Abstract
Rationale & Objective
The effect of apolipoprotein L1(APOL1) genotype on future risk of kidney disease among middle-aged individuals with good kidney function is not well established.
Study Design
Longitudinal cohort study.
Setting & Participants
In total, 5,886 healthy individuals (45-64 years old) enrolled in the Atherosclerosis Risk in Communities study with creatinine-based estimated glomerular filtration rate ≥ 80 mL/min who would be suitable kidney donors.
Exposures
Race and APOL1 genotype.
Outcomes
Creatinine- and cystatin C-based estimated glomerular filtration rate (eGFRcr-cys) using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) 2021 equation, urinary albumin-creatinine ratio (UACR), proportion with chronic kidney disease (CKD) 3a or worse, end-stage kidney disease (ESKD), and death.
Analytical Approach
Participants grouped based on race and APOL1 genotype. Compared eGFRcr-cys and UACR across groups. Multinomial logistic regression models were used compare odds of CKD. Kaplan–Meier survival curves were created to compare rates of ESKD and death at last follow-up.
Results
There were 5,075 Whites (86%), 701 Blacks carrying the low-risk APOL1 genotype (12%), and 110 Blacks carrying the high-risk APOL1 genotype (2%). The mean age at baseline was 53 ± 6 years. At 10 years, White participants had lower eGFRcr-cys than low-risk and high-risk groups (89 ± 16 vs 91 ± 16 and 92 ± 15 mL/min/1.73 m2, respectively; P < 0.001). At 25 years, White participants continued to have lower eGFRcr-cys than the low-risk group (70 ± 18 vs 72 ± 19 mL/min/1.73 m2; P < 0.001) but not compared with the high-risk APOL1 genotype (67±23 mL/min/1.73 m2). There was no difference in UACR among groups at 10 and 25 years (P = 0.87 and 0.91, respectively). The odds of developing CKD stage 3a or worse were not different between low-risk and high-risk APOL1 group in both unadjusted and adjusted models (P = 0.26 and P = 0.39, respectively). At last follow-up, <5% developed ESKD, and 45% of individuals either died or reached ESKD with no difference in outcomes between the groups.
Limitations
Low ascertainment because of death and long follow-up.
Conclusions
Among middle-aged individuals, APOL1 genotype does not appear to be a major driver of future risk of kidney disease.
Index Words: APOL1, Black, healthy, kidney function, living donors
Plain-Language Summary
Black patients with kidney disease carrying 2 variants of the apolipoprotein L1 (APOL1) gene, referred to as the high-risk genotype, experience an accelerated decline in kidney function than those with 0 or 1 risk variant. It is unknown whether the high-risk genotype negatively affects kidney function of healthy middle-aged individuals. We evaluated the effect of APOL1 genotype on kidney function of the Atherosclerosis Risk in Communities study participants (mean age 53 years) who had normal kidney function and blood pressure at baseline. At 25 years of follow-up, the APOL1 high-risk genotype did not appear to be a major driver of future risk of kidney disease. Our study findings are relevant for counseling older living donor candidates as well as family members of patients with APOL1-associated kidney disease.
Editorial, ●●●
In the United States, Black individuals carry a significantly greater burden of chronic kidney disease (CKD) than Whites.1 Variants in the apolipoprotein L1 (APOL1) gene have been reported to confer some part of this increased risk.2,3 Two APOL1 kidney risk variants are found in approximately 12%-15% of Black Americans and are virtually absent in those of European ancestry.4,5 Prior studies have reported a more rapid decline in kidney function among patients with CKD carrying 2 APOL1 kidney risk variants (high-risk genotype) than those carrying zero or one copy of the APOL1 risk variants (low-risk genotype).6 These findings have prompted physicians to test Black patients with CKD and proteinuria for APOL1 kidney risk variants. APOL1 genetic testing has also been used variably to evaluate Black living kidney donor (LKD) candidates with the intent to improve their risk stratification for future kidney disease.7,8 However, it is well established that most individuals carrying the high-risk APOL1 genotype will not develop kidney disease, and a second hit is required.9 Currently, it is unclear whether kidney donation is the instigating event for some LKD carrying high-risk APOL1 genotype to progress to CKD or end-stage kidney disease (ESKD). To date there is a single small study reporting no effect of donation on the course of APOL1-mediated kidney disease.10 To fully appreciate the effect of the high-risk APOL1 genotype on post donation outcomes, it is important to first establish baseline risk of kidney disease in healthy individuals carrying 2 APOL1 kidney risk variants.
The lifetime risk of developing ESKD varies by race and sex and decreases with age.11 The natural history of APOL1-mediated kidney disease also differs by age. For 18- to 30-year-old healthy Black individuals, a graded effect between the number of APOL1 kidney risk variants and 25-year risk of developing CKD has been reported.12 However, for 45- to 64-year-old Black individuals, the effect of APOL1 kidney risk variants was much smaller despite high prevalence of pre-existing diabetes and hypertension.13 The effect of the high-risk APOL1 genotype on future kidney function in middle-aged healthy individuals has not been evaluated to date. This information would be valuable for counseling Black middle-aged LKD candidates carrying high-risk APOL1 genotypes and unaffected family members of patients with APOL1-mediated kidney disease.
