Abstract
The use of antibiotics in animal production raises great public safety concerns; therefore, there is an urgent need for the development of substitutes for antibiotics. In recent decades, plant-derived feed additives have been widely investigated as antibiotic alternatives for use in animal health and production because they exert multiple biological functions and are less likely to induce resistance development. This review summarizes the research history and classification of phytogenic feed additives and their main functions, potential modes of action, influencing factors, and potential negative effects. Further, we highlight the challenges in developing sustainable, safe, and affordable plant-derived antibiotic alternatives for use in livestock production.
Keywords: Phytogenic feed additives, Antibiotic alternatives, Livestock animals, Health, Production
1. Introduction
Antibiotics have long been used as growth promoters to increase productivity in animal production (Hashemi and Davoodi, 2011). Long-term antibiotic use can result in the accumulation of antibiotic residues in meat and meat products, which may lead to antibiotic resistance in humans. In 2006, the European Union (Reg. No. 1831/2003/EC) banned the use of antibiotics as growth promoters in animals. Other countries have since developed policies to reduce or ban antibiotic use in animal production. Announcement No. 194 of the Ministry of Agriculture and Rural Affairs of the People's Republic of China states that from January 1, 2020, all types of growth-promoting pharmaceutical feed additives except traditional Chinese medicines will be withdrawn from animal production. Antibiotic-free animal products have improved market opportunities and have grown exponentially in recent years (Gadde et al., 2017). To achieve safe livestock and poultry production, new, natural, and safe feed additives are needed.
Various antibiotic alternatives, including enzymes, probiotics, prebiotics, inorganic acids, medicinal plants, immunostimulants, and management practices, have been used to enhance animal health and performance. Phytogenics are heterogeneous compounds with different biological activities considered to have some similar benefits as antibiotic growth promoters (Rossi et al., 2020; Kuralkar and Kuralkar, 2021). Numerous herbal products have been demonstrated to have beneficial effects and medicinal properties, including antimicrobial, antioxidant, anti-inflammatory, and immunomodulatory activities, without adversely affecting growth and feed efficiency, and are therefore used as growth-promoting feed additives in livestock production (Kumar et al., 2014; Lillehoj et al., 2018; Kuralkar and Kuralkar, 2021). There is an increasing preference for natural products because they are considered to have less undesirable side effects than synthetic products (Laudato and Capasso, 2013). The development of plant-based feed additives has become a research hotspot and is significant for the healthy development of animal husbandry and the improvement of animal product quality.
Phytogenic feed additives are pharmacological plant components that have various benefits for animals and animal production, including improved growth performance, health, reproduction, and product quality, and reduced emissions and toxicity. Herbal products are accessible, easy to prepare, and affordable. Moreover, they generate less residues, are less toxic, and have less side effects in animal product production, and are generally safe for humans. However, plants contain numerous pharmacologically active ingredients in various amounts that can moreover vary from batch to batch; therefore, the effectiveness and mechanisms of phytogenic feed additives have not been fully elucidated. Known mechanisms of action include antimicrobial, immunomodulatory, antioxidant, and intestinal microbiota-regulatory activities. The integration of metagenomics, transcriptomics, proteomics, and network pharmacology tools may help elucidate the characteristics and mechanisms of phytogenic feed additives and develop feasible and cost-effective methods to use plant-derived additives as effective antibiotic alternatives in animal production.
In this review, we summarize the definition and research history of phytogenic feed additives, as well as their classification and composition. The functions of phytogenic feed additives in livestock animals and their potential modes of action are systematically discussed. Additionally, we report influencing factors and potential negative effects of phytogenic feed additives. We aimed to provide a comprehensive overview of the current state of research on the effects of phytogenic feed additives in improving the health status and growth performance of livestock animals and an overall view of the usability of phytogenic feed additives as potential antibiotic alternatives and as a nutritional strategy in animals.
2. Definition and research history
Phytogenic feed additives (also referred to as phytobiotics or phytochemicals) are commonly defined as plant-derived natural compounds, herbal formulas, plant extracts, or bioactive compounds that have the capacity to ameliorate feed properties, promote animal production performance, and improve animal product quality (Hashemi and Davoodi, 2011; Yang et al., 2015a). Other terms commonly used to classify plant compounds in terms of their source and processing are, for example, Chinese herbal feed additives and plant extract feed additives.
Traditional herbal medicines have been used for more than 3000 years owing to their beneficial effects on human health (Huang et al., 2017). In livestock animals, they have been used for more than 2000 years (Gong et al., 2014). Producers gradually discovered the beneficial effects of phytogenic feed additives on animal performance, including increased body weight, feed conversion ratio, and meat quality. Later, researchers uncovered the antimicrobial, antiviral, antifungal, antioxidant, and anti-inflammatory properties of herbs that contributed to their beneficial effects. Therefore, herbs have traditionally been used as complementary or alternative antibiotics to improve health or cure diseases. Recently, their multifunctionality in modulating nutritional metabolism, immune responses, and the intestinal health of livestock animals has been recognized, expanding our understanding and use of these agents. With the progress of extraction techniques and the identification of active ingredients, research efforts to use phytogenic extracts/compounds to substitute antibiotics in animal diets have increased. Herbs used in animal production are generally nontoxic, economical, and ecofriendly, indicating their broad application potential. At present, most animal feed manufacturers include phytogenic feed additives in broiler, pig, and ruminant feed products.
3. Classification and composition
Traditional herbal medicines have various ingredients and exert complex, low-specific pharmacological effects. A clear classification standard and unified classification are lacking. Phytogenic feed additives are classified according to the plant part used (whole plant, root, stem, bark, leaf, flower, fruit, or seed), plant growth habit (grasses, sedges, herbs, shrubs, climbers, or trees), plant habitat (tropical, sub-tropical, or temperate), therapeutic action (antibacterial, antifungal, anti-inflammatory, antiulcer, antioxidant, antiviral, anticancer, or immunostimulatory), and administration route (tincture, decoction, maceration, syrup, inhalation, or tisane) (Hashemi and Davoodi, 2011). Additionally, they can be divided into glucosides, acids, polyphenols, polysaccharides, terpenoids, flavonoids, and alkaloids based on their main active ingredients and into vegetable oil, extract, powder, or lens based on the product form. Phytogenic feed additives can also be classified into immune enhancers, hormone regulators, anti-stress agents, antimicrobial agents, insect repellents, appetite stimulants, reproductive regulators, fattening agents, lactation regulators, disease control agents, and feed preservation agents based on their application characteristics.
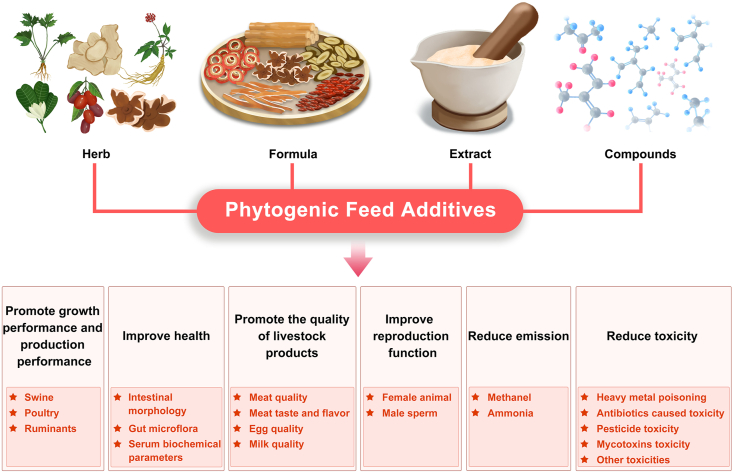
Traditional herbal products can be used as feed additives for their specific physiological actions. They can be supplemented after drying and crushing, or fed to animals freshly, particularly during the plant growing season (Fig. 1). In addition, they can be extracted as crude extract containing the main active herbal ingredients or individual compounds to be supplemented in animal feeds (Fig. 1). Further, multiple plant products or extracts can be combined in formulas to exert multiple beneficial effects (Fig. 1). However, even single herbs and formulas are complex products with multiple ingredients and multi-compound effects. They exert a unified effect through synergistic activities among the various components (Franz et al., 2010). Studies have emphasized that it is important to look at blends of components rather than single bioactive compounds (Rossi et al., 2020). However, the role of individual ingredients is also valued. Secondary plant metabolites as bioactive compounds elicit physiological and pharmacological effects in humans and animals (Gani et al., 2012). Major active secondary metabolites include alkaloids, glycosides, flavonoids, phenolic acids, saponins, tannins, terpenes, anthraquinones, and steroids. With the development of extraction and purification technologies, they can be separately extracted and used as additives for targeted functions. In recent years, such purified monomers have been found to exert specific physiological functions in animals by binding to specific receptors or activating specific signaling pathways.
Fig. 1.
Overview of phytogenic feed additives, including main classifications and functions.
4. Functions of phytogenic feed additives
The main aim of the livestock industry is to improve zootechnical performance. The use of phytogenic feed additives or herbal plants for livestock nutrition can improve animal health and performance (Rossi et al., 2020). Phytogenics can promote animal growth, production, and reproduction performance, and improve livestock product quality (Fig. 1). In addition, they have other beneficial effects, including the reduction of methane and ammonia emissions and of the toxicity of heavy metals, antibiotics, and mycotoxins (Fig. 1). The functions of phytogenic feed additives and potential modes of actions are summarized in Table 1. Accordingly, phytogenic feed additives have gained substantial attention in livestock production in recent years.
Table 1.
Function of phytogenic feed additives and potential modes of actions (representative references).
| Common name/species name | Major components/bioactive compounds | Animal species involved | Summary of findings/intended effect | Action and potential mechanism | References |
|---|---|---|---|---|---|
| Promotion of growth performance and production performance | |||||
| Essential oils from the Fabaceae, Laminaceae, Schisandraceae, and Zingiberaceae | Eucalyptol, p-cymene, linalool, anethole and thymol | Gestating and lactating sows | Piglets born alive↑; newborn piglets BW↓; colostrum protein and milk fat content↑; milk against Bacillus subtilis and Staphylococcus aureus↑; CAT, SOD, GSH-Px↑; MUC2↑; IDO↑; PPARGC1-α, TNF-α, TGF-β1, and IL-10↑ | Improve reproductive performance and suckling piglets gut heath attributed to its antioxidant activity and antimicrobial actions. | Reyes-Camacho et al. (2020) |
| Digestarom | Over 30 essential oils and phytogenic compounds | Broiler chickens | BWG↑; FCR↓; villus height↑; crypt depth↓; total tract digestibility of DM, CP, EE↑; total coliform count↓; coliforms↓; anaerobic bacteria↓; Lactobacillus spp.↑; Clostridium spp. ↓ | Stimulate growth performance of broilers through improving digestive function and modulating gut microbiota. | Murugesan et al. (2015) |
| Cinnamon (Cinnamomum verum) | Japanese quails | Body weight gain↑; feed conversion ratio↑; TBARS↓; water holding capacity↑ | Improve growth performance and positively influence meat quality, perhaps, because of its antioxidant properties. | Mehdipour and Afsharmanesh (2018) | |
| Peppermint (Mentha piperita L.) | Menthol, menthone, isomenthone, menthyl acetate, cineol | Laying hens | Egg weight↑; egg production↑; egg mass↑; feed intake↑; FCR↓; eggshell percentage↑; eggshell thickness↑; Haugh unit ↑; serum cholesterol↓; serum total proteins↑ | Improve performance of laying hens probably owing to its antimicrobial properties, antioxidant properties and enhance appetite. | Abdel-Wareth and Lohakare (2014) |
| Poplar (Populus deltoides) and eucalyptus (Eucalyptus citriodora) leaves | Total phenolics, condensed tannins, and other phytochemicals | Buffaloes (Bubalus bubalis) | Daily milk yield, 6% FCM (fat corrected milk), and FPCM (fat–protein corrected milk) yield↑; digestibility coefficient of dry matter, organic matter and neutral detergent fiber↑; antibody titer (log10) against Pasteurella multocida vaccine↑; skin thickness↑ | Enhance milk production and immunity of Murrah buffaloes. | Dey et al. (2021) |
| Golden-and-silver honeysuckle (Lonicera japonica Thunb), huangqi (Astragalus menbranaceus), duzhong leaves (Eucommia folium) and dangshen (Codonopsis pilosula) | Piglets | Crypt depth of duodenum↓; ratio of villus height to crypt depth (duodenum and jejunum)↑; the expression of nutrient transporters in ileum (SLC6A9, SLC15A1, and SLC5A1)↑; activities of maltase in ileum↓; ratio of small intestinal weight to BW↓ | Improve intestinal health by modulating intestinal morphology and increasing the mRNA expression of nutrients transporters. | Wang et al. (2020) | |
| Carvacrol, cinnamaldehyde, capsicum | Broiler chickens | Weight gain↑; feed efficiency↑; carcass energy retention↑; total heat loss↓; carcass protein retention↑; fat digestibility↑; net energy for production↑ | Improve growth performance through improving the metabolic efficiency of converting absorbed dietary energy into tissue. | Bravo et al. (2014) | |
| Essential oils of thymol and cinnamaldehyde | Weaning pigs | ADG↑; fecal score↑; DM and CP digestibility↑; lymphocyte proliferation↑; IGF-1 level↑; serum IL-6↓; serum TNF-α↑; T-AOC↑; villus height to crypt depth ratio↑; E. coli (cecum, colon and rectum)↓; Lactobacilli/E.coli ratio (colon)↑; total aerobe (rectum) ↓ | Improve growth performance and reduce the diarrhea probably by improving immune status, intestine ecology, and nutrient digestibility. | Li et al. (2012a) | |
| Health improvement | |||||
| Oregano, anise and citrus | Carvacrol, anethol and limonene | Broilers | Mucin composition↑; villus height:crypt depth ratio↑; duodenal mucus layer thickness↑ | Modulate intestinal mucin composition and morphology. | Tsirtsikos et al. (2012) |
| Brazilian red pepper (Schinus terebinthifolius Raddi) | Weaning pigs | Villi density↓; Lactobacillus counts↑; Enterobacteria counts↓; incidence of diarrhea↓ | Affect microbiota and histology without affecting performance and organ weights. | Cairo et al. (2018) | |
| Essential oils of eugenol, thymol, and piperine | Weaned piglets | ADG, ADFI and ATTD↑; normal feces frequency↑; goblet cells count↓; goblet cells count↓; small intestine and colon relative weights↓; Escherichia-Shigella↓; Campylobacter↓ | Improve growing performance, diets digestibility and gut health. | Silva Júnior et al. (2020) | |
| YGF251 (Phlomis umbrosa Turez, Cynancum wilfordii Hemsley, Zingiber officinale Rosc and Platycodi Radix) | Broiler chickens | Abdominal fat↓; femur length and weight↑; blood IgG counts↑; IGF-1 concentrations↑ | Enhance immune-related functions. | Begum et al. (2014) | |
