Abstract
Plants emit a range of volatile organic compounds (VOCs) as a way of interacting with their biotic and abiotic surroundings. These VOCs can have various ecological functions, such as attracting pollinators, repelling herbivores, or may be emitted in response to abiotic stress. For the present dataset, we used gas chromatography coupled ion mobility spectrometry (GC-IMS) to analyse the VOCs emitted by different plant species under controlled conditions. GC-IMS is a rapid and sensitive technique for gas phase analysis, that separates VOCs based on their retention time and drift time, resulting in characteristic heatmaps where the xy-position of a signal corresponds to compound identity, while signal intensity reflects its abundance.
In this dataset, rapid analysis by GC-IMS was used to record emission pattern of 140 plant species from different taxonomic groups. This includes both floral volatiles and emission from leaves after induced damage. The data was pre-evaluated and listed in one table, containing information on the plant material used, as well as information on the respective emission patterns (including already identified compounds). Thus, this dataset provides a broad overview over plant VOC emissions. These can be used to either check the distribution of knowns substances, or the specific emissions of plants for functional, ecological or physiological studies or as the starting point for chemotaxonomic studies. The extraordinary ease with which these data can be generated – with the suitable set-up – lends itself to larger scale systematic or ecological studies across plant (or animal) groups and even ecosystems.
Keywords: Gaseous phase analysis, VOC, Green leaf volatiles, GLV, Damaged induced emission
Specifications Table
| Subject | Plant Science: General, Analytical chemistry |
| Specific subject area | Gas phase analysis of plant volatile organic compounds from flowers and from damaged vegetative tissue via ion mobility spectrometry. |
| Data format | Analysed (pre-processed) and filtered data in .csv (labels and results are combined in one single document) |
| Type of data | .csv file (dataset with labels and numbers) |
| Data collection | Emission patterns of plants were recorded with gas chromatography coupled ion mobility spectrometry, an analytical technic for detecting and identifying trace compounds in gaseous phase. Data collection took place under controlled ambient conditions in the laboratory and includes both floral volatiles and emissions of vegetative tissue after mechanical damage. |
| Data source location | Institution: University of Bonn, Bonner Institut für Organismische Biologie, Department Biodiversität der Pflanzen City/Town/Region: Bonn, Germany |
| Data accessibility | Repository name: Zenodo Data identification number: 10.5281/zenodo.8413201 [1] Direct URL to data: Data of emission of floral volatiles and damage induced emissions of plants | Zenodo |
| Related research article | Losch et al. (2023): Evaluation of floral volatile patterns in the genus Narcissus using gas chromatography-coupled ion mobility spectrometry [2]. 10.1002/aps3.11506 https://bsapubs.onlinelibrary.wiley.com/doi/10.1002/aps3.11506 |
1. Value of the Data
-
•
These data provide a broad collection of plant volatile data, both floral volatiles as well as damage-induced emissions from foliage of different species, enabling researchers to study phenological adaptions in terms of volatile organic compounds across different plant taxa and families.
-
•
The data contains chemotaxonomic information for both floral and vegetative volatiles. This can give insights into the role of volatile organic compounds as biochemical features of plants.
-
•
Emission of floral volatiles permit a comparative analysis across different plant taxa. This can be used for e.g. comparative phylogenetic analysis on trait evolution. Floral volatiles attract or repel certain flower visitors. Therefore, such data can be used in combination with flower visitation data to study plant-pollinator interactions, pollinator preferences, or a comparison of floral volatile emissions based on similarity-metrics (such as Bray-Curtis Distance).
-
•
The dataset permits a comparative analysis of volatile emissions after foliage damage between plant taxa and families. Damage-induced emissions are mostly associated with the communication of plants in the context of biotic or abiotic (stress)-factors [3]. Hence, the data can give insights into protection mechanisms of plants in the form of VOC-emission across different families or genera, potentially revealing patterns or trends across different plant groups. Furthermore, this data can be used for comparative phylogenetic analyses.
-
•
The data obtained by GC-IMS reflect emission patterns of plants. The complex patterns recorded can be used to develop, train and evaluate algorithms or models for automated pattern recognition, using substance specific identification metrics of retention time and ion mobility.
-
•
The data have been obtained using an adjusted GC-IMS, which could be used for a variety of basic and applied fields. The present dataset can be used as a reference data set for these applications.
2. Data Description
The dataset describes the emissions of plant volatile organic (pVOCs) from a range of taxa. The data was collected using gas chromatography coupled ion mobility spectrometry (GC-IMS) and the raw data were pre-evaluated using the specially developed software (IONYsos, mad by ION-GAS GmbH, Dortmund, Germany).
