Abstract
In vivo recombination has been used to make a series of AroP-PheP chimeric proteins. Analysis of their respective substrate profiles and activities has identified a small region within span III of AroP which can confer on a predominantly PheP protein the ability to transport tryptophan. Site-directed mutagenesis of the AroP-PheP chimera, PheP, and AroP has established that a key residue involved in tryptophan transport is tyrosine at position 103 in AroP. Phenylalanine is the residue at the corresponding position in PheP. The use of PheP-specific antisera has shown that the inability of certain chimeras to transport any of the aromatic amino acids is not a result of instability or a failure to be inserted into the membrane. Site-directed mutagenesis has identified two significant AroP-specific residues, alanine 107 and valine 114, which are the direct cause of loss of transport activity in chimeras such as A152P. These residues replace a glycine and an alanine in PheP and flank a highly conserved glutamate at position 110. Some suggestions are made as to the possible functions of these residues in the tertiary structure of the proteins.
The aromatic amino acids phenylalanine, tyrosine, and tryptophan are actively transported across the inner membrane of the bacterium Escherichia coli by a number of distinct transport systems. A general aromatic transport system, encoded by the gene aroP, transports all three aromatic amino acids (1, 4). A closely related protein, PheP, is a high-affinity transporter of phenylalanine (23, 35). The PheP protein does not transport tryptophan and initially was not reported to be an active transporter of tyrosine. Tryptophan when present at a 20-fold excess can significantly inhibit phenylalanine transport by the AroP system but has only a slight effect on phenylalanine transport by the PheP protein. In the wild-type cell, expression of aroP is subject to control involving the TyrR protein (36), whereas the expression of pheP appears to be constitutive (23). The PheP protein is expressed to about one-third or less of the level of the AroP protein in derepressed strains. This weak expression can for the most part be attributed to the translational start codon GTG found in pheP. The AroP and PheP proteins are part of the amino acid transporter family within a ubiquitous superfamily of transporters referred to as the amino acid-polyamine-organocation superfamily (25, 38). Studies with uncouplers of oxidative phosphorylation and with strains deficient in F0F1-ATPase indicate that transport via the AroP and PheP systems is driven by the proton motive force (34).
The aroP and pheP genes show a high degree of sequence identity, encoding proteins composed of 457 and 458 amino acid residues, respectively (12, 23). In the predicted primary structure of the two proteins, 61% of the amino acid residues are identical. Secondary structure models for the orientation of the PheP and AroP proteins in the cytoplasmic membrane have been proposed based on their hydrophobicity profiles, distribution of charged amino acid residues, and a study of alkaline phosphatase sandwich fusions (6, 22). The topological models for the AroP and PheP permeases are very similar and involve hydrophobic nonpolar residues arranged in 12 transmembrane-spanning regions connected by hydrophilic segments. The two permeases show the greatest level of identity in transmembrane spans 1 and 2 and are least conserved in the amino and carboxyl termini and in transmembrane spans 11 and 12 (Fig. 1).
FIG. 1.
Topological models of AroP (a) and PheP (b) permeases. The amino acid residues which are identical between AroP and PheP are shaded.
Despite the high degree of similarity between the AroP and PheP permeases referred to above, their substrate specificities and affinities differ. To investigate whether there might be distinct domains within their common structural arrangements which could be identified as being responsible for these differences, a number of AroP-PheP chimeric proteins were constructed and studied for their transport characteristics. In other studies, the characterization of chimeric proteins has proved very useful in investigating the structure-function relationship of a number of transporters (5, 9, 10, 11, 17, 30). A simple and efficient method for the construction of a series of AroP-PheP chimeric proteins via in vivo homologous recombination of their genes was suggested from the previous work of Tommassen et al. (30). In this study, we report the creation and the characteristics of a number of chimeric proteins involving both AroP and PheP and identify a residue present in AroP but not in PheP that is required for tryptophan transport. We also demonstrate that the inactivity of some chimeras is not a consequence of failure to be inserted into the membrane.
MATERIALS AND METHODS
Bacterial strains, plasmids, and phages.
The E. coli K-12 strains, plasmids, and phages used in this study are described in Table 1.
TABLE 1.
E. coli K-12 strains, plasmids, and phages used in this study
| Strain, plasmid, or phage | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| JM101 | Δ(lac-pro) supE thi (F′ traD36 proA+B+ lacIq ΔlacZM15) | 15 |
| JP7910 | JP777 aroP1000 pheP367 tyrP mtr-24 tnaAB2 tyrR366 | 6 |
| JP8442 | W3110 (ΔphoA8 tsx) | 28 |
| Plasmids and phages | ||
| M13mp18 | lacPOZ′ | 18 |
| M13tg130 (aroP) | lacPOZ′ 2.8-kb EcoRV-HindIII fragment carrying aroP in M13tg130 | This study |
| pLG339 | Tcr Kmr, low-copy-number vector, pSC101 ori | 29 |
| pMU2149 | Kmr, 2.3-kb SalI-EcoRI pheP fragment cloned into pLG339 | 20 |
| pMU2195 | Kmr, 3.2-kb SalI-EcoRI aroP fragment cloned in pLG339 | 20 |
| pMU3138 | Derivative of pMU2149 containing pheP with an ATG start codon | This study |
| pMU3322 | Kmr, 2.8-kb SalI-XbaI aroP fragment of M13tg130 (aroP) cloned into pMU2149 | This study |
| pMU3767 | pheP gene with ′phoA inserted at aspartate 432 | 22 |
| pMU6542 | AroP-PheP chimera A94P in pLG339 | This study |
| pMU6543 | AroP-PheP chimera A107P in pLG339 | This study |
| pMU6557 | Derivative of pMU3138 encoding a PheP protein with a phenylalanine 111-to-tyrosine alteration | This study |
| pMU6558 | Derivative of pMU2195 encoding an AroP protein with a tyrosine 107-to-phenylalanine alteration | This study |
| pSWFII | Apr, phoA sandwich fusion construction vector | 8 |
The genetic nomenclature is that described by Bachmann (2). Allele numbers are indicated where known.
Growth media and reagents.
