Abstract
Purpose
Emerging data suggest that metastasis-directed therapy (MDT) improves outcomes in patients with oligometastatic castration-sensitive prostate cancer (omCSPC). Prostate-specific membrane antigen positron emission tomography (PSMA-PET) can detect occult metastatic disease, and PSMA response has been proposed as a biomarker for treatment response. Herein, we identify and validate a PSMA-PET biomarker for metastasis-free survival (MFS) following MDT in omCSPC.
Methods and Materials
We performed an international multi-institutional retrospective study of patients with omCSPC, defined as ≤3 lesions, treated with metastasis-directed stereotactic ablative radiation who underwent PSMA-PET/computed tomography (CT) before and after (median, 6.2 months; range, 2.4-10.9 months) treatment. Pre- and post-MDT PSMA-PET/CT maximum standardized uptake value (SUVmax) was measured for all lesions, and PSMA response was defined as the percent change in SUVmax of the least responsive lesion. PSMA response was both evaluated as a continuous variable and dichotomized into PSMA responders, with a complete/partial response (at least a 30% reduction in SUVmax), and PSMA nonresponders, with stable/progressive disease (less than a 30% reduction in SUVmax). PSMA response was correlated with conventional imaging-defined metastasis-free survival (MFS) via Kaplan-Meier and Cox regression analysis.
Results
A total of 131 patients with 261 treated metastases were included in the analysis, with a median follow-up of 29 months (IQR, 18.5-41.3 months). After stereotactic ablative radiation, 70.2% of patients were classified as PSMA responders. Multivariable analysis demonstrated that PSMA response as a continuous variable was associated with a significantly worse MFS (hazard ratio = 1.003; 95% CI, 1.001-1.006; P = .016). Patients classified as PSMA responders were found to have a significantly improved median MFS of 39.9 versus 12 months (P = .001) compared with PSMA nonresponders. Our study is limited as it is a retrospective review of a heterogenous population.
Conclusions
After stereotactic ablative radiation, PSMA-PET response appears to be a radiographic biomarker that correlates with MFS in omCSPC. This approach holds promise for guiding clinical management of omCSPC and should be validated in a prospective setting.
Introduction
Globally, prostate cancer represents the third most common malignancy and is responsible for nearly 400,000 deaths annually.1 Despite advances in technologies and treatment of both castration-sensitive and castration-resistant prostate cancer, metastatic disease remains largely incurable.2, 3, 4, 5, 6, 7 Although androgen deprivation therapy (ADT) is used to treat metastatic castration-sensitive prostate cancer (mCSPC), the majority of patients will eventually develop castration resistance, which is associated with higher rates of mortality.
Given this trajectory, there is significant interest in metastasis-directed therapy (MDT), used to improve outcomes by delaying progression and initiation of long-term systemic therapy. Several clinical trials have evaluated the role of MDT with stereotactic ablative radiation (SABR) in patients with limited metastatic disease, known as oligometastatic disease.8, 9, 10, 11, 12 These trials have defined the oligometastatic state as no more than 3 to 5 metastases. For metachronous oligorecurrent mCSPC, the STOMP and ORIOLE trials demonstrated improved ADT-free and progression-free survival, respectively, with MDT, compared with observation.13,14
Importantly, numerical definitions of oligometastasis rely heavily upon the sensitivity of the imaging used.15 With the advent of molecular imaging, the sensitivity of prostate cancer imaging has improved dramatically. Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein expressed on the surface of prostate cancer cells and is overexpressed by both local and metastatic prostate cancer.16,17 PSMA-based positron emission tomography (PET) imaging has demonstrated high sensitivity and specificity in detecting occult metastatic disease,18,19 and the PSMA response has been proposed as a potential biomarker for the response to systemic or local therapy.20 Herein, we evaluated the post-SABR PSMA-PET response to MDT in patients with oligometastatic mCSPC (omCSPC) to assess the correlation between PSMA response and clinical outcomes.
