Abstract
The dense stroma is one cause of poor efficacy of T cell-mediated immunotherapy in pancreatic ductal adenocarcinoma (PDAC). Carbohydrate sulfotransferase 15 (CHST15) is a proteoglycan-synthetic enzyme responsible for remodeling tumor stroma. Intra-tumoral injection of CHST15 small interfering RNA (siRNA) has been shown to increase the tumor-infiltrating T cells (TILs) in patients with unresectable PDAC. However, the mechanism underlying the enhanced accumulation of TILs is not fully explored. Here, we demonstrate that intra-tumoral injection of CHST15 siRNA locally and remotely diminishes myeloid-derived suppressor cells (MDSCs) and enhances TILs in mice. CHST15 was expressed by tumor cells and MDSCs in both tumor and tumor-draining lymph nodes (TDLNs), and CHST15 siRNA repressed stromal density, neutrophil extracellular traps, and Ly6C/G+ MDSCs in vivo. Remarkably, tumor growth inhibition was only observed in the immunocompetent KPC model, which is associated with enhanced TILs. In vitro, CHST15 siRNA significantly downregulated the levels of CHST15 and indoleamine 2,3-dioxygenase mRNA in CD33+ MDSCs derived from human peripheral blood mononuclear cells. These results suggest a dual role for intra-tumorally injected CHST15 siRNA on modulating the tumor immune microenvironment for T cell entry and remotely diminishing CHST15+ MDSCs, decreasing T cell suppression and expanding T cells in the TDLN, ultimately leading to an enhanced accumulation of TILs.
Keywords: MT: Regular Issue, pancreatic ductal adenocarcinoma, PDAC, oligonucleotide, tumor-draining lymph node, TDLN, myeloid-derived suppressor cells, MDSCs, T cell, carbohydrate sulfotransferase 15, CHST15
Graphical abstract
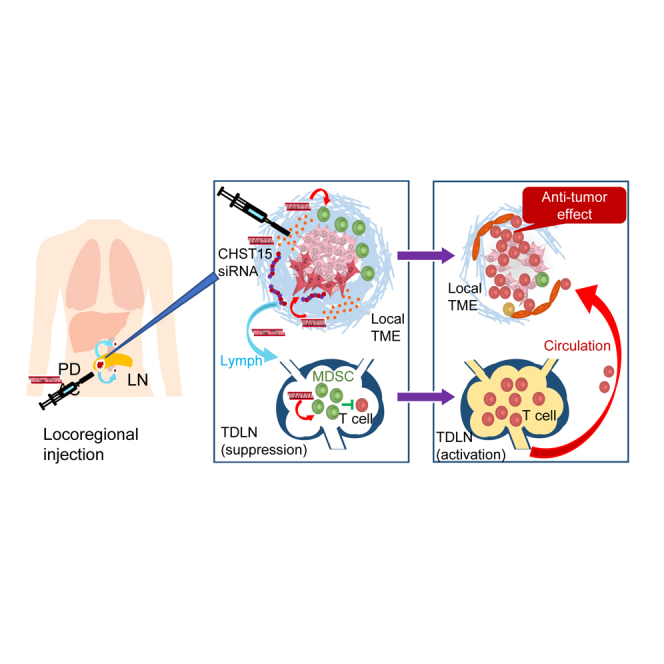
Kadota, Ye, and colleagues demonstrated that intra-tumoral injection of CHST15 siRNA locally and remotely diminishes MDSCs and enhances TILs. CHST15 was expressed by tumor cells and MDSCs in tumor and TDLNs, and CHST15 siRNA repressed stromal density, NETs, and Ly6C/G + MDSCs in vivo.
Introduction
Dense stromal remodeling and poor tumor-infiltrating T cells (TILs) are hurdles for the treatment of pancreatic ductal adenocarcinoma (PDAC), especially in patients with unresectable PDAC who did not respond sufficiently or lost response to the first-line chemotherapy. Tumor matrix remodeling has been reported to act as a physical barrier to diminish the efficiency of cancer therapy by interfering with the abilities of systemic drugs and anti-tumor T cells to enter, diffuse, and be activated in the tumor.1,2,3,4,5,6,7,8 Stroma-modifying agents would thus provide a novel therapeutic strategy to overcome immune suppression and are anticipated to augment effective treatment regimens by combination with chemotherapy and/or immune checkpoint inhibitors.1,2,3,4,5
Carbohydrate sulfotransferase 15 (CHST15) is a type II transmembrane Golgi protein that highly biosynthesizes sulfated disaccharide units (E-units) of chondroitin sulfate (CS), which is responsible for tumor matrix remodeling.9,10 CHST15 and CS-E are involved in the multi-process of tumor progression of a wide range of cancers, including PDAC,11,12,13,14,15,16,17,18,19,20,21,22,23,24 but their role in immune suppression is largely unexplored. In biopsy specimens from unresectable PDAC patients who showed progression despite first-line gemcitabine and nab-paclitaxel therapy, higher CHST15 expression correlated with lower numbers of tumor-infiltrating CD3+ and CD8+ T cells,25 suggesting the involvement of CHST15 in T cell immune suppression in tumor.
We previously reported that specific knockdown of tumor-intrinsic CHST15 enhanced tumor-infiltrating CD4+ and CD8+ T cells and eliminated tumors in a T cell-dependent manner in a mouse model of PDAC.26 Comprehensive RNA expression analyses revealed that tumoral CHST15 knockdown up-regulated genes involved in the recognition and killing of cancer cells by T cells, while down-regulated genes related to stromal remodeling and metastasis.26 The role of tumor-intrinsic CHST15 in the suppression of anti-tumor activity by T cells was shown in mice.26
In a phase 1/2a clinical study, intra-tumoral administration of STNM01, the RNA oligonucleotide to suppress CHST15 gene expression through an RNA interference mechanism,25,27,28,29,30,31,32,33,34 significantly increased tumor-infiltrating CD3+ and CD8+ T cells in patients with chemotherapy-refractory, unresectable PDAC as a second-line therapy.25 An increase in CD3+ T cells at week 4 after the first dose of STNM01 correlated with longer survival, indicating that treatment-associated rapid increase of TILs contributed to favorable outcomes.25 One possibility is that STNM01 acted locally and broke the physiological matrix barrier, leading to accelerated recruitment of anti-tumor T cells into the tumor. However, questions arose if anti-tumor T cells were sufficiently induced or expanded before entering into the tumor site, as T cells were poorly detectable in immunosuppressive PDAC patients of second-line setting. Thus, in the present study, we investigated how locally injected human CHST15 small interfering RNA (siRNA), which has the same sequence as STNM01, enhances TILs in vivo in mouse models of PDAC.
