polymers nanofibers scaffolds for vascular tissue engineering. J. Ind. Textil. 2022;52 [Google Scholar]
- 40.Soffer L., Wang X., Zhang X., Kluge J., Dorfmann L., Kaplan D.L., Leisk G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008;19:653–664. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salifu A.A., Nury B.D., Lekakou C. Electrospinning of nanocomposite fibrillar tubular and flat scaffolds with controlled fiber orientation. Ann. Biomed. Eng. 2011;39:2510–2520. doi: 10.1007/s10439-011-0350-1. [DOI] [PubMed] [Google Scholar]
- 42.Aldana A.A., Abraham G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017;523:441–453. doi: 10.1016/j.ijpharm.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 43.McKenna K.A., Hinds M.T., Sarao R.C., Wu P.C., Maslen C.L., Glanville R.W., Babcock D., Gregory K.W. Mechanical property characterization of electrospun recombinant human tropoelastin for vascular graft biomaterials. Acta Biomater. 2012;8:225–233. doi: 10.1016/j.actbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu E., Mi H.Y., Zhang J., Thomson J.A., Turng L.S. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J. Biomed. Mater. Res. 2018;106:985–996. doi: 10.1002/jbm.a.36297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackstone B.N., Gallentine S.C., Powell H.M. Collagen-based electrospun materials for tissue engineering: A systematic review. Bioengineering. 2021;8:39. doi: 10.3390/bioengineering8030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karimi A., Navidbakhsh M., Shojaei A., Faghihi S. Measurement of the uniaxial mechanical properties of healthy and atherosclerotic human coronary arteries. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33:2550–2554. doi: 10.1016/j.msec.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Fu W., Liu Z., Feng B., Hu R., He X., Wang H., Yin M., Huang H., Zhang H., Wang W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomedicine. 2014;9:2335–2344. doi: 10.2147/IJN.S61375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park S., Kim J., Lee M.-K., Park C., Jung H.-D., Kim H.-E., Jang T.-S. Fabrication of strong, bioactive vascular grafts with PCL/collagen and PCL/silica bilayers for small-diameter vascular applications. Mater. Des. 2019;181 doi: 10.1016/j.matdes.2019.108079. [DOI] [Google Scholar]
- 49.Zhu T., Gu H., Zhang H., Wang H., Xia H., Mo X., Wu J. Covalent grafting of PEG and heparin improves biological performance of electrospun vascular grafts for carotid artery replacement. Acta Biomater. 2021;119:211–224. doi: 10.1016/j.actbio.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Ahn H., Ju Y.M., Takahashi H., Williams D.F., Yoo J.J., Lee S.J., Okano T., Atala A. Engineered small diameter vascular grafts by combining cell sheet engineering and electrospinning technology. Acta Biomater. 2015;16:14–22. doi: 10.1016/j.actbio.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Mun C.H., Jung Y., Kim S.H., Lee S.H., Kim H.C., Kwon I.K., Kim S.H. Three-dimensional electrospun poly(lactide-co-varepsilon-caprolactone) for small-diameter vascular grafts. Tissue Eng. Part A. 2012;18:1608–1616. doi: 10.1089/ten.TEA.2011.0695. [DOI] [PubMed] [Google Scholar]
- 52.Soletti L., Hong Y., Guan J., Stankus J.J., El-Kurdi M.S., Wagner W.R., Vorp D.A. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010;6:110–122. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieponice A., Soletti L., Guan J., Hong Y., Gharaibeh B., Maul T.M., Huard J., Wagner W.R., Vorp D.A. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng. Part A. 2010;16:1215–1223. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Touroo J.S., Dale J.R., Williams S.K. Bioengineering human blood vessel mimics for medical device testing using serum-free conditions and scaffold variations. Tissue Eng. Part C Methods. 2013;19:307–315. doi: 10.1089/ten.TEC.2012.0311. [DOI] [PubMed] [Google Scholar]