The minimal media used were the half-strength buffer 56 of Monod et al. (16) and the 121 salts medium of Torriani (31), both supplemented with 0.2% glucose and the required growth factors. Kanamycin was added to nutrient and minimal media at a final concentration of 25 μg/ml. All enzymes were purchased from AMRAD Pharmacia Biotech, Melbourne, Australia, unless otherwise indicated. [α-35S]dATP (1,200 Ci/mmol; 10 mCi/ml) for use in DNA sequencing and l-[14C]tyrosine (493 mCi/mmol; 50 μCi/ml), l-[14C]phenylalanine (493 mCi/mmol; 50 μCi/ml), and l-[14C]tryptophan (52 Ci/mmol; 20 μCi/ml) for use in transport assays were obtained from NEN/DuPont. The chromogenic substrate 5-bromo-4-chloro-3-indolylphosphate-toluidine salt (XP) from Sigma Chemical Co. was dissolved in dimethyl formamide and used at 40 μg/ml in solid medium. ProtoGel 30% (wt/vol) acrylamide for protein gels was purchased from National Diagnostics. Oligonucleotides were synthesized on a Gene Assembler Plus (Pharmacia-LKB) or obtained commercially from Bresatec.
Recombinant DNA techniques.
Standard recombinant DNA techniques were used essentially as described by Sambrook et al. (26).
Site-directed mutagenesis.
The method of Vandeyar et al. (32) was carried out using a commercially available kit from United States Biochemical Corporation.
Construction of aroP-pheP chimeric genes.
The strategy adopted for creating AroP-PheP chimeric proteins is summarized in Fig. 2. Plasmid pMU3322 used for the construction of aroP-pheP chimeric genes contains the aroP and pheP genes in tandem. This plasmid is a derivative of the low-copy-number vector pLG339 (copy number, ∼6) (29). The vector M13tg130 (aroP) was digested with SalI and XbaI and cloned into the same sites of pMU2149 carrying the pheP+ gene. The resulting plasmid pMU3322 contains the aroP gene located upstream of the pheP gene, with unique XbaI and EcoRV sites located on the vector between the two genes. The plasmid was digested with both of these enzymes, the linear DNA was transformed into strain JP7910, and then kanamycin-resistant colonies were selected. The amount of linear DNA required to produce between 10 and 100 kanamycin-resistant transformants was determined to be between 0.5 and 1.0 μg per 200 μl of competent cells. The percentage of transformants containing chimeric genes ranged from 30 to 75% between individual experiments. A total of 11 transformants were characterized which contained plasmids harboring chimeric genes. To allow nucleotide sequencing of the chimeric genes, the 2.2-kb SalI-EcoRI fragment of pMU3322 derivatives harboring aroP-pheP chimeric genes was cloned into the same sites of M13mp18, and sequencing was performed using the dideoxy method of Sanger et al. (27).
FIG. 2.
Construction of aroP-pheP chimeric genes by in vivo homologous recombination. The homologous genes aroP (black arrows) and pheP (gray arrows) are located in tandem on plasmid pMU3322. Situated between these genes are unique XbaI and EcoRV restriction enzyme sites. The plasmid is cleaved with these restriction enzymes, and the linear DNA is transformed into the E. coli strain JP7910. The linear plasmid DNA cannot be maintained in the host, but stable circular plasmids can arise by a recombination event between homologous regions in the two genes.
Construction of aroP-pheP-aroP chimeric genes.
Additional chimeric proteins between AroP and PheP were constructed by exchanging different regions of the chimeric genes generated by in vivo homologous recombination. A pheP-aroP chimeric gene (P365A) in which the site of recombination between the pheP and aroP genes occurred at nucleotide position 1110 was isolated by in vivo homologous recombination using a starting plasmid in which the order of the aroP and pheP genes was reversed to that of pMU3322. This pheP-aroP chimera was used to generate aroP-pheP-aroP chimeric genes. A unique SphI site is present at nucleotides 951 to 955 in the pheP gene (Fig. 3). Digestion of this pheP-aroP chimera with SphI and EcoRI (located downstream of the chimeric gene coding sequence) enables this 800-bp fragment to be cloned into the equivalent sites of the aroP-pheP chimeric genes, thus generating aroP-pheP-aroP chimeras. Three such AroP-PheP-AroP chimeras were constructed between chimera P365A and chimeras A80P, A152P, and A286P.
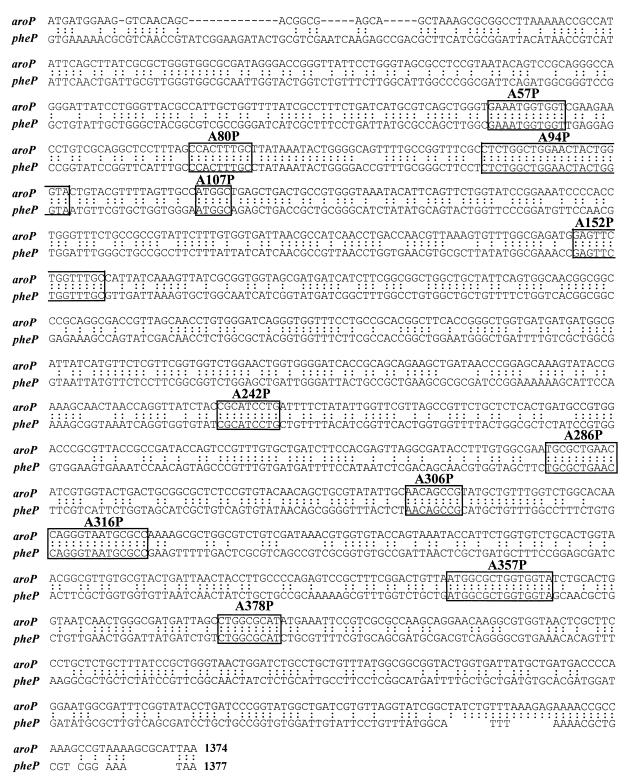
FIG. 3.
Sites of recombination of the aroP-pheP chimeric genes. The nucleotide sequences of the aroP (upper sequence) and pheP (lower sequence) genes are shown. Homologous nucleotide sequences are indicated by two dots. The sequences determined to be the site of genetic recombination for individual chimeric genes are boxed, with the corresponding chimeric gene numbers shown.
Chimeric AroP-PheP-alkaline phosphatase fusions.
Plasmid pMU3767, which contains a pheP-alkaline phosphatase sandwich fusion at position aspartate 432, was digested with SphI and BamHI restriction enzymes. The 2.5-kb SphI-BamHI partial fragment containing the pheP-phoA fusion was cloned into the equivalent sites of pMU3322-derived aroP-pheP chimeras A80P, A152P, and A286P. The resulting aroP-pheP-phoA fusions contain alkaline phosphatase inserted at position aspartate 432, which is a proposed periplasmic location. Additional alkaline phosphatase fusions were constructed with chimera A286P. In this case, oligonucleotide-mediated site-directed mutagenesis (32) was used to create individual unique BglII restriction sites within the chimeric A286P gene present on M13mp18. The ′phoA gene from pSWFII (8) was excised on a BamHI fragment and inserted in frame into the chimeric gene at each of the BglII sites. The detailed procedure for the generation of site-specific alkaline phosphatase sandwich fusions has been previously reported (6). Alkaline phosphatase sandwich fusions were constructed at positions isoleucine 169, alanine 224, proline 261, leucine 279, and leucine 361 in chimera A286P. An alkaline phosphatase sandwich fusion was also generated by this method at position leucine 279 in chimera A80P.