Results
Comparison of human CHST15 siRNA’s binding affinity to and silencing efficacy on human and mouse mRNAs
To investigate the in vivo mechanism underlying the increased TILs by intra-tumoral STNM01 application found in clinical studies in patients with unresectable PDAC, the present study was conducted under several conditions in mice. First, we used human CHST15 siRNA (hCHST15 siRNA), which has the same sequence as STNM01, but not mouse CHST15 siRNA. Second, both T cell-deficient nude mice and immunocompetent mice were used as a hosts, to examine the differences in tumor lesions in the presence or absence of T cells. Third, we selected PDAC cell lines whose proliferation was not affected by CHST15 silencing in vitro, to examine immune-mediated tumor growth inhibition by excluding the influence of in vivo anti-proliferative effects as much as possible. Therefore, human BxPC-3 cells and mouse KPC cells were selected, as the proliferation of these cells was not affected by several CHST15 silencing methods in vitro in our preliminary and past studies (Figure S2).26
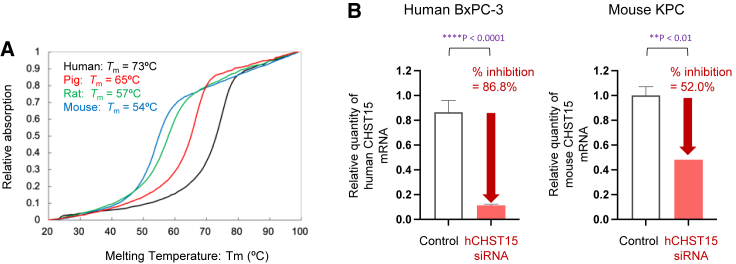
Melting temperature analysis showed that antisense oligonucleotides of hCHST15 siRNA designed to be fully complementary to the Homo sapiens (human) RNA sequence hybridized most strongly with it. In addition, the antisense oligonucleotide hybridized with the corresponding RNA sequences of Sus scrofa domesticus (pig), Rattus norvegicus (rat), and Mus musculus (mouse), even though these hybridized complexes include several base mismatches (Figure 1A; Table S2). The melting temperature tended to decrease as the number of base mismatches increased. The decreased binding affinity of hCHST15 siRNA to mouse CHST15 mRNA was consistent with decreased silencing efficacy in vitro that hCHST15 siRNA showed 86.8% knockdown against human CHST15 mRNA in BxPC-3 cells and 52.0% against mouse CHST15 mRNA in KPC cells (Figure 1B). However, the inhibition rate of mouse CHST15 mRNA by hCHST15 siRNA was significant compared with that by control.
Figure 1.
Comparison of hCHS15 siRNA’s binding affinity to and silencing efficacy on human and mouse mRNAs
(A) Binding affinity of hCHST15 siRNA to human (black), pig (red), rat (green), and mouse (blue) CHST15 mRNAs. Melting temperatures are shown. (B) In vitro silencing efficacy of hCHST15 siRNA on human CHST15 mRNA by human BxPC-3 cells and mouse CHST15 mRNA by mouse KPC cells are shown. Relative quantity was measured compared with control by qPCR. ∗∗∗∗p < 0.0001, ∗∗p < 0.01, Welch’s independent t test.
hCHST15 siRNA showed tumor growth inhibition in immunocompetent mice
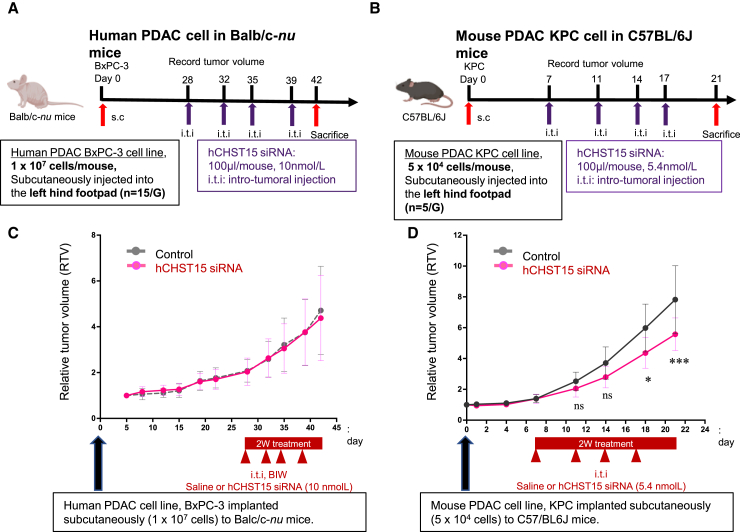
In human BxPC-3-implantation model in T cell-deficient nude mice, hCHST15 siRNA or control vehicle was injected intra-tumorally twice a week from day 28, and mice were sacrificed at day 42 (Figure 2A). In a murine KPC-implantation model in immunocompetent mice, hCHST15 siRNA or control vehicle was injected intra-tumorally twice a week from day 7 and mice were sacrificed at day 21 (Figure 2B).
Figure 2.
hCHST15 siRNA showed tumor growth inhibition in immunocompetent mice
(A and C) Human BxPC-3 cells (1 × 107 cell/mouse) were implanted subcutaneously into Balb/c-nu mice on day 0, and macroscopic tumor volume was monitored (n = 15). ns, not significant by two-way ANOVA with Bonferroni multiple comparison test. (B and D) Mouse KPC cells (5 × 104 cell/mouse) were implanted subcutaneously into syngeneic C57/BL6 mice on day 0, and macroscopic tumor volume was monitored (n = 5). ∗p < 0.05, ∗∗∗p < 0.001. ns, not significant by two-way ANOVA with Bonferroni multiple comparison test.
In T cell-deficient mice, there was no significant difference in tumor size (Figure 2C); in contrast, tumor growth was inhibited in the immunocompetent mice (Figures 2C and 2D).