The dataset generated in this way focuses exclusively on plant volatile emissions. This includes flower volatiles from various plant species, but also emissions from vegetative tissue (leaves) after mechanical damage. In total, the data set consists of one table, which contains floral emission patterns from 86 different species and emission patterns after leaf damage from 54 different species.
The relevant experimental parameters are tabulated. The data contains information about the taxon sampled, sample type, emission patterns recorded, and the specific compounds identified (compare Table 1).
Table 1.
Summary of variable names, data types and brief explanation of the variables for the presented dataset.
| Column name | Value | Description |
|---|---|---|
| Species | Character | Name of species |
| Family | Character | Belonging to family |
| order | Character | Belonging to order |
| Genus | Character | Belonging to Genus |
| Plant part | Character | Refers to the part of a plant that was measured (leaves, flower etc.) |
| Plant status | Character | damaged means the plant was mechanically damaged. Undamaged means the plant was intact. |
| Sample_location | String | Origin of the plant material |
| Accession | Integer | ID for plants from the Bonn University Botanical Gardens |
| compound | String | Name of the compound. UNK is used for unidentified signals |
| Retention_time | Numeric | Separation time from the GC-Preseperation. Used for identification of compounds |
| Ion_mobility | Numeric | Drift time from the IMS-separation. Normalized to the reactant ion peak. Used for identification of compounds. |
| Signal_type | Character | Some compounds can form Ion clusters. Thus, a single compound can form several signals which are listed here as Signal type. |
| Signal_intensity | Numeric | Intensity of a signal calculated from the measured peak volume |
| Rel. intensity | Numeric | Relative distribution of compounds in an individual Taxa compared to the most intense signal |
The first variables (species, family, order, genus and if available subgenus and section) are self-explanatory and contain information on the classification of the species examined.
The sample state is described by 4 variables. Plant_status refers to the condition of the plant -mechanically damaged (damaged) or undamaged. Plant_part refers to the parts of the plant that was examined, mostly flowers or leaves. Sample_location contains information about the origin of the plant material. Samples from the collection of Bonn University Botanic Gardens are also provided with an accession number. These can be traced in the database of the Bonn University Botanical Gardens to get additional information about the plant material (https://bonnubg.gardenexplorer.org).
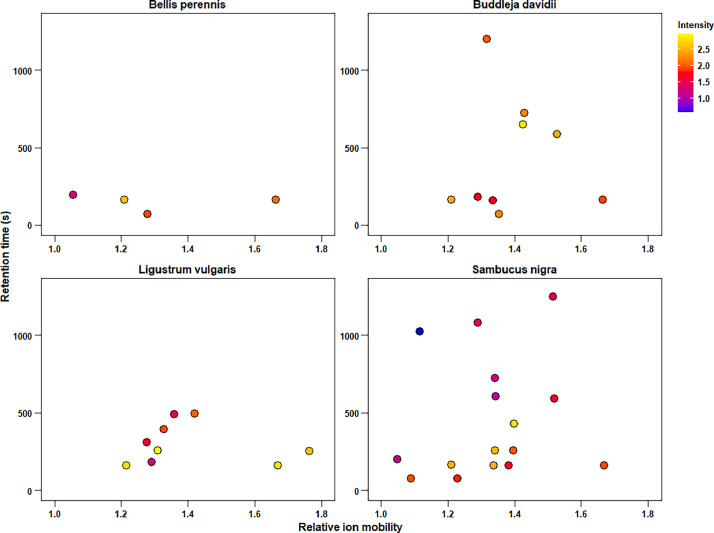
The recorded emission patterns of plants can be visualized by two-dimensional heatmaps, where each substance is characterized by its position on the xy-coordinates, with the retention time on the y-axis and the relative ion mobility on the x-axis, yielding characteristic emission patterns for each plant (Fig. 1). Signal intensity, which is a semi-quantitative measure for the abundance of a substance, can be displayed via colour code. . As a part of pre-procession of raw data, several compounds were identified by comparing the metrics to our own database. The name of identified substances is displayed under compound. Distinct unidentified substances are reported with their identification metrics (retention time and relative ion mobility) as UNK1, UNK2…UNKN.
Fig. 1.
Emission patterns of floral volatiles for Bellis perennis, Buddleia davidii, Ligustrum vulgaris and Sambucus nigra.
For some substances, the formation of “so-called” ion clusters can occur, if they are present in high concentrations. Whether a signal is referred to an ion clusters is indicated by the signal type. M stands for monomer (ion with single analyte molecule). D (dimer), T (trimer), Q (Tetramer) and P (pentamer) describe the respective ion clusters of an individual substance observed. However, signal type can only be assigned with certainty for previously identified substances and is therefore not given for unidentified compounds.