We hypothesized that the presence of the APOL1 high-risk genotype would not adversely affect the long-term kidney function of those who survive to middle-age with good health and normal kidney function. We used the Atherosclerosis Risk in Communities (ARIC) study data set to select a cohort of healthy individuals age 45-64 years and assessed the effect of self-reported race and APOL1 genotype on long-term kidney function.
Methods
Design and Setting
The ARIC database was queried to identify healthy middle-aged individuals. In brief, the ARIC study, initiated in 1987, enrolled 15,792 participants age 45-64 years from 4 communities in North Carolina, Mississippi, Maryland, and Minnesota to investigate the causes of atherosclerosis and its clinical outcomes.14 Participants completed their baseline visit between 1987 and 1989 (visit 1) and were contacted at least annually to assess their health status including hospitalizations. Clinic examinations occurred from 1990 to 1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), 2011–2013 (visit 5), 2016-2017 (visit 6), and 2018-2019 (visit 7). Serum creatinine levels were measured at all clinic visits except visit 3, and cystatin C levels were measured at visits 5, 6 and 7. Data on death were ascertained from various sources including but not limited to the National Death Index, proxy interviews, and hospital surveillance in the local catchment areas.
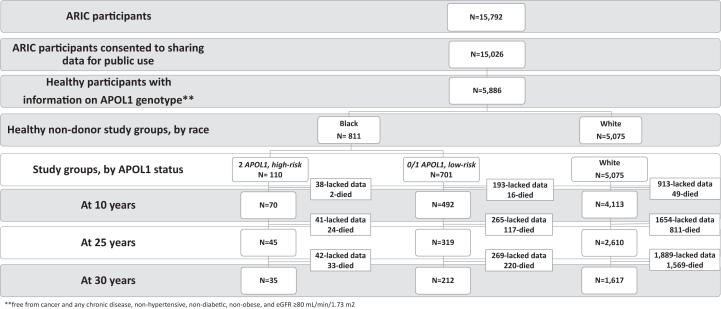
Of the 15,792 participants enrolled in ARIC, 15,026 (95%) consented to public use of their data. We used the publicly available ARIC data set and restricted our study cohort to healthy participants (free from cancer and heart, liver, and kidney disease) and those who met acceptable criteria for LKD selection, ie, blood pressure < 140/90 mm Hg, fasting blood sugars < 126 mg/dL, not using antihypertensive and diabetic medications, an estimated glomerular filtration (eGFR) ≥ 80 mL/min/1.73 m2 and body mass index (BMI) < 35 kg/m2.15,16 The participants self-reported their race and those reporting Black race were genotyped for APOL1 kidney risk variants. Persons with race other than Black or White (n = 34), missing serum creatinine at baseline (n = 150) or missing genotyping data (the latter requirement for Blacks only, n = 68) were excluded. Baseline kidney function was estimated using serum creatinine values and the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula for the creatinine-based estimated glomerular filtration rate (eGFRcr).17 This study was approved by the institutional review board at the University of Michigan (Approval Number HUM00212938). The reporting of this study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STREGA) guidelines for observational research.18
Genotyping of APOL1
Only Black participants who had consented to genetic studies were genotyped for APOL1 using TaqMan assays.19
Participants
Selected participants were divided into 3 groups based on race and APOL1 status: White participants, Black participants with the APOL1 high-risk genotype, and Black participants with the APOL1 low-risk genotype.
Outcomes
The primary outcome was eGFR at 10 and 25 years, proportion of participants with CKD stage 3a or worse and (creatinine- and cystatin C-based estimated glomerular filtration rate [eGFRcr-cys] ≤ 60 mL/min/1.73 m2), and microalbuminuria (urinary albumin-creatinine ratio [UACR] > 30 mg/g) in each group). Kidney function at follow-up was assessed using measurements of serum creatinine and plasma cystatin C concentration, and urine albumin excretion was estimated using spot UACR (mg/g). GFR was estimated with the race-free CKD-EPI 2021 equation using serum creatinine at the baseline visit (eGFRcr) and the combined creatinine and cystatin C values (eGFRcr-cys) at follow-up visits.17
The secondary outcome was the proportion of participants reaching ESKD, death ,or both among healthy participants from the main ARIC cohort (including those who declined public use of their data, n = 6,980). Death and ESKD events were captured using the National Death Index and United States Renal Data System.20 Both these sources have a near complete ascertainment of outcomes independent of study visits.
Creatinine levels were assayed using the modified kinetic Jaffé method and were standardized and calibrated across visits.21 Cystatin C levels were measured in plasma specimens using a particle-enhanced immune-nephelometric assay (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). Urinary albumin levels were measured in urine samples that were frozen and stored at −70°C using a nephelometric method either with the Dade Behring BN100 (assay sensitivity, 2.0 mg/L) or the Beckman Image Nephelometer. Urinary creatinine levels were measured using the Jaffé method.