| Ashwagandha (Withania somnifera) | Broiler chicks | Feed intake and BW↑; values of hemoglobin, packed cell volume and total leukocyte count↑; antibodies titers against IB and IBD↑ | Improve feed intake, BW gain, hematological profile and immunological status. | Mushtaq et al. (2012) | |
| Ashwagandha (Withania somnifera) | Chicken embryo fibroblast (CEF) cell | Virus titer (against IBD virus)↓ | Antiviral property | Pant et al. (2012) | |
| Eugenol and cinnamaldehyde | Growing pigs | Lymphocyte count↑; fecal E. coli concentration↓; NH3 and H2S concentration↓ | Exhibited lymphocyte-enhancing activity, fecal microbial shedding and noxious gas emission. | Yan and Kim (2012) | |
| Essential oils of thymol and cinnamaldehyde | Weaned pigs | Occurrence of diarrhea↓; E. coli counts↓; lymphocyte transformation↑; leucocyte phagocytosis rates↑; IgA and IgM↑; C3 and C4↑; ADG and FCR↑ | Improve performance, immunity and gut microflora. | Li et al. (2012b) | |
| Promotion of livestock product quality | |||||
| Oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract | Pigs | GSH-Px and GR activities in the Longissimus lumborum muscle↑; lipid oxidation; L∗ and H° values↓, and a∗ values↑ in cooked meat; cores for color, taste and overall liking↑ | Increase the pig antioxidant status, to prevent lipid oxidation and increase meat shelf-life. Higher taste score and liking degree after cooked. | Ranucci et al. (2014) | |
| Commercial products (AVHGP, SCP, BHGP, AVSSL, SG) | Broiler chicks | Slaughter weights↑; breast weights↑; fat pad size↓; L∗↓; b∗↓; green herb flavor↑; SOD2↑; ERK1/2↑; JNK↑ | Improve meat quality of broilers through modulation of stress- and antioxidant-related pathways. | Orlowski et al. (2018) | |
| Liquorice extract from Glycyrrhiza uralensis | Flavonoids | Tan sheep | Total flavonoid↑; VE and GSH contents of meat↑; DPPH and ABTS free radical scavenging activity (in vitro)↑; ROS and TBARS level of lambs meat↓ | Protect fresh meat against lipid oxidation by increasing non-enzymatic antioxidant content to scavenge free radical. | Zhang et al. (2015) |
| Greek Origanum vulgare subsp. hirtum; Sophora japonica L. | Oregano essential oil; quercetin | Finishing pigs | After 5 h transportation, BW loss↓; hot carcass weight↑; dressing percentage↑; pH value and Opto-star value↑; drop values↓; TBARS↓; ROS↓; Gpx↑; T-SOD↑ | Reduce transportation-induced oxidative stress and improving meat quality by improving antioxidant status. | Zou et al. (2016) |
| Huangqi (Astragali Radix), Baizhu (Rhizoma Atractylodis Macrocephalae), and FangFeng (Saposhnikoviae Radix) | Black goats | Palmitic acid, octadecanoic acid, and arachidonic acid in the longissimus dorsi muscle↑; flavor fatty acid↑, SFA↑ and PUFA concentration↑; PUFA/SFA↑; PPARγ and CD36 expression↑; Ruminococcus↑; Alistipes↑ | Improve the meat quality and flavor through regulating the fat metabolism and beneficial microbes. | Yang et al. (2021) | |
| Yizhi extract (Alpiniae oxyphyllae) | Ducks | Serum LDL-C level↓; total amino acids, essential amino acids, branched-chain amino acids, nonessential amino acid, and umami taste amino acids concentration in breast muscle↑; Se and Zn in breast muscle↓; flavor amino acid (Arg, Asp, Glu, His, Phe, and Ser)↑; unclassified Bacteroidales and Ruminococcaceae abundance↑ | Increase meat nutrition profile and flavor, maintain intestine integrity and modulate the microbial composition. | Ji et al. (2022) | |
| Plantain (Plantago lanceolata L.) and/or garlic leaf (Allium sativum) | Sheep | Mutton ether extract↓; rib eye area↑; saturated fatty acid↓; polyunsaturated fatty acids↑; linoleic acid contents↑; IgA, IgG1, IgG2 and IgM concentrations of serum↑; level of TAC, SOD, GPx and CAT↑ | Promote growth performance and health status as well as lean mutton production and mutton fatty acid profile. | Redoy et al. (2020) | |
| Yucca schidigera extract | Laying hens | Egg number and egg mass↑; shell thickness↑; albumin and IgG↑; SOD↑; GSH↑; MDA↓; total cholesterol↓; lipid peroxidation↓ | Improve egg production and egg quality as well as immunity functions and antioxidant status. | Alagawany et al. (2016) | |
| 70% pine needle and 30% Artemisia annua | Laying hens | Lay rate↑; egg-shell color and egg yolk color↑; cracked-egg rate↓; egg yolk cholesterol↓; blood serum cholesterol, triglyceride, LDL-C and ALT↓; blood serum HDL-C↑ | Improve egg production and egg quality and produce low cholesterol and higher egg yolk phospholipid eggs. | Li et al. (2016) | |
| Menthol, levomenthol, β-linaloolm, anethole, hexadecanoic acid and p-menthane | Lactating cows | Milk production↑; milk content of total solids, protein, lactose and fat↑; ruminal pH, total volatile fatty acids, propionate and acetate↑; serum total protein and antioxidant capacity↑; serum urea-N, triglycerides, total lipids, cholesterol and MDA↓ | Improve feed utilization, ruminal fermentation and milk production probably through enhancing antioxidant status. | Kholif et al. (2020) | |
| Mustard and cumin seeds | Goats | Digestibility of DM, OM, non-structural carbohydrates, and fiber fractions↑; milk yield↑; energy corrected milk↑; milk contents of total solids, solids not fat, fat, lactose and ash↑; milk SFA↓; total UFA↑; total CLA contents↑; ruminal SCFA, molar proportion of propionate↑; serum total proteins, globulin, glucose↑; serum cholesterol↓ | Improve nutrient digestibility, ruminal fermentation and milk yield. Positively affect milk FA profile as the relative percentage of UFA and CLA are increased. | Morsy et al. (2018) | |
| Improvement of livestock reproduction | |||||
| Aegle marmelos, Murraya koenigii | Buffaloes heifers | Estrus response↑; serum calcium↑; ovulation rate↑; conception rate↑ | Effective in fertility improvement in delayed pubertal buffalo heifers by increasing ovulation and conception rate. | Baitule et al. (2016) | |
| Yucca schidigera powder | Steroids, saponins, Glycocomponents | Dairy goats | Live BW↑; conception rate↑; fertility rate↑; oestrus resumption↓; oestrus duration↑; blood concentration of cholesterol, triglyceride and urea↓; glucose and calcium level↓ | Provide a positive adjustment on BW, favor high reproductive performance and contribute in regulating metabolic disturbances, not only through reinstituting the homeostatic balance but also affecting the productive characteristics. | Khalifa et al. (2014) |
| Sheng Hua Tang (consisting of RadixAngelicae Sinensis, Rhizome Ligustici, Semen Persicae, Rhizoma Zingiberis, and Radix Glycyrrhizae) | Dairy cows | Placental retention proportion↓; calving-to-first-service interval↓; calving-to-conception interval↑; service per conception↓; first artificial insemination conception proportion↑; pregnant within 180 days postpartum↑ | Reduce the incidence of retained placenta and improve subsequent reproductive performance. Provides a preventive strategy for bovine postpartum care. | Cui et al. (2014) | |
| Moringa oleifera leaf extract | Rabbit bucks | Rectal temperature↓; serum albumin↑; total antioxidant capacity↑; testosterone↑; seminal plasma initial fructose↑; sperm concentration↑; forward motility↑; live sperm↑; normal sperm↑; sperm progressive motility↑; sperm viability↑; sperm normal morphology↑; intact acrosome sperm↑; sperm with integrated cell membrane↑ | Improve heat tolerance, oxidative status and semen quality during summer season. | El-Desoky et al. (2017) | |
| Albizia harveyi | Myricetin, quercetin, and kaempferol glycosides | Bull semen | Sperm motility, viability, and membrane integrity↑; percentage of viable sperm cells↑; percentages of early apoptotic and apoptotic sperm cells↓; damage in sperm ultra-structure↓; total antioxidant capacity↑; MDA↓ | Ameliorate the damaging effects of the frozen-thawing process in cryopreserved bull semen because of its antioxidant activities. | Sobeh et al. (2017) |
| Murtilla (Ugni molinae Turcz) | Gallic acid, catechin, quercetin-3-β-D-glucoside, myricetin, quercetin, kaempferol | Boar semen | Intracellular O2−/peroxides↓; lipid peroxidation↓; sperm motility↑; sperm cells with fragmented DNA↓; membrane damage↓; ROS↓ | Have antioxidant capacity, improve sperm motility decay and reduce membrane damage. | Jofré et al. (2019) |
| Moringa oleifera seed | Ascorbic acid, flavonoids, polyphenolics, carotenes | Ram semen | Antioxidant activity↑; viability and progressive motility↑; sperm membrane damage↓ | Improve the outcome of semen cryopreservation used as an antioxidant. | Carrera-Chávez et al. (2020) |
| Emission reduction | |||||
| Resveratrol | In vitro fermentation system | Methane emission↓; Methanobrevibacter abundance↓ | Reduce methane emission through improving rumen fermentation and regulating prokaryotic community. | Ma et al. (2020) | |
| Oregano essential oil | In vitro fermentation system | Digestibility of DM, NDF, and ADF↑; ammonia nitrogen concentrations↓; VFA concentrations↓; total gas and methane production↓; Prevotella and Dialister abundance↑ | Modify ruminal fermentation to alter VFA concentrations and reduce methane emissions by extensively altering the ruminal bacterial community. | Zhou et al. (2020) | |
| Mangosteen peel powder | Tannins and saponins | Swamp buffaloes | Propionic acid↑; acetic acid/propionic acid↓; methane↓; total bacteria population↑; Ruminococcus flavefaciens and methanogens population↓; efficiency of microbial protein synthesis↑ | Mitigate methane production through influencing rumen methanogenic population. | Wanapat et al. (2014) |
| Oregano extract, green tea extracts | Lactating cows | Digestible fraction of the ingested DM↑; gas emission↓; CH4↓ | Reduce methane emission without affecting performance. | Kolling et al. (2018) | |
| Cablin patchouli herb, Atractylodes rhizome, Amur Cork-tree | In vitro fermentation | Total VFA concentration↓; acetate molar proportion↓; acetate to propionate ratio↓; gas and methane productions↓; hydrogen (H) produced and consumed↓; methanogens and total fungi populations↓; propionate molar proportion↑; R. flavefaciens↓ | Suppress methanogenesis, probably mediated via indirect mode by channeling H2 utilized for methanogenesis to synthesis of propionate and direct action against the rumen microbes involved in methane formation. | Ma et al. (2017) | |
| Mulberry leaf | Flavonoids | Ewes | Digestibility of N and NDF↑; fecal N↓; urinary N output↑; CH4 emissions↓; total VFA concentrations↑; methanogens↓; protozoans↓; Fibrobacter succinogenes↑ | Improve the digestibility of organic matter and reduce CH4 output by inhibiting the populations of microbes involved in methanogenesis. | Wang et al. (2019) |
| Mootral (garlic and citrus extracts) | In vitro fermentation | Total VFA↑; propionate↑; acetate↓; methane↓; Prevotellaceae and Veillonellaceae↑; hydrogen-producing bacteria↓; Methanobacteriaceae↓; Methanomassiliicoccaceae↑ | Reduce methane by altering the ruminal microbial community, produce more propionate, and reduce microbial groups associated with methane production. | Ahmed et al. (2021) | |
| Corymbia citriodora leaf extract | Holstein calves | Degradation of DM, CP, NDF and ADF↓; acetate and butyrate↓; propionate↑; ruminal NH3-N concentration↓; protozoan population↓; methane production↓; plasma antioxidant enzymatic activities↑; faecal pathogenic bacterial counts↓ | Improve the health status and manipulate rumen fermentation in mitigating methane production. | Hassan et al. (2020a) | |
| Liquorice extract | Isoflavonoids | The rumen simulation technique | Ammonia production↓; methane↓; total VFA production↓; propionate molar proportion↑; protozoa numbers↓; diverse bacteria population↓ | Decrease ammonia production through changing the bacterial and archaeal communities and improve the efficiency of the feed utilization. | Ramos-Morales et al. (2018) |
| Yucca schidigera extract | Saponin and resveratrol | Sows | Number of stillbirth piglets↓; weak piglets↓; pre-weanling mortality↓; diarrhea↓; apparent digestibility of DM↑; catalase activity↑; MDA↓; loss of total nitrogen, urea nitrogen, and ammonia nitrogen↓ | Improve sow and litter performance, nutrient digestibility, and reduce ammonia emission. | Chen et al. (2021) |
| Essential oils of cinnamaldehyde, thymol and carvacrol | In vitro model and growing pigs | Nitrogen digestibility↑; ammonia production↓; activity of microbial enzymes↓; emission of ammonia↓; total fecal nitrogen↓; microbial protease↓; urease activities↓; indole↓; 3-methylindole↓; p-cresol↓ | Increase nitrogen utilization and reduce waste emission and odorous compounds. | Hsu et al. (2021) | |
| Toxicity reduction | |||||
| Garlic (Allium sativum) | Broiler chickens | Live weight↑; carcass weight↑; eviscerated fresh carcass↑; FCR↑ | Might play an active role to antagonize lead toxicity. | Hossain et al. (2014) | |
| Yucca schidigera extract | Japanese quails | Quail performance parameters↑; fertility and hatchability percentages↑; triglycerides↓, cholesterol↓; LDL↓; catalase activity↑; superoxide dismutase activity↑; MDA↓; total protein, albumin, and globulin↑; triglycerides, cholesterol, and LDL contents↓; HDL↑; immunoglobulins↑; lead residues↓ | Reverse the lead-induced toxic impacts on the productive and reproductive performances probably owing to enhanced antioxidant status and immune function. | Alagawany et al. (2018) | |
| Turmeric (Curcuma longa) | Broiler chicks | Cortex thickness of bursal tissue↑; medulla zone↓; lymphocytic necrosis↓; fibrosis of interstitium↓; edema of medulla zone↓ | Protect bursa of Fabricius against toxicity induced by salinomycin probably through increasing antioxidant potential and immune function. | Sayrafi et al. (2017) | |
| Astragali Radix | Calycosin | Rat cardiomyocyte line H9c2 and Kunming mice cell | H9c2 cell viability↑; apoptosis induced by doxorubicin↓; reactive oxygen species↓; GSH-Px↑, CAT and SOD↑; AST↓, LDH↓; MDA↓; NLRP3 and TXNIP expression↓ | Ameliorate doxorubicin-induced toxicity by Sirt1–NLRP3 pathway, antioxidant activity and immune modulate. | Zhai et al. (2020) |
| Licorice extract | Broiler chickens | FCR↑; serum TP, TG, LDL↓; abdominal fat↓; meat MDA↓; ALP↓; serum albumin↑; serum uric acid↓; liver pathological damages↓ | Ameliorate the negative effects of aflatoxin B1 on broiler chicken probably owing to its antioxidant protection properties. | Rashidi et al. (2020) | |
CAT = catalase; SOD = superoxide dismutase; GSH-Px = glutathione peroxidase; MUC2 = mucin2; IDO = indoleamine 2, 3- dioxygenase; PPARGC1-α = peroxisome proliferative activated receptor gamma coactivator 1 alpha; TGF = transforming growth factor; IL = interleukin; BWG = body weight gain; FCR = feed conversion rate; DM = dry matter; CP = crude protein; EE = ether extract; TBARS = thiobarbituric acid reactive substances; ADG = average daily gain; T-AOC = total antioxidant capacity; ADFI = average daily feed intake; ATTD = apparent total tract digestibility; IGF-1 = insulin-like growth factor 1; BW = body weight; IB = infectious bronchitis; IBD = infectious bursal disease; GR = glutathione reductase; ERK1/2 = extracellular signal-regulated protein kinases1and 2; JNK = c-Jun N-terminal kinase; VE = vitamin E; DPPH = α,α-diphenyl-β-picrylhydrazyl; ABTS = 2,2′-azino-bis-3-ethyl-benzthiazoline-6-sulfonic acid; ROS = reactive oxygen species; SFA = saturated fatty acid; PUFA = polyunsaturated fatty acid; PPARγ = peroxisome proliferator-activated receptor γ ; LDL-C = low-density lipoprotein cholesterol; TAC = total antioxidant capacity; MDA = malondialdehyde; ALT = alanine aminotransferase; HDL-C = high-density lipoprotein cholesterol; OM = organic matter; UFA = unsaturated fatty acids; CLA = conjugated linoleic acid; SCFA = short-chain fatty acid; NDF = neutral detergent fiber; ADF = acid detergent fiber; VFA = volatile fatty acid; LDL = low density lipoprotein; HDL = high density lipoprotein; AST = aspartate transaminase; NLRP3 = NOD-like receptors family pyrin domain containing 3; TXNIP = thioredoxin-interacting protein; TP = total protein; TG = triglycerides; ALP = alkaline phosphatase.