In addition to the presence-absence of characteristic substances, the data set also contains information about the abundance of a respective substance. Signal intensity is given without dimensions (artificial unit or a.u.) and is a measure of the abundance of the respective substance, permitting semi-quantitative statements and an approximate comparison between species. Relative abundance is also reported in addition to signal intensity. This shows the proportion of the signal intensity of a single signal in relation to the strongest signal of the sample. The strongest signal is set to 1.00 (or 100 percent). Signal intensities often differ dramatically between across species, relative abundance offers the possibility for a better interspecific comparison in terms of emission patterns, independent of overall emissions. Alternatively, other types of normalisations might also be applied to signal intensity (min-max normalisation, Log10, etc.).
3. Experimental Design, Materials and Methods
3.1. Device
Data acquisition was performed using Gas-Chromatography coupled Ion Mobility Spectrometry (GC-IMS). GC-IMS itself is a selective and sensitive technique able to detect trace compounds in the gaseous phase with common detection limits (depending on the substance) down to the concentration range of parts per trillion by volume. With the integration of pre-concentration steps, detection limits down to parts per quadrillion per volume are possible. [4] Furthermore, the orthogonal separation (GC and IMS) permits the unequivocal identification of previously documented signals.
The data acquisition was part of the AMMOD-Project (Automated Multisensor station for Monitoring of Species Diversity) for continuous monitoring of plant volatiles at an observation site. Within the AMMOD-project low concentrations were expected, two GC-IMS were used (one in the monitoring station, the other for reference measurements in the lab and field). To meet these requirements, we used a customized ppq-tec-GC-IMS (ION-GAS GmbH, Dortmund, Germany) based on hardware provided by STEP (Pockau-Lengefeld, Germany). The commonly used sample loop was substituted with an internal, in-line Micro-Electro-Mechanical-System based pre-concentrator chip from CNR-IMN (Bologna, Italy). Carbograph 4 was used as filling as adsorbent material for enrichment. The pre-concentrator chip permits higher sample volumes, increasing the sensitivity, without interfering with fully automated sampling. Second, the system is equipped with a refined filter systems and utilizes filtered air as operational gas.
Although other GC-carrier gases (e.g. Helium or Hydrogen) would yield a better GC-resolution, here the orthogonal analysis setup with the downstream IMS compensates for the lower GC-separation. The use of filtered air from the internal gas circuit for both modules (GC and IMS) permit mobile measurements without the need of handling bulky gas bottles and thus allowing a maintenance-free operation for months, independently of external gas sources.
3.2. Sampling and analysis
The plant material for this study was provided by the Bonn University Botanic Gardens (University of Bonn, Germany). GC-IMS sampling and subsequent analysis took place in a ventilated room under ambient conditions. All measurements were accompanied by appropriate background measurements of the ambient air to identify potential background signals.
Two separate sampling protocols were applied for floral volatiles and damage-induced emissions of leaf tissue, each sample was measured in three replicate measurements.
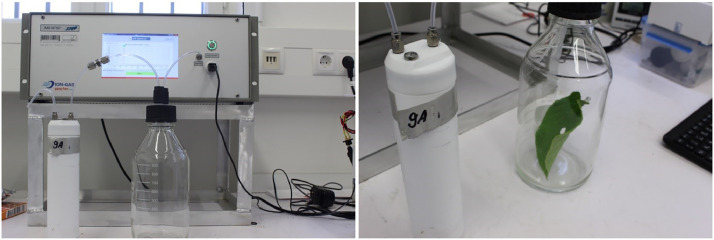
Floral volatiles were sampled by immobilizing the flowers at approx. 2 cm distance from the sample inlet of the ppq-tec-GC-IMS (no flower enclosure). A sample volume of 10 mL was used for flowers. damage-induced leaf emissions were sampled by stamping a 1 cm-diameter hole into the respective leaf. The leaf was then placed in a 1 L glass bottle, sealed with an PFA screw cap and concentrated for 5 min. at ambient temperature (20 – 25 °C). Then, 5 mL of sample were directly taken from the static headspace in the glass bottle via PFA-tubing (Fig 2).
Fig. 2.
Experimental set-up for detection of emission patterns of vegetative tissue after mechanical damage. Damaged leaves were placed in a flushed and sealed 1 L glass bottle. The sample volume was directly and automatically taken from the static headspace and transferred to the GC-IMS for analysis.
The sample volume with pVOCs was then drawn into the ppq-tec-GC-IMS and automatically transferred to the internal in-line pre-concentration chip for enrichment (adsorption temperature: 45 °C).