Methods and Materials
Following institutional review board approval, we performed an international multi-institutional cohort study of men with newly diagnosed omCSPC treated with metastasis-directed SABR who underwent pre- and posttreatment PSMA-PET/computed tomography (CT). Patients included those treated at Johns Hopkins Hospital as part of the ORIOLE trial14 (conventionally staged cohort) and those treated at Baskent University (PSMA-PET staged cohort). Inclusion criteria were patients with omCSPC, defined as ≤3 metastases on either conventional (CT/radionuclide bone scan) or PSMA-targeted (PET) molecular imaging. Patients with oligometastatic disease per conventional imaging but with polymetastatic disease per PET (range, 4-25 lesions) were eligible for inclusion, as patients in the ORIOLE trial were defined on conventional imaging but underwent pre-MDT PSMA-PET, to which investigators were blinded. Patients treated with concurrent ADT were also included in the analysis.
Before MDT, all patients received either 68Ga-PSMA-HBED-CC or 18F-DCFPyL-PSMA PET/CT. All patients underwent CT-based simulation with personalized immobilization specific to the metastasis location. Gross tumor volume and organs at risk (OARs) were identified by the treating physician. A variable planning target volume expansion of 2 to 5 mm was performed based on metastasis location. A SABR plan was generated with dose and fractionation based on tumor size and location, while maintaining normal tissue constraints to OARs per AAPM Task Group 101 recommendations.21 Prescription doses ranged from 16 to 60 Gy in 1 to 5 fractions (1 patient was treated with hypofractionated radiation therapy in 15 fractions). Image guidance with cone beam CT was used to confirm patient set-up before treatment. A follow-up PSMA-PET/CT was performed to evaluate disease response after SABR. Following MDT, patients did not receive any systemic therapy until evidence of disease progression was observed (with the exception of the limited duration of concurrent ADT delivered with MDT). Available follow-up data from serial physical examinations, imaging, and prostate-specific antigen (PSA) measurements were obtained by chart review.
Summary statistics were calculated for patients and lesions. Each lesion was characterized as having a complete response (CR; no residual PSMA activity), partial response (PR; at least 30% reduction in SUVmax), stable disease (SD; <30% reduction or <20% increase in SUVmax), or progressive disease (PD; at least 20% increase in SUVmax). Discrete cutoff values were determined to reflect commonly used radiographic thresholds of response.22 Multivariable binary logistic regression was used to identify clinical/treatment features associated with PSMA response (CR/PR). The primary outcome was metastasis-free survival (MFS) following SABR, stratified into PSMA responders (all lesions with CR/PR) versus PSMA nonresponders (at least 1 lesion with SD/PD); PSMA response was evaluated as a continuous variable for the worst responding lesion. MFS was defined as the time from MDT to development of new distant metastasis on conventional imaging, or death from any cause.23 Given the clinical heterogeneity of the cohort, several subgroup analyses were also performed to evaluate PSMA response as a biomarker for MFS in more clinically homogenous groups, including those treated with and without concurrent ADT, metachronous and synchronous metastatic disease, conventional and PSMA staging, total and subtotal disease consolidation, and lymph node only and bone/visceral metastases. Our secondary outcomes included lesion local control (LLC) after MDT. Lesion local failure was defined as radiographic growth of a lesion on conventional imaging within the SABR-treated field in conjunction with a rising PSA level. Survival analyses were calculated with the Kaplan-Meier method and compared using a log-rank test. Multivariable Cox regression analysis was conducted for MFS. Variables included in the multivariable analysis were selected a priori based on characteristics known to be associated with prognosis.14,24, 25, 26 Proportional hazards assumption was validated for PSMA response and the timing of the post-MDT PSMA-PET (Fig. E1). Interaction terms for PSMA response and covariates included in the multivariable Cox regression were calculated. All statistical analyses were conducted using IBM SPSS Statistics, version 27, and a 2-sided P value <0.05 was considered statistically significant.