4.1. Promotion of growth performance and production performance
Antibiotics have long been used as growth promoters to enhance productivity in animal production (Hashemi and Davoodi, 2011). Because of antimicrobial resistance development, the use of antibiotics as growth promoters has been gradually banned and therefore, alternative growth promoters have received increasing attention. Numerous herbal products having multiple biological functions are used as growth promoters to improve production efficiency in the animal industry (Gong et al., 2014; Rossi et al., 2020). Currently, phytogenic feed additives are widely used in feeding programs for swine, poultry, and ruminants.
In swine, the effect of phytogenic feed additives has been widely demonstrated. Yan et al. (2011a) investigated a mixture of herbal extracts of buckwheat, thyme, curcuma, black pepper, and ginger as a feed additive in growing pigs and suggested it as an antibiotic alternative because it increased feed intake and growth performance. A herbal feed additive consisting of benzoic acid and essential oils of thymol, eugenol, and piperine promoted piglet growth performance by improving average daily gain, average daily feed intake, apparent total tract digestibility of nutrients, and energy (Silva Júnior et al., 2020). A blend of Cinnamomum zeylanicum and Trachyspermum copticum essential oils and the blend plus plant extracts of Mikania micrantha and Garcinia lanceifolia did not affect growth performance when compared with no-additive and antibiotic controls; however, it did improve some lipid profiles, immune responses, and intestinal microbial populations in piglets (Samanta et al., 2021). Dietary supplementation of commercial phytogenics in sows during gestation/lactation improved litter size and live births as well as the composition and bioactivity of colostrum and milk, which may benefit offspring health and performance (Reyes-Camacho et al., 2020; Nowland et al., 2021).
The effects of plant extract feed additives on poultry growth performance have been extensively studied, with relatively consistent results. Numerous plant extract additives have been demonstrated to improve growth performance, nutrient digestibility, feed efficiency, and intestinal health in poultry (Abdelli et al., 2021; Seidavi et al., 2022). Dietary supplementation of Digestarom Poultry, which comprises more than 30 essential oils and phytogenic compounds, significantly increased body weight gain, while lowering the feed-to-gain ratio in broiler chicks (Murugesan et al., 2015). Along with positive effects on total-tract digestibility, intestinal function, and beneficial microbial colonization, this product has demonstrated efficacy as a substitute for antibiotic growth promoter in poultry. Plant extracts have also yielded positive results in other poultry species. Cinnamon (Cinnamomum verum), garlic (Allium sativum), ginger (Zingiber officinale), oregano (Origanum vulgare), and other plant extracts significantly improved body weight gain and feed availability in turkey and quail (Al-Shuwaili et al., 2015; Mehdipour and Afsharmanesh, 2018; Gernat et al., 2021). Positive effects were also observed in laying hens. The inclusion of peppermint (Mentha piperita L.) leaves in a Hy-Line brown laying hen diet significantly increased egg weight, egg production, and egg mass in a dose-dependent manner (Abdel-Wareth and Lohakare, 2014).
While most studies on plant additives in ruminants focused on the regulation of rumen fermentation and the prevention or treatment of mastitis, some studies have demonstrated beneficial effects on production performance. Dietary supplementation of a blend of poplar (Populus deltoides) and eucalyptus (Eucalyptus citriodora) leaves in Murrah buffaloes increased daily milk yield, 6% fat-corrected milk yield, and fat-protein-corrected milk yield, as well as the digestibility of dry matter, organic matter, and neutral detergent fiber (Dey et al., 2021). Akbarian-Tefaghi et al. (2018) investigated thyme, eucalyptus, and celery and a commercial essential oil in calf starter; they found that these additives improved starter intake, daily gain, and feed efficiency and concluded that eucalyptus may be a superior alternative to monensin for improving post-weaning performance. The positive effect of herbal feed additives on ruminant performance may be attributed to the modulation of ruminal fermentation and improvement of nutrient utilization (Kholif et al., 2020).
Phytogenic feed additives can improve animal growth and production performance via several mechanisms. First, they can improve palatability, thus increasing feed intake. Adding sensory feed additives, such as Stevia rebaudiana, Citrus sinensis, saponin-rich plants, and hot-flavored spice extracts to the diet of weaned piglets significantly improved feed palatability, edibility, and intake (Clouard and Val-Laillet, 2014). Second, plant extracts can improve the secretion and activity of endogenous enzymes and the nutrient utilization rate. Supplementation of Chinese herbal extract mixtures in piglet and beef cattle diets promoted digestive enzyme secretion and activity in the gastrointestinal tract, increasing digestibility and nutrient absorption (Wang et al., 2011; 2020). Third, they can improve nutrient transformation and utilization for growth. Plant extracts containing carvacrol, cinnamaldehyde, and capsaicin added to a broiler diet significantly increased body weight gain, feed utilization, and carcass energy retention, and reduced heat loss (Bravo et al., 2014). This indicated that plant extracts can improve the energy conversion efficiency in broiler organs, i.e., they reduce the energy used for maintenance and increase the net energy for production, thus improving overall performance. Fourth, they can regulate the secretion of growth-related hormones and thus promote animal performance. Insulin-like growth factor (IGF) promotes intestinal development (Li et al., 2012a). Adding thymol and cinnamaldehyde in the diet of weaned piglets significantly increased plasma IGF-1 levels and body weight gain, thus improving performance (Ariza-Nieto et al., 2011).
4.2. Health improvement
Herbs and herbal medicines are potential sources of nutrients and therapeutics and can have significant health benefits in humans and animals (Kuralkar and Kuralkar, 2021). Phytogenic feed additives have been demonstrated to strengthen animal health defense, mainly by improving the physiological status of the intestinal ecosystem and enhancing immune system functions.
Intestinal health is extremely important for overall animal health. Herbs and extracts can exert beneficial effects on the digestive tract, including stimulating digestive function, reducing pathogenic stress, and promoting the establishment of a beneficial intestinal microbiota. Intestinal crypts and villi are lined by epithelial cells that play important roles in digestive function. Herbal feed additives can increase the villus height-to-crypt depth ratio in the ileum, jejunum, and duodenum, indicating their beneficial effects in maintaining intestinal health and improving nutrient digestion and absorption (Lin et al., 2020). The intestinal mucus layer governs nutrient absorption and protects the underlying epithelium against enteric pathogens. A phytogenic feed additive containing carvacrol, anethol, and limonene modulated broiler intestinal mucin composition and mucosal morphometry and increased duodenal mucus layer thickness (Tsirtsikos et al., 2012). Dietary cinnamon stimulated the gene expression of mucin 2, an important mucus layer component (Ali et al., 2021). Intestinal microbiota abundance and composition are regulated by nutrients. Phytogenic feed additives can exert health and growth-promoting effects by maintaining intestinal microbiota equilibrium. Dietary supplementation of an essential oil from Brazilian red pepper increased intestinal Lactobacillus counts and lowered the incidence of diarrhea in weaning pigs (Cairo et al., 2018). Diets containing essential oils of eugenol, thymol, and piperine combined with benzoic acid (Silva Júnior et al., 2020) reduced the numbers of pathogenic bacteria Campylobacter and Escherichia-Shigella in the intestines of weaned piglets, indicating their potential to improve intestinal health in piglets soon after weaning.
Phytogenic feed additives exert positive effects on several serum biochemical parameters, which are animal health indicators. Chinese herbal feed additives positively influenced serum biochemical parameters of protein synthesis and metabolism, liver synthetic function, and pancreatic and renal health in swine, indicating their potential in sustaining a healthy host environment, which contributes to better growth performance (Lin et al., 2020). Additionally, phytogenic feed additives can improve immune function as reflected by certain serum biochemical parameters. Inclusion of 0.1% of a mixture of herbal extracts (YGF251) in a broiler diet increased the serum immunoglobulin (Ig) G content (Begum et al., 2014). Ashwagandha (Withania somnifera) administration in broiler chickens improved hemoglobin, packed cell volume, total leukocyte count, and antibody titers against viral diseases, such as infectious bursal disease and bronchitis, suggesting an improved hematological profile and immunological status (Mushtaq et al., 2012; Pant et al., 2012). In growing pigs, eugenol and cinnamaldehyde exhibited lymphocyte-enhancing activity, although growth performance was not improved (Yan and Kim, 2012). Essential oil products containing thymol and cinnamaldehyde reduced inflammatory mediator and interleukin (IL)-6 levels and increased the lymphocyte proliferation rate, phagocytic rate, and IgA and IgM levels in the plasma of weaning pigs (Li et al., 2012a, Li et al., 2012b).
It has to be pointed out that animal health assessment is not standardized. It is difficult to evaluate animal health status based on a few simple indicators. The actual efficacy of phytogenic feed additives in maintaining animal health and their potential to substitute and reduce the application of antibiotics have to be investigated under practical animal production conditions.
4.3. Promotion of livestock product quality
Phytogenic feed additives have been investigated for their effects in improving meat quality in pork, chicken, and lamb. Herbal additives have been demonstrated to improve the flavor and health effects of meats as well as egg and milk quality.
There has been a growing interest in supplementing animal feeds with plant extracts or raw materials to boost the nutritional value and health benefit of meat and meat products. Supplementation of Chinese herb feed additives in a pig diet improved meat quality, including increased loin eye area, decreased drip loss, and increased muscle protein and amino acid contents (Lin et al., 2020). Oregano essential oil and sweet chestnut (Castanea sativa Mill.) wood extract (Ranucci et al., 2014), fish wort (Houttuynia cordata) and dandelion (Taraxacum officinale) extracts (Yan et al., 2011b), and Coptis chinensis extract (Zhou et al., 2013a) had positive effects on pig meat quality, which were attributed to the antioxidant properties of these feed additives. Phytogenic feed additives can improve the meat quality of broilers. Several commercial phytobiotics have been shown to increase slaughter weight and breast weight and decrease fat pad size and L∗ (lightness) and b∗ (yellowness) in broiler chickens, possibly via the modulation of stress- and antioxidant-related pathways (Orlowski et al., 2018). In ruminants, thyme (Nieto et al., 2010), rosemary (Salvia rosmarinus) leaves (Nieto et al., 2011), and licorice extract (Zhang et al., 2015) decreased lipid oxidation and improved lamb meat quality parameters. Furthermore, herbs can alleviate the negative effects of transport stress on meat quality. Dietary supplementation with oregano essential oil or quercetin effectively reduced drip loss due to transportation stress (Zou et al., 2017) and other meat traits, including cold carcass weight, dressing percentage, pH, and color (Zou et al., 2016).
Phytogenic feed additives have also been considered for improving meat flavor and health effects. Orlowski et al. (2018) evaluated the effects of several phytogenic supplements on the flavor of broiler breast fillets. Most of them induced a “green herb flavor” in breast meat as compared with a control diet. Supplementation of fermented and unfermented Yupingfeng composed of Huangqi (Radix Astragali), Baizhu (Rhizoma Atractylodis Macrocephalae), and FangFeng (Radix Saposhnikoviae) in a Qingyuan black goat diet increased the concentrations of palmitic acid, octadecanoic acid, and arachidonic acid in the meat, which are related to meat flavor (Yang et al., 2021). Amino acids not only provide key nutritional value, they also contribute to meat taste and flavor (Zhao et al., 2016). Yizhi (Alpiniae oxyphyllae) supplementation in a Jiaji duck diet improved the meat nutritional profile and flavor by increasing total, essential, and flavor amino acid levels in the breast muscle (Ji et al., 2022). Phytogenic feed additives may modulate broiler muscle flavor and acceptability by altering the intestinal microbiota composition or by modulating stress- and antioxidant-related pathways (Shirzadegan and Falahpour, 2014; Orlowski et al., 2018; Ji et al., 2022). Consumers pay increasing attention to the fatty acid composition of meat because of its health implications. There has been a continuing effort to manipulate the fatty acid composition of meat through feed regimens. Plantain (Plantago lanceolata L.) and/or garlic (A. sativum) leaf supplementation in a sheep diet lowered caul fat, pelvic fat, mutton ether extract, and saturated fatty acid levels, while increasing polyunsaturated fatty acid levels, indicating their beneficial effects for lean and healthy mutton meat production (Redoy et al., 2020). Herbal additives can also affect the quality of processed meat. The addition of herb and spice combinations in pork sausages during processing improved their aroma, taste, and sensory qualities (Huynh et al., 2020). Most authors mentioned that the taste improvement was highly correlated with the antioxidant potential of the plants used.
Phytogenic feed additives affect egg quality; however, research results are variable. Supplementation of YGF251 dose-dependently positively affected egg quality parameters, including egg strength, eggshell thickness, and Haugh unit, likely by enhancing the IGF-1 levels in laying hens (Dang et al., 2021). Supplementation of a yucca (Yucca schidigera) extract in a laying hen diet at 100 mg/kg significantly improved egg number and egg mass, and shell thickness increased quadratically with increasing extract amount (Alagawany et al., 2016). Lonicera confusa and Radix Astragali extract supplementation during the late laying period did not affect egg production, egg weight, and the feed conversion ratio in laying hens when compared with a basal diet, but it did improve yolk color and sensory quality (Xie et al., 2019). Yucca supplementation did not improve egg quality parameters in Hy-Line Brown laying hens as compared to a basal diet and antibiotic control (Wang and Kim, 2011). Dietary supplementation with Lavandula angustifolia and/or Mentha spicata essential oils had no beneficial effects on egg weight, egg index, yolk index, Haugh unit, egg shell weight, and egg shell thickness when compared with a basal diet (Torki et al., 2020). The reasons for the differences in the effects on egg quality have to be analyzed, and more studies on the specific effects of herbal additives on egg quality characteristics are needed. Health-conscious consumers desire healthy eggs with lower cholesterol levels and higher yolk phospholipid levels. Supplementation of a 1.0% Chinese herbal mixture composed of 70% pine needle and 30% Artemisia annua in the layer diet may be a feasible means of producing eggs with lower cholesterol and higher egg yolk phospholipid contents (Li et al., 2016). Black cumin, garlic, and turmeric lowered egg yolk cholesterol and triglyceride levels when supplemented in the diet of White Leghorn hens during the first phase of the laying period (Singh et al., 2019).