Transport assays.
Cultures of JP7910 harboring the chimeric genes were grown in half-strength buffer 56 containing 0.2% glucose, the appropriate growth factors, and kanamycin at 37°C to an optical density at 600 nm of 0.45 to 0.55. Transport activity was assayed as previously described (37) in the presence of 10 μM l-[14C]tyrosine, l-[14C]phenylalanine, or l-[14C]tryptophan. Competition studies were performed by measuring transport activity in the presence of a 20-fold excess of unlabeled amino acid.
Alkaline phosphatase assays.
Cultures of JP8442 harboring aroP-pheP-phoA gene fusions were grown under the same conditions described for the transport assay, and alkaline phosphatase activity was assayed as described by Manoil (14). Each assay was performed in duplicate on at least three separate occasions.
Immunoprecipitation.
Preparation of cell extracts and immunoprecipitation conditions were performed and set, respectively, according to the method specific for integral membrane proteins described by Ito and Akiyama (13), using anti-PheP serum TTP7 (21). Samples were electrophoresed on a sodium dodecyl sulfate–12% polyacrylamide gel. The dried gel was exposed to X-ray film for at least 72 h. Broad-range protein molecular weight standards (Bio-Rad) were used to estimate the molecular weights of PheP and chimeric proteins. The densities of the radioactive bands were measured by scanning the autoradiographs with a Molecular Dynamics scanning densitometer, and these values were used to estimate the levels of chimeric proteins relative to that of the wild type.
RESULTS
Localization of the sites of recombination in the chimeric genes.
A range of AroP-PheP chimeras were produced as described in Materials and Methods, and the fusion sites in the 11 chimeric genes chosen for study were mapped using restriction enzymes that cleave in either aroP (DdeI, PvuII, AccI, and NdeI) or pheP (HpaI, BssHII, and AatII), but not in both. By determining the presence or absence of these cleavage sites in each chimeric gene, it was possible to localize the approximate sites of recombination of the individual chimeric genes. The exact sites at which recombination between the two permease genes had occurred were subsequently identified by nucleotide sequencing of each of the chimeric genes. The results are shown in Fig. 3.
In all cases, recombination has taken place between corresponding homologous regions of the aroP and pheP genes. Each chimera has a single junction site, and no deletions or insertions of bases were observed. The regions at which recombination occurred varied from 5 to 22 bp of uninterrupted homology.
Altered properties of the PheP system when expressed from aroPP.
Before describing the transport activities of the various chimeric proteins, it is necessary to describe an unexpected observation involving strains in which the pheP gene is transcribed from the aroP promoter and the pheP message is translated using the aroP translation initiation region involving an ATG instead of a GTG initiation codon. Since this will be the situation with each of the AroP-PheP chimeras, we made this strain as an appropriate control along with wild-type aroP for comparing transport activities.
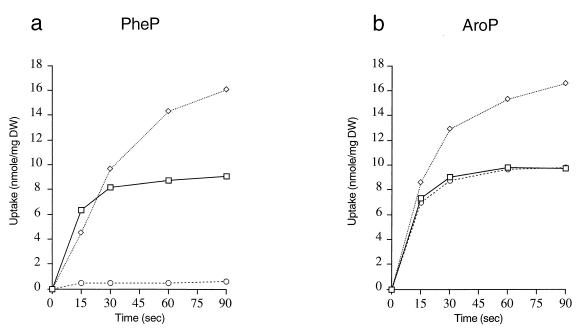
Site-directed mutagenesis was used to change the PheP start codon from GTG to ATG and to introduce an NdeI cut site over the translation initiation codon. A similar site had been introduced over the ATG start codon of aroP to allow the exchange of fragments to create the required aroPpromoter-TIR-pheP construct. This construct was present on the plasmid pLG339 and was introduced into the tyrR, aromatic amino acid transport-negative strain JP7910. The transport characteristics of this new strain and of a corresponding strain which has the wild-type aroP gene on pLG339 are shown in Fig. 4.
FIG. 4.
Uptake of phenylalanine (□), tyrosine (◊), and tryptophan (○) (10 μM) by E. coli strain JP7910 carrying pMU4784 with pheP expressed from the aroP transcription and translation regions (a) and pMU2195 carrying aroP (b). DW, dry weight.
Whereas strains possessing the wild-type pheP gene had previously been characterized as primarily phenylalanine transporters because the steady-state levels of tyrosine transport were both very low and also significantly less than the levels obtained for phenylalanine transport, as can be seen in Fig. 4, strains expressing PheP protein at higher-than-normal levels exhibit a transport profile in which, as in AroP, steady-state levels of tyrosine transport exceed those of phenylalanine. The initial rate of tyrosine transport by PheP in these experiments is noticeably less than that for phenylalanine, and we have determined that this probably reflects the lesser affinity of the PheP protein for tyrosine (Km, 30 μm) than for phenylalanine (Km, 2 μm) (data not shown). In contrast, the AroP protein transports each of the aromatic amino acids with a Km of 1 μM (4). The strain with the PheP protein still fails to transport tryptophan, and tryptophan is unable to significantly inhibit the transport of phenylalanine. In the light of these results, we have concentrated in this paper on the ability of the chimeric proteins to transport tryptophan and the ability of tryptophan to inhibit phenylalanine transport. Studies of more subtle effects influencing Km values for tyrosine transport will be reported separately.
Nomenclature.
In order to simplify the description of the various chimeras, we have used the AroP residue number at each of the fusion sites. In the AroP-PheP chimeras, we have indicated after “A” the last AroP-specific residue in the chimera. In some cases, the actual recombination may have taken place a little further into the gene, but the additional AroP DNA sequence, although different from PheP, did not result in any changes at the amino acid level. In the case of the AroP-PheP-AroP chimeras, the fusion point for the distal AroP segment is indicated by the first AroP-specific residue after the PheP sequence.
Overall transport activity of the various chimeras.