Results
A total of 131 patients with 315 metastases (261 treated with MDT) were included in the analysis, with a median follow-up of 35.4 months (IQR, 21.3-49.9 months). Baseline demographic, clinical, and treatment characteristics at initial diagnosis (Table E1) and oligometastasis (Table 1) are reported. Characteristics of the conventionally staged and PSMA-PET-staged cohorts can be seen in Table E2. The majority of patients had metachronous disease (74.0%) and received MDT to all PSMA-positive lesions (87.8%). The characteristics of all 315 observed PSMA-positive lesions are summarized in Table E3. Among the 261 treated lesions, bone (52.5%) and lymph node (45.2%) metastases were most common. The median pre-MDT SUVmax for all lesions was 8.7 (IQR, 4.0-16.7) and was similar among metastasis locations (Table 1). Lymph node and bone lesions were both treated with a median biologically effective dose (α/β = 3) (BED3) of 116.7 Gy (IQR: bone, 90-126; node, 90-124), while visceral metastases received a median BED3 of 378 Gy (IQR, 234-419). A detailed list of radiation prescriptions used can be found in Supplemental Table E4.
Table 1.
Demographic, treatment, and lesion characteristics at time of oligometastasis
| Oligometastatic characteristics | N = 131 | Treated lesion characteristics | N = 261 |
|---|---|---|---|
| Median age at oligomet (IQR) | 66 (60.75-66) | Location | |
| Median PSA at oligomet (IQR) | 4.5(1.9-11.8) | Node | 118 (45.2%) |
| Bone | 137 (52.5%) | ||
| Visceral | 3 (1.1%) | ||
| Prostate/Local recurrence | 1 (0.4%) | ||
| Timing | |||
| Metachronous | 97 (74.0%) | ||
| Synchronous/de novo | 34 (25.2%) | Median pre-MDT SUVmax (IQR) | |
| All Lesions | 8.7 (4.0-16.7) | ||
| Staging imaging | Node | 9.1(4.0-18.5) | |
| Conventional (CT/Bone Scan) | 35 (26.7%) | Bone | 8.6 (4.2-15.7) |
| PSMA-PET | 96 (73.3%) | Visceral | 7.8 (6.2-10.2) |
| Number of PSMA lesions | Median BED3 Gy (IQR) | ||
| 1 | 62 (47.3%) | All Lesions | 116.7(90-126) |
| 2 | 42 (32.1%) | Node | 116.7 (90-124) |
| 3 | 12 (9.2%) | Bone | 116.7 (90-126) |
| ≥ 4 | 15 (11.5%) | Visceral | 378 (234-419) |
| Total PSMA consolidation | PSMA SUV response | ||
| Yes | 115 (87.8%) | Complete response | 72 (27.6%) |
| No | 16 (12.2%) | Partial response | 135 (51.7%) |
| ADT with MDT | Stable disease | 37 (14.2%) | |
| Yes | 86 (65.6%) | Progressive disease | 17 (6.5%) |
| No | 45 (34.4%) | ||
| Median duration of ADT (IQR) | 2 (1.0-3.75) mo. | ||
| Mode of failure | |||
| Long-term disease free | 64 (48.9%) | ||
| Oligoprogressor | 32 (24.4%) | ||
| Polyprogressor | 35 (26.7%) |
Abbreviations: ADT = androgen deprivation therapy; MDT = metastasis-directed therapy; PSA = prostate-specific antigen; PSMA-PET = prostate-specific membrane antigen positron emissssion tomography; SUV = standardized uptake value.
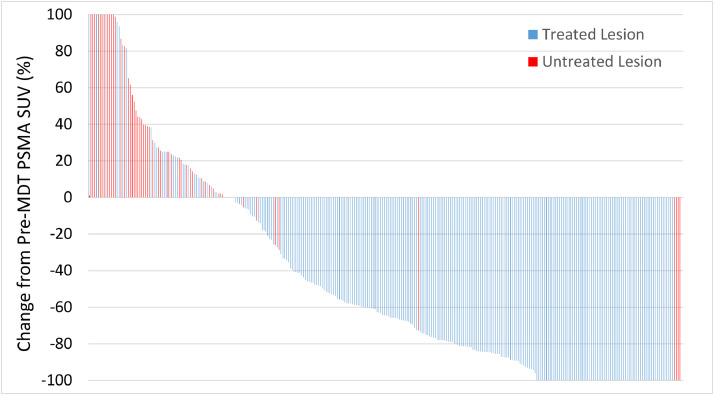
A post-PSMA PET scan was performed a median of 6.2 months (IQR, 4.6-8.7; range, 2.4-10.9) after MDT. The per lesion response rates after SABR were as follows: CR, 27.6%; PR, 51.7%; SD, 14.2%; and PD, 6.5%. In contrast, 90.7% of the 54 untreated lesions had SD/PD. Percent change from baseline SUVmax can be seen for treated and untreated lesions (Fig. 1). Multivariable logistic regression only identified that concurrent ADT was associated with an increased likelihood of lesion PSMA response (CR/PR) (OR = 3.04; 95% CI, 1.38-6.70; P = .006) (Table 2).