Milk yield, composition, and fatty acid profiles can be altered through phytogenic supplementation. Phytogenic feed additives increased milk production and total solid, protein, lactose, and fat contents in milk of lactating Friesian cows, probably via modulating ruminal fermentation and enhancing feed utilization (Kholif et al., 2020). The addition of shatavari (Asparagus racemosus) extract in milk altered its functional properties and stability, including decreased pH, rennet coagulation time, and L∗ value, and increased acidity, viscosity, heat stability, and a∗ (redness) and b∗ values, indicating the potential of herb-fortified functional dairy foods (Veena et al., 2015). The demand for healthy dairy products, including unsaturated fatty acids and conjugated linoleic acids, which are considered to have a preventive effect on cardiovascular diseases, is increasing. Supplementation of mustard and cumin seeds or cumin seed extract in a goat diet lowered milk saturated fatty acids and increased polyunsaturated fatty acid and total conjugated linoleic acid contents (Miri et al., 2013; Morsy et al., 2018). Cumin secondary metabolites may exert antimicrobial effects on biohydrogenating bacterial species in the rumen, resulting in the accumulation of biohydrogenation intermediates and increases in milk polyunsaturated fatty-acid and conjugated linoleic-acid contents (Morsy et al., 2018).
4.4. Improvement of livestock reproduction
Reproductive efficiency is the major determinant of economic animal production systems. Herbs have been used to treat human reproductive diseases for centuries, and even today, phytogenic herbal additives are used to effectively treat reproductive disorders in livestock animals (Kuralkar and Kuralkar, 2021; Swelum et al., 2021).
Reproductive disorders in female animals negatively affect their production potential. Numerous herbs have remarkable medicinal properties for the treatment of various reproductive disorders, particularly in cattle (Kumar et al., 2018). Tripathi (2015) found that several traditional phytogenic compounds exert beneficial effects in inducing uterine contractions, restoring the oestrus cycle, preventing postpartum infections, and synchronizing the release of reproductive hormones in buffalo heifers. Murraya koenigii alone or in combination with Aegle marmelos improved fertility in delayed pubertal buffalo heifers by increasing the ovulation and conception rates (Baitule et al., 2016). Yucca powder supplementation increased the conception rate in Zaraibi dairy goats (Khalifa et al., 2014). Tinospora cordifolia plus Cassia fistula bark and Artocarpus heterophyllus L. leaves alleviated postpartum anestrus in cattle (Talukdar et al., 2015). Herbal extracts have shown curative efficacy in treating subclinical endometritis, a common cause of subfertility and infertility in highly productive dairy cattle. Intrauterine administration of a hydromethanolic extract of Achyranthes aspera or Azadirachta indica showed good therapeutic efficacy in postpartum cows and enhanced the first service conception rate (Nikhade et al., 2019). Placental retention is a common problem during the puerperium, mainly in dairy cattle and buffalo. Traditional herbs that stimulate uterine contraction and placenta expulsion have been suggested for the treatment of placental retention and to help reduce the incidence of animal reproductive disorders (Perumal et al., 2013; Saxena et al., 2019; Kuralkar and Kuralkar, 2021). Sheng Hua Tang, a classical herbal formula consisting of Radix Angelicae Sinensis, Rhizoma Ligustici, Semen Persicae, Rhizoma Zingiberis, and Radix Glycyrrhizae, reduced the incidence of retained placenta and improved reproductive performance in Holstein dairy cows (Cui et al., 2014). Whether herbal extracts or formulas can be developed as a general preventive approach in postpartum cows is worth further investigation.
In male livestock animals, herbs and plants can improve semen production, ejaculation, sperm count, semen volume, and sexual health. A herbal extract of Eurycoma longifolia, Tribulus terrestris, and Leuzea carthamoides improved sex libido and semen quality in boars (Frydrychová et al., 2011). Oral administration of a Moringa oleifera leaf extract in rams increased the semen volume and sperm concentration, motility, viability, and membrane integrity (El-Desoky et al., 2017). Supplementation of T. cordifolia in Muzzafarnagari rams had no effect on physico-morphological and biochemical semen attributes and serum testosterone concentrations (Jayaganthan et al., 2013). However, cholesterol, superoxide dismutase (SOD), and catalase (CAT) levels in seminal plasma were increased, suggesting that T. cordifolia supplementation may protect spermatozoa during cryopreservation and thus may enhance fertility in livestock animals. The positive effects of herbal extracts on male reproductive functions may be owing to the suppression of oxidative stress (Yun et al., 2016; El-Desoky et al., 2017).
Recent studies on the effects of herbs and plants on sperm quality have mainly focused on semen preservation (e.g., through refrigeration and cryopreservation). Semen preservation is a crucial factor for the success of assisted reproductive technology in livestock. Ros-Santaella and Pintus (2021) systematically reviewed studies on the use of extracts and active substances from 45 plant species belonging to 28 families as alternative additives for sperm preservation in 13 animal species. More than half of the studies were in pigs, sheep, and cattle. They concluded that many plant extracts can be used to effectively improve sperm viability and motility and to avoid membrane damage, thus maintaining sperm function during semen storage. The protective effect on sperm preservation likely is mediated by their antioxidant activity and through the enhancement of antioxidant enzyme activity (Carrera-Chávez et al., 2020; Jofré et al., 2019; Sobeh et al., 2017). Moreover, some natural compounds have antimicrobial properties that can be exploited to prevent the deleterious effects of pathogens on the liquid phase of refrigerated swine seminal doses (Elmi et al., 2019). The use of plant extracts as preservatives for semen storage is promising and is expected to develop into a natural and efficient sperm preservation strategy.
4.5. Emission reduction
Ruminal and intestinal fermentation results in a waste of feed energy and the production of methane and ammonia, which contribute to the greenhouse effect and environmental pollution. To improve energy use and address concerns about environmental effects, ways to reduce methane and ammonia emissions are being researched. Herbal products have great potential in this regard. Certain plant extracts and active compounds can improve ruminal and intestinal fermentation and function and suppress deamination and methanogenesis to reduce ammonia and methane production (Groot et al., 2011; Sun et al., 2021).
Methane is a major greenhouse gas mainly produced by ruminants during ruminal fermentation, which accounts for 8% to 14% of the total feed energy consumption. Tannins, saponins, essential oils, polyphenols, and resveratrol alter ruminal fermentation and suppress methanogenesis and thus, methane production, in vitro (Belanche et al., 2016; Cieslak et al., 2016; Jafari et al., 2020; Ma et al., 2020; Zhou et al., 2020). The in vivo effects of plant compounds and products such as tannins, mangosteen peel powder, and green tea (Camellia sinensis) extract as feed additives on methane emissions have been confirmed in different ruminants (Bhatta et al., 2013; Kolling et al., 2018; Wanapat et al., 2014). Phytogenic feed additives reduce methane emission via various mechanisms. Methane production is highly associated with methanogens, which convert primary fermentation products into methane. Supplementation with ivy fruit saponins (Belanche et al., 2016), condensed tannins (Jayanegara et al., 2015), a mixture of patchouli (Pogostemon cablin), Atractylodes rhizome, Amur cork tree, and Cypsum (Wang et al., 2019), and mulberry leaf flavonoids (Ma et al., 2017) significantly decreased the amount and activity of methanogens and methane production, suggesting that the reduction effect of phytogenic compounds on methane production can be partially attributed to a decrease in methanogen activity. A commercial phytogenic feed additive, Mootral (garlic and citrus extracts), significantly reduced the amount of Methanobacteriaceae, a major methane-producing family, thus reducing methane production, in vitro (Ahmed et al., 2021). Another major emission-reducing mechanism is the defaunation of ruminal protozoa. The hydrogenosomes of rumen protozoa are associated with the production of H2, which is converted to methane by methanogens via the hydrogenotrophic pathway (Belanche et al., 2014). The positive correlation between ruminal protozoa and methane production has been demonstrated (Hassan et al., 2020a). Corymbia citriodora leaf extract (Hassan et al., 2020b), licorice extract (Ramos-Morales et al., 2018), mulberry leaf flavonoids (Ma et al., 2017), and moringa seed oil (Ebeid et al., 2020) reduce the number of ruminal protozoa as well as methane production.
Long-term exposure to high concentrations of ammonia can cause severe toxicity stress, especially in young animals. Additionally, ammonia is an atmospheric and environmental pollutant. Thus, suppressing ammonia production is essential for improving animal health and environmental quality. Yucca herb or extract reduced fecal odor and ammonia emissions in porcine and poultry farms (Chen et al., 2021; Saeed et al., 2018), likely by lowering the activity of intestinal urease and urea cycle enzymes. Saponins are the main active component of yucca that contributes to ammonia emission reduction and are used in the feed industry to overcome ammonia emission and odor (Saeed et al., 2018). Certain other herbs and extracts are also effective in reducing ammonia emissions. Supplementation of YGF251 in the diet of laying hens dose-dependently decreased excreta ammonia emission, possibly by improving feed protein utilization (Dang et al., 2021). In growing pigs, dietary supplementation of YGF251 was not effective in improving growth performance and nutrient digestibility nor in decreasing fecal ammonia and hydrogen sulfide emissions (Lei et al., 2019a). Dietary supplementation with essential oils from cinnamaldehyde, thymol, and carvacrol in growing pigs decreased the emissions of ammonia and total fecal nitrogen, likely by inhibiting microbial protease and urease activities (Hsu et al., 2021). Essential oils (including thymol, eugenol, and piperine) interacted with proteases, improving total-tract nitrogen retention and decreasing ammonia emission in growing broiler chicks (Park and Kim, 2018). Ammonia and odor emissions negatively affect animal production and farm workers; therefore, suppressing ammonia production using plant products is important to maintain and improve animal performance and animal and human health.
4.6. Toxicity reduction
Accumulated heavy metals, antibiotics, and mycotoxins can cause toxicity, which is a critical health issue. Some herbal plants and their extracts can decrease the deleterious effects of toxic substances in livestock animals, including heavy metal poisoning, antibiotic toxicity, pesticide toxicity, and mycotoxin toxicity.
Exposure to lead (Pb) at high levels has a wide range of deleterious effects on multiple organs in animals, affecting feed consumption and the growth rate (Taha et al., 2019). Garlic, bitter cola, coriander, and yucca protected against Pb toxicity and improved the reduction in growth performance and alterations in blood parameter values caused by Pb toxicity (Alagawany et al., 2018; Hossain et al., 2014; Osemwegie et al., 2017; Téllez-López et al., 2017). The restorative properties of herbal plants on Pb toxicity have been attributed to their antioxidant activities and/or chelating efficacy, because the main mechanism of Pb poisoning is the induction of oxidative stress (Abd El-Hack et al., 2019). Antibiotic and pesticide toxicity can also be alleviated by certain plants and their extracts. Turmeric protected the bursa of Fabricius against salinomycin toxicity in chickens (Sayrafi et al., 2017). Calycosin alleviated cardiotoxicity induced by doxorubicin in vitro (H9c2 cells) and in vivo (male Kunming mice) by reducing oxidative stress via the sirtuin 1-NOD-like receptor protein 3 (NLRP3) pathway (Zhai et al., 2020). Dietary inclusion of a Thymus vulgaris oil extract protected catfish against thiamethoxam-induced toxicity, likely partially owing to its anti-inflammatory, antioxidant, antiapoptotic, and immune-stimulatory activities (El Euony et al., 2020).
Mycotoxins are common feed contaminants. Mycotoxin exposure can affect animal health and performance, lower weight gain, productivity, and immune responses, and even lead to death. Licorice extract alleviated liver damage caused by aflatoxin B1 in broiler chickens by decreasing alkaline phosphatase, aspartate aminotransferase, and alanine transaminase activities as well as the breast meat malondialdehyde (MDA) concentration (Rashidi et al., 2020). Curcumin alleviated the adverse effects of aflatoxins, likely by inhibiting cytochrome p450 isozymes, in chicken liver (Zhang et al., 2016). Some other toxicities can also be mitigated by herbs. For example, polysaccharide from Dendrobium nobile alleviated ethanol-induced toxicity (Zhang et al., 2018), and andrographolide alleviated CCl4-induced toxicity (Krithika et al., 2013). However, these forms of toxicity are rarely observed in animals, and few studies are available.
5. Modes of action of phytogenic feed additives
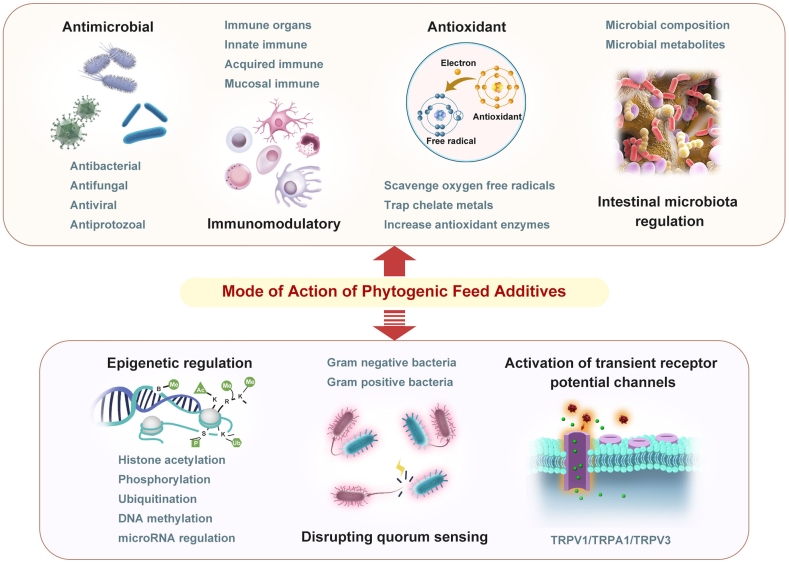
Exploring the potential action mechanisms of phytogenic products is complicated by their complex composition. Different active components have different functional properties and may act on different targets and play different roles via multiple pathways. Phytogenic additives can exert beneficial effects by directly suppressing the proliferation of pathogens, regulating intestinal microbial composition, enhancing immune functions, and alleviating oxidative stress, thus improving phenotypic traits, such as growth and production performance, and physical health (Fig. 2). Some molecular mechanisms of phytogenic additives, such as epigenetic regulation, transient receptor potential channel activation, and quorum sensing disruption, have been gradually revealed by molecular biology and cell biology studies in recent years (Fig. 2). However, the mechanisms of numerous plant-derived additives in livestock animals are still being explored.
Fig. 2.