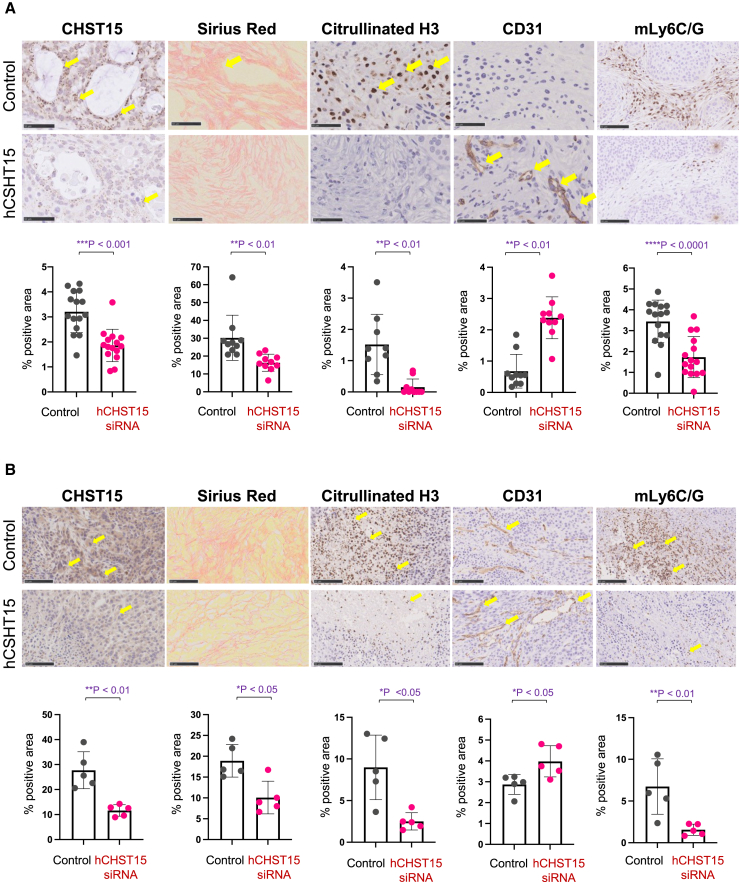
Intra-tumoral injection of hCHST15 siRNA altered tumor stromal components in both human BxPC-3 and murine KPC tumor models
We first investigated the stromal components by histological examination. CHST15 protein was positive for ductal tumor cells in control mice (Figure 3A) and hCHST15 siRNA significantly repressed CHST15 positive area (Figure 3A). The dense deposit of collagen fibers and fibrils was seen and this was associated with massive citrullinated histone H3 staining, a hallmark of neutrophil extracellular trap (NET) formation, and poor CD31+ vascularity (Figure 3A). In contrast, collagen formation was sparse in hCHST15 siRNA-treated mice (Figure 3A). hCHST15 siRNA markedly decreased the citrullinated histone H3+ NET formation while increasing the CD31+ vascularity (Figure 3A). Accumulation of Ly6C/G+ myeloid-derived suppressor cells (MDSCs) within the fibrotic area was also detected in control, and this was reduced by hCHST15 siRNA (Figure 3A).
Figure 3.
Silencing of hCHST15 siRNA alters stromal components in human BxPC-3 and mouse KPC models
(A and B, top) Immunostaining for CHST15, citrullinated histone H3 (Citrullinated H3), CD3 or mLy6C/G (brown) and Sirius red in the tumor of mice treated with vehicle control or hCHST15 siRNA. Scale bars, 20 μm. Yellow arrows indicate positive signals. (A and B) Quantitative analysis for stained tissues. Percentage positive areas for CHST15, Sirius red, citrullinated H3, CD31, and mLy6C/G are shown. Mean ± SD (n = 5). ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 by Wilcoxon rank-sum test.
CHST15 was strongly expressed by tumor cells (Figure 3B) and stromal remodeling, such as dense fibrotic architecture, NETosis, and aberrant vascularity was seen in control mice (Figure 3B). Massive accumulation of MDSCs was seen (Figure 3B) and greater positivity of CHST15 and MDSC was observed in immunocompetent control mice compared with T cell-deficient control mice (Figures 3A, bottom, and 3B, bottom). hCHST15 siRNA significantly reduced percent positive areas of CHST15 and this was associated with reduced fibrotic structure, NETosis, and MDSCs (Figure 3B). Part of the accumulated MDSCs expressed CHST15, and hCHST15 siRNA repressed these cells (Figure S3). These results indicated that suppressed stromal remodeling by hCHST15 siRNA was a common feature in both immunodeficient and immunocompetent mice.
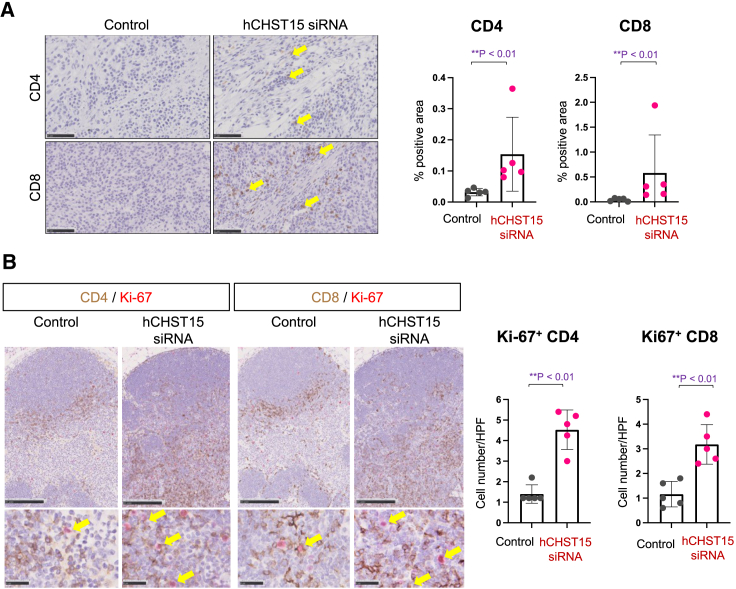
hCHST15 siRNA showed enhancement of T cells in both the tumor and TDLN in the KPC model
In the KPC model, tumor-infiltrating CD4+ and CD8+ T cells were rarely detectable in control mice (Figure 4A). In contrast, we found a significant increase in the number of tumor-infiltrating CD4+ and CD8+ T cells by intra-tumoral injection of hCHST15 siRNA (Figure 4A). In addition, Ki-67+ proliferating CD4+ and CD8+ T cells were rarely detectable in control mice as well, while significantly increased by locally injected hCHST15 siRNA (Figure 4B).
Figure 4.
hCHST15 siRNA showed enhancement of T cells in both the tumor and TDLN in KPC model
(A) Immunostaining for CD4 or CD8 (brown) in the tumor of mice treated with vehicle control or hCHST15 siRNA in KPC model (left). Original magnification ×200. Yellow arrows indicate positive signals. Quantitative analysis for stained tissues (right). Percentage positive areas for CD4 and CD8 are shown. Mean ± SD (n = 5). ∗∗p < 0.01 by Mann-Whitney test. (B) Double immunostaining for Ki-67 (red) and CD4 (brown) or CD8 (brown) in the TDLN with vehicle control or hCHST15 siRNA in KPC model (left). Original magnification ×100 (top left), ×630 (bottom left). Yellow arrows indicate double-positive signals. Quantitative analysis for stained tissues (right). Percentage doubly positive areas for Ki-67+CD4+ and Ki-67+CD8+ are shown. Mean ± SD (n = 5). ∗∗p < 0.01 by Mann-Whitney test.