- 55.Ratner B. Vascular Grafts: Technology Success/Technology Failure. BME Front. 2023;4 doi: 10.34133/bmef.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Yu Y., Akkouch A., Dababneh A., Dolati F., Ozbolat I.T. In vitro study of directly bioprinted perfusable vasculature conduits. Biomater. Sci. 2015;3:134–143. doi: 10.1039/C4BM00234B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Q., Dong H., Su J., Han J., Song B., Wei Q., Shi Y. A Review of 3D Printing Technology for Medical Applications. Engineering. 2018;4:729–742. doi: 10.1016/j.eng.2018.07.021. [DOI] [Google Scholar]
- 58.Masood S.H. In: Comprehensive Materials Processing. Hashmi S., Batalha G.F., Van Tyne C.J., Yilbas B., editors. Elsevier; 2014. 10.04 - Advances in Fused Deposition Modeling; pp. 69–91. [DOI] [Google Scholar]
- 59.Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S., Bliley J.M., Campbell P.G., Feinberg A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 60.Shahrubudin N., Lee T.C., Ramlan R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019;35:1286–1296. doi: 10.1016/j.promfg.2019.06.089. [DOI] [Google Scholar]
- 61.Wadnap S., Krishnamoorthy S., Zhang Z., Xu C. Biofabrication of 3D cell-encapsulated tubular constructs using dynamic optical projection stereolithography. J. Mater. Sci. Mater. Med. 2019;30:36. doi: 10.1007/s10856-019-6239-5. [DOI] [PubMed] [Google Scholar]
- 62.Kjar A., McFarland B., Mecham K., Harward N., Huang Y. Engineering of tissue constructs using coaxial bioprinting. Bioact. Mater. 2021;6:460–471. doi: 10.1016/j.bioactmat.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui H., Zhu W., Huang Y., Liu C., Yu Z.X., Nowicki M., Miao S., Cheng Y., Zhou X., Lee S.J., et al. In vitro and in vivo evaluation of 3D bioprinted small-diameter vasculature with smooth muscle and endothelium. Biofabrication. 2019;12 doi: 10.1088/1758-5090/ab402c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 65.Xu L., Varkey M., Jorgensen A., Ju J., Jin Q., Park J.H., Fu Y., Zhang G., Ke D., Zhao W., et al. Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step. Biofabrication. 2020;12 doi: 10.1088/1758-5090/aba2b6. [DOI] [PubMed] [Google Scholar]
- 66.Freeman S., Ramos R., Alexis Chando P., Zhou L., Reeser K., Jin S., Soman P., Ye K. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019;95:152–164. doi: 10.1016/j.actbio.2019.06.052. [DOI] [PubMed] [Google Scholar]
- 67.Melchiorri A.J., Hibino N., Best C.A., Yi T., Lee Y.U., Kraynak C.A., Kimerer L.K., Krieger A., Kim P., Breuer C.K., Fisher J.P. 3D-Printed Biodegradable Polymeric Vascular Grafts. Adv. Healthc. Mater. 2016;5:319–325. doi: 10.1002/adhm.201500725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elomaa L., Pan C.C., Shanjani Y., Malkovskiy A., Seppälä J.V., Yang Y. Three-dimensional fabrication of cell-laden biodegradable poly(ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J. Mater. Chem. B. 2015;3:8348–8358. doi: 10.1039/c5tb01468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou X., Nowicki M., Sun H., Hann S.Y., Cui H., Esworthy T., Lee J.D., Plesniak M., Zhang L.G. 3D Bioprinting-Tunable Small-Diameter Blood Vessels with Biomimetic Biphasic Cell Layers. ACS Appl. Mater. Interfaces. 2020;12:45904–45915. doi: 10.1021/acsami.0c14871. [DOI] [PubMed] [Google Scholar]
- 70.Zhou X., Gao Q., Yu D., Shao Y., Wang Z., Liu X., Wang W., Chang L., Ma T., Mok H., et al. 3D-bioprinted vascular scaffold with tunable mechanical properties for simulating and promoting neo-vascularization. Smart Materials in Medicine. 2022;3:199–208. doi: 10.1016/j.smaim.2022.01.003. [DOI] [Google Scholar]