Potential modes of action of phytogenic feed additives.
5.1. Antimicrobial activities
Herbs and plant extracts exhibit broad-spectrum antibacterial activities against both gram-negative and gram-positive bacteria (Mahfuz et al., 2021; O'Bryan et al., 2015). In vitro, condensed and hydrolyzable tannin-rich extracts showed strong antimicrobial activity against Campylobacter jejuni (Anderson et al., 2012), and ethanolic cinnamon extract against Salmonella aureus (Bonilla and Sobral, 2017). Brachyspira intermedia, a poultry pathogen, and Brachyspira hyodysenteriae, a swine pathogen, were highly sensitive to cinnamaldehyde in vitro (Verlinden et al., 2013; Vande Maele et al., 2016). Numerous studies have reported on the in vivo antimicrobial effects of phytogenic additives in animal feeds, particularly the inhibition of intestinal pathogens, and their health-promoting effects. Salmonella infection is an ongoing issue in poultry farming and causes extensive economic losses. Cinnamaldehyde feed supplementation effectively reduced Salmonella colonization, spread, and egg contamination in layer chickens and broiler chickens (Upadhyaya et al., 2013, 2015). In vivo studies in broilers have demonstrated the antimicrobial efficacy of quercetin in inhibiting the growth of bacteria such as Salmonella enterica serotype Typhimurium, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa in the intestine (Iqbal et al., 2020). E. coli and Salmonella typhimurium are frequently isolated in pig farms and cause intestinal diseases in piglets. Dietary resveratrol significantly reduced fecal Salmonella and E. coli counts and reversed the adverse effects of weaning stress in piglets challenged with these two pathogens (Ahmed et al., 2013). As enteric diseases caused by intestinal pathogen infection adversely affect animal growth and production performance, phytogenic feed additives may play serve as antibiotic alternatives to reduce the use of antimicrobials in animal production.
Another implication of the antimicrobial action of phytogenic feed additives lies in improving the microbial hygiene of animal carcass and meat products (Friedman, 2017). Alali et al. (2013) reported an essential oil blend (carvacrol, thymol, eucalyptol, and lemon) that may control Salmonella Heidelberg contamination in crops of broilers and reduce carcass cross-contamination. Supplementation of quaternary benzo[c]phenanthridine alkaloids in an herbal extract in finishing pigs reduced Salmonella shedding during transportation and positively impacted pork safety (Artuso-Ponte et al., 2015). However, data on the possible efficacy of certain phytogenic feed additives in improving carcass hygiene are too limited to draw a definite conclusion.
Because of the variety of plants used in plant extracts and phytogenic feed additives, they contain numerous active ingredients and thus, may have various antibacterial mechanisms. Further, individual extract ingredients can have a single target or multiple targets, rendering their antibacterial mechanisms more comprehensive. Membrane perforation and membrane adhesion are considered to be the primary antibacterial action modes for the majority of herbal feed additives (Kumar et al., 2014). Plant extracts and their active ingredients are generally hydrophobic and damage the phospholipid bilayer structures of cell membranes and mitochondria, increasing membrane permeability, leading to the leakage of ions and other substances, collapse of proton pumps, and depletion of the ATP pool (O'Bryan et al., 2015; Rossi et al., 2020). This leads to the disruption of cellular processes, resulting in the loss of chemical osmotic control and bacterial death (O'Bryan et al., 2015). Additionally, active compounds may increase bacterial nuclear envelope permeability and inhibit DNA topoisomerase or RNA polymerase activities involved in DNA and RNA synthesis, leading to cell damage (Mahfuz et al., 2021). Other possible mechanisms of bacteriostasis are interference with membrane proteins and intracellular targets, inhibition of nutrient absorption and enzyme activity, and disruption of protein, lipid, and polysaccharide synthesis (Rossi et al., 2020).
Besides antibacterial activity, some phytogenic feed additives exhibit antiviral, antifungal, and antiprotozoal activities. Caesalpinia sappan bioactive compounds inhibited porcine reproductive and respiratory syndrome virus replication in a MARC-145 cell monolayer and therefore have antiviral activity potential (Arjin et al., 2021). Puerarin inhibited porcine epidemic diarrhea virus replication and the expression of several cytokines in vitro and in vivo and attenuated the growth performance reduction in infected piglets (Wu et al., 2020). The antifungal activities of plant ingredients have also been demonstrated (Atiphasaworn et al., 2017; Oubihi et al., 2020). Acetone and cold-water extracts of Alchornea laxiflora, Ficus exasperata, Morinda lucida, Jatropha gossypiifolia, Ocimum gratissimum, and Acalypha wilkesiana exhibited different levels of antifungal activity, and A. laxiflora extract showed good overall antifungal activity against all organisms tested, warranting further study in an in vivo chicken feed trial (Olawuwo et al., 2022). Some phytogenic feed additives act against coccidiosis (coccidian infection). Curcumin (diferuloylmethane) showed a strong inhibitory effect on Eimeria tenella sporozoites in vitro by inducing morphological changes and reducing sporozoite viability and infectivity (Khalafalla et al., 2011). The inclusion of curcumin (Yadav et al., 2020), cinnamaldehyde and Echinacea purpurea plant extract (Orengo et al., 2012), or an herbal formula (Pop et al., 2019) in broiler diet decreased gross coccidiosis lesion scores and reduced Eimeria aceryulina oocyst infection, without negatively affecting production parameters. However, extract from E. purpurea, betaine (Betain), curcumin, and carvacrol failed to reduce coccidiosis lesion scores and oocyst shedding in broilers (Peek et al., 2013).
5.2. Immunomodulatory activity
The immune response is a specific physiological response of the body to maintain homeostasis by recognizing and eliminating antigenic foreign bodies. Dietary immunomodulation is an important strategy to enhance immune system integrity in farm animals to ensure health and productivity in the absence of antibiotics (Kumar et al., 2011). Traditional herbs and natural feed additives have various immunomodulatory effects, including improving the activity of lymphocytes, macrophages, and natural killer cells, thus modulating cytokine secretion, histamine release, Ig secretion, class switching, cellular co-receptor expression, lymphocyte expression, phagocytosis, and interferon synthesis (Kiczorowska et al., 2017; Mahima Rahal et al., 2012).
The immunomodulatory effects of many phytogenic feed additives and herbal ingredients have been evaluated in vitro. Extracts of dandelion, mustard (Brassica juncea), safflower (Carthamus tinctorius), thistle (Silybum marianum), turmeric (Curcuma longa), reishi mushroom (Ganoderma lucidum), and shiitake mushroom (Lentinus edodes) have been evaluated using chicken lymphocytes and macrophages, and most extracts exhibited immune-activating properties, including tumor cell growth inhibition and innate immunity stimulation (Lee et al., 2007, 2010). The commercial plant-derived feed additive Digestarom DC significantly inhibited nuclear factor kappa beta (NF-κB) activation upon tumor necrosis factor α (TNFα) treatment and significantly reduced the gene expression of IL-6, IL-8, and C-C motif chemokine ligand 2, and the release of IL-6 in porcine intestinal epithelial cells (Kaschubek et al., 2018). Several active ingredients of traditional Chinese medicines, including deoxyshikonin, isorhapontigenin, and calycosin (Wang et al., 2021a), and phytochemicals such as cynaroside, sanguinarine, and tamarixetin (Deng et al., 2018; Lyu et al., 2018) have been found to induce endogenous host defense peptide expression and synthesis in pigs and broilers and thus may contribute to immune modulation in these animals.
In broilers, dietary supplementation of a phytogenic blend consisting of carvacrol, cinnamaldehyde, and capsicum oleoresin reduced inflammation levels under nonchallenging conditions as demonstrated by downregulated interferon-γ and IL-6 cytokine levels (Pirgozliev et al., 2019). In broilers challenged by necrotic enteritis or with lipopolysaccharide (LPS), a mixture of capsicum oleoresin and turmeric oleoresin (Lee et al., 2013a) and Allium hookeri (Lee et al., 2017) stimulated the immune system to fight pathogen infection and prevent intestinal dysbiosis. Supplementation of A. annua (Song et al., 2018), thyme powder (Hassan and Awad, 2017) or oregano essential oil (Mohiti-Asli and Ghanaatparast-Rashti, 2017) improved Ig levels in broilers. Several phytogenic feed additives promote the immune-stimulating response to some chicken virus vaccines, such as Newcastle disease or infectious bursal disease vaccines (El-Shall et al., 2020). The immune-regulatory effects of phytogenic feed additives have also been reported in swine. Phytogenic feed additives rich in the alkaloid sanguinarine or polyphenols improved production performance and had a regulatory effect on pro- and anti-inflammatory activities in weaning piglets (Fiesel et al., 2014; Kantas et al., 2015). In LPS-challenged weaning piglets, dietary supplementation with cinnamon oil ameliorated LPS-induced mucosal barrier dysfunction and mucosal damage in the small intestine by suppressing the inflammatory response (Wang et al., 2015a). Supplementation of a ginger extract in a sow diet enhanced the immune function of piglets (Lee et al., 2013b), likely by enhancing Ig levels in the colostrum. This demonstrated the beneficial immune effects of phytogenic feed additives on progeny via mother-offspring transmission. In ruminants, phytogenic feed additives rich in tannins, saponins, and essential oils positively affected the immune status of buffalo calves as reflected by decreased cortisol concentrations and enhanced cellular and humoral immune responses (Lakhani et al., 2018). Murrah buffaloes supplemented with a blend of poplar and eucalyptus leaves exhibited enhanced cell-mediated and humoral immune responses (Dey et al., 2021). In lactating Holstein cows, supplementation of a fermented Chinese herbal medicine mixture enhanced immune functions, including the blood lymphocyte apoptosis rate, serum biochemical parameters, and gene expression in lymphocytes, under heat stress (Shan et al., 2018). In sheep, plantain and/or garlic leaf supplementation significantly increased serum IgA, IgG1, IgG2, and IgM concentrations (Redoy et al., 2020).
Dietary immunomodulation is an important means to strengthen the immune system and improve the productivity of farm animals raised in the absence of antibiotics. Phytogenic feed additives can modulate animal immune responses through different pathways owing to their complex active ingredients, mainly via enhancing the secretion of macrophages, T and B lymphocytes, and dendritic cells and regulating immune cytokine production by binding with specific receptors on immune cell membranes, thus enhancing the immune response and eliminating inflammation. The modulation of cytokine expression is a key mechanism by which phytogenic feed additives regulate adaptive and innate immunity. Treatment of LPS-challenged porcine peripheral blood mononuclear cells with an Agrimonia procera extract significantly decreased IL1B and TNF mRNA levels and TNFα release, which may be due to the inhibition of transcription factor NF-κB activity (Gräber et al., 2014). NF-κB is a master regulator of genes involved in immune and inflammatory responses, particularly cytokines. Yang et al. (2015a) reviewed numerous phytochemicals that reduce or inhibit NF-κB activation, thus improving animal immune function and health.
5.3. Antioxidant activity
In many herbs and spices used in animal nutrition, antioxidation is the most important biological activity. Oxidative stress causes numerous health problems and diseases in animals. Antioxidants help relieve oxidative stress by reducing free-radical intermediates and are therefore effective agents to prevent diseases. Certain herbal additives have been suggested to be useful as antioxidants in animal feeds to protect animals from oxidative stress at specific physiological stages or in certain environments.
In animal nutrition, antioxidants are appreciated to act mainly as health stabilizers. The administration of carvacrol or thymol enhanced the antioxidant capacity in broilers as observed by increased antioxidant enzyme activity and reduced lipid oxidation and MDA levels, improving production performance (average daily gain and feed efficiency) (Hashemipour et al., 2013; Hoffman-Pennesi and Wu, 2010). Thyme essential oil improved intestinal function and epithelial integrity in broilers by decreasing the MDA content in the duodenal mucosa via its antioxidant activity (Placha et al., 2014). The antioxidant capacities of quercetin (Sohaib et al., 2015), resveratrol (Sridhar et al., 2015), allicin (Wang et al., 2014), and curcumin (Wang and Zhang, 2014) and their associated benefits for body health and production in broilers have also been reported. Compared with the number of studies in broilers, studies on improving production and health through the use of plant additives to enhance antioxidant capacity in pigs and ruminants are limited in number. Resveratrol supplementation in piglets attenuated intestinal barrier dysfunction induced by oxidative stress by protecting intestinal mitochondrial functionality, decreasing reactive oxygen species production, and increasing the mitochondrial DNA content (Cao et al., 2019). Supplementation of a blend of phytogenic additives improved the reproductive performance of sows and intestinal health in suckling piglets, which was partly attributed to its antioxidant activity and antimicrobial action (Reyes-Camacho et al., 2020). Supplementation of mulberry leaf flavonoids in Murrah buffaloes improved the antioxidant capacity by decreasing MDA concentrations and increasing serum heat shock protein, glutathione peroxidase, catalase, and insulin concentrations, thus enhancing lactation performance and alleviating heat-induced oxidative stress during summer (Li et al., 2020a). The recently developed phytogenic additive mixture Aromix improved feed utilization, ruminal fermentation, and milk production in lactating cows by improving their antioxidant status (Kholif et al., 2020). As mentioned above, the antioxidant properties of plant herbal additives are mainly reflected in improved meat quality. The potential of plant-based feed additives to improve the oxidative stability of animal products has been widely demonstrated for pork, poultry, and lamb meat (Cherian et al., 2013; Lin et al., 2020; Zhang et al., 2015).
The antioxidant activity of plant additives is highly correlated with their phenolic constituents, including phenolic acids, phenolic diterpenes, flavonoids, and volatile oils, which contribute the most to the antioxidant activity because they are able to donate hydrogens and electrons to free radicals to interrupt oxidative chain reactions in tissues. Generally, these plant extracts scavenge excess oxygen free radicals and chelate metals, depending on the location and number of hydroxyl groups and their capacity to donate hydrogens to metals to inhibit their pro-oxidant activity (Brewer, 2011; de Castilho et al., 2018; Manuelian et al., 2021). Further, plant-derived antioxidants can bind to host cell-surface receptors and enhance antioxidant enzyme secretion at the transcriptional and translational levels, thus enhancing the antioxidant capacity of the body and relieving oxidative stress. Pretreatment with resveratrol significantly increased cell viability and SOD, CAT, glutathione peroxidase activities and decreased intracellular reactive oxygen species levels and apoptosis in porcine IPEC-J2 cells under H2O2-induced oxidative stress in vitro via PI3K/Akt-mediated Nrf2 signaling (Zhuang et al., 2019). In vivo, supplementation of piceatannol, puerarin, or Eucommia ulmoides flavones increased the levels of SOD, glutathione peroxidase, CAT, heme oxygenase 1, NAD(P)H:quinone oxidoreductase 1, and glutamate-cysteine ligase catalytic subunit in the small intestinal mucosa, serum, and liver of diquat-challenged piglets, also via Nrf2 signaling (Jia et al., 2020; Li et al., 2020b; Xiao et al., 2019; Yuan et al., 2017). Although the antioxidant activity of many phytogenic additives has been widely demonstrated, it remains unclear whether newly developed phytogenic antioxidants can replace current antioxidants used in animal feeds (e.g., α-tocopherols), and their exact antioxidant mechanisms and roles in animal health and production require further investigation.