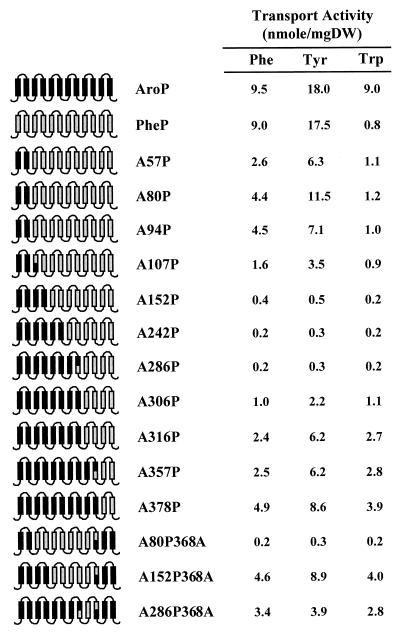
The overall transport activities of AroP, PheP, and each of the chimeric proteins are shown in Fig. 5, along with a diagram indicating the composition of the chimeras (the exact fusion point for each chimera can be established by consulting Fig. 1).
FIG. 5.
Schematic representation of the AroP permease (black), the PheP permease (grey), and each chimeric protein, illustrating the composition of AroP and PheP regions (black and grey). Based on topological information, the proteins are represented as possessing 12 membrane-spanning domains with their amino and carboxyl termini within the cytoplasm. The steady-state levels of phenylalanine, tyrosine, and tryptophan transport by derivatives of the transport-minus strain JP7910 carrying plasmids with these chimeric genes are also presented. Background levels of transport into the transport-minus strain have been subtracted. DW, dry weight.
Inspection of the results shows that none of the chimeras exhibit overall transport activities equivalent to more than 50% of either of the parent proteins. In general, the highest values are obtained in chimeras that are predominantly AroP, such as A378P, or predominantly PheP, such as A80P. There are exceptions to this rule, as A57P has lower overall transport activity than does A80P. Three chimeras, A152P, A242P, and A286P, appear to have lost all ability to transport any of the aromatic amino acids. In the case of A152P, this activity is restored to a high level in the composite chimera A152P368A, and this result is in stark contrast with the loss of activity when A80P is converted to A80P368A.
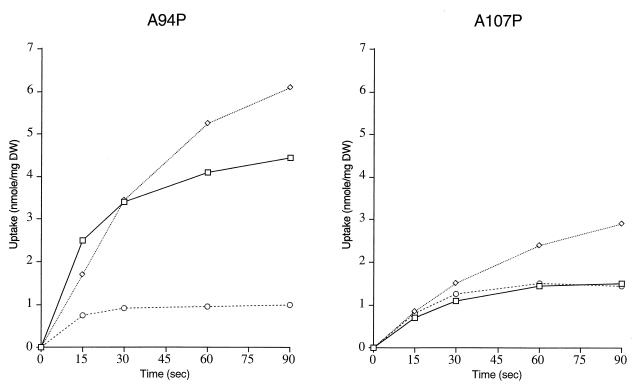
Wild-type AroP and the chimeras A316P, A357P, A378P, A152P368A, and A286P368A are characterized by the ability to transport both phenylalanine and tryptophan to the same steady-state levels. Wild-type PheP and chimeras A57P, A80P, and A94P, on the other hand, transport phenylalanine to significantly higher levels than they do tryptophan, which remains at around background levels. In the case of A107P and A306P, the situation is less clear. Although the results could resemble the AroP rather than the PheP profile, the fact that the level of phenylalanine transport is so low makes interpretation uncertain. However, another way of looking at tryptophan transport is to measure the ability of cold tryptophan when in excess to inhibit the uptake of phenylalanine. In the case of AroP, when radiolabeled phenylalanine is at 10 μm and cold tryptophan inhibitor is 200 μm, inhibition of phenylalanine uptake is about 90%. By contrast, in the case of PheP, such inhibition is only 7% (data not shown). When the ability of tryptophan to inhibit phenylalanine transport was tested in A94P and A107P, the results were 18 and 80% inhibition, respectively. Detailed transport studies were carried out, and the kinetics of transport by these two chimeras is shown in Fig. 6, where it can be seen that even though overall transport activity is low, that of A94P is definitely PheP-like and that of A107P is AroP-like. On these grounds, we conclude that key residues for tryptophan transport present in AroP but absent in PheP are to be found between residues 94 and 107 of AroP.
FIG. 6.
Uptake of phenylalanine (□), tyrosine (◊), and tryptophan (○) (10 μM) by E. coli strain JP7910 carrying plasmids pMU6542 and pMU6543, encoding chimeric proteins A94P and A107P, respectively. DW, dry weight.
Identification of a residue critical for tryptophan transport.
Reference to the AroP and PheP sequences in Fig. 1 reveals that the only differences between the two proteins in the region between positions 94 and 107 in AroP are leucine 102, tyrosine 103, and alanine 107, which in PheP are replaced by methionine, phenylalanine, and glycine, respectively.
As shown in Fig. 6, A94P and A107P differ from each other in two important characteristics. Firstly, A94P is twice as active as A107P in transporting tyrosine and phenylalanine, and secondly, whereas A94P cannot transport tryptophan, A107P has acquired this activity. To investigate this further, we used site-directed mutagenesis to change individual amino acids in A107P into the corresponding residue present in PheP. The results are shown in Table 2.
TABLE 2.
Transport activities of AroP-PheP chimeric proteins A94P and A107P and mutant derivatives
| Chimeric protein | Amino acid replacementa | Uptake (nmol of amino acid/mg [DW])b
|
||
|---|---|---|---|---|
| Phenylalanine | Tryptophan | Tyrosine | ||
| A107P | 1.3 (0.27)c | 0.6 | 2.3 | |
| A107P | Ala 107 Gly | 3.3 (0.38) | 1.9 | 5.7 |
| Tyr 103 Phe | 1.0 (0.7) | 0.1 | 1.4 | |
| Leu 102 Met | 1.4 (0.2) | 1.1 | 2.5 | |
| Tyr 103 Phe + Ala 107 Gly | 2.9 (2.7) | 0.4 | 4.8 | |
| Leu 102 Met + Ala 107 Gly | 1.9 (0.2) | 1.7 | 3.9 | |
| Leu 102 Met + Tyr 103 Phe | 1.8 (1.3) | 0.4 | 2.8 | |
| A94P | 3.3 (2.7) | 0.2 | 4.6 | |
| PheP | 10.9 (10.4) | 0.9 | 15.4 | |
| AroP | 9.4 (0.9) | 6.8 | 18.3 | |
AroP residue numbering.
The steady-state level of uptake taken at 60 s for phenylalanine and tryptophan and 90 s for tyrosine with substrate at 10 μM. DW, dry weight.
Phenylalanine uptake in the presence of 200 μM cold tryptophan.