Figure 1.
Waterfall plot demonstrating percent change of PSMA-PET SUVmax for both treated and untreated lesions.
Abbreviations: PSMA-PET = prostate-specific membrane antigen positron emission tomography.
Table 2.
Binary logistic regression of characteristics predictive of SUV response (CR/PR) to MDT
| Multivariate |
||
|---|---|---|
| Predictor of SUV response | OR (95% CI) | P value |
| Lymph node (vs bone) | 1.21 (0.56-2.62) | 0.6 |
| Pre-MDT SUVmax (continuous) | 1.022 (0.99-1.05) | 0.15 |
| BED3 (continous) | 1.01 (0.99-1.02) | 0.4 |
| ADT with MDT | 3.04 (1.38-6.70) | 0.006 |
Abbreviations: BED3 = biologically effective dose (α/β = 3); CR = complete response; MDT = metastasis-directed therapy; PR = partial response; SUV = standardized uptake value.
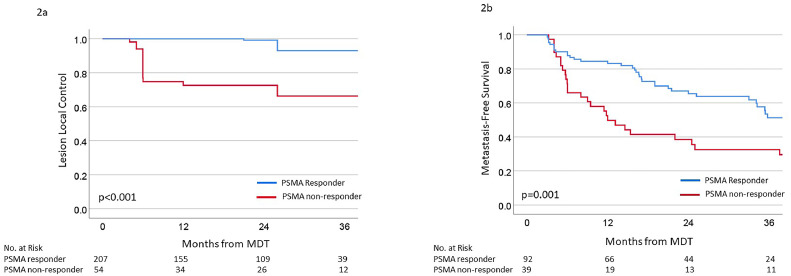
Within the entire cohort, the 3-year LLC was 87%, and the median LLC was not reached. When stratified by lesional PSMA response, 3-year LLC rates were 92% versus 66% for PSMA responders and nonresponders, respectively (Fig. 2A). Per multivariable Cox regression, PSMA response, as a continuous variable, was associated with LLC (HR = 1.003; 95% CI, 1.001-1.006; P = .016) after accounting for BED3 of SABR, lesion location, ADT, and pre-MDT SUVmax (Table 3).
Figure 2.
Kaplan-Meier survival curves of (A) lesion local control for treated lesions and (B) metastasis-free survival stratified by PSMA responders and nonresponders.
Abbreviations: PSMA = prostate-specific membrane antigen.
Table 3.
Multivariable Cox regression analysis of variables associated with lesion local control for treated lesions
| Clinical variable | HR (95% CI) | P value |
|---|---|---|
| PSMA response* | 1.003 (1.001-1.006) | 0.016 |
| BED3 (continuous) | 0.97 (0.96-0.99) | 0.005 |
| Lymph node (vs bone) | 1.44 (0.29-7.15) | 0.7 |
| Concurrent ADT (vs no ADT) | 0.19 (0.03-1.07) | 0.06 |
| Pre-MDT SUVmax (continuous) | 1.04 (1.01-1.06) | 0.002 |
Abbreviations: ADT = androgen deprivation therapy; BED3 = biologically effective dose (α/β = 3); MDT = metastasis-directed therapy; PSMA = prostate-specific membrane antigen; SUV = standardized uptake value.
PSMA response defined as percent change in SUVmax after MDT, evaluated as a continuous variable.