5.4. Intestinal microbiota regulation
Intestinal microbes modulate host physiological activities and therefore, any change in the intestinal microbiota affects animal physiology. Many plant ingredients are not absorbed directly by the host, but are first transformed by the intestinal microbiota. Increasing evidence indicates that plant-derived additives have a regulatory effect on the intestinal microbiota composition, which may contribute to the conversion of plant ingredients into active metabolites (Lin et al., 2021). On the one hand, phytogenic additives can directly affect microbial abundance and diversity, and on the other hand, they can be converted by the intestinal microbiota into bioavailable, bioactive, or even toxic metabolites that have various biological roles. Both the regulated microbiota and transformed metabolites may contribute to health maintenance and disease suppression.
Herbal feed additives and their bioactive compounds contribute to the development and maintenance of the intestinal microbiota in livestock animals, thus exerting positive health effects. Supplementation of an extract from green tea leaves and pomegranate (Punica granatum) rinds in a broiler diet strongly promoted the relative abundance of lactic acid bacteria (Perricone et al., 2020), which positively affect intestinal health and are correlated with improved performance and health. Inclusion of a phytogenic mixture (garlic, cinnamon, peppermint, green tea, black cumin) (Rashid et al., 2020), polyphenol-rich products (Viveros et al., 2011), or thymol and carvacrol (Yin et al., 2017) in a broiler diet increased bacterial diversity and expanded the populations of Lactobacillus, Enterococcus, and Clostridium, while lowering the counts of Clostridium perfringens, Campylobacter, and E. coli. In swine, dietary supplementation of enokitake mushrooms (Flammulina velutipes) or resveratrol and essential oils from medicinal plants increased the amounts of beneficial bacteria such as Bifidobacterium and Lactobacillus in the intestines and reduced the counts of pathogenic bacteria, including enterobacteria, Salmonella, and E. coli (Ahmed et al., 2013; Tlabela et al., 2015). Beneficial microbiota establishment in phytobiotics-fed piglets or chickens was associated with reduced intestinal damage, diarrhea, and necrotic enteritis (Cairo et al., 2018; Xu et al., 2021; Yin et al., 2017). In addition to balancing beneficial and harmful bacteria in the intestines, phytogenic feed additives play a beneficial role through microbiota-transformed active metabolites. Supplementation of a feed additive containing carvacrol increased cecal mucosa-associated levels of Clostridium clusters IV and XIVa (Paraskeuas and Mountzouris, 2019), which as butyrate-producing bacteria can produce butyrate (Lopetuso et al., 2013), thus contributing to a reduction in the shedding of pathogenic bacteria such as Salmonella spp. and E. coli (El-Saadony et al., 2022). Short-chain fatty acids (SCFA) are important metabolites produced by the intestinal microbiota. Supplementation of Gegen-Qinlian decoction, a Chinese herbal formula, in diarrheal piglets significantly increased the relative abundances of SCFA-producing bacteria, including Akkermansia, Bacteroides, Clostridium, Ruminococcus, and Phascolarctobacterium, which helped attenuate mucosal inflammation and diarrhea, through the production of SCFA (Liu et al., 2019). Furthermore, phytogenic feed additives can affect intestinal digestion and absorption by regulating bacteria with digestive enzyme activity. Dietary supplementation of oregano essential oil in laying hens increased Deinococcus, Bacillaceae, and Caulobacteriales in ileal digesta, which may have increased digestive enzyme activity, leading to improved feed utilization (Feng et al., 2021). Additionally, the intestinal microbiota contributes to the host immune system, nutrition, and metabolism (Shang et al., 2018). Therefore, the microbiota composition regulated by phytogenic feed additives may benefit immunity regulation, nutrient production, and host metabolism, thus improving animal health status.
In ruminants, phytogenic feed additives have been shown to modulate the ruminal microbiota and fermentation kinetics, leading to enhanced animal production and reduced methane and ammonia emissions. Supplementation of Murrah buffaloes with a phytogenic mixture substantially altered the ruminal bacteriome composition (i.e., it increased the abundances of Firmicutes, Proteobacteria, and Spirochaetes and decreased those of Bacteroidetes and Prevotella), which was positively associated with milk and fat yields (Hassan et al., 2020c). Supplementation of the Chinese traditional herb medicine Yu-Ping-Feng in Qingyuan black goats significantly increased the ruminal relative abundances of Ruminococcus and Alistipes, which are related to fiber degradation and SCFA production and likely contributed to the improved fatty acid profile of supplemented goat meat (Yang et al., 2021). Saponins, tannins, and essential oils have been widely used to manipulate rumen fermentation dynamics to mediate methane emissions from livestock, as comprehensively reviewed by Hassan et al. (2020a). Mootral, a commercial mixture of nine parts garlic powder to one part citrus powder, increased the abundance of propionate-producing families such as Prevotellaceae and Veillonellaceae, reducing certain hydrogen-producing bacteria and regulating the archaeal community by decreasing Methanobacteriaceae and increasing Methanomassiliicoccaceae, resulting in reduced methane production (Ahmed et al., 2021). Essential oils-cobalt (Lei et al., 2019b) and a traditional herbal medicine mixture (Wang et al., 2019) have been reported to modulate ruminal fermentation and ruminal bacterial and archaeal communities to lower methane and ammonia emissions.
5.5. Epigenetic regulation
Epigenetics refers to heritable changes in gene expression from causes other than genome sequence changes. It is the result of interactions between environmental factors and the genetic material and mainly involves histone acetylation, phosphorylation, ubiquitination, DNA methylation, and microRNA regulation (Katsnelson, 2010). Epigenetic modifications are essential in gene expression regulation and involved in various cellular processes, including differentiation, development, and tumorigenesis (Wilkinson and Gozani, 2017). In recent years, significant developments have been achieved in epigenomics studies of phytogenic medicines and active compounds (Guo et al., 2021; Hsieh et al., 2013), which has contributed to the unraveling of their mechanisms. Numerous herbal drugs, compounds, and formulae, including ginsenoside (Wang et al., 2021b), tanshinone (Li et al., 2019), curcumin (Wu et al., 2013), Yisui Shengxue granules (Cheng et al., 2019), and Jieduquyuziyin (Li et al., 2018), have been found to modulate DNA methylation levels. Astragaloside (Dou et al., 2021), curcumin (Wang et al., 2015b), the flavone chrysin (Ganai et al., 2021), and Acanthopanax senticosus (Wang et al., 2016) can act as histone deacetylase inhibitors to modulate gene expression, thus affecting cell proliferation, differentiation, metabolism, and programmed death. Most studies focused on human diseases and mouse models, and although researchers have recognized the importance of epigenetic modifications in livestock animals (Ma and Diao, 2015), epigenetic modulation of cellular bioprocesses by phytogenic herbs, extracts, and compounds in livestock animals is understudied.
5.6. Activation of transient receptor potential (TRP) channels
TRP channels are expressed throughout the digestive tract and play important physiological roles, including stimulus perception, inflammatory molecule production and secretion, migration, and phagocytosis, likely through the maintenance of intracellular calcium homeostasis (Clement et al., 2020; Feske et al., 2015; Khalil et al., 2018). Growing evidence suggests that herbal products can affect the chemosensory system by activating TRP channels, including TRPV1 (curcumin, allicin, capsaicin), TRPA1 (allicin, thymol, cinnamaldehyde), and TRPV3 (thymol, carvacrol, vanillin) (Rossi et al., 2020). The importance of TRP channel activation by phytogenic products and natural compounds in livestock animals has been reviewed by Rossi et al. (2020) and Firmino et al. (2021). However, few studies have directly demonstrated the beneficial effects of phytogenic active compounds via TRP channel activation in animals. In zebrafish, TRP channel activation has been demonstrated to modulate inflammatory processes through the activation of TRPV4/Ca2+/TAK1/NF-κB signaling (Galindo-Villegas et al., 2016). In fish mucosal tissues, phytogenic bioactive compounds can activate TRP channels, leading to an intracellular Ca2+ increase and TAK1/MAPK/NF-κB signaling pathway activation, modulating inflammatory processes in both immune and epithelial cells (Firmino et al., 2021). This suggests that TRP channel-mediated cellular activation may underlie the immunomodulatory effect of bioactive compounds in animals.
5.7. Disruption of quorum sensing
Quorum sensing refers to bacterial cell–cell communication through chemical signals. It plays an important role in the regulation of virulence factors in several enteric pathogens (Zhu et al., 2011). Therefore, disrupting quorum sensing has been suggested to be a promising tool for controlling enteric pathogens in food-producing animals (Yang et al., 2015a). The use of small molecules to disrupt quorum sensing-regulated virulence gene expression and limit the colonization of enteric pathogens has been extensively investigated (Galloway et al., 2011). Various phytogenic compounds have been shown to have anti-quorum sensing activity in vitro (Koh and Tham, 2011; Salini and Pandian, 2015; Zhou et al., 2013b). Nazzaro et al. (2013) reviewed 15 phytochemicals with anti-quorum sensing activity. To date, quorum sensing suppression has mostly been employed to control bacterial infections in aquaculture (Defoirdt et al., 2013). Pyrogallol derived from tea polyphenols show quorum sensing-inhibitory activity and protected brine shrimp (Artemia franciscana) and giant river prawn (Macrobrachium rosenbergii) larvae from Vibrio harveyi infection (Defoirdt et al., 2013). Cinnamaldehyde and its derivatives reportedly are effective against Vibrio spp. in Artemia shrimp and freshwater prawn, likely by disrupting protein-DNA interaction of the quorum sensing master regulator, LuxR (Brackman et al., 2008; Pande et al., 2013). Thiophenes and indoles show strong quorum sensing-disrupting activity and protected brine shrimp larvae against V. harveyi (Yang et al., 2015b; Zhang et al., 2022). Studies on the use of phytogenic compounds as quorum sensing inhibitors to control bacterial infections in other food-producing animals are lacking. However, a better understanding the potential of phytogenic compounds to inhibit quorum sensing is significant for identifying novel anti-quorum sensing agents to prevent bacterial infection in livestock animals.
6. Influencing factors and potential negative effects
The effects of phytogenic feed additives on livestock animal health and production range from neutral to beneficial for many different reasons. The composition and concentration of bioactive substances are the most important factors influencing the effects of plant-derived additives. For a single plant species, the plant parts used (e.g., root, bark, stem, leaf, flower, pollen, fruit, seeds), geographical location (altitude, soil), harvesting weather (sunlight, rainfall), and environmental factors (temperature, humidity) can lead to obvious differences in the derived products. Different origins and different sources may also affect the phytochemical profile of a phytogenic product and thus, its effect on livestock animals, even when using the same amount of plant material. Plant extraction methods, including steam distillation, maceration, cold pressing, and solvent extraction, also influence the composition and ingredient concentrations of a plant product. Further, batch-to-batch variability also influences the active component composition of phytogenic products (Pan et al., 2020). As the potential benefits of plant-derived feed additives can differ because of the large variability in product composition, it is difficult to compare their efficacy. Therefore, the truly active ingredient and its concentration in plants and plant-derived products have to be considered when using phytogenic products as antibiotic alternatives in animal production. Moreover, some herbal products are rapidly degraded in the stomach, which also affects their effectiveness. Appropriate release techniques, such as encapsulation or other coating technologies, should be considered when using these compounds as feed additives (El Asbahani et al., 2015; Sherry et al., 2013). Furthermore, the efficacy of phytogenic feed additives depends on the diet, growth stage, microbiota, and health status of an animal. Animal management and rearing conditions (age, genetics, challenges, environmental conditions) may also contribute to the inconsistency of results obtained with phytogenic feed additives (Abdelli et al., 2021; Yang et al., 2015a). To ensure the effectiveness of phytogenic feed additives in animals, several factors and conditions need to be considered to determine which product to use and how to use it.
Most phytogenic feed additives studies have shown positive effects in livestock animals. Nevertheless, several negative effects have been reported. Some herbs have drug-like actions that can interact with other dietary components. Some herbs contain prohibited substances such as salicylates, heroin, caffeine, and steroids (Williams and Lamprecht, 2008). These may have side effects or toxicities, ranging from mild to severe. The high doses of phytogenic feed additives generally cause more negative effects. To their positive effects, tannins may have antinutritional effects; high tannin levels in livestock animal feeds negatively affect appetite and protein digestibility because of their binding effect (Gilani et al., 2012; Faehnrich et al., 2016). In the context of sperm preservation, high concentrations of oregano, splinter bean (Entada abyssinica), rosemary, and pomegranate exerted negative effects on some sperm parameters or were even detrimental to sperm function (Ros-Santaella and Pintus, 2021). Therefore, attention has to be paid to the safety of phytogenic products and the conditions in which they exert biological effects when considering them as feed additives in livestock animals.
7. Conclusions and final remarks
This review focused on phytogenic feed additives as potential antibiotic alternatives in livestock animal production. As natural products, phytogenic products have several advantages over antibiotic agents, including cost-effectiveness and a lower likelihood of resistance development. Therefore, they are perceived as ideal substitutes for antibiotics, and research interest in and the application of phytogenic products are increasing. Phytogenic feed additives have well demonstrated beneficial effects on growth, performance, health, production, reproduction, and emission reduction and toxicity reduction in both monogastric animals and ruminants. While, given the process influence and batch-to-batch variation, more research on effect comparison between purified monomers and extracted products and evaluation of batch-to-batch differences are recommended in the future. Moreover, considering quality control, safety concerns, and inconsistent results, more in vitro and in vivo studies are required to elucidate their quality, safety, and effectiveness. Phytogenic feed additives belong to different chemical classes and generally are a mixture of bioactive compounds; therefore, various mechanisms of action or synergistic effects may underlie their beneficial effects. Several potential mechanisms of action, including antimicrobial, immunomodulatory, antioxidant, intestinal microbiota-regulatory activities, have been well established. The mechanisms of phytogenic products in epigenetic regulation, TRP channel activation, and quorum sensing are still in an early stage of investigation and therefore, not yet fully understood. Future research will have to explore more widely available, inexpensive, and effective phytogenic feed additives and comprehensively elucidate their biological mechanisms. Another aspect that needs to be highlighted here is the standardization of the evaluation systems for phytogenic feed additives, which is difficult to achieve because of the wide variety in extracts and formulas. However, standardization is essential for more efficient and conclusive evaluation of phytogenic feed additives for use in livestock animals. In summary, phytogenic feed additives have strong potential to replace antibiotics in animal feeds, but more studies are needed to expand the understanding and utilization of phytogenic feed additives to promote animal health and production.
Author contributions
Jing Wang: conceptualization, resources, writing - original draft preparation. Lufang Deng: resources and methodology. Meixia Chen: resources and visualization. Yuyan Che: resources and visualization. Lu Li: formal analysis and visualization. Longlong Zhu: formal analysis and visualization. Guoshun Chen: conceptualization, writing - review & editing. Tao Feng: conceptualization, supervision, writing - review & editing. The author(s) read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This work was supported by the National Key R&D Program of China (grant No. 2022YFD130040311), the National Natural Science Foundation of China (grant No. 31972576, 31972575), the Special Program on Science and Technology Innovation Capacity Building of BAAFS (grant No. KJCX 20240339), and the innovation and development project of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (grant No. XMS202303).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Guoshun Chen, Email: chengs@gsau.edu.cn.