After enrichment, analytes were thermally desorbed at 290 °C for 12 s and injected automatically into the GC-IMS for analysis.
GC pre-separation was performed under isothermal conditions (80 °C) for 1500s on a MXT-200 capillary column (30 m x 0.53 mm, 1.5 µm coating, RESTEK Corp., USA) with a carrier gas flow (filtered air from internal gas circuit) of 21 mL min−1. Ionization of pVOCs for mobility separation was performed in positive ionization mode using a tritium source of β-radiation (100 MBq). Mobility separation and subsequent detection were performed with a drift-tube IMS (drift length of 5.61 cm) at 70 °C and at a field strength of 300 V cm−1. After each measurement, the pre-concentrator chip was automatically purged (290 °C for 1 min), to eliminate carry-over [4].
3.3. Data procession and evaluation
Data processing and evaluation of raw data was performed with IONysos (v1.4.1, https://www.ion-gas.de/products/ionysos.html), a custom-made software by ION-GAS GmbH (Dortmund, Germany), providing the pre-processed data. The drift time in the tD,rel is normalized to the reactant ion peak (RIP), which is proportional to the inverse reduced ion mobility [5] and the retention times were aligned to a known standard.
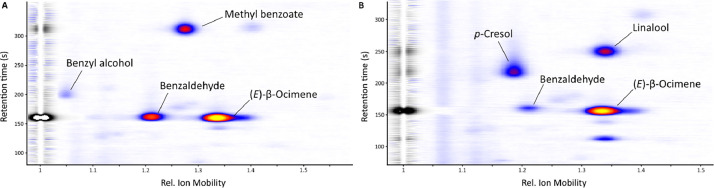
The data is visualized as 2-dimensional heatmaps in IONysos. Signals are manually annotated. Signal intensity of annotated signals for each plant was then calculated by integration of the respective peak volume and if possible, signals detected were identified by a comparison of retention times and relative ion mobilities with data of pure reference compounds from our in-house database (Fig 3). The in-house database was built by directly injecting pure reference compounds into the ppq-tec-GC-IMS. A list of substances identified (including the corresponding retention times and relative ion mobilities) is also deposited at Zenodo.
Fig. 3.
Two-dimensional heatmaps obtained from IONysos (v1.4.1) for A) Narcissus broussonetii and B) Narcissus cantabricus ssp. cantabricus. Prominent signals were annotated manually with the respective compounds by compare rel. Ion mobility and retention time with data from pure reference compounds.
Limitations
Signal identification of unknown substances requires additional analytical efforts and many signals have not yet been identified. Identification of unknown signals is an ongoing process and the data recorded can be refined based in an expansion of the reference data base. Additional data sets and reference data will be incorporated as they become available.
Ethics statement
The authors declare that the present dataset meets the ethical requirements of Data in Brief and that no animal or human study was involved.
CRediT authorship contribution statement
Florian Losch: Methodology, Data curation, Writing – original draft. Sascha Liedtke: Methodology, Data curation. Wolfgang Vautz: Conceptualization, Supervision. Maximilian Weigend: Conceptualization, Writing – review & editing.
Acknowledgements
This research was funded by the German Bundesministerium für Bildung und Forschung and was conducted in the context of the AMMOD-Project (Grant Number: 01LC1903B). The authors thank the Bonn University Botanical Gardens for supplying the plant material.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
References
- 1.Losch F. Data of emission of floral volatiles and damage induced emissions of plants (1.0.1) [Data set] Zenodo. 2023 https://zenodo.org/records/10808039 [Google Scholar]
- 2.Losch F., Liedtke S., Vautz W., Weigend M. Evaluation of floral volatile patterns in the genus Narcissus using gas chromatography-coupled ion mobility spectrometry. Appl. Plant Sci. 2023;11(1):e11506. doi: 10.1002/aps3.11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouwmeester H., Schuurink RC., Bleeker PM., Schiestl F. The role of volatiles in plant communication. Plant J. 2019;100(5):892–907. doi: 10.1111/tpj.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liedtke S., Zampolli S., Elmi I., Masini L., Barboza T., Dalcanale E., et al. Hyphenation of a MEMS based pre-concentrator and GC-IMS. Talanta. 2019;191:141–148. doi: 10.1016/j.talanta.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 5.Vautz W., Bödeker B., Baumbach J.I., Bader S., Westhoff M., Perl T. An implementable approach to obtain reproducible reduced ion mobility. Int. J. Ion Mobil. Spec. 2009;12(2):47–57. doi: 10.1007/s12127-009-0018-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.