Within the entire cohort, the 3-year MFS was 46%, and the median MFS was 35.4 months (95% CI, 23.6-47.2 months). When stratified by treatment response, the 3-year MFS was 51% versus 33% for PSMA responders and nonresponders, respectively (Fig. 2B). Similarly, the median MFS was significantly prolonged among PSMA responders versus nonresponders (39.9 vs 12 months, respectively; P = .001). Although death was considered an MFS event, only 2 patients died before developing a new, conventionally detected metastasis, neither of whom had PSMA progression nor died of prostate cancer. Given the heterogeneity of the cohort, which included patients with different staging imaging, timing of disease, use of ADT, and metastatic location, we evaluated whether PSMA response was associated with MFS within these subsets. PSMA response was significantly associated with MFS in patients treated with and without ADT (Fig. E2), in patients with metachronous disease (Fig. E3), in those staged with conventional and PSMA-PET imaging (Fig. E4), in patients with total PSMA consolidation (Fig. S5), and in patients with lymph node only and bone/visceral metastases (Fig. E6). Notably, PSMA response was only not significantly associated with MFS among patients with synchronous disease (only 2 PSMA nonresponders) and those with subtotal PSMA consolidation (only 1 PSMA responder). Per multivariable Cox regression, PSMA response, as a continuous variable, was associated with MFS (HR = 1.003; 95% CI, 1.001-1.004; P < .001) when accounting for total PSMA consolidation, disease timing, Gleason grade group, ADT, pre-MDT PSA, and staging imaging (Table 4). None of the covariates included in the multivariable Cox regression analysis, nor the time from the end of MDT to post-MDT PSMA-PET, had a significant P value for the interaction with the PSMA response (Table E5).
Table 4.
Multivariable cox regression of variables associated with conventionally defined metastasis-free survival
| Clinical variable | HR (95% CI) | P value |
|---|---|---|
| PSMA response* | 1.003 (1.001-1.004) | <0.001 |
| Total PSMA consolidation | 0.59 (0.22-1.59) | 0.29 |
| Metachronous (vs synchronous) | 3.35 (1.62-6.91) | 0.001 |
| Gleason grade group | 1.13 (0.91-1.41) | 0.28 |
| Concurrent ADT (vs no ADT) | 1.61 (0.37-6.70) | 0.5 |
| Pre-MDT PSA | 1.003 (0.997-1.009) | 0.3 |
| Conventionally staged (vs PSMA staged) | 0.52 (0.10-2.59) | 0.4 |
Abbreviations: ADT = androgen deprivation therapy; MDT = metastasis-directed therapy; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen.
PSMA response defined as percent change in the SUVmax of the worst responding lesions, evaluated as a continuous variable.
Discussion
We report an association between post-MDT PSMA-PET response dynamics and important clinical outcomes. MDT induces a PSMA-PET response that is not observed in untreated metastases, and PSMA progression is associated with MFS, both as a continuous variable and a clinically valid dichotomization. Taken together, these findings suggest that PSMA-PET response may be an effective radiographic biomarker for distant control following MDT in omCSPC. The identification of a PSMA-PET response biomarker that correlates with MFS following MDT in omCSPC is of particular interest, as MFS has been shown to be a strong surrogate for overall survival in localized CSPC.23 These findings allow for the early identification of patients (PSMA responders) who can remain off of any systemic therapy for a meaningful period of time, versus those that are likely to progress quickly (PSMA nonresponders) and potentially benefit from treatment intensification with early initiation of systemic therapy. Further work in elucidating which type and duration of systemic therapy for these rapidly progressing patients with omCSPC should be pursued.
Two previous studies compared MDT using PSMA- or choline-PET to identify lesions, and both found that PSMA-guided treatment improved ADT-free survival.27,28 This improvement is likely due to the increased detection of occult metastatic disease with PSMA-PET and is consistent with our results of improved distant MFS with total PSMA-PET consolidation.29 Additional reports with PSMA-PET-directed SBRT in both castration-sensitive and resistant oligometastatic prostate cancer have demonstrated similar 2-year local control rates ranging between 95% to 100%, but with a median PFS ranging from 12 to 41 months.30, 31, 32 Glicksman et al.33 reported that in a single-arm phase 2 trial of 37 patients with PSMA-PET-defined oligorecurrent prostate cancer (negative by conventional imaging) treated with MDT with either SABR or surgery, there was a biochemical response rate of 60%, and there was no biochemical evidence of disease in 22% of patients following MDT.