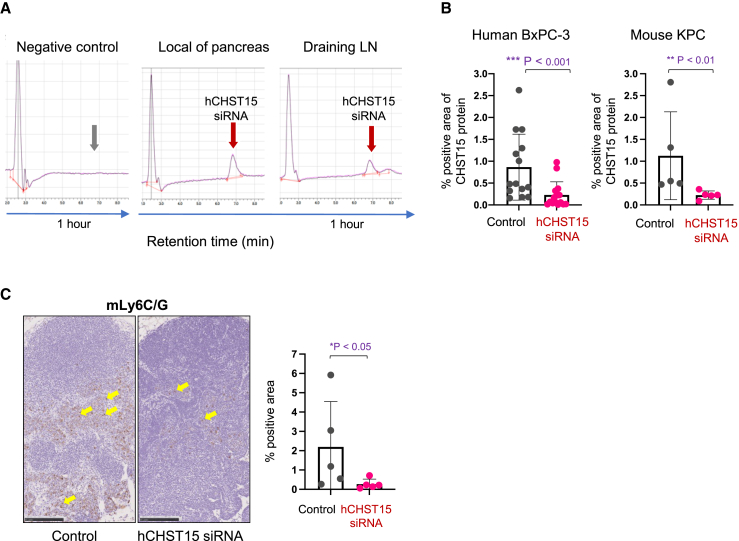
hCHST15 siRNA entered into the draining lymph node after local injection, suppressed CHST15, and diminished MDSCs
To investigate whether locally injected hCHST15 siRNA possesses any effect on TDLN components directly or indirectly, we examined the pharmacokinetics of locally injected hCHST15 siRNA. Although locally injected CHST15 siRNA has been reported to be degraded rapidly after entering into the blood circulation,30,34 hCHST15 siRNA was unexpectedly detected in not only the local sites, but also the draining lymph node after local injection (Figure 5A). In the KPC model, MDSCs were abundantly detectable in the TDLN, but this was significantly diminished by locally injected hCHST15 siRNA (Figures 5B and 5C). hCHST15 siRNA also suppressed CHST15 in the TDLN (Figure 5B). Part of the MDSCs also expressed CHST15 in the TDLN, and this was reduced by locally injected hCHST15 siRNA (Figure S4A). In the BxPC-3 model, similar findings were observed, although the absolute numbers of MDSCs were low compared with the KPC model (Figure S4B).
Figure 5.
hCHST15 siRNA diminishes MDSCs in the TDLN
(A) Concentrations of hCHST15 siRNA within mouse tissues were detected by HLPC. Typical histograms of hCHST15 siRNA in the local tissue (pancreas) and its draining LN (paraaortic LN) 1 h after single local injection of hCHST15 siRNA are shown. Negative control shows HPLC result in the pancreas 1 h after single local injection of saline. (B) Quantitative analysis for CHST15-immunostained stained tissues of TDLN from human BxPC-3 model (left) or mouse KPC model (right). Percentage positive areas for CHST15 are shown. Mean ± SD (n = 15 for BxPC-3 model, n = 5 for KPC model). ∗∗p < 0.01, ∗∗∗p < 0.001 by Mann Whitney test. (C) Immunostaining for Ly6C/G (brown) in the TDLN of KPC model (left panels). Original magnification ×100. Yellow arrows indicate positive signals. Quantitative analysis for stained tissues (right). Percentage positive areas for Ly6C/G are shown. Mean ± SD (n = 5). ∗∗p < 0.01 by Mann-Whitney test. LN, lymph node.
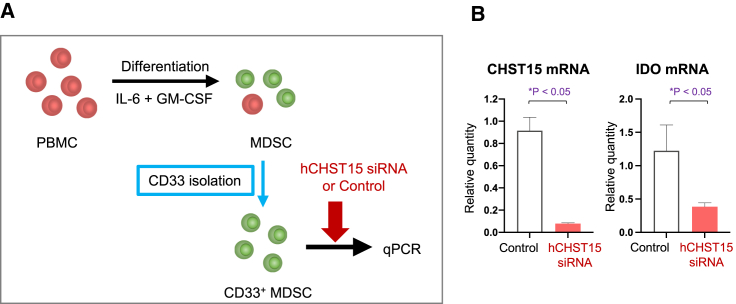
Expression of CHST15 by human peripheral blood mononuclear cell-derived MDSC and direct action by hCHST15 siRNA
Finally, we investigated whether hCHST15 siRNA directly acts on MDSCs (Figure 6A). Human peripheral blood mononuclear cell (PBMC)-derived, IL-6, and granulocyte macrophage colony stimulating factor (GM-CSF)-induced CD33+ MDSCs actually expressed CHST15 mRNA in vitro (Figure 6B). hCHST15 siRNA significantly suppressed CHST15 mRNA by CD33+ MDSCs (Figure 6B). In addition, indoleamine 2,3-dioxygenase (IDO) mRNA was suppressed by hCHST15 siRNA (Figure 6B).
Figure 6.
Expression of CHST15 by human PBMC-derived MDSC and direct action by hCHST15 siRNA
(A) In vitro, the efficacy of hCHST15 siRNA on the expression of human CHST15 mRNA and IDO mRNAs by human PBMC-derived CD33+ MDSCs are shown. (B) Relative quantity was measured compared with control by qPCR. ∗p < 0.05, Independent t test.
Discussion
Intra-tumoral injection of STNM01 was shown to enhance TILs in chemotherapy-refractory patients with unresectable PDAC who showed a poor T cell immune suppressive condition.25 In the present study, we investigated how intra-tumorally injected hCHST15 siRNA enhances TILs using xenogeneic and syngeneic mouse models of PDAC. We found that intra-tumorally injected hCHST15 siRNA acted not only locally, but also remotely. In a BxPC-3 xenograft model, depletion of local dense stroma alone was not effective in inducing tumor regression. In an immunocompetent KPC model, which showed tumor progression with poor TILs, hCHST15 siRNA achieved significant enhancement of TILs and this was associated with a significant increase in CD4+ and CD8 T+ cells in the TDLN compared with the control. Effective accumulation of TILs is thus considered to be achieved when an abundant number of T cells are generated in the secondary lymphoid organs, especially TDLN. This work is also the first report illustrating that CHST15 is expressed by tumor-associated MDSCs and the direct action of hCHST15 siRNA on MDSCs.
Locally, the major effects of hCHST15 siRNA are considered to be the suppression of CHST15 expression and the inhibition of tumor cell invasion, as observed in the present study. In addition, hCHST15 siRNA diminished Ly6C/G+ MDSCs. In both BxPC-3 and KPC models, hCHST15 siRNA monotherapy by intra-tumoral injection inhibited stromal remodeling as evidenced by an altered fibrotic structure from dense to sparse, decreased NET formation, and increased vascularity. NET is recently reported to be a critical structure to interfere with CD8+ cytotoxic T cell infiltration into cancer epithelium and is induced by PMN-MDSCs and neutrophils.35 Decreased Ly6C/G+ MDSCs by hCHST15 siRNA may thus lead to decreased NET formation. Hypovascularity is one of the unique features of PDAC, which leads to insufficient systemic drug delivery, blocks T cell entry, and is associated with a worse prognosis.36 Although the molecular mechanisms remain unexplored, hCHST15 siRNA-mediated alteration of stromal structure may contribute to the maturation of vascularity, as fibrotic stroma is considered to inhibit the formation and function of blood vasculature.37 Therefore, hCHST15 siRNA conditions the local tumor microenvironment to less NETosis with mature vascularity, which is a prerequisite for T cell entry.