Changing leucine to methionine affects neither overall transport activity nor the specific transport of tryptophan. Changing alanine 107 to glycine, however, restores transport activity for phenylalanine and tyrosine to levels obtained with A94P, and tryptophan transport is still observed at levels similar to those for phenylalanine. In the mutants which transport tryptophan, the transport of phenylalanine is strongly inhibited by a 20-fold excess of cold tryptophan. Changing tyrosine 103 to phenylalanine, on the other hand, while not changing the level of phenylalanine transport appears to eliminate tryptophan transport and to severely reduce the ability of tryptophan to inhibit phenylalanine transport. In summary, then, these results indicate that the presence of alanine instead of glycine at PheP position 107 causes the overall decrease in transport activity seen in A107P and that the presence of tyrosine instead of phenylalanine at AroP position 103 enables this chimera to transport tryptophan.
Site-directed mutagenesis of PheP.
To determine whether the substitution for phenylalanine 111 (equivalent to position 103 in AroP) of tyrosine was all that was required to enable PheP protein to transport tryptophan, site-directed mutagenesis was used to change phenylalanine 111 to tyrosine in the wild-type PheP protein. The pheP-carrying plasmid pMU3138 was the target for the mutagenesis, and the plasmid with the mutated pheP gene was introduced, as before, into the transport-negative strain JP7910. The transport properties of this new strain are shown in Fig. 7.
FIG. 7.
Uptake of phenylalanine (□), tyrosine (◊), and tryptophan (○) (10 μM) by E. coli strain JP7910 carrying plasmid pMU3138 carrying wild-type pheP with an ATG start codon (a), pMU6557 carrying pheP with an ATG start codon and phenylalanine 111-to-tyrosine alteration (b), and pMU6558 carrying aroP with a tyrosine 103-to-phenylalanine alteration (c). DW, dry weight.
As can be seen, changing phenylalanine 111 to tyrosine enables the altered PheP protein to transport tryptophan to almost the same steady-state level as that of phenylalanine. The steady-state level for tyrosine remains high, and the initial rate for tyrosine uptake appears to have increased. A 20-fold excess of unlabeled tryptophan inhibits phenylalanine transport by 60%. This is significantly higher than the 7% inhibition seen with wild-type PheP protein but also significantly less than the 90% or higher levels seen in A107P and wild-type AroP. This may indicate that other AroP-specific residues in the region of the protein preceding span III contribute also to tryptophan binding or transport.
In a similar fashion, tyrosine inhibition of phenylalanine transport is also increased from about 15% in the wild-type PheP to 40% in the mutant (data not shown).
Computer modeling of the tyrosine-substituted span III reveals that either a single molecule of tryptophan or a single molecule of tyrosine can simultaneously form hydrogen bonds with glutamate 118 and with a tyrosine residue located at position 111. Glutamate 118, whose possible function is discussed below, is a highly conserved residue whose replacement by residues other than aspartate leads to loss of function (24).
Further modeling of span III reveals a similar relationship between tyrosine 125 and glutamate 118. To test whether tyrosine 125 is also essential for tryptophan transport, site-directed mutagenesis was used to change tyrosine 125 to phenylalanine in the PheP-encoding gene already carrying the mutation changing phenylalanine 111 to tyrosine. In spite of the modeling results, the introduction of the second substitution into the PheP protein failed to affect either tryptophan transport or the inhibition of phenylalanine transport by tryptophan. Unexpectedly, this mutation did, however, cause some overall reduction in steady-state level for phenylalanine transport (data not shown).
The significance of tyrosine 103 in AroP.
To determine the role of tyrosine 103 in the AroP protein, site-directed mutagenesis was used to change tyrosine 103 to phenylalanine in the wild-type aroP gene carried on plasmid pMU2195. The mutated gene was again introduced into the transport-negative strain JP7910, and the new strain was tested for its ability to transport each of the three aromatic amino acids. The results are shown in Fig. 7. Although the tyrosine 103-to-phenylalanine alteration does not completely abolish tryptophan transport, it does specifically decrease it to less than 50% of wild-type levels, and it also reduces tryptophan inhibition of phenylalanine transport from 95 to 62%. From this result, it would appear that tyrosine 103 does play an important role in tryptophan transport but that in AroP protein it is supported by other AroP-specific residues beyond span III. It should also be noted that whereas tyrosine at a 20-fold excess can inhibit phenylalanine transport by more than 90% in wild-type AroP, in the case of the tyrosine 103-to-phenylalanine mutation this inhibition is reduced to only 50% (data not shown).
The failure of some chimeras to transport any amino acids.
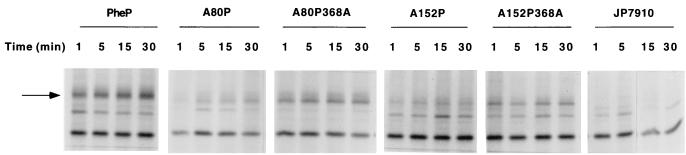
As previously stated, a number of chimeras fail to transport any amino acids, and in particular, whereas chimeras A152P and A80P368A fail to show any significant transport activity, chimeras A152P368A and A80P are two of the most active chimeric transporters. By using an anti-PheP specific polyclonal serum that had been prepared against residues 231 to 245 of PheP (see Materials and Methods), we carried out pulse-chase experiments to test whether (a) the nontransporters failed to insert into the membrane or (b) whether such insertions were inherently unstable. The results are shown in Fig. 8.
FIG. 8.
Stability of chimeric proteins. E. coli strain JP7910 expressing wild-type PheP, AroP-PheP, and AroP-PheP-AroP chimeric proteins was pulse-labeled with [35S]methionine-cysteine for 1 min followed by chase periods of 1, 5, 15, and 30 min. A 0.5-ml aliquot of each sample was taken at the times indicated and immunoprecipitated with the PheP-specific antibody TTP7. The samples were separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and visualized by autoradiography. The intensity of each band was quantified by densitometry as described in Materials and Methods. The arrow indicates the location of bands corresponding to wild-type PheP and AroP-PheP chimeric proteins.
Although the overall strength of the signal is weaker with the chimeras than with wild-type PheP, the chimeras with no transport activity appear to be present in the membrane to the same extent as the chimeras with high transport activity. None of the proteins show any marked instability. A densitometric measurement of the signals from the chimeric proteins and from wild-type PheP indicates that if the affinity between the chimeric proteins and the antibody is the same as that for PheP, then the membrane contains chimeric proteins at approximately 40% of the levels of wild-type PheP protein. This calculation would imply that the in situ, specific activities of the two most active chimeric proteins, A80P and A152P368A, were approximately the same as that of the wild-type PheP protein.