Timing of posttreatment PSMA-PET imaging remains of critical importance. ADT initially increases PSMA expression in the short term through abrogation of androgen-related downregulation of FOLH1 gene expression, leading to increased FOLH1 transcription and subsequent increased PSMA expression.34, 35, 36 This, however, is contrasted by a subsequent decrease in PSMA expression through tumor cell killing. Onal et al demonstrated a median decrease in PSMA-PET SUVmax of 66% following 3 months of ADT.37 This decrease is likely due to the treatment effect of ADT reducing tumor mass, despite upregulation of PSMA on the remaining viable cells.38 Given the above-noted time dependence of PSMA-PET avidity on ADT, post-SABR imaging should be performed at least 3 months following treatment, particularly if concurrent ADT was delivered, to avoid misclassifying patients with an ADT flare as having progressive lesions.
Surprisingly, within this study, concurrent ADT was not found to be significantly associated with MFS, despite it showing an increased likelihood of a PSMA response. This is likely due to the fact that patients with more aggressive disease characteristics are more likely to receive concurrent ADT. Given that the duration of the concurrent ADT was very short, it is possible that this short duration of ADT was able to overcome these more aggressive features and show an MFS benefit. Importantly, however, PSMA response was shown to be prognostic regardless of whether patients received concurrent ADT.
Although we demonstrated a correlation between SUVmax and clinical outcomes at the post-SABR time point, the optimal time for posttreatment imaging remains unknown, as the PSMA-PET response to MDT is seldom reported. Greco et al reported a single-institution phase 2 study evaluating the PET response following MDT in oligometastatic disease across multiple histologies. This study included 147 patients with prostate cancer (who underwent 68Ga-PSMA PET) and demonstrated that a change in the PET SUVmax was associated with locoregional failure.39 This study included a 3- and 6-month posttreatment PET, which showed similar results, though specific SUVmax changes over time were not reported. Sadestski et al reported a retrospective review of 12 patients (15 lesions) with bone metastases treated with SABR with pre- and posttreatment PSMA-PET. Posttreatment imaging was performed at a median of 17 months following treatment, and 93% of lesions demonstrated a complete SUVmax response. No lesion local failures were observed at a median follow-up of 26.5 months.40 This report demonstrated a significantly higher complete SUVmax response rate than what we demonstrated here, which may be because of the earlier time point used for posttreatment imaging in our study. Notably, neither of these prior studies associated PET response with either MFS or overall survival, which is a major strength of the present study.
This study has several limitations. First, this was a retrospective review that included a heterogeneous cohort of patients. Although we attempted to control for the nuanced different clinical features apparent in our cohort, there may be confounding variables not accounted for. Second, post-MDT imaging was also performed at variable times (IQR, 4.6-8.8 months; range, 2.4-10.9), which may affect the degree of PSMA response observed. Although we have correlated outcomes with SUVmax percent change, this may not be the most biologically appropriate response assessment, as other markers of metabolic response, such as SUVmean (which was not available for these cohorts) may serve as a more robust biomarker. Additionally, 2 different PSMA tracers were used in the cohort, which may have influenced our findings.41 Another limitation is that detailed PSA kinetics were unavailable for the cohort, so the interaction between PSMA response and PSA kinetics could not be evaluated, which may provide an additional dimension for understanding the risk of disease progression. Finally, the ICECaP definition of MFS has been strongly correlated with overall survival within localized disease, and it is unknown if this association retains its significance in the oligometastatic setting. Despite these limitations, we have identified a novel radiographic biomarker of PSMA-PET change after SABR as a response indicator correlating with MFS.
Conclusion
In this multi-institutional, international patient series, PSMA-PET response in patients with omCSPC after MDT was associated with MFS. Pending prospective validation, our findings suggest that PSMA-PET should be considered for MDT targeting, evaluating treatment response, and guiding subsequent intervention. Future work is required to further refine our understanding of when post-SABR PSMA-PET imaging should be performed and how best to characterize the PSMA response.