Statistical Analyses
The normally distributed data were summarized using mean (standard deviation [SD]). The non-normally distributed data were summarized using medians (interquartile range [IQR]), and proportions were used for categorical data. The baseline characteristics were compared between Black and White participants and among Black persons between APOL1 high-risk and low-risk groups using independent sample t tests and χ2 - χ2 test. The follow-up data were compared across 3 groups using analysis of variance, Mann–Whitney U tests, and χ2 - χ2 test or Fisher exact tests as appropriate. To examine the association of APOL1 and the risk of CKD stage 3a or worse, we performed a multinomial logistic regression on the categorical outcome to estimate the odds of having CKD stage 3a or worse at follow-up with the APOL1 high-risk group serving as a reference. The analyses were repeated after adjusting for baseline characteristics, including eGFRcr, age, systolic and diastolic blood pressure, BMI, sex, employment status, education, smoking status, follow-up with primary care, family history of diabetes, and/or hypertension. We did not impute for missing values. Those who died or were lost to follow-up did not contribute to the analysis. In addition, we used a linear mixed-effect model to estimate annual decline in eGFR from enrollment to last follow-up, considering the repeated measures of eGFRcr at baseline and 3, 10, 25 and 30 years of follow-up. We tested whether the annual rate of decline differed by groups in both unadjusted and adjusted analyses. We did not provide a figure showing slope over time or a linear curve given the variable length of follow-up and lack of measurements for every participant at each time point. Cystatin C values were not available at baseline and at 3 years, so eGFR values at these time points were calculated using creatinine values alone with the CKD-EPI 2021 formula. We used SAS version 9.4 (SAS Institute, Cary, NC) to perform the analyses. All tests were two-sided tests, and we interpreted P < 0.05 as statistically significant.
Results
Baseline Characteristics Based on Race and APOL1 Risk Status
Of the 15,026 participants who enrolled in the ARIC study and consented for public use of data, 5,886 (39%) participants met the eligibility criteria at the time of enrollment. There were 5,075 (86%) White and 811 (14%) Black participants. Of the 811 Black persons, 337 (42%) carried 0, 364 (45%) carried 1, and 110 (13%) carried 2 APOL1 kidney risk variants. Participants carrying 0 or 1 APOL1 kidney risk variants were grouped as “low risk” (n = 701), and those carrying 2 APOL1 kidney risk variants were called “high risk” (n = 110) (Fig 1). Compared with Whites, Black participants were younger with higher BMI, higher systolic and diastolic blood pressures, and lower eGFRcr. The proportion of women, employed participants, and of smokers was greater among Black participants than Whites. Fewer Black participants reported having a primary care physician than Whites (Table 1). Baseline characteristics of Black participants grouped by APOL1 risk status were similar except for age (Table 1).
Figure 1.
Flow Chart of the Study. Healthy participants were defined as freedom from cancer, hypertension, and diabetes, with BMI < 35 kg/m2, and eGFR ≥ 80 mL/min/1.73 m2 (using the CKD-EPI 2021 GFR calculator with serum creatinine). GFR, glomerular filtration rate.
Table 1.
Comparison of Baseline Characteristics of Participants by Race and APOL1 Status
| Black (n = 811, 14%) | White (n = 5,075, 86%) | P value | Black high-risk (n = 110, 14%) | Black low-risk (n = 701, 86%) | P value | |
|---|---|---|---|---|---|---|
| Age, y | 52 ± 6 | 53 ± 5 | <0.01 | 51 ± 5 | 52 ± 6 | 0.03 |
| Women | 471 (58%) | 2,715 (54%) | 0.02 | 66 (60%) | 405 (58%) | 0.7 |
| Body mass index, kg/m2 | 27 ± 4 | 26 ± 4 | <0.01 | 27 ± 4 | 27 ± 4 | 0.6 |
| Systolic blood pressure, mm Hg | 116 ± 11 | 112 ± 12 | <0.01 | 116 ± 12 | 116 ± 11 | 0.6 |
| Diastolic blood pressure, mm Hg | 74 ± 8 | 69 ± 8 | <0.01 | 75 ± 8 | 74 ± 8 | 0.2 |
| eGFR-creatinine, mL/min per 1.73 m2a | 104 ± 9 | 105 ± 9 | <0.01 | 104 ± 10 | 105 ± 9 | 0.3 |
| College graduates | 327 (40%) | 2,132 (42%) | 0.4 | 42 (38%) | 285 (41%) | 0.6 |
| Employed full- or part-time | 655 (81%) | 3,750 (74%) | <0.01 | 90 (82%) | 565 (81%) | 0.8 |
| Current smoker | 267 (33%) | 1,303 (26%) | <0.01 | 40 (36%) | 227 (32%) | 0.4 |
| Family history of diabetes | 108 (13%) | 699 (14%) | 0.8 | 14 (13%) | 94 (13%) | 0.8 |
| Family history of hypertension | 222 (27%) | 1,296 (26%) | 0.3 | 26 (24%) | 196 (28%) | 0.3 |
| Established care with a primary care physician | 661 (82%) | 4,521 (89%) | <0.01 | 90 (82%) | 571 (82%) | 0.9 |
Data presented as mean ± standard deviation and n (%) for categorical data.
Cystatin C was not measured at baseline.