Unexpectedly, we found that locally injected hCHST15 siRNA was detectable in the TDLNs. Remotely, a major effect of hCHST15 siRNA is considered to expand CD4+ and CD8+ T cells in the TDLN of the immunocompetent host. Direct action of hCHST15 siRNA on T cells is unlikely, since CHST15 was not expressed by LN T cells in the KPC model. In contrast, CHST15 was expressed by both human PBMC-derived and mouse MDSCs. hCHST15 siRNA acted on MDSCs and inhibited IDO expression in vitro. Direct action of hCHST15 siRNA on MDSCs in the TDLN is thus considered. Therefore, intra-tumorally injected hCHST15 siRNA enters into the TDLN through afferent lymphatics, diminishes MDSCs, removes MDSC-mediated T cell suppression, and expands CD4+ and CD8+ T cells in the TDLN. As it remains a challenge to convert an immune suppressive condition toward activation in PDAC, reactivation of T cells in secondary lymphoid organs would be a reasonable approach. The intra-tumoral route of administration is suggested to be an effective route for RNA oligonucleotide to reactivate T cells in the TDLNs.
We previously reported that TDLN-expanded T cells enter into the circulation, migrate to the tumor sites, and are further activated within the tumor sites, probably upon antigen encounter in the KPC implantation model.26 Similarly, in the present study, hCHST15 siRNA-expanded T cells can accumulate in the tumor, contributing to T cell-dependent tumor growth inhibition. In PDAC patients, intra-tumoral administration of STNM01 was also reported to increase TILs.25 The rate of RECIST-based progression disease (22.7%) was not so high considering reported clinical studies ranging from 46.8% to 91.6% for second-line setting,38,39,40,41,42,43 and one complete response was observed.25 This implies that locally acting STNM01 kept stable disease status for not only primary, but also metastatic, lesions and was able to eliminate metastatic tumors. Our results in the present study explain why local injection influences remote lesions. Since TDLN-expanded T cells enter the circulation, and then migrate to primary and metastatic lesions, it is also considered that these T cells play roles in preventing the progression or promoting the regression of tumors systemically.
There are some limitations in our study. First, the silencing efficacy of hCHST15 siRNA on mouse CHST15 mRNA was not strong (Figure 1B). This might influence the extent of anti-tumor efficacy in the mouse KPC model, although we found significant tumor growth inhibition by hCHST15 siRNA compared with controls (Figure 4B). Second, although CHST15 was expressed by parts of MDSCs in both tumor and TDLN, hCHST15 siRNA broadly diminished MDSCs in vivo, including CHST15-negative MDSCs. It is possible that other mechanisms besides siRNA silencing of hCHST15 are responsible for this phenomenon. Since tumoral CHST15-derived CS-E is released into the circulation and leads to MDSC activation by binding to its functional receptor, CXCL4,44,45 one possibility is considered that hCHST15 siRNA inhibits CS-E synthesis and indirectly diminishes MDSCs. Further investigations including MDSC mobilization will be required to explore the roles of hCHST15 siRNA on the modulation of tumor-emergent MDSCs. Third, our models did not show visible metastases, so the effect of hCHST15 siRNA on metastatic lesions needs to be investigated in other animal models. Forth, molecules related to immune checkpoint inhibition were not investigated. However, considering the findings that TILs increased in number by hCHST15 siRNA treatment, it will be of great interest to examine the detailed phenotype of T cells and the anti-tumor efficacy by combination with immune checkpoint inhibitors in mouse models. Fifth, the extent of MDSCs in the tumor-bearing control mice of C57BL/6 background was relatively higher than that of Balb/c background (Figure 3). Further investigations are needed into whether MDSCs with different strains46,47 would modify the anti-tumor effect of hCHST15 siRNA.
In conclusion, in vivo coordinated actions of intra-tumorally injected hCHST15 siRNA to achieve effective TIL enhancement were documented in murine PDAC. Locally, hCHST15 siRNA suppresses stromal remodeling, which is a pivotal prerequisite for T cell entry, diffusion, recognition, and killing of cancer. At the same time, locally injected hCHST15 siRNA moves to the TDLN and remotely diminishes MDSCs while expanding T cells in the TDLN. A novel concept for an intra-tumoral route for RNA oligonucleotide is suggested to alter the suppressive microenvironment toward activation in addition to breaking dense stroma in this hard-to-treat PDAC.
Materials and methods
BxPC-3 xenograft model in T cell-deficient nude mice
A human PDAC cell line of BxPC-3 cells (ECACC 93120816) was used. Cells were grown in RPMI 1640 with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C under a humidified 5% CO2 atmosphere.
BxPC-3 cells (1 × 107 cell/mouse) were implanted subcutaneously in T cell-deficient Balb/c-nu mice on day 0. CHST15 siRNA26,27,28,29,30,31,32 (10 nmol/L; n = 15) or physiological saline as control vehicle (n = 15) was injected intra-tumorally (100 μL/mouse) twice a week from day 28 to day 42 and mice were sacrificed at day 42. The physiological saline was selected as a negative control to conduct experiments under the same conditions as a clinical trial.34 Scrambled siRNA was not used, as we previously reported that there were no significant differences between scrambled siRNA and physiological saline in several murine models, including tumor implantation models to Balb/c-nu and C57BL/6J mice.26,29,30,31 The dosing interval was determined by in situ hybridization and stem-loop PCR (Supporting materials and Figure S1). This study was approved by the Institutional Animal Care and Use Committee (Approval No. IACUC649-003) and was performed in accordance with the animal welfare bylaws of Shin Nippon Biomedical Laboratories, Ltd., which is accredited by AAALAC International.
KPC syngeneic model in immunocompetent mice
Mouse PDAC cell line of KPC cells (C57BL/6J background) were purchased from Ximbio (London, UK) and used as previously described.26
C57BL/6J mice (5-week-old males) were purchased from CLEA Japan, Inc. (Tokyo, Japan). KPC cells (5 × 104 cell/mouse) were subcutaneously injected into the left hind footpad of C57BL/6J mice. Tumor size was measured twice weekly using a caliper, and the tumor volume was determined using the formula, width2 × length × 0.5. CHST15 siRNA27,28,29,30,31,32 (5.4 nmol/L, n = 5) or physiological saline as a control vehicle (n = 5) was intra-tumorally injected (100 μL/mouse) twice a week from day 7 to day 17, and mice were sacrificed at day 21. This study was approved by the Institutional Animal Care and Use Committee (Approval No. HKD49008) and was performed in accordance with the animal welfare by laws of HOKUDO, Co., Ltd. At the end of experiment, mice were euthanized and tumors and left popliteal lymph nodes (as TDLNs) were excised.