Disclosures
Theodore L Deweese: Digital Harmonics (stock); Daniel Song: Isoray, BioProtect (consulting), Candel Therapeutics, BioProtect (research funding); Ana Kiess: Novartis, Merck, Bayer (research funding); Kenneth Pienta: CUE Biopharma; Keystone Biopharma (leadership), CUE Biopharma; Keystone Biopharma; Medsyn Biopharma; Oncopia Therapeutics (stocks/ownership), Akrevia Therapeutics; CUE Biopharma; GloriousMed Technology (consulting), Progenics (research Funding); Felix Feng: Artera (stocks/ownership), Astellas Pharma; Bayer; BlueStar Genomics; Bristol Myers Squibb; Exact Sciences; Foundation Medicine; Janssen Biotech; Myovant Sciences; Novartis; Roivant; SerImmune; Varian Medical Systems (consulting), Zenith Epigenetics (research funding); Martin Pomper: Precision Molecular (leadership), D&D Pharmatech; PlenaryAI (stocks/ownership), Jubilant Biosys; Precision Molecular; Progenics; Reflexion Medical (consulting), Precision Molecular; Roche; Sanofi (research funding), D&D Pharmatech; Progenics (intellectual property); Phuoc Tran: Reflexion Medical (honoraria), Astellas Pharma; AstraZeneca; Dendreon; GenomeDx; Janssen; Myovant Sciences; Noxopharm; Reflexion Medical; Reflexion Medical; Regeneron (consulting), Astellas Pharma, Bayer Health, Reflexion Medical (research funding).
Footnotes
Sources of support: P.T.T. was funded by an anonymous donor, Movember Foundation-Distinguished Gentlemen's Ride-Prostate Cancer Foundation, Babara's Fund, National Capitol Cancer Research Fund, and the NIH/NCI (U01CA212007, U01CA231776, and U54CA273956) and DoD (W81XWH-21-1-0296). M.D. was funded by the DoD (W81XWH-22-1-0579). M.P. was funded by the NIH/NCI (R01CA134675). The funders had no role in the design, analysis, or publication of the study.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2024.101507.
Contributor Information
Cem Onal, Email: hcemonal@hotmail.com.
Ryan M. Phillips, Email: phillips.ryan@mayo.edu.
Appendix. Supplementary materials
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–158. doi: 10.1016/j.eururo.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 5.Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30:1992–2003. doi: 10.1093/annonc/mdz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hope TA, Eiber M, Armstrong WR, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A multicenter prospective phase 3 imaging trial. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellman S, Oligometastases Weichselbaum RR. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/s1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 10.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 11.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutera P, Clump DA, Kalash R, et al. Initial results of a multicenter phase 2 trial of stereotactic ablative radiation therapy for oligometastatic cancer. Int J Radiat Oncol Biol Phys. 2019;103:116–122. doi: 10.1016/j.ijrobp.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. Journal of Clinical Oncology. 2020;38 10-10. [Google Scholar]
- 14.Phillips R, Shi WY, Deek M, et al. Outcomes of observation versus stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–659. doi: 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutera P, Phillips RM, Deek M, Ozyigit G, Onal C, Tran PT. The promise of metastasis-directed therapy for oligometastatic prostate cancer: Going beneath the surface with molecular imaging. J Nucl Med. 2022;63:339–341. doi: 10.2967/jnumed.121.263684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 17.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 18.Calais J, Ceci F, Eiber M, et al. 18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294. doi: 10.1016/S1470-2045(19)30415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 20.Fanti S, Goffin K, Hadaschik BA, et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:469–476. doi: 10.1007/s00259-020-04934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35:3097–3104. doi: 10.1200/JCO.2017.73.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali A, Hoyle A, Haran ÁM, et al. Association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2021;7:555–563. doi: 10.1001/jamaoncol.2020.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC) Prostate. 2018;78:889–895. doi: 10.1002/pros.