We also inserted a leaderless alkaline phosphatase gene into different sites in the chimeras A80P, A152P, A286P, and A378P. As shown in Table 3, the alkaline phosphatase activities obtained showed strong correlation with those of similar fusions involving either PheP or AroP.
TABLE 3.
Alkaline phosphatase activities of AroP, PheP, and AroP-PheP chimeric proteins
From these sets of results, we conclude that failure of these transporters to actively transport the amino acids is not a consequence of failure to be inserted in the membrane or of greatly enhanced instability. The observation that A80P and A152P368A are both strong transporters, whereas A80P368P and A152P failed to transport amino acids, suggested that possible interactions between some proximal and distal spans of the protein may be central to activity. The high activity of A80P and A378P, however, would appear to exclude spans I and II and spans XI and XII from this requirement. A shared characteristic of the three strongest transporters, A80P, A152P368A, and A378P, is that spans III and IV and associated loops and span X come from the same protein, either PheP in the case of A80P or AroP in the case of the other two. If close packing or critical interactions need to occur between these regions of the protein to create a functional transporter, variations in amino acid sequences between AroP and PheP could prevent these interactions in the heterologous constructs.
Identification of residues affecting transport activity of different chimeras.
We have used site-directed mutagenesis to investigate the particular amino acid substitutions responsible for major changes in the transport activities of the different chimeras, and the results are shown in Table 4. Chimera A80P has twice the transport activity of A57P and differs from it by the substitution of an alanine for serine at position 76 and a substitution of a serine for an alanine at position 80. When we separately changed these residues in A57P, the change of serine to alanine at position 76 resulted in a doubling of activity, whereas the change of alanine to serine at position 80 had no effect. Chimera A94P shows a specific decrease in the steady-state levels of tyrosine accumulation and differs from A80P at two positions. Single-site substitutions in A80P reveal that this change is a consequence of the substitution of serine for proline at position 89 and that the other change of leucine to alanine at position 94 has no effect (data not shown). As we have already described, the significant loss of activity in A107P can be attributed to the change of glycine to alanine at position 107.
TABLE 4.
Transport activities of AroP-PheP chimeric proteins and their mutant derivatives
| Chimeric protein | Amino acid replacementa | Uptake (nmol of amino acid/mg [DW])b
|
|
|---|---|---|---|
| Phenylalanine | Tyrosine | ||
| A57P | 2.6 | 6.3 | |
| Ser 76 Ala | 4.2 | 10.9 | |
| Ala 80 Ser | 2.8 | 7.0 | |
| A80P | 4.4 | 11.5 | |
| Ala 76 Ser | 2.9 | 7.2 | |
| Pro 89 Ser | 4.3 | 7.2 | |
| A94P | 4.5 | 7.1 | |
| Ser 89 Pro | 4.7 | 11.1 | |
| A152P | 0.7 | 1.2 | |
| Lys 116 Ile | 0.8 | 1.8 | |
| Ala 107 Gly | 2.4 | 6.4 | |
| Val 114 Ala | 1.7 | 3.3 | |
AroP residue numbering.
The steady-state level of uptake was taken as the level of amino acid accumulation when uptake had leveled off after 2 min, with substrate at 10 μM. DW, dry weight.
The low activity in A107P is further decreased in A152P, which appears to have lost almost all of its transport activity. Since we had already shown that the glycine-to-alanine alteration at PheP position 115 (AroP position 107) had contributed to the loss of activity in A107P, we decided to concentrate on AroP-specific residues in span III to see if we could restore activity to the A152P chimera. Site-directed mutagenesis was used on the A152P template, and mutants were isolated and checked for a restoration of activity. The substitution of lysine for isoleucine at AroP position 116 seemed a possible cause of major disturbance. However, as can be seen from Table 4, the reverse change of lysine 116 to isoleucine failed to restore activity. On the other hand, the change of alanine 107 to glycine and the change of valine 114 to alanine both resulted in significant increases in transport activity. The chimera A152P also recovers significant transport activity when AroP sequences are substituted for PheP from amino acid 368 to the carboxy terminus, perhaps suggesting a role for homologous distal spans in forming some functional tertiary structure. The chimera A378P, which has the first 10 spans of AroP and the last 2 of PheP, also shows high transport activity and shares only the single distal span X with A152P368A. We tested for the presence of AroP-specific residues in span X which might provide complementary functions to alanine 107 and valine 114 by changing each of the PheP-specific residues in span X of A152P individually into the corresponding amino acid present in AroP. We also made double and triple substitutions and finally changed all of the PheP-specific residues. None of these changes, however, restored the activity of A152P to above 10% of that of the wild type (data not shown). This means that, if our hypothesis is correct, the activity of span X must be able to be influenced either by homologous spans XI and XII or by IX or the preceding spans. Transport activity also increases significantly between A306P and A316P. This change, however, involves only two substitutions in the cytoplasmic loop between spans VIII and IX, and other studies have suggested that changes in this region can have major consequences for tertiary structures involving the distal spans (J. Pi and A. J. Pittard, unpublished results).
DISCUSSION
Identification of an amino acid of major importance for tryptophan transport.
A system of in vivo homologous recombination has been successfully used to generate a family of AroP-PheP chimeras. Examination of these different chimeras coupled with some site-directed mutagenesis has identified an amino acid, phenylalanine 111 in span III of PheP, which, when changed to tyrosine (the corresponding residue in AroP), enables the protein to transport tryptophan and enables tryptophan to inhibit the transport of phenylalanine. The observation that cold tryptophan can inhibit the transport of both phenylalanine and tyrosine would suggest that the substrate binding sites for each of these amino acids overlap and certainly involve span III. We have not measured the Km for tryptophan transport in this PheP mutant, but the observation that a 20-fold excess of cold tryptophan causes a 60% inhibition of phenylalanine transport rather than the >90% seen in AroP and in the chimera A107P indicates that other AroP-specific amino acids in A107P may also contribute to tryptophan binding or transport. The finding that the conversion of a phenylalanine to tyrosine is necessary for tryptophan transport implies some essential function for the hydroxyl of the tyrosine residue. The observation that it is possible, in computer modeling, to position either a tryptophan or a tyrosine molecule so that it is simultaneously hydrogen bonded to both glutamate 118 and tyrosine 111 may be relevant to the elaboration of a molecular mechanism for the transport of these amino acids, although it should be noted that similar hydrogen bonds can be formed with glutamate 118 and tyrosine 125 and yet changing tyrosine 125 to phenylalanine does not interfere with either tryptophan or tyrosine transport.