- 71.Jang E.H., Kim J.H., Lee J.H., Kim D.H., Youn Y.N. Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers. 2020;12 doi: 10.3390/polym12030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sai T.V., Vinod T., Sowmya G. A critical review on casting types and defects. Eng. Technol. 2017;3:463–468. [Google Scholar]
- 73.Altan T., Lilly B., Yen Y.C., Altan T. Manufacturing of Dies and Molds. CIRP Annals. 2001;50:404–422. doi: 10.1016/s0007-8506(07)62988-6. [DOI] [Google Scholar]
- 74.Singh G., Cordero J., Wiles B., Tembelis M.N., Liang K.L., Rafailovich M., Simon M., Khan S.U., Bui D.T., Dagum A.B. Development of In Vitro Bioengineered Vascular Grafts for Microsurgery and Vascular Surgery Applications. Plast. Reconstr. Surg. Glob. Open. 2019;7 doi: 10.1097/GOX.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebhota W.S., Karun A.S., Inambao F.L. Centrifugal casting technique baseline knowledge, applications, and processing parameters: overview. Int. J. Mater. Res. 2016;107:960–969. doi: 10.3139/146.111423. [DOI] [Google Scholar]
- 76.Helms F., Lau S., Aper T., Zippusch S., Klingenberg M., Haverich A., Wilhelmi M., Böer U. A 3-Layered Bioartificial Blood Vessel with Physiological Wall Architecture Generated by Mechanical Stimulation. Ann. Biomed. Eng. 2021;49:2066–2079. doi: 10.1007/s10439-021-02728-9. [DOI] [PubMed] [Google Scholar]
- 77.Syedain Z.H., Meier L.A., Bjork J.W., Lee A., Tranquillo R.T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32:714–722. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huynh T.N., Tranquillo R.T. Fusion of concentrically layered tubular tissue constructs increases burst strength. Ann. Biomed. Eng. 2010;38:2226–2236. doi: 10.1007/s10439-010-0045-z. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez C.E., Yen R.W., Perez S.M., Bedell H.W., Povsic T.J., Reichert W.M., Truskey G.A. Human Vascular Microphysiological System for in vitro Drug Screening. Sci. Rep. 2016;6 doi: 10.1038/srep21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olive M., Harten I., Mitchell R., Beers J.K., Djabali K., Cao K., Erdos M.R., Blair C., Funke B., Smoot L., et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atchison L., Abutaleb N.O., Snyder-Mounts E., Gete Y., Ladha A., Ribar T., Cao K., Truskey G.A. iPSC-Derived Endothelial Cells Affect Vascular Function in a Tissue-Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome. Stem Cell Rep. 2020;14:325–337. doi: 10.1016/j.stemcr.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X., Xu J., Nicolescu C.T., Marinelli J.T., Tien J. Generation, Endothelialization, and Microsurgical Suture Anastomosis of Strong 1-mm-Diameter Collagen Tubes. Tissue Eng. Part A. 2017;23:335–344. doi: 10.1089/ten.TEA.2016.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoerstrup S.P., Cummings Mrcs I., Lachat M., Schoen F.J., Jenni R., Leschka S., Neuenschwander S., Schmidt D., Mol A., Günter C., et al. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation. 2006;114:I159–I166. doi: 10.1161/CIRCULATIONAHA.105.001172. [DOI] [PubMed] [Google Scholar]
- 84.Sohn S.H., Kim T.H., Kim T.S., Min T.J., Lee J.H., Yoo S.M., Kim J.W., Lee J.E., Kim C.H., Park S.H., Jo W.M. Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease. Materials. 2021;14 doi: 10.3390/ma14051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wegst U.G.K., Schecter M., Donius A.E., Hunger P.M. Biomaterials by freeze casting. Philos. Trans. A Math. Phys. Eng. Sci. 2010;368:2099–2121. doi: 10.1098/rsta.2010.0014. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z., Liu C., Xiao Y., Gu X., Xu Y., Dong N., Zhang S., Qin Q., Wang J. Remodeling of a Cell-Free Vascular Graft with Nanolamellar Intima into a Neovessel. ACS Nano. 2019;13:10576–10586. doi: 10.1021/acsnano.9b04704. [DOI] [PubMed] [Google Scholar]