Kidney Function, CKD Events by Race, and APOL1 Risk Status
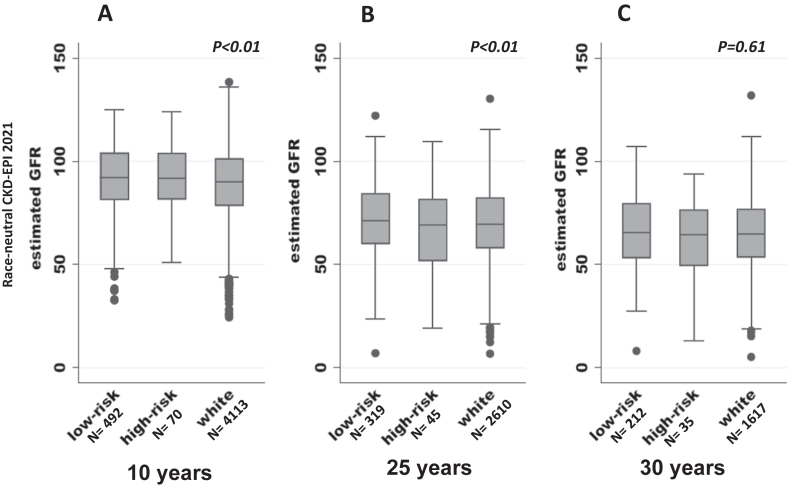
At 10 years, 67 participants died. Of the remaining 5,819 surviving participants, 4,675 (80%) had an assessment of kidney function at follow-up. Of these, 4,226 (90%) also had urine tested for albumin excretion. The proportion of participants who did not have kidney function assessed at 10 years differed significantly by group, ie, 913 (18%) in Whites, 193 (28%) APOL1 low-risk group, and 38 (35%) in APOL1 high-risk group (P < 0.01). Figure 2A compares kidney function among participants at 10 years of follow-up. White participants had lower mean eGFRcr-cys than low-risk and high-risk groups (89 ± 16 vs 91 ± 16 and 92 ± 15 mL/min/1.73 m2, respectively; P < 0.01). The proportion of participants with CKD stage 3a or worse was highest in White and low-risk Black individuals compared with the high-risk group (3.4% and 3.9% vs 1.4%, respectively; P = 0.02). Only 1 participant in the high-risk group developed CKD stage 3a or worse, so the odds ratio was not calculated. There was no difference in UACR among White, low-risk, and high-risk participants in each group (median [IQR] is 4 [2-9], 4 [2-7], and 4 [2-8], respectively; P = 0.87). The proportion of participants developing microalbuminuria was similar across the 3 groups (6% in Whites, 4% in low-risk participants, and 6% in high-risk participants; P = 0.08).
Figure 2.
Comparison of kidney function at follow-up based on race and APOL1 status of the participants using race-neutral CKD-EPI 2021 creatinine-cystatin C GFR calculator. eGFR (mL/min/1.73 m2) calculated using the CKD-EPI 2021 calculator using creatinine and cystatin was used to assess kidney function at follow-up. (A) At 10 years of follow-up, White participants had lower mean eGFRcr-cys than low-risk and high-risk groups (P < 0.01). (B) At 25 years of follow-up, eGFRcr-cys was lower in White participants than Black participants with the low-risk APOL1 genotype (P < 0.01), and there was no difference between Black participants in the low- and high-risk groups. (C) At 30 years of follow-up, there was no difference in eGFRcr-cys among the 3 groups. The box plot indicates median and interquartile range. eGFRcr-cys, creatinine- and cystatin C-based estimated glomerular filtration rate; GFR, glomerular filtration rate.
At 25 years of follow-up, 952 (16%) participants were reported dead, and the proportion of deaths were similar across the 3 groups (P = 0.51). Of the remaining 4,934 reported alive, 2,974 (60%) participants had information on kidney function. The proportion of participants lacking data on kidney function was lowest in White participants compared with the low-risk and high-risk APOL1 groups (39%, 45%, vs 48%; P = 0.01). Kidney function (eGFRcr-cys) remained lower in White participants than Black participants with low-risk APOL1 genotype (70 ± 18 vs 72 ± 19 mL/min/1.73 m2; P < 0.01) but did not differ from participants carrying the high-risk APOL1 genotype (67 ± 23 mL/min/1.73 m2, see Fig 2, panel B). The proportion of participants with CKD stage 3a or worse was similar across 3 groups (30%, 25%, and 33% in Whites, low-risk participants, and high-risk participants, respectively, P = 0.22). The odds of developing CKD stage 3a or worse were not different in White participants or the low-risk participants as compared with the high-risk group in both unadjusted and adjusted models (P = 0.61 and P = 0.91, respectively, Table 2). The UACR (median [IQR]) was similar across 3 groups (11 [6-24] White participants, 12 [7-32] low-risk Black individuals, and 10 [7-39] high-risk Black individuals; P = 0.91). The prevalence of microalbuminuria was also similar (7% in Whites, 10% low-risk participants, and 9% in high-risk participants; P = 0.21).
Table 2.