The replacement of the corresponding tyrosine residue at position 103 in AroP by phenylalanine did not result in complete loss of tryptophan transport but did cause a 50% reduction and reduced the ability of tryptophan to inhibit phenylalanine transport from 95 to 62%. This result suggests that, in addition to tyrosine at position 103, the AroP protein has other AroP-specific residues which contribute to tryptophan transport. As the chimera A94P is unable to transport tryptophan, it seems likely that such residues will be found beyond position 94 of AroP.
Although we have not yet carried out a detailed kinetic analysis of tyrosine transport in these various chimeras and mutants, the observation that the tyrosine 103-to-phenylalanine alteration in AroP and the phenylalanine 111-to-tyrosine alteration at the equivalent position in PheP each cause changes in the degree of inhibition of phenylalanine transport by tyrosine suggests that a tyrosine residue at position 103 in AroP is also involved in tyrosine-specific transport.
Reevaluation of span III.
The composition of span III in both PheP and AroP has not been as clearly defined as that of most other spans. Hydropathy algorithms such as GES or von Heijne's Top Pred select a sequence from 98 to 116 in PheP and from 91 to 109 in AroP. However, a study of alkaline phosphatase sandwich fusions with both PheP and AroP produced results indicating that position 116 in PheP and 109 in AroP were located well within the membrane, and on the basis of these results, the sequences 106 to 126 and 98 to 118 have been selected for span III in PheP and AroP, respectively (Fig. 1) (6, 22). In the PheP sequence from 98 to 126, there are seven aromatic amino acids. If this is represented as a helix, all of these residues, phenylalanine 98, phenylalanine 101, tryptophan 105, tyrosine 107, tryptophan 108, phenylalanine 111, and tyrosine 125, are located on the same side of the helix, which also includes an essential and highly conserved residue, glutamate 118, and the two important residues glycine 115 and alanine 122 (Fig. 9).
FIG. 9.
A helical wheel plot of PheP amino acid residues 98 to 126, assuming a periodicity of 3.6 residues per turn. Aromatic amino acid residues (boxed) and residues found to be important for activity (underlined) cluster on one side of the helix.
When six of the aromatic residues were changed individually to leucine, mutants involving phenylalanine 98 or tyrosine 107 showed no change in phenylalanine transport, but changes to either phenylalanine 101, tryptophan 105, tryptophan 108, or phenylalanine 111 resulted in a loss of between 40 and 60% of transport activity (24). In considering whether or not these amino acids had an important role to play in a putative channel for the aromatic amino acids, we had concluded that this loss of activity was not a sufficient indication. However, recent results may warrant further consideration of this possibility. A double mutant with alteration of phenylalanine 101 to leucine and phenylalanine 111 to leucine has been created and shown to have no ability to transport phenylalanine (J. Pi and A. J. Pittard, personal communication). Structural studies of three transmembrane proteins reveal that helix lengths can range from 14 to 36 residues with an average length of 26.4 residues (3), and on the basis of the above results, the possibility that the membrane span III of PheP extends from 98 to about 126 and involves a face of aromatic amino acids as part of a channel should be kept open. Such channels have been proposed for other systems (7, 19).
The basis for loss of activity in some chimeras.
An examination of chimeras which had lost all activity revealed that this was not a consequence of failure to be inserted into the membrane. A detailed study of one such chimera, A152P, revealed two amino acid substitutions, alanine for glycine 115 and valine for alanine 122, which contributed to loss of activity. Activity could be partially restored by reversing either of these substitutions. These residues are located above and below glutamate 118 slightly to one side (Fig. 9). Since alanine and valine at these positions are no encumbrance to activity in the case of AroP and since, when distal AroP spans are changed in A152P to produce A152P368A, activity is restored, we propose that important interactions occur between these residues and others located in distal spans of the protein. One consequence of this interaction could be the positioning of the negatively charged residue glutamate 118. At the position corresponding to 118 in PheP, other bacterial members of the family contain either glutamate or aspartate. Converting glutamate 118 of the PheP protein to glycine, valine, leucine, tryptophan, or asparagine has been shown to completely destroy transport activity, whereas changing it to aspartate reduces transport activity to 36% of wild-type level (24). These results indicate the importance of glutamate 118, which is presumed to have a critical role either in proton translocation or in substrate binding or both. The observation that its replacement by aspartate causes a partial reduction in activity suggests that span III has some flexibility to adjust to changes in side chain length. We have not yet identified the residue with which glutamate 118 interacts, but it should be noted that lysine 168 is also highly conserved and critical for transport activity. The replacement of lysine 168 of the PheP protein by arginine results in a reduction of transport activity to about 30% of wild-type level. It is possible that increasing the side chain length of glycine 115 and alanine 122 interferes with transport activity because it perturbs the positioning of glutamate 118 in the tertiary configuration of the PheP protein. Neither the glycine nor the alanine in span III at position 115 or 122, respectively, is highly conserved throughout other members of the family, and this may support the hypothesis that their significance is more related to packing arrangements with other helices than to providing, in the case of glycine 115, the sort of flexibility that has been recently demonstrated to be of importance in the Lac permease (33).
The abilities of some of the chimeras and some of the mutants to transport tyrosine show significant variations from those of each of the parent proteins and merit detailed kinetic studies to elaborate changes in Km. Furthermore, the dramatic change in relative steady-state levels of phenylalanine and tyrosine when the PheP protein is more highly expressed also requires an explanation.
Hama and Wilson have also used chimeras of the closely related melibiose transporters of E. coli and Klebsiella pneumoniae to study Na coupling in transport (11). It is of interest that in this case chimeras involving the first six transmembrane regions of one protein and the last six of the other show strong transport activity, perhaps reflecting the greater overall similarity between these two proteins (78% identity) than is the case with AroP and PheP.
ACKNOWLEDGMENTS
This work was supported by a grant from the Australian Research Council Large Grants Scheme. A. J. Cosgriff and J. P. Sarsero were each a recipient of an Australian Postgraduate Research Award.
We thank Frank Gibson for the modeling work and helpful discussion. We also thank Judyta Praszkier for reading the manuscript. We are grateful to J.-H. An, Y. Jiang, and M. Pont for technical assistance. Some of the oligonucleotides used in this study were synthesized by V. Athanasopoulos, J. Praszkier, and J. Yang.