- 87.Lin C.-H., Hsia K., Ma H., Lee H., Lu J.-H. In vivo performance of decellularized vascular grafts: a review article. Int. J. Mol. Sci. 2018;19:2101. doi: 10.3390/ijms19072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leal B.B.J., Wakabayashi N., Oyama K., Kamiya H., Braghirolli D.I., Pranke P. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front. Cardiovasc. Med. 2020;7 doi: 10.3389/fcvm.2020.592361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weekes A., Bartnikowski N., Pinto N., Jenkins J., Meinert C., Klein T.J. Biofabrication of small diameter tissue-engineered vascular grafts. Acta Biomater. 2022;138:92–111. doi: 10.1016/j.actbio.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 90.Li Y., Zhou Y., Qiao W., Shi J., Qiu X., Dong N. Application of decellularized vascular matrix in small-diameter vascular grafts. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu Y., Wang F., Wang R., Li J., Wang C., Li L., Xu Z., Zhang J. Preparation and evaluation of decellularized porcine carotid arteries cross-linked by genipin: the preliminary results. Cell Tissue Bank. 2018;19:311–321. doi: 10.1007/s10561-017-9675-9. [DOI] [PubMed] [Google Scholar]
- 92.Humacyte . 2023. Platform Technology.https://humacyte.com/platform-technology/ [Google Scholar]
- 93.Lauria A.L., Sen I., Rasmussen T.E. The Human Acellular Vessel for Vascular Reconstruction or Bypass: A Novel Biologic Conduit for Vascular Bypass and Repair. JAMA Surg. 2022;157:731–732. doi: 10.1001/jamasurg.2022.1214. [DOI] [PubMed] [Google Scholar]
- 94.Negishi J., Hashimoto Y., Yamashita A., Zhang Y., Kimura T., Kishida A., Funamoto S. Evaluation of small-diameter vascular grafts reconstructed from decellularized aorta sheets. J. Biomed. Mater. Res. 2017;105:1293–1298. doi: 10.1002/jbm.a.36017. [DOI] [PubMed] [Google Scholar]
- 95.Ma X., He Z., Li L., Liu G., Li Q., Yang D., Zhang Y., Li N. Development and in vivo validation of tissue-engineered, small-diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J. Cardiothorac. Surg. 2017;12:101. doi: 10.1186/s13019-017-0661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dahl S.L.M., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., Manson R.J., Tente W.E., DiBernardo L., Hensley M.T., et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 97.Kristofik N.J., Qin L., Calabro N.E., Dimitrievska S., Li G., Tellides G., Niklason L.E., Kyriakides T.R. Improving in vivo outcomes of decellularized vascular grafts via incorporation of a novel extracellular matrix. Biomaterials. 2017;141:63–73. doi: 10.1016/j.biomaterials.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li M.-X., Wei Q.-Q., Mo H.-L., Ren Y., Zhang W., Lu H.-J., Joung Y.K. Challenges and advances in materials and fabrication technologies of small-diameter vascular grafts. Biomater. Res. 2023;27:58. doi: 10.1186/s40824-023-00399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoenig M.R., Campbell G.R., Rolfe B.E., Campbell J.H. Tissue-engineered blood vessels: alternative to autologous grafts? Arterioscler. Thromb. Vasc. Biol. 2005;25:1128–1134. doi: 10.1161/01.ATV.0000158996.03867.72. [DOI] [PubMed] [Google Scholar]
- 100.Niklason L.E., Lawson J.H. Bioengineered human blood vessels. Science. 2020;370 doi: 10.1126/science.aaw8682. [DOI] [PubMed] [Google Scholar]
- 101.Song L., Wang L., Shah P.K., Chaux A., Sharifi B.G. Bioengineered vascular graft grown in the mouse peritoneal cavity. J. Vasc. Surg. 2010;52:994–1002.e10022. doi: 10.1016/j.jvs.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geelhoed W.J., van der Bogt K.E.A., Rothuizen T.C., Damanik F.F.R., Hamming J.F., Mota C.D., van Agen M.S., de Boer H.C., Restrepo M.T., Hinz B., et al. A novel method for engineering autologous non-thrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting. Biomaterials. 2020;229 doi: 10.1016/j.biomaterials.2019.119577. [DOI] [PubMed] [Google Scholar]