Immunohistochemistry
The tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and were sliced serially into sections (3 μm thick) for hematoxylin and eosin, Sirius red, immunohistochemical staining, and immunofluorescence staining. Immunohistochemical staining was performed as previously reported26,48,49 using CHST15, CD4, CD8α, mLy6C/G, CD31, CD11b, citrullinated histone H3, and Ki-67. All antibodies used in the present study are listed in Table S1. For quantification of immunostained areas, bright field images were captured using a digital camera (DFC280, Leica Microsystems, Wetzlar, Germany) at 400-fold magnification and the positive areas or cell counts in five fields per section were measured using ImageJ software (National Institute of Health, Bethesda, MD, USA).26,48,49
In vitro silencing experiments of human PBMC-derived MDSCs, human PDAC, and murine PDAC cell lines
Human PBMC were purchased from Fujifilm Wako Pura Chemical Corporation (Richmond, VA, USA; Lot #2010113900) and were cultured in RPMI 1640 with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin for 7 days, supplemented with recombinant human IL-6 (10 ng/mL, Fujifilm Wako Pura Chemical Corporation) and GM-CSF (10 ng/mL, Fujifilm Wako Pura Chemical Corporation) under humidified conditions in a CO2 incubator set at 5% CO2 conc1entration and 37°C.50,51 After 1 week, all cells were collected from PBMC cultures. Adherent cells were removed using non-protease cell detachment solution Detachin (Genlantis, San Diego, CA, USA). CD33+ cells were isolated from each culture using anti-CD33 magnetic microbeads and LS column separation (Miltenyi Biotec, Bergisch Gladbach, Germany) per the manufacturer’s instructions.
The concentration of hCHST15 siRNA was 50 nM. We incubated 500 μL/well of Opti-MEMI (GIBCO, Waltham, MA, USA), siRNA, and 7.5 μL/well of RNAiMAX-Reagent (Invitrogen, Waltham, MA, USA) on a six-well plate at 25°C for 20 min. Isolated CD33+ cells were suspended in 2.5 mL basic culture medium in a CO2 incubator for 48 h (2.5 mL/well). Total RNA was extracted from each transfected cell using a FastPure RNA kit (Takara Bio Inc., Kusatsu, Japan) according to the manufacturer’s instructions. The cDNA was synthesized and real-time RT-PCR was performed using SYBR premix Taq (Takara Bio Inc.). The expression of the human CHST15 gene or IDO was normalized by the expressed amount of RNA of human TATA Box Binding Protein. Human BxPC-3 cells and mouse KPC cells were treated in the same method and the expressions of human CHST15 genes and mouse CHST15 genes were measured as previously described.26,30,31,32,33,34
Melting temperature analysis
Binding affinities of the antisense strand of hCHST15 siRNA to human, pig, rat, and mouse mRNAs were analyzed by melting temperature using a UV/VIS absorption spectrophotometer UV-1650PC (Shimadzu, Kyoto, Japan) connected to a temperature controller. The thermal melting curve was prepared by measuring the absorbance of UV light at a wavelength of 260.0 nm in the temperature range of 20°C–100°C (measurement interval of 1°C, temperature increase rate of 1°C/min). A buffer solution containing 10 mM MOPS (pH 7.0) and 100 mM NaNO3 was used in this experiment. Thermal melting curves of hybridized complexes between an antisense oligonucleotide (2 μL) and its target RNAs (2 μL) were measured. The melting temperatures (Tm) were determined by the least-squares method. The melting curves are calculated using the following equation.
Detection of hCHST15 siRNA in mouse tissues
C57BL/6J mice (8 weeks old male) were purchased from CLEA Japan, Inc. We injected 30 μL 100 nM hCHST15 siRNA directly subcutaneously or into the pancreas tail of normal mice. One hour and 4 h later, mice were sacrificed (n = 2 per time point) and local tissues as well as draining lymph nodes were snap-frozen in liquid nitrogen for further analyses. The control group (n = 2 per time point) received PBS. Oligonucleotides were purified from frozen tissue samples using Clarity OTX methodology (Phenomenex, Inc., Torrance, CA, USA) according to the manufacturer’s instructions.52 The concentration of isolated oligonucleotides was then analyzed by HPLC using YMC-Triart C18 (YMC Co., Ltd., Kyoto, Japan).
Statistical analysis
Statistical analysis was performed using GraphPad PRISM version 9.0 software. For comparison variables, data was analyzed by Wilcoxon rank-sum test or Welch independent t test. A p value of less than 0.05 was considered to indicate statistical significance. Results were expressed as mean ± SD.
Data and code availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
We would like to acknowledge the significant contribution of our coauthor, Yoko Matsuda, who was the supervising author for this study. Prof. Matsuda, died on September 17, 2022.We also would like to express our sincere gratitude to Prof. Masayuki Nashimoto and Dr. Masayuki Takahashi (Research Institute for Healthy Living, Niigata University of Pharmacy and Applied Life Sciences, Niigata-shi, Niigata, Japan) for providing suggestions for stem-loop PCR methodology and design of primers. We thank Erina Momohara for her assistance in preparing the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number JP: 16H06277 to Y.M.; 19H03447, 23H02702 to F.S.) and Taiju Life Social Welfare Foundation to Y.M. and F.S.
Author contributions
Data curation: J.Y., F.S., H.Y, K.K., and Y.M.; funding acquisition: F.S., and K.K.; investigation: J.Y., F.S., K.Y., Y.M., M.K., and H.Y.; methodology: J.Y., F.S., K.Y., H.Y., J.K., M.K., N.Y., and Y.M.; supervision: N.Y., F.S., and K.K.; writing – original draft, J.Y., H.Y., F.S., A.N., and K.K.; writing – review and editing: J.Y., H.Y., F.S., A.N., and K.K.
Declaration of interests
H.Y. is the founder of TME Therapeutics Inc. M.K. received payments for lectures from Boston Scientific Japan, Olympus Japan, AstraZeneca, Takeda Pharmaceutical, EA Pharma, and Daiichi Sankyo, and supports for attending meetings from Olympus Japan. N.Y. received free provision of test agents from TME therapeutics, and a research grant for the present study from the Japan Agency for Medical Research and Development (AMED), research grants from Sanwa Kagaku Kenkyujo and Kaigen Pharma, and consulting fees from Olympus Japan, Top corporation, Fujifilm and Boston Scientific Japan, and payments for lectures from Olympus Japan, AstraZeneca, Daiichi Sankyo. Takeda Pharmaceuticals, Ohtsuka Pharmaceutical and EA Pharma.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omton.2024.200812.
Contributor Information
Futoshi Suizu, Email: suizu.futoshi@kagawa-u.ac.jp.