Odds of Developing CKD stage 3a or Higher Based on Race and APOL1 Status
| 25 Years (Only Those Who Are Alive) |
30 Years (Only Those Who Are Alive) |
|||||||
|---|---|---|---|---|---|---|---|---|
| At risk | Events, N (%) | UnadjustedOR (95% CI), P Value | Adjusteda OR (95% CI), P value | At Risk | Events, N (%) | Unadjusted OR (95% CI), P Value | Adjusteda OR (95% CI), P Value | |
| Black high-risk | 45 | 15 (33%) | REF | REF | 35 | 15 (43%) | REF | REF |
| Black low-risk | 319 | 81 (25%) | 0.7 (0.4-1.3), 0.3 | 0.7 (0.4-1.5), 0.4 | 212 | 77 (36%) | 0.8 (0.4-1.5), 0.5 | 0.9 (0.4-2), 0.9 |
| White | 2,610 | 779 (30%) | 0.9 (0.5-1.6), 0.6 | 1.04 (0.5-2), 0.9 | 1,617 | 642 (40%) | 0.9 (0.5-1.7), 0.7 | 1.3 (0.6-2.9), 0.5 |
CKD stage 3a or worse is defined as eGFRcr-cys ≤ 60 mL/min/1.73 m2.
Abbreviations: CI, confidence interval; OR, odds ratio; REF, reference.
Adjusted for eGFRcr, age, systolic and diastolic blood pressure, BMI, sex, employment status, education, smoking status, follow-up with primary care, family history of diabetes and hypertension.
At 30 years, 1,822 (31%) participants had died, and proportion of deaths was similar across 3 groups (P = 0.96). Of the 4,064 alive at the last follow-up visit, 1,864 (46%) had information on kidney function, and the proportion of participants without information on follow-up kidney function was similar (54% in White participants, 56% in low-risk participants, and 55% in high-risk participants; P = 0.33). Kidney function (eGFRcr-cys) was similar across all 3 groups (65 ± 17, 66 ± 18, and 64 ± 20 mL/min/1.73 m2 in Whites, low-risk participants, and high-risk participants, respectively; P = 0.61; Fig 2C). The proportion with CKD stage 3a or worse was similar across all 3 groups, and the odds of developing CKD stage 3a or worse were not different in White participants or Black low-risk participants compared with the high-risk Black group (Table 2). Although the UACR was similar across 3 groups (median [IQR], (6 [3-15] Whites, 6 [3-15] low-risk Black individuals, and 7 [4-34] high-risk Black individuals; P = 0.91), there were a greater proportion of participants with microalbuminuria in the high-risk group than in White and low-risk group (29% vs 14% and 13%, respectively; P = 0.04).
Annual Rate of Decline in Kidney Function by Race and APOL1 Risk Status
Table 3 shows that Whites had lower annual rate of decline in eGFRcr than the high-risk group in unadjusted analyses (estimate [standard error], 1.15 [0.01] vs 1.33 [0.05] mL/min/1.73 m2; P < 0.01), but there was no difference between the high-risk and low-risk group (1.33 (0.05) vs 1.26 (0.02) mL/min/1.73 m2/year; P = 0.13). The results remained unchanged in the adjusted analyses.
Table 3.
Annual Rate of Decline in Kidney Function Based on Race and APOL1 Status
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| Estimate of eGFR Rate of Decline (95% CI) | P Value | Estimate of eGFR Rate of Decline (95% CI) | P Value | |
| Black low-risk | −1.25 (−1.29 to −1.22) | 0.1b | −1.26 (−1.30 to −1.23) | 0.2b |
| Black high-risk | −1.33 (−1.42 to −1.24) | - | −1.33 (−1.42 to −1.24) | - |
| White | −1.15 (−1.16 to −1.14) | <0.001c | −1.16 (−1.18 to −1.15) | <0.001c |
Kidney function was assessed using the CKD-EPI 2021 GFR calculator using serum creatinine values available at enrollment and at 3, 10, 25 and 30 years of follow-up. Cystatin C was not used because it was not available at enrollment and 3-year follow-up.
Adjusted for age, systolic and diastolic blood pressure, BMI, sex, employment status, education, smoking status, follow-up with primary care, family history of diabetes and hypertension.
Compared with Black high-risk group.
Compared with Black high-risk group.
Secondary Outcomes
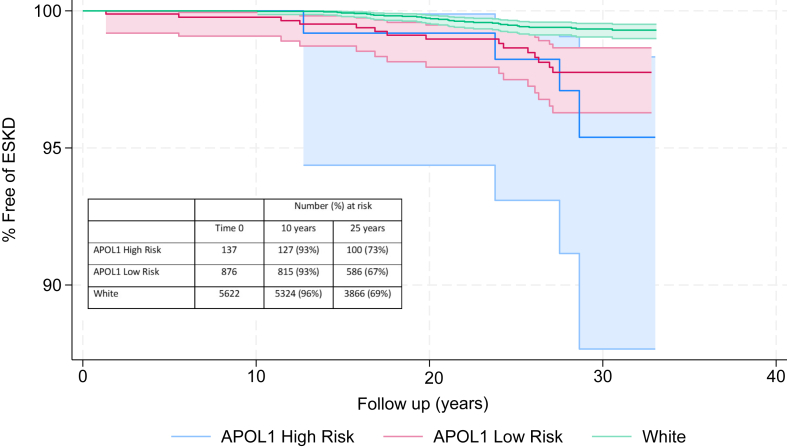
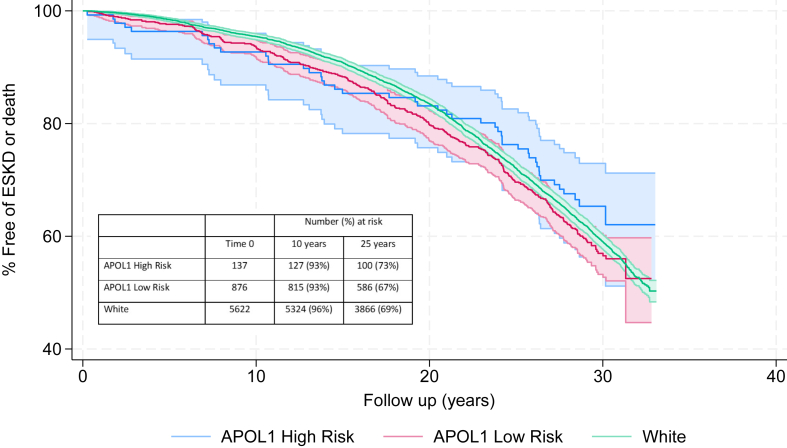
After applying the study inclusion criteria to all 15,792 original participants enrolled in ARIC (including those who declined public use of their data), 6,980 (44%) met our eligibility criteria. Of these 54 developed ESKD, and 3,014 had died or developed ESKD at mean follow-up of 25 years (maximum follow-up of 33 years). The ESKD rates were <5% at 30 years for the entire cohort, and the composite ESKD and death rate for entire cohort was ∼45% at 30 years. The ESKD rates and death rates were equal among all 3 groups with significant overlap in the 95% confidence interval (Figs 3 and 4).