- 103.Furukoshi M., Moriwaki T., Nakayama Y. Development of an in vivo tissue-engineered vascular graft with designed wall thickness (biotube type C) based on a novel caged mold. J. Artif. Organs. 2016;19:54–61. doi: 10.1007/s10047-015-0859-4. [DOI] [PubMed] [Google Scholar]
- 104.Nakayama Y., Furukoshi M., Terazawa T., Iwai R. Development of long in vivo tissue-engineered "Biotube" vascular grafts. Biomaterials. 2018;185:232–239. doi: 10.1016/j.biomaterials.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 105.Rothuizen T.C., Damanik F.F.R., Lavrijsen T., Visser M.J.T., Hamming J.F., Lalai R.A., Duijs J.M.G.J., van Zonneveld A.J., Hoefer I.E., van Blitterswijk C.A., et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials. 2016;75:82–90. doi: 10.1016/j.biomaterials.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 106.Tseng Y.C., Roan J.N., Ho Y.C., Lin C.C., Yeh M.L. An in vivo study on endothelialized vascular grafts produced by autologous biotubes and adipose stem cells (ADSCs) J. Mater. Sci. Mater. Med. 2017;28:166. doi: 10.1007/s10856-017-5986-4. [DOI] [PubMed] [Google Scholar]
- 107.Fayon A., Menu P., El Omar R. Cellularized small-caliber tissue-engineered vascular grafts: looking for the ultimate gold standard. NPJ Regen. Med. 2021;6:46. doi: 10.1038/s41536-021-00155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mallis P., Kostakis A., Stavropoulos-Giokas C., Michalopoulos E. Future perspectives in small-diameter vascular graft engineering. Bioengineering. 2020;7:160. doi: 10.3390/bioengineering7040160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carrabba M., Madeddu P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front. Bioeng. Biotechnol. 2018;6:41. doi: 10.3389/fbioe.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ercolani E., Del Gaudio C., Bianco A. Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2015;9:861–888. doi: 10.1002/term.1697. [DOI] [PubMed] [Google Scholar]
- 111.Rickel A.P., Deng X., Engebretson D., Hong Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;129 doi: 10.1016/j.msec.2021.112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khorshidi S., Solouk A., Mirzadeh H., Mazinani S., Lagaron J.M., Sharifi S., Ramakrishna S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016;10:715–738. doi: 10.1002/term.1978. [DOI] [PubMed] [Google Scholar]
- 113.Zohdi N., Yang R.C. Material Anisotropy in Additively Manufactured Polymers and Polymer Composites: A Review. Polymers. 2021;13 doi: 10.3390/polym13193368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boularaoui S., Al Hussein G., Khan K.A., Christoforou N., Stefanini C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting. 2020;20 doi: 10.1016/j.bprint.2020.e00093. [DOI] [Google Scholar]
- 115.Nikolova M.P., Chavali M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019;4:271–292. doi: 10.1016/j.bioactmat.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Puertas-Bartolomé M., Mora-Boza A., García-Fernández L. Emerging biofabrication techniques: A review on natural polymers for biomedical applications. Polymers. 2021;13:1209. doi: 10.3390/polym13081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herrick S., Blanc-Brude O., Gray A., Laurent G. Fibrinogen. Int. J. Biochem. Cell Biol. 1999;31:741–746. doi: 10.1016/s1357-2725(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 118.Raut B.K., Patil V.N., Cherian G. Coronary artery dimensions in normal Indians. Indian Heart J. 2017;69:512–514. doi: 10.1016/j.ihj.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marieb E.N., Hoehn K. Pearson Education; 2019. Human Anatomy & Physiology. [Google Scholar]