Kyuichi Kadota, Email: kadota.kyuichi@kagawa-u.ac.jp.
Supplemental information
References
- 1.Ho W.J., Jaffee E.M., Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosein A.N., Brekken R.A., Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020;17:487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halbrook C.J., Lyssiotis C.A., Pasca di Magliano M., Maitra A. Pancreatic cancer: Advances and challenges. Cell. 2023;186:1729–1754. doi: 10.1016/j.cell.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flies D.B., Langermann S., Jensen C., Karsdal M.A., Willumsen N. Regulation of tumor immunity and immunotherapy by the tumor collagen extracellular matrix. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1199513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Z., Todd L., Huang L., Noguera-Ortega E., Lu Z., Huang L., Kopp M., Li Y., Pattada N., Zhong W., et al. Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat. Commun. 2023;14:5110. doi: 10.1038/s41467-023-40850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Conejo-Garcia J.R., Katsaros D., Gimotty P.A., Massobrio M., Regnani G., Makrigiannakis A., Gray H., Schlienger K., Liebman M.N., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 7.Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y., Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiryu S., Ito Z., Suka M., Bito T., Kan S., Uchiyama K., Saruta M., Hata T., Takano Y., Fujioka S., et al. Prognostic value of immune factors in the tumor microenvironment of patients with pancreatic ductal adenocarcinoma. BMC Cancer. 2021;21:1197. doi: 10.1186/s12885-021-08911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habuchi O., Moroi R., Ohtake S. Enzymatic synthesis of chondroitin sulfate E by N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase purified from squid cartilage. Anal. Biochem. 2002;310:129–136. doi: 10.1016/s0003-2697(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 10.Mizumoto S., Yamada S., Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015;34:35–42. doi: 10.1016/j.sbi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 11.ten Dam G.B., van de Westerlo E.M.A., Purushothaman A., Stan R.V., Bulten J., Sweep F.C.G.J., Massuger L.F., Sugahara K., van Kuppevelt T.H. Antibody GD3G7 selected against embryonic glycosaminoglycans defines chondroitin sulfate-E domains highly up-regulated in ovarian cancer and involved in vascular endothelial growth factor binding. Am. J. Pathol. 2007;171:1324–1333. doi: 10.2353/ajpath.2007.070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallen M.J.E., Massuger L.F.A.G., ten Dam G.B., Bulten J., van Kuppevelt T.H. Highly sulfated chondroitin sulfates, a novel class of prognostic biomarkers in ovarian cancer tissue. Gynecol. Oncol. 2012;127:202–209. doi: 10.1016/j.ygyno.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda Y., Fujii Y., Matsukawa M., Ishiwata T., Nishimura M., Arai T. Overexpression of carbohydrate sulfotransferase 15 in pancreatic cancer stroma is associated with worse prognosis. Oncol. Lett. 2019;18:4100–4105. doi: 10.3892/ol.2019.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito Z., Takakura K., Suka M., Kanai T., Saito R., Fujioka S., Kajihara M., Yanagisawa H., Misawa T., Akiba T., et al. Prognostic impact of carbohydrate sulfotransferase 15 in patients with pancreatic ductal adenocarcinoma. Oncol. Lett. 2017;13:4799–4805. doi: 10.3892/ol.2017.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugahara K.N., Hirata T., Tanaka T., Ogino S., Takeda M., Terasawa H., Shimada I., Tamura J.I., ten Dam G.B., van Kuppevelt T.H., Miyasaka M. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 2008;68:7191–7199. doi: 10.1158/0008-5472.Can-07-6198. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T., Yan H., Kurahashi Y., Ito Y., Maeda H., Tada T., Hongo K., Nakayama J. Role of GalNAc4S-6ST in astrocytic tumor progression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumoto S., Takahashi J., Sugahara K. Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J. Biol. Chem. 2012;287:18985–18994. doi: 10.1074/jbc.M111.313437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Cheng G., Zhang T., Deng L., Xu K., Xu X., Wang W., Zhou Z., Feng Q., Chen D., et al. CHST15 promotes the proliferation of TE-1 cells via multiple pathways in esophageal cancer. Oncol. Rep. 2020;43:75–86. doi: 10.3892/or.2019.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito Y., Watanabe M., Nishizawa T., Omachi T., Kobayashi T., Kasama S., Habuchi O., Nakayama J. The utility of formalin-fixed and paraffin-embedded tissue blocks for quantitative analysis of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase mRNA expressed by colorectal cancer cells. Acta Histochem. Cytoc. 2007;40:53–59. doi: 10.1267/ahc.07004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadanaka S., Kinouchi H., Kitagawa H. Chondroitin sulfate-mediated N-cadherin/β-catenin signaling is associated with basal-like breast cancer cell invasion. J. Biol. Chem. 2018;293:444–465. doi: 10.1074/jbc.M117.814509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L.C., Wang Y.L., Lin P.L., Zhang X., Cheng W.C., Liu S.H., Chen C.J., Hung Y., Jan C.I., Chang L.C., et al. Long noncoding RNA HOTAIR promotes invasion of breast cancer cells through chondroitin sulfotransferase CHST15. Int. J. Cancer. 2019;145:2478–2487. doi: 10.1002/ijc.32319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizumoto S., Watanabe M., Yamada S., Sugahara K. Expression of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase involved in chondroitin sulfate synthesis is responsible for pulmonary metastasis. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/656319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawashima H., Hirose M., Hirose J., Nagakubo D., Plaas A.H., Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J. Biol. Chem. 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 24.Cooney C.A., Jousheghany F., Yao-Borengasser A., Phanavanh B., Gomes T., Kieber-Emmons A.M., Siegel E.R., Suva L.J., Ferrone S., Kieber-Emmons T., Monzavi-Karbassi B. Chondroitin sulfates play a major role in breast cancer metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011;13 doi: 10.1186/bcr2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisawa T., Tsuchiya T., Kato M., Mizuide M., Takakura K., Nishimura M., Kutsumi H., Matsuda Y., Arai T., Ryozawa S., et al. STNM01, the RNA oligonucleotide targeting carbohydrate sulfotransferase 15, as second-line therapy for chemotherapy-refractory patients with unresectable pancreatic cancer: An open label, phase I/IIa trial. EClinicalMedicine. 2023;55 doi: 10.1016/j.eclinm.2022.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye J., Suizu F., Yamakawa K., Mukai Y., Kato M., Yoneyama H., Yahagi N., Matsuda Y. Silencing of tumoral carbohydrate sulfotransferase 15 reactivates lymph node pancreatic cancer T cells in mice. Eur. J. Immunol. 2023;53 doi: 10.1002/eji.202250160. [DOI] [PubMed] [Google Scholar]