Tao Feng, Email: fengtao@baafs.net.cn.
References
- Abd El-Hack M.E., Abdelnour S.A., Abd El-Moneim A.E.E., Arif M., Khafaga A., Shaheen H., et al. Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ Sci Pollut Res Int. 2019;26:23209–23218. doi: 10.1007/s11356-019-05805-8. [DOI] [PubMed] [Google Scholar]
- Abdel-Wareth A.A.A., Lohakare J.D. Effect of dietary supplementation of peppermint on performance, egg quality, and serum metabolic profile of Hy-Line Brown hens during the late laying period. Anim Feed Sci Technol. 2014;197:114–120. [Google Scholar]
- Abdelli N., Solà-Oriol D., Pérez J.F. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animals. 2021;11:3471. doi: 10.3390/ani11123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed E., Yano R., Fujimori M., Kand D., Hanada M., Nishida T., et al. Impacts of Mootral on methane production, rumen fermentation, and microbial community in an in vitro study. Front Vet Sci. 2021;7 doi: 10.3389/fvets.2020.623817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.T., Hossain M.E., Kim G.M., Hwang J.A., Ji H., Yang C.J. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian-Australas J Anim Sci. 2013;26:683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian-Tefaghi M., Ghasemi E., Khorvash M. Performance, rumen fermentation and blood metabolites of dairy calves fed starter mixtures supplemented with herbal plants, essential oils or monensin. J Anim Physiol Anim Nutr. 2018;102:630–638. doi: 10.1111/jpn.12842. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E., El-Kholy M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ Sci Pollut Res Int. 2016;23:6774–6782. doi: 10.1007/s11356-015-5919-z. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M., Farag M., Elnesr S., El-Kholy M., Saadeldin I., et al. Dietary supplementation of Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult Sci. 2018;97:3126–3137. doi: 10.3382/ps/pey186. [DOI] [PubMed] [Google Scholar]
- Alali W.Q., Hofacre C.L., Mathis G.F., Faltys G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poult Sci. 2013;92:836–841. doi: 10.3382/ps.2012-02783. [DOI] [PubMed] [Google Scholar]
- Ali A., Ponnampalam E.N., Pushpakumara G., Cottrell J.J., Suleria H.A.R., Dunshea F.R. Cinnamon: a natural feed additive for poultry health and production—a review. Animals. 2021;11:2026. doi: 10.3390/ani11072026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shuwaili M.A., Ibrahim I.E., Al-Bayati M.T. Effect of dietary herbal plants supplement in Turkey diet on performance and some blood biochemical parameters. Global J BioSci Biotechnol. 2015;4:153–157. [Google Scholar]
- Anderson R.C., Vodovnik M., Min B.R., Pinchak W.E., Krueger N.A., Harvey R.B., et al. Bactericidal effect of hydrolysable and condensed tannin extracts on Campylobacter jejuni in vitro. Folia Microbiol (Praha) 2012;57:253–258. doi: 10.1007/s12223-012-0119-4. [DOI] [PubMed] [Google Scholar]
- Ariza-Nieto C., Bandrick M., Baidoo S.K., Anil L., Molitor T.W., Hathaway M.R. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J Anim Sci. 2011;89:1079–1089. doi: 10.2527/jas.2010-3514. [DOI] [PubMed] [Google Scholar]
- Artuso-Ponte V., Moeller S., Rajala-Schultz P., Medardus J.J., Munyalo J., Lim K., et al. Supplementation with quaternary benzo(c)phenanthridine alkaloids decreased salivary cortisol and Salmonella shedding in pigs after transportation to the slaughterhouse. Foodborne Pathog Dis. 2015;12:891–897. doi: 10.1089/fpd.2015.2009. [DOI] [PubMed] [Google Scholar]
- Arjin C., Hongsibsong S., Pringproa K., Seel-Audom M., Ruksiriwanich W., Sutan K., et al. Effect of ethanolic caesalpinia sappan fraction on in vitro antiviral activity against porcine reproductive and respiratory syndrome virus. Vet Sci. 2021;8:106. doi: 10.3390/vetsci8060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiphasaworn P., Monggoot S., Pripdeevech P. Chemical composition, antibacterial and antifungal activities of Cinamomum bejolghota bark oil from Thailand. J Appl Pharm Sci. 2017;7:69–73. [Google Scholar]
- Baitule M.M., Gawande A.P., Kumar U., Sahatpure S.K., Patil M.S., Baitule M.M. Effect of Aegle marmelos and Murraya koenigii in treatment of delayed pubertal buffaloes heifers. Vet World. 2016;9:1375–1380. doi: 10.14202/vetworld.2016.1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanche A., de la Fuente G., Newbold C.J. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol Ecol. 2014;90:663–677. doi: 10.1111/1574-6941.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum M., Hosain M.M., Kim I.H. Effects of the plant extract YGF251 on growth performance, meat quality, relative organ weight, nutrient digestibility and blood profiles in broiler chickens: possible role of insulin-like growth factor 1. Vet Med. 2014;59:415–423. [Google Scholar]
- Belanche A., Pinloche E., Preskett D., Newbold C.J. Effects and mode of action of chitosan and ivy fruit saponins on the microbiome, fermentation and methanogenesis in the rumen simulation technique. FEMS Microbiol Ecol. 2016;92 doi: 10.1093/femsec/fiv160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta R., Enishi O., Yabumoto Y., Nonaka I., Takusari N., Higuchi K., et al. Methane reduction and energy partitioning in goats fed two concentration of tannin from Mimosa spp. J Agric Sci. 2013;151:119–128. [Google Scholar]
- Bonilla J., Sobral P.J.A. Antioxidant and antimicrobial properties of ethanolic extracts of guarana, boldo, rosemary and cinnamon. Braz J Food Technol. 2017;20 [Google Scholar]
- Brackman G., Defoirdt T., Miyamoto C., Bossier P., Callenbergh S.V. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8:149. doi: 10.1186/1471-2180-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo D., Pirgozliev V., Rose S.P. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J Anim Sci. 2014;92:1531–1536. doi: 10.2527/jas.2013-6244. [DOI] [PubMed] [Google Scholar]
- Brewer M.S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247. [Google Scholar]
- Cairo P.L.G., Gois F.D., Sbardella M., Silveira H., de Oliveira R.M., Allaman I.B., et al. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J Sci Food Agric. 2018;98:541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- Carrera-Chávez J.M., Jiménez-Aguilar E.E., Acosta-Pérez T.P., Núñez-Gastélum J.A., Quezada-Casasola A., Escárcega-Ávila A.M., et al. Effect of Moringa oleifera seed extract on antioxidant activity and sperm characteristics in cryopreserved ram semen. J Appl Anim Res. 2020;48:114–120. [Google Scholar]
- Cao S., Shen Z., Wang C., Zhang Q., Hong Q., He Y., et al. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets. Food Funct. 2019;10:344–354. doi: 10.1039/c8fo02091d. [DOI] [PubMed] [Google Scholar]
- Chen F., Lv Y., Zhu P., Cui C., Wu C., Chen J., et al. Dietary Yucca schidigera extract supplementation during late gestating and lactating sows improves animal performance, nutrient digestibility, and manure ammonia emission. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.676324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.L., Zhang X.H., Sun Y.W., Wang W.J., Huang J., Chu N.L., et al. Genomewide DNA methylation responses in patients with β-thalassemia treated with Yisui Shengxue Granules. Chin J Integr Med. 2019;25:490–496. doi: 10.1007/s11655-018-2777-9. [DOI] [PubMed] [Google Scholar]
- Cherian G., Orr A., Burke I.C., Pan W. Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poult Sci. 2013;92:1085–1090. doi: 10.3382/ps.2012-02752. [DOI] [PubMed] [Google Scholar]
- Cieslak A., Zmora P., Matkowski A., Nawrot-Hadzik I., Pers-Kamczyc E., El-Sherbiny M., et al. Tannins from Sanguisorba officinalis affect in vitro rumen methane production and fermentation. J Anim Plant Sci. 2016;26:54–62. [Google Scholar]
- Clement D., Goodridge J.P., Grimm C., Patel S., Malmberg K.J. TRP channels as interior designers: remodeling the endolysosomal compartment in natural killer cells. Front Immunol. 2020;11:753. doi: 10.3389/fimmu.2020.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouard C., Val-Laillet D. Impact of sensory feed additives on feed intake, feed preferences, and growth of female piglets during the early postweaning period. J Anim Sci. 2014;92:2133–2140. doi: 10.2527/jas.2013-6809. [DOI] [PubMed] [Google Scholar]
- Cui D., Wang X., Wang L., Wang X., Zhang J., Qin Z., et al. The administration of Sheng Hua Tang immediately after delivery to reduce the incidence of retained placenta in Holstein dairy cows. Theriogenology. 2014;81:645–650. doi: 10.1016/j.theriogenology.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Dang X., Chung Y.H., Kim I.H. Effects of dietary supplementation of herbal active ingredients promoting insulin-like growth factor-1 secretion on production performance, egg quality, blood hematology, and excreta gas emission in laying hens. Anim Biosci. 2021;34:1802–1810. doi: 10.5713/ab.20.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castilho T.S., Matias T.B., Nicolini K.P., Nicolini J. Study of interaction between metal ions and quercetin. Food Sci Hum Wellness. 2018;7:215–219. [Google Scholar]
- Defoirdt T., Pande G.S., Baruah K., Bossier P. The apparent quorum-sensing inhibitory activity of pyrogallol is a side effect of peroxide production. Antimicrob Agents Chemother. 2013;57:2870–2873. doi: 10.1128/AAC.00401-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Wang J., Lyu W., Wieneke X., Matts R., Ma X., et al. Development of a cell-based high-throughput screening assay to identify porcine host defense peptide-inducing compounds. J Immunol Res. 2018;2018 doi: 10.1155/2018/5492941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., Attri K., Dahiya S.S., Paul S.S. Influence of dietary phytogenic feed additives on lactation performance, methane emissions and health status of Murrah buffaloes (Bubalus bubalis) J Sci Food Agric. 2021;101:4390–4397. doi: 10.1002/jsfa.11080. [DOI] [PubMed] [Google Scholar]
- Dou B., Li S., Wei L., Wang L., Zhu S., Wang Z., et al. Astragaloside IV suppresses post-ischemic natural killer cell infiltration and activation in the brain: involvement of histone deacetylase inhibition. Front Med. 2021;1:79–90. doi: 10.1007/s11684-020-0783-8. [DOI] [PubMed] [Google Scholar]
- Ebeid H.M., Li M., Kholif A.E., Faiz-Ul H., Yang C., Peng L., et al. Moringa oleifera oil modulates rumen microflora to mediate in vitro fermentation kinetics and methanogenesis in total mix rations. Curr Microbiol. 2020;77:1271–1282. doi: 10.1007/s00284-020-01935-2. [DOI] [PubMed] [Google Scholar]
- Elmi A., Prosperi A., Zannoni A., Bertocchi M., Scorpio D.G., Forni M., et al. Antimicrobial capabilities of non-spermicidal concentrations of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on the liquid phase of refrigerated swine seminal doses. Res Vet Sci. 2019;127:76–81. doi: 10.1016/j.rvsc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- El Asbahani A., Miladi K., Badri W., Sala M., Aït Addi E.H., Casabianca H., et al. Essential oils: from extraction to encapsulation. Int J Pharm. 2015;483:220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- El Euony O.I., Elblehi S.S., Abdel-Latif H.M., Abdel-Daim M.M., El-Sayed Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus) Environ Sci Pollut Res Int. 2020;27:23108–23128. doi: 10.1007/s11356-020-08588-5. [DOI] [PubMed] [Google Scholar]
- El-Desoky N.I., Hashem N.M., Elkomy A., Abo-elezz Z.R. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal. 2017;11:1549–1557. doi: 10.1017/S1751731117000088. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Umar M., Hassan F.U., Alagawany M., Arif M., Taha A.E., et al. Applications of butyric acid in poultry production: the dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim Health Res Rev. 2022;14:1–11. doi: 10.1017/S1466252321000220. [DOI] [PubMed] [Google Scholar]
- El-Shall N.A., Shewita R.S., Abd El-Hack M.E., AlKahtane A., Alarifi S., Alkahtani S., et al. Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poult Sci. 2020;29:2944–2954. doi: 10.1016/j.psj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faehnrich B., Lukas B., Humer E., Zebeli Q. Phytogenic pigments in animal nutrition: potentials and risks. J Sci Food Agric. 2016;96:1420–1430. doi: 10.1002/jsfa.7478. [DOI] [PubMed] [Google Scholar]
- Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G., et al. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J Anim Sci Biotechnol. 2021;12:72. doi: 10.1186/s40104-021-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S., Wulff H., Skolnik E.Y. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiesel A., Gessner D.K., Most E., Eder K. Effect of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet Res. 2014;10:96. doi: 10.1186/s12917-014-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmino J.P., Galindo-Villegas J., Reyes-López F.E., Gisbert E. Phytogenic bioactive compounds shape fish mucosal immunity. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.695973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C., Baser K.H.C., Windisch W. Essential oils and aromatic plants in animal feeding - a European perspective. A review. Flavour Frag J. 2010;25:327–340. [Google Scholar]
- Friedman M. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J Agric Food Chem. 2017;65:10406–10423. doi: 10.1021/acs.jafc.7b04344. [DOI] [PubMed] [Google Scholar]
- Frydrychová S., Opletal L., Macáková K., Lustykov′a A., Rozkot M., Lipenský J. Effects of herbal preparation on libido and semen quality in boars. Reprod Domest Anim. 2011;46:573–578. doi: 10.1111/j.1439-0531.2010.01703.x. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gani A., Wani S.M., Masoodi F.A., Hameed G. Whole-grain cereal bioactive compounds and their health benefits: a review. J Food Process Technol. 2012;3 [Google Scholar]
- Galindo-Villegas J., Montalban-Arques A., Liarte S., de Oliveira S., Pardo-Pastor C., Rubio-Moscardo F., et al. TRPV4-Mediated detection of hyposmotic stress by skin keratinocytes activates developmental immunity. J Immunol. 2016;196:738–749. doi: 10.4049/jimmunol.1501729. [DOI] [PubMed] [Google Scholar]
- Galloway W., Hodgkinson J.T., Bowden S.D., Welch M., Spring D.R. Quorum sensing in gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- Ganai S.A., Sheikh F.A., Baba Z.A. Plant flavone chrysin as an emerging histone deacetylase inhibitor for prosperous epigenetic-based anticancer therapy. Phytother Res. 2021;35:823–834. doi: 10.1002/ptr.6869. [DOI] [PubMed] [Google Scholar]
- Gernat A.A., Santos F.B., Grimes J.L. Alternative approaches to antimicrobial use in the Turkey industry: challenges and perspectives. Ger J Vet Res. 2021;1:37–48. [Google Scholar]
- Gilani G.S., Xiao C.W., Cockell K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr. 2012;108:S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Gong J., Yin F., Hou Y., Yin Y. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: potential and challenges in application. Can J Anim Sci. 2014;94:223–241. [Google Scholar]