Figure 3.
Rates of ESKD based on race and APOL1 status. ESKD was ascertained for the ARIC Cohort who met our study inclusion criteria, using the United States Renal Data System. The ESKD rates were low and equal across all 3 groups. ESKD, end-stage kidney disease.
Figure 4.
Comparison of composite outcomes of ESKD and death based on race and APOL1 status. Deaths and ESKD events were ascertained for the ARIC Cohort who met our study inclusion criteria, using the National Death Index and United States Renal Data System, respectively. Composite outcomes of ESKD and death were much higher than ESKD alone and were equal across all groups. ARIC, Atherosclerosis Risk in Communities; ESKD, end-stage kidney disease.
Discussion
We report that middle-aged Black individuals (45-64 years) carrying the high-risk APOL1 genotype had similar kidney function at 10 and 25 years of follow-up compared with Blacks with low-risk genotype as well as Whites. The proportion of participants with CKD stage 3a or worse at 25 years of follow-up was similar across 3 groups. Average annual rate of eGFRcr decline was statistically significantly lower in White participants than Black participants, but the difference was not clinically meaningful and was similar between Black participants regardless of APOL1 genotype. We report similar rates microalbuminuria at 10 and 25 years among the 3 groups. Our results remained unchanged when we evaluated kidney function using the race-inclusive CKD-EPI 2012 equation using serum creatinine and cystatin C (Fig S1).22 We report no difference in rates of death and ESKD among participants grouped by race and APOL1 status. Therefore, our findings suggest that the APOL1 high-risk genotype does not increase the long-term risk of kidney disease or death in middle-aged healthy Black individuals with normal kidney function at baseline.
Several case-control studies have reported a strong association between the APOL1 high-risk genotype and the development of certain types of progressive CKD.2,23 The presence of 2 APOL1 kidney risk variants were reported to confer 17-fold higher odds of developing FSGS and 29-fold higher odds of developing human immunodeficiency virus-associated nephropathy.24 The magnitude of the association is considerably less in population-based studies and varies by age of the participants. For cohorts with younger participants, such as the Coronary Artery Risk Development in Young study (participant age 18-30 years) or the Dallas Heart Study (participant age 45 ± 10 years), Black participants carrying 2 APOL1 kidney risk variants were 3- to 6-fold more likely to have microalbuminuria and CKD stage 3a or worse than Black participants carrying 0 or 1 risk variants.12,25 In contrast, in the ARIC cohort with participants age 45-64 years (unscreened for good health), individuals carrying 2 APOL1 kidney risk variants had only a 1.5-fold increased risk of developing CKD stage 3a or worse and a 1.9-fold increased risk of developing ESKD compared with individuals with 0 or 2 risk variants.19 Similarly, in the Reasons for Geographic and Racial Differences in Stroke study in which all participants were older than 45 years in age and the baseline prevalence of diabetes was nearly 30% and prevalence of hypertension over 70%, the adjusted hazard ratio for ESKD was 1.77 in Blacks with the high-risk APOL1 genotype compared with Blacks with the low-risk genotype at a median follow-up of 6.5 years.26
Unlike previous studies, we did not find a significant association between the APOL1 high- risk genotype and the subsequent decline in kidney function, which may be attributed to the older age of our study participants and the absence of hypertension, diabetes, and kidney disease at the time of study entry. Prior studies reporting an association between the APOL1 high-risk genotype and accelerated progression of kidney disease were mainly limited to CKD cohorts.6,27, 28, 29 Meta-analysis of 10 prospective studies consisting of 53,976 participants ranging from 35 to 62 years of age with a median follow-up of 10 years (range, 4-25) reported a modest association between the APOL1 genotype and the annual decline in GFR. The high-risk APOL1 genotype-associated decline in kidney function was more pronounced in individuals with CKD at baseline compared with those without CKD.30
Our study findings are relevant for counseling middle-aged Black persons with normal kidney function who may be considering the relevance of APOL1 test results in assessing their risk of kidney disease. These include potential LKDs as well as unaffected family members of patients with CKD attributed to APOL1 kidney risk variants. Among Black persons, 80% of LKDs are biologically related to their intended recipient, and 78% are first-degree relatives.31 An estimated 23% of first-degree relatives of Blacks with non-diabetic kidney disease will carry 2 APOL1 kidney risk variants.32 Although a small study demonstrated an association between APOL1 high-risk genotypes and reduced kidney function after living kidney donation, not all donors carrying the high-risk APOL1 genotype experienced a more rapid decline in GFR after donation.10 Of note, the mean age of LKD at the time of donation was 37 ± 9 years, much younger than our study group.10 Our study is not designed to assess the effect of APOL1 high-risk genotype on postdonation kidney function. There are several National Institutes of Health-supported prospective studies underway, ie, “APOL1 Long-term Kidney Transplantation Outcomes Network (APOLLO)” and “Living Donor Extended Time Outcomes,” and their results should provide information on association between APOL1 genotype and postdonation kidney function.33