- 120.Lee J.M., Wilson G.J. Anisotropic tensile viscoelastic properties of vascular graft materials tested at low strain rates. Biomaterials. 1986;7:423–431. doi: 10.1016/0142-9612(86)90029-3. [DOI] [PubMed] [Google Scholar]
- 121.Stoiber M., Grasl C., Moscato F., Schima H. Mechanical Testing of Vascular Grafts. Springer, Cham; 2020. pp. 35–61. [DOI] [Google Scholar]
- 122.Rahman R., Zhafer Firdaus Syed Putra S. In: Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. Jawaid M., Thariq M., Saba N., editors. Woodhead Publishing; 2019. 5 - Tensile properties of natural and synthetic fiber-reinforced polymer composites; pp. 81–102. [DOI] [Google Scholar]
- 123.ANSI/ISO 7198:2016 - Cardiovascular Implants and Extracorporeal Systems – Vascular Prostheses – Tubular Vascular Grafts and Vascular Patches. International Organization for Standardization; 2016. [Google Scholar]
- 124.Bergmeister H., Podesser B.K. Preclinical In Vivo Assessment of Tissue Engineered Vascular Grafts and Selection of Appropriate Animal Models. Tissue-Engineered Vascular Grafts. 2020:63–93. [Google Scholar]
- 125.Swartz D.D., Andreadis S.T. Animal models for vascular tissue-engineering. Curr. Opin. Biotechnol. 2013;24:916–925. doi: 10.1016/j.copbio.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Byrom M.J., Bannon P.G., White G.H., Ng M.K.C. Animal models for the assessment of novel vascular conduits. J. Vasc. Surg. 2010;52:176–195. doi: 10.1016/j.jvs.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 127.Rashid S.T., Salacinski H.J., Hamilton G., Seifalian A.M. The use of animal models in developing the discipline of cardiovascular tissue engineering: a review. Biomaterials. 2004;25:1627–1637. doi: 10.1016/s0142-9612(03)00522-2. [DOI] [PubMed] [Google Scholar]
- 128.Koch S.E., de Kort B.J., Holshuijsen N., Brouwer H.F.M., van der Valk D.C., Dankers P.Y.W., van Luijk J.A.K.R., Hooijmans C.R., de Vries R.B.M., Bouten C.V.C., Smits A.I.P.M. Animal studies for the evaluation of in situ tissue-engineered vascular grafts - a systematic review, evidence map, and meta-analysis. NPJ Regen. Med. 2022;7:17. doi: 10.1038/s41536-022-00211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ong C.S., Fukunishi T., Liu R.H., Nelson K., Zhang H., Wieczorek E., Palmieri M., Ueyama Y., Ferris E., Geist G.E., et al. Bilateral Arteriovenous Shunts as a Method for Evaluating Tissue-Engineered Vascular Grafts in Large Animal Models. Tissue Eng. Part C Methods. 2017;23:728–735. doi: 10.1089/ten.TEC.2017.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walpoth B.H., Bergmeister H., Bowlin G.L., Kong D., Rotmans J.I., Zilla P. Springer; 2020. Tissue-Engineered Vascular Grafts. [Google Scholar]
- 131.Thilak A.P., Thacker D., Shales S., Das D., Behera S.K., Ghosh A.K., Narayan P. Timing of coronary artery bypass grafting after acute myocardial infarction: does it influence outcomes? Kardiochirurgia i Torakochirurgia Polska. Pol. J. Thorac. Cardiovasc. Surg. 2021;18:27–32. doi: 10.5114/kitp.2021.105184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lanza R., Russell D.W., Nagy A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019;19:723–733. doi: 10.1038/s41577-019-0200-1. [DOI] [PubMed] [Google Scholar]
- 133.Mitchell G.R., Tojeira A. Role of Anisotropy in Tissue Engineering. Procedia Eng. 2013;59:117–125. doi: 10.1016/j.proeng.2013.05.100. [DOI] [Google Scholar]
- 134.Geelhoed W.J., Lalai R.A., Sinnige J.H., Jongeleen P.J., Storm C., Rotmans J.I. Indirect Burst Pressure Measurements for the Mechanical Assessment of Biological Vessels. Tissue Eng. Part C Methods. 2019;25:472–478. doi: 10.1089/ten.TEC.2019.0133. [DOI] [PubMed] [Google Scholar]