REFERENCES
- 1.Ames G F-L. Uptake of amino acids by Salmonella typhimurium. Arch Biochem Biophys. 1964;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann B J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990;54:130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowie J U. Helix packing in membrane proteins. J Mol Biol. 1997;272:780–789. doi: 10.1006/jmbi.1997.1279. [DOI] [PubMed] [Google Scholar]
- 4.Brown K D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970;104:177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck K, Amara S. Chimeric dopamine-norepinephrine transporters delineate structural domains influencing selectivity for catecholamines and 1-methyl-4-phenylpyridinium. Proc Natl Acad Sci USA. 1994;91:12584–12588. doi: 10.1073/pnas.91.26.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgriff A, Pittard A J. A topological model for the general aromatic amino acid permease, AroP, of Escherichia coli. J Bacteriol. 1997;179:3317–3323. doi: 10.1128/jb.179.10.3317-3323.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn M F, Aguilar V, Brzovic P, Drewe J W F, Houben K F, Leja C A, Roy M. The tryptophan synthase bienzyme complex transfers indole between the α- and β-sites via a 25-30 A long tunnel. Biochemistry. 1990;29:8598–8607. doi: 10.1021/bi00489a015. [DOI] [PubMed] [Google Scholar]
- 8.Ehrmann M, Boyd D, Beckwith J. Genetic analysis of membrane protein topology by a sandwich gene fusion approach. Proc Natl Acad Sci USA. 1990;87:7574–7578. doi: 10.1073/pnas.87.19.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisele J-L, Bertrand S, Galzi J-L, Devillers-Thiery A, Changeux J-P, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 10.Giros B, Wang Y, Suter S, McLeskey S, Pifl C, Caron M. Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J Biol Chem. 1994;269:15985–15988. [PubMed] [Google Scholar]
- 11.Hama H, Wilson T H. Cation-coupling in chimeric melibiose carriers derived from Escherichia coli and Klebsiella pneumoniae. J Biol Chem. 1993;268:10060–10065. [PubMed] [Google Scholar]
- 12.Honoré N, Cole S T. Nucleotide sequence of the aroP gene encoding the general aromatic amino acid transport protein of Escherichia coli K-12: homology with yeast transport proteins. Nucleic Acids Res. 1990;18:653. doi: 10.1093/nar/18.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Akiyama Y. In vivo analysis of integration of membrane proteins in Escherichia coli. Mol Microbiol. 1991;55:2243–2253. doi: 10.1111/j.1365-2958.1991.tb02154.x. [DOI] [PubMed] [Google Scholar]
- 14.Manoil C. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase gene fusions. Methods Cell Biol. 1992;34:61–75. doi: 10.1016/s0091-679x(08)61676-3. [DOI] [PubMed] [Google Scholar]
- 15.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 16.Monod J, Cohen-Bazire G, Cohn M. Sur la biosynthese de la β-galactosidase (lactase) chez Escherichia coli. La specificite de l'induction. Biochim Biophys Acta. 1951;7:585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- 17.Nishizawa K, Shimoda E, Kasahara M. Substrate recognition domain of the Gal2 galactose transporter in yeast Saccharomyces cerevisiae as revealed by chimeric galactose-glucose transporters. J Biol Chem. 1995;270:2423–2426. doi: 10.1074/jbc.270.6.2423. [DOI] [PubMed] [Google Scholar]
- 18.Norrander J, Kempe T, Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983;26:101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- 19.Pawag A, Wang J, Silverman M, Reithmeier R, Deber C. Transmembrane aromatic amino acid distribution in P-glycoprotein. A functional role in broad substrate specificity. J Mol Biol. 1994;235:554–564. doi: 10.1006/jmbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 20.Pi J. Ph.D. thesis. Melbourne, Australia: University of Melbourne; 1991. [Google Scholar]
- 21.Pi J, Dogovski C, Pittard A J. Functional consequences of changing proline residues in the phenylalanine-specific permease of Escherichia coli. J Bacteriol. 1998;180:5515–5519. doi: 10.1128/jb.180.21.5515-5519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi J, Pittard A J. Topology of the phenylalanine-specific permease of Escherichia coli. J Bacteriol. 1996;178:2650–2655. doi: 10.1128/jb.178.9.2650-2655.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pi J, Wookey P J, Pittard A J. Cloning and sequencing of the pheP gene, which encodes the phenylalanine-specific transport system of Escherichia coli. J Bacteriol. 1991;173:3622–3629. doi: 10.1128/jb.173.12.3622-3629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pi J, Wookey P J, Pittard A J. Site-directed mutagenesis reveals the importance of conserved charged residues for the transport activity of the PheP permease of Escherichia coli. J Bacteriol. 1993;175:7500–7504. doi: 10.1128/jb.175.22.7500-7504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reizer J, Finley K, Kakuda D, MacLeod C L, Reizer A, Saier M H., Jr Mammalian integral membrane receptors are homologous to facilitators and antiporters of yeast, fungi, and eubacteria. Protein Sci. 1993;2:20–30. doi: 10.1002/pro.5560020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarsero J P. Ph.D. thesis. Melbourne, Australia: University of Melbourne; 1994. [Google Scholar]
- 29.Stoker N G, Fairweather N F, Spratt B G. Versatile low copy number plasmid vectors for cloning in Escherichia coli. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- 30.Tommassen J, van der Ley P, van Zeijl M, Agterberg M. Localization of functional domains in E. coli K-12 outer membrane porins. EMBO J. 1985;4:1583–1587. doi: 10.1002/j.1460-2075.1985.tb03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torriani A. Alkaline phosphatase from Escherichia coli. In: Cantoni G L, Davies R, editors. Procedures in nucleic acid research. New York, N.Y: Harper & Row, Publishers, Inc.; 1966. pp. 224–234. [Google Scholar]
- 32.Vandeyar M A, Weiner M P, Hutton C J, Batt C A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988;65:129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]
- 33.Weinglass A B, Kaback H R. Conformational flexibility at the substrate binding site in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:11178–11182. doi: 10.1073/pnas.96.20.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whipp M J. Ph.D. thesis. Melbourne, Australia: University of Melbourne; 1977. [Google Scholar]
- 35.Whipp M J, Halsall D M, Pittard A J. Isolation and characterization of an Escherichia coli K-12 mutant defective in tyrosine- and phenylalanine-specific transport systems. J Bacteriol. 1980;143:1–7. doi: 10.1128/jb.143.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whipp M J, Pittard A J. Regulation of aromatic amino acid transport systems in Escherichia coli. J Bacteriol. 1977;132:453–461. doi: 10.1128/jb.132.2.453-461.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wookey P J, Pittard J, Forrest S M, Davidson B E. Cloning of the tyrP gene and further characterization of the tyrosine-specific transport protein in Escherichia coli K-12. J Bacteriol. 1984;160:169–174. doi: 10.1128/jb.160.1.169-174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young G B, Jack D L, Smith D W, Saier M H., Jr The amino acid/auxin:proton symport permease family. Biochim Biophys Acta. 1999;1415:306–322. doi: 10.1016/s0005-2736(98)00196-5. [DOI] [PubMed] [Google Scholar]