This study’s notable strengths include a well-phenotyped cohort at entry with at least 25 years of follow-up and complete ascertainment of ESKD and death. The ARIC data set ascertained important social and family history that is known to affect future risk of CKD. The selection criteria for this study were rigorous, resulting in only one-third of ARIC participants being eligible for the study. Both serum creatinine and cystatin C measurements were standardized and calibrated over time. Kidney function at follow-up was assessed with the race-neutral and race-inclusive GFR calculator equation using both creatinine and cystatin C (eGFRcr-cys), providing a more accurate estimate of GFR.17 Of note, the choice of equation used to calculate GFR may affect comparisons between Blacks and White participants and not within Black participants.
The major limitations are large and variable intervals between study visits resulting in greater loss to follow-up, particularly among Black individuals rather than Whites. This is likely because of the older age of our study participants and long duration of follow-up. To overcome differential loss to follow-up rates over time across the groups, we ascertained ESKD and vital status from the United States Renal Data System and National Death Index. We report similar low ESKD rates (<5%) among individuals of both races and APOL1 genotype. However, the composite rate of death and ESKD was 45%, supporting the notion that healthy middle-aged individuals are far more likely to die than reach ESKD over a 30-year time span.34 We also compared the baseline characteristics of the participants carrying the high-risk genotype who did and did not have data on kidney function at 25 years and report similar characteristics except for history of smoking and lack of follow-up with primary care physician (Table S1). Lastly, the data set lacked information on family history of ESKD in first-degree relatives, a significant risk factor for advanced kidney disease.35,36
In conclusion, we report a lack of association of the APOL1 genotype with long-term kidney function among middle-aged Black individuals screened for good health and absence of kidney disease at baseline. In the general population, it is known that the risk of ESKD decreases with age and is lowest among individuals older than 60 years. We hypothesize that older Black individuals who are healthy are also beyond the years in which ESKD typically manifests.34 Given the data we have to date, despite the study limitations, we recommend that Black middle-aged and older individuals considering kidney donation or unaffected family members of patients with CKD attributed to APOL1 should be educated about APOL1 kidney risk variants and the availability of APOL1 testing. In addition, these individuals should be counseled that APOL1 kidney risk variants are not major drivers of their future risk of kidney disease.
Article Information
Authors’ Full Names and Academic Degrees
Mona D. Doshi, MD, Lihua Li, PhD, Abhijit S. Naik, MD, MPH, Christie P. Thomas, MD
Authors’ Contributions
Research idea and study design: MDD, ASN; data acquisition: MDD; data analysis/interpretation: MDD, LL, ASN, CPT; statistical analysis: MDD, LL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
MDD was supported by NIH grant U01DK116041-01. ASN was supported by the University of Michigan George O'Brien Kidney Translational Core Center P30 DK081943 and an NIH grant K23DK125529.
Financial Disclosures
CPT is a site investigator for a Vertex Pharmaceuticals trial for patients with APOL1 risk variants. MDD, ASN, and LL have no conflicts of interest to disclose.
Acknowledgements
This manuscript was prepared using ARIC Research Materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ARIC or the NHLBI.
Disclaimer
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Data Sharing
Publicly available ARIC data were used for this study. Information on ESKD and death for eligible ARIC cohort was provided by the USRDS.
Peer Review
Received November 19, 2023 as a submission to the expedited consideration track with 1 external peer review. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form April 12, 2024.
Footnotes
Complete author and article information provided before references.
Figure S1: Comparison of kidney function at follow-up based on race and APOL1 status of the participants using the race-inclusive CKD-EPI 2012 creatinine-cystatin C GFR calculator.
Table S1: Comparison of Baseline Characteristics of Participants With the High-Risk APOL1 Genotype Who Did and Did Not Have Follow-Up Data on Kidney Function at 25 Years.
Descriptive Text for Online Delivery
Figure S1; Table S1.