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutera P, Song Y, Van der Eecken K, et al. Clinical and genomic differences between advanced molecular imaging-detected and conventional imaging-detected metachronous oligometastatic castration-sensitive prostate cancer. Eur Urol. 2023;84:531–535. doi: 10.1016/j.eururo.2023.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzola R, Francolini G, Triggiani L, et al. Metastasis-directed therapy (SBRT) guided by PET-CT (18)F-CHOLINE Versus PET-CT (68)Ga-PSMA in castration-sensitive oligorecurrent prostate cancer: A comparative analysis of effectiveness. Clin Genitourin Cancer. 2021;19:230–236. doi: 10.1016/j.clgc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Deijen CL, Vrijenhoek GL, Schaake EE, et al. PSMA-11-PET/CT versus choline-PET/CT to guide stereotactic ablative radiotherapy for androgen deprivation therapy deferral in patients with oligometastatic prostate cancer. Clin Transl Radiat Oncol. 2021;30:1–6. doi: 10.1016/j.ctro.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zacho HD, Nielsen JB, Dettmann K, et al. 68Ga-PSMA PET/CT in patients with biochemical recurrence of prostate cancer: A prospective, 2-center study. Clin Nucl Med. 2018;43:579–585. doi: 10.1097/rlu.0000000000002169. [DOI] [PubMed] [Google Scholar]
- 30.Onal C, Ozyigit G, Oymak E, et al. Stereotactic radiotherapy to oligoprogressive lesions detected with (68)Ga-PSMA-PET/CT in castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2021;48:3683–3692. doi: 10.1007/s00259-021-05298-z. [DOI] [PubMed] [Google Scholar]
- 31.Onal C, Ozyigit G, Akgun Z, et al. Oligometastatic bone disease in castration-sensitive prostate cancer patients treated with stereotactic body radiotherapy using 68Ga-PSMA PET/CT: TROD 09-004 study. Clin Nucl Med. 2021;46:465–470. doi: 10.1097/RLU.0000000000003558. [DOI] [PubMed] [Google Scholar]
- 32.Hurmuz P, Onal C, Ozyigit G, et al. Treatment outcomes of metastasis-directed treatment using (68)Ga-PSMA-PET/CT for oligometastatic or oligorecurrent prostate cancer: Turkish Society for Radiation Oncology group study (TROD 09-002) Strahlenther Onkol. 2020;196:1034–1043. doi: 10.1007/s00066-020-01660-6. [DOI] [PubMed] [Google Scholar]
- 33.Glicksman RM, Metser U, Vines D, et al. Curative-intent metastasis-directed therapies for molecularly-defined oligorecurrent prostate cancer: A prospective phase II trial testing the oligometastasis hypothesis. Eur Urol. 2021;80:374–382. doi: 10.1016/j.eururo.2021.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Wright GL, Jr., Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 35.Hope TA, Truillet C, Ehman EC, et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: First human experience. J Nucl Med. 2017;58:81–84. doi: 10.2967/jnumed.116.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murga JD, Moorji SM, Han AQ, Magargal WW, DiPippo VA, Olson WC. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate. 2015;75:242–254. doi: 10.1002/pros.22910. [DOI] [PubMed] [Google Scholar]
- 37.Onal C, Guler OC, Torun N, Reyhan M, Yapar AF. The effect of androgen deprivation therapy on (68)Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:632–641. doi: 10.1007/s00259-019-04581-4. [DOI] [PubMed] [Google Scholar]
- 38.Vaz S, Hadaschik B, Gabriel M, Herrmann K, Eiber M, Costa D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:9–15. doi: 10.1007/s00259-019-04529-8. [DOI] [PubMed] [Google Scholar]
- 39.Greco C, Pares O, Pimentel N, et al. Positron emission tomography-derived metrics predict the probability of local relapse after oligometastasis-directed ablative radiation therapy. Adv Radiat Oncol. 2022;7 doi: 10.1016/j.adro.2021.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadetski I, Eshet Y, Kaidar-Person O, et al. PSMA PET/CT to evaluate response to SBRT for prostate cancer bone metastases. Rep Pract Oncol Radiother. 2021;26:528–534. doi: 10.5603/RPOR.a2021.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evangelista L, Maurer T, van der Poel H, et al. [(68)Ga]Ga-PSMA versus [(18)F]PSMA positron emission tomography/computed tomography in the staging of primary and recurrent prostate cancer. A systematic review of the literature. Eur Urol Oncol. 2022;5:273–282. doi: 10.1016/j.euo.2022.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.