- 27.Kiryu H., Terai G., Imamura O., Yoneyama H., Suzuki K., Asai K. A detailed investigation of accessibilities around target sites of siRNAs and miRNAs. Bioinformatics. 2011;27:1788–1797. doi: 10.1093/bioinformatics/btr276. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura M., Matsukawa M., Fujii Y., Matsuda Y., Arai T., Ochiai Y., Itoi T., Yahagi N. Effects of EUS-guided intratumoral injection of oligonucleotide STNM01 on tumor growth, histology, and overall survival in patients with unresectable pancreatic cancer. Gastrointest. Endosc. 2018;87:1126–1131. doi: 10.1016/j.gie.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Takakura K., Shibazaki Y., Yoneyama H., Fujii M., Hashiguchi T., Ito Z., Kajihara M., Misawa T., Homma S., Ohkusa T., Koido S. Inhibition of Cell Proliferation and Growth of Pancreatic Cancer by Silencing of Carbohydrate Sulfotransferase 15 In Vitro and in a Xenograft Model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K., Arumugam S., Yokoyama J., Kawauchi Y., Honda Y., Sato H., Aoyagi Y., Terai S., Okazaki K., Suzuki Y., et al. Pivotal Role of Carbohydrate Sulfotransferase 15 in Fibrosis and Mucosal Healing in Mouse Colitis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kai Y., Tomoda K., Yoneyama H., Kitabatake M., Nakamura A., Ito T., Yoshikawa M., Kimura H. Silencing of Carbohydrate Sulfotransferase 15 Hinders Murine Pulmonary Fibrosis Development. Mol. Ther. Nucleic Acids. 2017;6:163–172. doi: 10.1016/j.omtn.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K., Arumugam S., Sreedhar R., Thandavarayan R.A., Nakamura T., Nakamura M., Harima M., Yoneyama H., Suzuki K. Small interfering RNA therapy against carbohydrate sulfotransferase 15 inhibits cardiac remodeling in rats with dilated cardiomyopathy. Cell. Signal. 2015;27:1517–1524. doi: 10.1016/j.cellsig.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Sato H., Sagara S., Nakajima N., Akimoto T., Suzuki K., Yoneyama H., Terai S., Yahagi N. Prevention of esophageal stricture after endoscopic submucosal dissection using RNA-based silencing of carbohydrate sulfotransferase 15 in a porcine model. Endoscopy. 2017;49:491–497. doi: 10.1055/s-0042-123189. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K., Yokoyama J., Kawauchi Y., Honda Y., Sato H., Aoyagi Y., Terai S., Okazaki K., Suzuki Y., Sameshima Y., et al. Phase 1 Clinical Study of siRNA Targeting Carbohydrate Sulphotransferase 15 in Crohn's Disease Patients with Active Mucosal Lesions. J. Crohns Colitis. 2017;11:221–228. doi: 10.1093/ecco-jcc/jjw143. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Chandra V., Riquelme Sanchez E., Dutta P., Quesada P.R., Rakoski A., Zoltan M., Arora N., Baydogan S., Horne W., et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsuta E., Qi Q., Peng X., Hochwald S.N., Yan L., Takabe K. Pancreatic adenocarcinomas with mature blood vessels have better overall survival. Sci. Rep. 2019;9:1310. doi: 10.1038/s41598-018-37909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., Wientjes M.G., Au J.L.S. Pancreatic cancer: pathobiology, treatment options, and drug delivery. AAPS J. 2010;12:223–232. doi: 10.1208/s12248-010-9181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagrial A.M., Chin V.T., Sjoquist K.M., Pajic M., Horvath L.G., Biankin A.V., Yip D. Second-line treatment in inoperable pancreatic adenocarcinoma: A systematic review and synthesis of all clinical trials. Crit. Rev. Oncol. Hematol. 2015;96:483–497. doi: 10.1016/j.critrevonc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Morizane C., Okusaka T., Furuse J., Ishii H., Ueno H., Ikeda M., Nakachi K., Najima M., Ogura T., Suzuki E. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2009;63:313–319. doi: 10.1007/s00280-008-0741-7. [DOI] [PubMed] [Google Scholar]
- 40.Wang-Gillam A., Hubner R.A., Siveke J.T., Von Hoff D.D., Belanger B., de Jong F.A., Mirakhur B., Chen L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer. 2019;108:78–87. doi: 10.1016/j.ejca.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly E.M., Oh D.Y., Dhani N., Renouf D.J., Lee M.A., Sun W., Fisher G., Hezel A., Chang S.C., Vlahovic G., et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1431–1438. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.P., Geva R., Gottfried M., Penel N., Hansen A.R., et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/jco.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bockorny B., Macarulla T., Semenisty V., Borazanci E., Feliu J., Ponz-Sarvise M., Abad D.G., Oberstein P., Alistar A., Muñoz A., et al. Motixafortide and Pembrolizumab Combined to Nanoliposomal Irinotecan, Fluorouracil, and Folinic Acid in Metastatic Pancreatic Cancer: The COMBAT/KEYNOTE-202 Trial. Clin. Cancer Res. 2021;27:5020–5027. doi: 10.1158/1078-0432.Ccr-21-0929. [DOI] [PubMed] [Google Scholar]
- 44.Petersen F., Brandt E., Lindahl U., Spillmann D. Characterization of a neutrophil cell surface glycosaminoglycan that mediates binding of platelet factor 4. J. Biol. Chem. 1999;274:12376–12382. doi: 10.1074/jbc.274.18.12376. [DOI] [PubMed] [Google Scholar]
- 45.Joseph R., Soundararajan R., Vasaikar S., Yang F., Allton K.L., Tian L., den Hollander P., Isgandarova S., Haemmerle M., Mino B., et al. CD8(+) T cells inhibit metastasis and CXCL4 regulates its function. Br. J. Cancer. 2021;125:176–189. doi: 10.1038/s41416-021-01338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe H., Numata K., Ito T., Takagi K., Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 47.Youn J.I., Nagaraj S., Collazo M., Gabrilovich D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneyama H., Matsuno K., Zhang Y., Murai M., Itakura M., Ishikawa S., Hasegawa G., Naito M., Asakura H., Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 2001;193:35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitazawa Y., Ueta H., Sawanobori Y., Katakai T., Yoneyama H., Ueha S., Matsushima K., Tokuda N., Matsuno K. Novel Targeting to XCR1(+) Dendritic Cells Using Allogeneic T Cells for Polytopical Antibody Responses in the Lymph Nodes. Front. Immunol. 2019;10:1195. doi: 10.3389/fimmu.2019.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lechner M.G., Liebertz D.J., Epstein A.L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bronte V., Brandau S., Chen S.H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7 doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown K.M., Nair J.K., Janas M.M., Anglero-Rodriguez Y.I., Dang L.T.H., Peng H., Theile C.S., Castellanos-Rizaldos E., Brown C., Foster D., et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 2022;40:1500–1508. doi: 10.1038/s41587-022-01334-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.