References
- 1.Tarver-Carr M.E., Powe N.R., Eberhardt M.S., et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 2.Genovese G., Friedman D.J., Ross M.D., et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman D.J., Pollak M.R. APOL1 Nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol. 2021;16(2):294–303. doi: 10.2215/CJN.15161219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer H.J., Stilp A.M., Laurie C.C., et al. African ancestry-specific alleles and kidney disease risk in Hispanics/Latinos. J Am Soc Nephrol. 2017;28(3):915–922. doi: 10.1681/ASN.2016030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke J.N., Bostrom M.A., Hicks P.J., et al. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant. 2012;27(4):1505–1511. doi: 10.1093/ndt/gfr522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsa A., Kao W.H., Xie D., et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg N., Lentine K.L., Inker L.A., et al. The kidney evaluation of living kidney donor candidates: US practices in 2017. Am J Transplant. 2020;20(12):3379–3389. doi: 10.1111/ajt.15951. [DOI] [PubMed] [Google Scholar]
- 8.Lentine K.L., Mannon R.B. Apolipoprotein L1: role in the evaluation of kidney transplant donors. Curr Opin Nephrol Hypertens. 2020;29(6):645–655. doi: 10.1097/MNH.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman B.I., Skorecki K. Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol. 2014;9(11):2006–2013. doi: 10.2215/CJN.01330214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi M.D., Ortigosa-Goggins M., Garg A.X., et al. APOL1 genotype and renal function of black living donors. J Am Soc Nephrol. 2018;29(4):1309–1316. doi: 10.1681/ASN.2017060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grams M.E., Sang Y., Levey A.S., et al. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374(5):411–421. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke J.E., Sawinski D., Reed R.D., et al. Apolipoprotein L1 and chronic kidney disease risk in young potential living kidney donors. Ann Surg. 2018;267(6):1161–1168. doi: 10.1097/SLA.0000000000002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams M.E., Rebholz C.M., Chen Y., et al. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27(9):2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 15.Delmonico F., Council of the Transplantation S A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: data and medical guidelines. Transplantation. 2005;79(6 Suppl):S53–S66. [PubMed] [Google Scholar]
- 16.Lentine K.L., Kasiske B.L., Levey A.S., et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101(8S Suppl 1):S1–S109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E., Moher D., Little J., STREGA collaboration Reporting genetic association studies: the STREGA statement. Lancet. 2009;374(9684):98–100. doi: 10.1016/S0140-6736(09)61265-4. [DOI] [PubMed] [Google Scholar]
- 19.Foster M.C., Coresh J., Fornage M., et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; 2022. 2022 USRDS annual data report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 21.Parrinello C.M., Grams M.E., Couper D., et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61(7):938–947. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker L.A., Schmid C.H., Tighiouart H., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. Jul 5 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasembeli A.N., Duarte R., Ramsay M., et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in Black South Africans. J Am Soc Nephrol. 2015;26(11):2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp J.B., Nelson G.W., Sampath K., et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman D.J., Kozlitina J., Genovese G., Jog P., Pollak M.R. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22(11):2098–2105. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik R.P., Irvin M.R., Judd S., et al. Sickle cell trait and the risk of ESRD in Blacks. J Am Soc Nephrol. 2017;28(7):2180–2187. doi: 10.1681/ASN.2016101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen A., Suen S.C., Lin E. APOL1 genotype, proteinuria, and the risk of kidney failure: a secondary analysis of the AASK (African American Study of Kidney Disease and Hypertension) and CRIC (Chronic Renal Insufficiency Cohort) studies. Kidney Med. 2022;4(12) doi: 10.1016/j.xkme.2022.100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D.P., Henderson C.D., Anguiano J., et al. Kidney disease progression in membranous nephropathy among Black participants with high-risk APOL1 genotype. Clin J Am Soc Nephrol. 2023;18(3):337–343. doi: 10.2215/CJN.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallash M., Wang Y., Smith A., et al. Rapid progression of focal segmental glomerulosclerosis in patients with high-risk APOL1 genotypes. Clin J Am Soc Nephrol. 2023;18(3):344–355. doi: 10.2215/CJN.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagannathan R., Rajagopalan K., Hogan J., et al. Association between APOL1 genotype and kidney diseases and annual kidney function change: a systematic review and meta-analysis of the prospective studies. Int J Nephrol Renovasc Dis. 2021;14:97–104. doi: 10.2147/IJNRD.S294191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lentine K.L., Schnitzler M.A., Garg A.X., et al. Race, relationship and renal diagnoses after living kidney donation. Transplantation. 2015;99(8):1723–1729. doi: 10.1097/TP.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman B.I., Langefeld C.D., Turner J., et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int. 2012;82(7):805–811. doi: 10.1038/ki.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman B.I., Moxey-Mims M.M., Alexander A.A., et al. APOL1 Long-term Kidney Transplantation Outcomes Network (APOLLO): design and rationale. Kidney Int Rep. 2020;5(3):278–288. doi: 10.1016/j.ekir.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu C.Y., Iribarren C., McCulloch C.E., Darbinian J., Go A.S. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massie A.B., Muzaale A.D., Luo X., et al. Quantifying postdonation risk of ESRD in living kidney donors. J Am Soc Nephrol. 2017;28(9):2749–2755. doi: 10.1681/ASN.2016101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainright J.L., Robinson A.M., Wilk A.R., Klassen D.K., Cherikh W.S., Stewart D.E. Risk of ESRD in prior living kidney donors. Am J Transplant. 2018;18(5):1129–1139. doi: 10.1111/ajt.14678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Table S1.