- Gräber T., Kluge H., Granica S., Horn G., Brandsch C., Stangl G.I. Studies on the health impact of Agrimonia procerain in piglets. BMC Vet Res. 2014;10:210. doi: 10.1186/s12917-014-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot M., Kleijer-Ligtenberg G., van Asseldonk T., KoeNatuurlijk H.H. RIKILT-Wageningen UR; 2011. Natural dairy cow health: a guide to keeping your herd healthy with herbs and other natural products; p. 29. [Google Scholar]
- Guo W., Ma H., Wang C.Z., Wan J.Y., Yao H., Yuan C.S. Epigenetic studies of Chinese herbal medicine: pleiotropic role of DNA methylation. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.790321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi S., Davoodi H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet Res Commun. 2011;35:169–180. doi: 10.1007/s11259-010-9458-2. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Hassan A., Abu Hafsa S.H., Elghandour M.M.Y., Kanth Reddy P.R., Salem M.Z.M., Anele U.Y., et al. Influence of Corymbia citriodora leaf extract on growth performance, ruminal fermentation, nutrient digestibility, plasma antioxidant activity and faecal bacteria in young calves. Anim Feed Sci Technol. 2020;261 [Google Scholar]
- Hassan F., Arshad M.A., Ebeid H.M., Rehman M.S., Khan M.S., Shahid S., et al. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet-microbe interaction. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.575801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F., Ebeid H.M., Tang Z., Li M., Peng L., Peng K., et al. A mixed phytogenic modulates the rumen bacteria composition and milk fatty acid profile of water buffaloes. Front Vet Sci. 2020;7:569. doi: 10.3389/fvets.2020.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F.A.M., Awad A. Impact of thyme powder (Thymus vulgaris L.) supplementation on gene expression profiles of cytokines and economic efficiency of broiler diets. Environ Sci Pollut Res. 2017;24:15816–15826. doi: 10.1007/s11356-017-9251-7. [DOI] [PubMed] [Google Scholar]
- Hoffman-Pennesi D., Wu C. The effect of thymol and thyme oil feed supplementation on growth performance, serum antioxidant levels, and cecal Salmonella population in broilers. J Appl Poult Res. 2010;19:432–443. [Google Scholar]
- Hossain M., Mostofa M., Alam M., Sultana M., Rahman M. The ameliorating effects of garlic (Allium Sativum) against lead (Pb) intoxication on body weight, dressing percentages, feed consumption and feed conversion ratio in lead induced broiler chickens. Bangladesh J Vet Med. 2014;12:1–7. [Google Scholar]
- Hsieh H.Y., Chiu P.H., Wang S.C. Histone modifications and traditional Chinese medicinals. BMC Complement Altern Med. 2013;13:115. doi: 10.1186/1472-6882-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.E., Lo S.H., Lin Y.Y., Wang H.T., Chen C.Y. Effects of essential oil mixtures on nitrogen metabolism and odor emission via in vitro simulated digestion and in vivo growing pig experiments. J Sci Food Agric. 2021;102:1939–1947. doi: 10.1002/jsfa.11531. [DOI] [PubMed] [Google Scholar]
- Huang K.C., Yen H.R., Chiang J.H., Su Y.C., Sun M.F., Chang H.H., et al. Chinese herbal medicine as an adjunctive therapy ameliorated the incidence of chronic hepatitis in patients with breast cancer: a nationwide population-based cohort study. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/1052976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh D.B.T., Krickmeier J., Schnaeckel W. Effects of treating temperatures on redox potential and sensory evaluation of different spices and herbs on meat product application. J Sci Food Agric. 2020;100:2898–2904. doi: 10.1002/jsfa.10316. [DOI] [PubMed] [Google Scholar]
- Iqbal Y., Cottrell J.J., Suleria H.A.R., Dunshea F.R. Gut microbiota-polyphenol interactions in chicken: a review. Animals. 2020;10:1391. doi: 10.3390/ani10081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S., Goh Y.M., Rajion M.A., Ebrahimi M. Effects of polyphenol rich bamboo leaf on rumen fermentation characteristics and methane gas production in an in vitro condition. Indian J Anim Res. 2020;54:322–326. [Google Scholar]
- Jayanegara A., Goel G., Makkar H.P.S., Becker K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim Feed Sci Technol. 2015;209:60–68. [Google Scholar]
- Jayaganthan P., Perumal P., Balamurugan T.C., Verma R.P., Singh L.P., Pattanaik A.K., et al. Effects of Tinospora cordifolia supplementation on semen quality and hormonal profile in rams. Anim Reprod Sci. 2013;140:47–53. doi: 10.1016/j.anireprosci.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Ji F., Gu L., Rong G., Hu C., Sun W., Wang D., et al. Using extract from the stems and leaves of Yizhi (Alpiniae oxyphyllae) as feed additive increases meat quality and intestinal health in ducks. Front Vet Sci. 2022;8 doi: 10.3389/fvets.2021.793698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P., Ji S., Zhang H., Chen Y., Wang T. Piceatannol ameliorates hepatic oxidative damage and mitochondrial dysfunction of weaned piglets challenged with diquat. Animals. 2020;10:1239. doi: 10.3390/ani10071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofré I., Cuevas M., de Castro L.S., De Agostini L.J.D., Torres M.A., Alvear M., et al. Antioxidant effect of a polyphenol-rich murtilla (Ugni molinae Turcz.) extract and its effect on the regulation of metabolism in refrigerated boar sperm. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/2917513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantas D., Papatsiros V.G., Tassis P.D., Athanasiou L.V., Tzika E.D. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim Sci J. 2015;86:92–98. doi: 10.1111/asj.12240. [DOI] [PubMed] [Google Scholar]
- Kaschubek T., Mayer E., Rzesnik S., Grenier B., Bachinger D., Schieder C., et al. Effects of phytogenic feed additives on cellular oxidative stress and inflammatory reactions in intestinal porcine epithelial cells. J Anim Sci. 2018;96:3657–3669. doi: 10.1093/jas/sky263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson A. Genomics goes beyond DNA sequence. Nature. 2010;465:145. doi: 10.1038/465145a. [DOI] [PubMed] [Google Scholar]
- Khalafalla R.E., Müller U., Shahiduzzaman M., Dyachenko V., Desouky A.Y., Alber G., et al. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitol Res. 2011;108:879–886. doi: 10.1007/s00436-010-2129-y. [DOI] [PubMed] [Google Scholar]
- Kholif A.E., Hassan A.A., El Ashry G.M., Bakr M.H., El-Zaiat H.M., Olafadehan O.A., et al. Phytogenic feed additives mixture enhances the lactational performance, feed utilization and ruminal fermentation of Friesian cows. Anim Biotechnol. 2020;32:708–718. doi: 10.1080/10495398.2020.1746322. [DOI] [PubMed] [Google Scholar]
- Khalifa E.I., Hanan A.M.H., Mohamed A.H., Hussein A.M. Effects of using Yucca schidigera powder as feed additive on productive and reproductive efficiency of Zaraibi dairy goats. Egypt J Sheep Goat Sci. 2014;9:9–21. [Google Scholar]
- Khalil M., Alliger K., Weidinger C., Yerinde C., Wirtz S., Becker C., et al. Functional role of transient receptor potential channels in immune cells and epithelia. Front Immunol. 2018;9:174. doi: 10.3389/fimmu.2018.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiczorowska B., Samolińska W., Al-Yasiry A.R.M., Kiczorowski P., Winiarska-Mieczan A. The natural feed additives as immunostimulants in monogastric animal nutrition-a review. Ann Anim Sci. 2017;17:3605–3625. [Google Scholar]
- Koh K.H., Tham F.Y. Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J Microbiol Imunol Infect. 2011;44:144–148. doi: 10.1016/j.jmii.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kolling G.J., Stivanin S.C.B., Gabbi A.M., Machado F.S., Ferreira A.L., Campos M.M., et al. Performance and methane emissions in dairy cows fed oregano and green tea extracts as feed additives. J Dairy Sci. 2018;101:4221–4234. doi: 10.3168/jds.2017-13841. [DOI] [PubMed] [Google Scholar]
- Krithika R., Verma R.J., Shrivastav P.S. Antioxidative and cytoprotective effects of andrographolide against CCl4-induced hepatotoxicity in HepG2 cells. Hum Exp Toxicol. 2013;32:530–543. doi: 10.1177/0960327112459530. [DOI] [PubMed] [Google Scholar]
- Kumar A., Katiyar R., Ahmad F., Deepak D., Prasad J.K. Current treatment aspects of bovine reproductive disorders. Theriogenol Insight. 2018;8:101–109. [Google Scholar]
- Kumar M., Kumar V., Roy D., Kushwaha R., Vaswani S. Application of herbal feed additives in animal nutrition - a review. Int J Livest Res. 2014;4:1–8. [Google Scholar]
- Kumar S., Ciraci C., Redmond S.B., Chuammitri P., Andreasen B., Palić D., et al. Immune response gene expression in spleens of diverse chicken lines fed dietary immunomodulators. Poult Sci. 2011;90:1009–1013. doi: 10.3382/ps.2010-01235. [DOI] [PubMed] [Google Scholar]
- Kuralkar P., Kuralkar S.V. Role of herbal products in animal production–an updated review. J Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114246. [DOI] [PubMed] [Google Scholar]
- Lakhani N., Kamra D.N., Lakhani P., Alhussien M.N. Immune status and haemato-biochemical profile of buffalo calves supplemented with phytogenic feed additives rich in tannins, saponins and essential oils. Trop Anim Health Prod. 2018;51:565–573. doi: 10.1007/s11250-018-1727-z. [DOI] [PubMed] [Google Scholar]
- Laudato M., Capasso R. Useful plants for animal therapy. OA Altern Med. 2013;1:1–6. [Google Scholar]
- Lee S.D., Kim J.H., Jung H.J., Kim Y.H., Kim I.C., Kim S.B., et al. The effect of ginger extracts on the antioxidant capacity and IgG concentrations in the colostrum and plasma of neo-born piglets and sows. Livest Sci. 2013;154:117–122. [Google Scholar]
- Lee S.H., Lillehoj H.S., Chun H.K., Tuo W., Park H.J., Cho S.M., et al. In vitro treatment of chicken peripheral blood lymphocytes, macrophages, and tumor cells with extracts of Korean medicinal plants. Nutr Res. 2007;27:362–366. doi: 10.1016/j.nutres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Hong Y.H., Jang S.I., Lillehoj E.P., Ionescu C., et al. In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Br Poult Sci. 2010;51:213–221. doi: 10.1080/00071661003745844. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Jang S.I., Lillehoj E.P., Min W., Bravo D.M. Dietary supplementation of young broiler chickens with capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br J Nutr. 2013;110:840–847. doi: 10.1017/S0007114512006083. [DOI] [PubMed] [Google Scholar]
- Lee Y., Lee S., Gadde U.D., Oh S., Lee S., Lillehoj H.S. Dietary Allium hookeri reduces inflammatory response and increases expression of intestinal tight junction proteins in LPS-induced young broiler chicken. Res Vet Sci. 2017;112:149–155. doi: 10.1016/j.rvsc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Lei Z., Zhang K., Li C., Jiao T., Wu J., Wei Y., et al. Ruminal metagenomic analyses of goat data reveals potential functional microbiota by supplementation with essential oil-cobalt complexes. BMC Microbiol. 2019;19:30–39. doi: 10.1186/s12866-019-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X.J., Lee S.I., Kim I.H. Effects of different levels of dietary protein with or without plant extract YGF251 on growth performance, nutrient digestibility, blood profiles, fecal microbial shedding, and fecal gas emission in growing pigs. Anim Sci J. 2019;90:547–553. doi: 10.1111/asj.13162. [DOI] [PubMed] [Google Scholar]
- Li M., Hassan F., Tang Z., Peng L., Liang X., Li L., et al. Mulberry leaf flavonoids improve milk production, antioxidant, and metabolic status of water buffaloes. Front Vet Sci. 2020;7:599. doi: 10.3389/fvets.2020.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Yuan D., Liu Y., Jin H., Tan B. Dietary puerarin supplementation alleviates oxidative stress in the small intestines of diquat-challenged piglets. Animals. 2020;10:631. doi: 10.3390/ani10040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Piao X., Ru Y., Han X., Xue L., Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas J Anim Sci. 2012;25:1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhuang A., Ma J., Ji L., Hou X., Chen H., et al. Effect of Jieduquyuziyin prescription-treated rat serum on MeCP2 gene expression in Jurkat T cells. In Vitro Cell Dev Biol Anim. 2018;54:692–704. doi: 10.1007/s11626-018-0295-x. [DOI] [PubMed] [Google Scholar]
- Li S.Y., Ru Y.J., Liu M., Xu B., Peron A., Shi X.G. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest Sci. 2012;145:119–123. [Google Scholar]
- Li W., Sargsyan D., Wu R., Li S., Wang L., Cheng D., et al. DNA methylome and transcriptome alterations in high glucose-induced diabetic nephropathy cellular model and identification of novel targets for treatment by tanshinone IIA. Chem Res Toxicol. 2019;32:1977–1988. doi: 10.1021/acs.chemrestox.9b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.L., He W.L., Wang Z.B., Xu T.S. Effects of Chinese herbal mixture on performance, egg quality and blood biochemical parameters of laying hens. J Anim Physiol Anim Nutr. 2016;100:1041–1049. doi: 10.1111/jpn.12473. [DOI] [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Chi F., Cravens R.L., et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.L., Lu C.C., Lai W.F., Wu T.S., Lu J.J., Chen Y.M., et al. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell. 2021;12:394–410. doi: 10.1007/s13238-020-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.N., Ye L., Li Z.W., Huang X.S., Lu Z., Yang Y.Q., et al. Chinese herb feed additives improved the growth performance, meat quality, and nutrient digestibility parameters of pigs. Animal Model Exp Med. 2020;3:47–54. doi: 10.1002/ame2.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.S., Liang X., Wei X.H., Jin Z., Chen F.L., Tang Q.F., et al. Gegen Qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids. Front Microbiol. 2019;10:825. doi: 10.3389/fmicb.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu W., Deng Z., Sunkara L.T., Becker S., Robinson K., Matts R., et al. High throughput screening for natural host defense peptide-inducing compounds as novel alternatives to antibiotics. Front Cell Infect Microbiol. 2018;8:191. doi: 10.3389/fcimb.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Diao Q.Y. Roles of butyrate and plant extracts on histone acetylation in animals. Chin J Anim Nutr. 2015;27:1028–1033. [in Chinese] [Google Scholar]
- Ma T., Chen D.D., Tu Y., Zhang N.F. Dietary supplementation with mulberry leaf flavonoids inhibits methanogenesis in sheep. Anim Sci J. 2017;88:72–78. doi: 10.1111/asj.12556. [DOI] [PubMed] [Google Scholar]
- Ma T., Wu W., Tu Y., Zhang N., Diao Q. Resveratrol affects in vitro rumen fermentation, methane production and prokaryotic community composition in a time- and diet-specific manner. Microb Biotechnol. 2020;13:1118–1131. doi: 10.1111/1751-7915.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuz S., Shang Q., Piao X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J Anim Sci Biotechnol. 2021;12:48. doi: 10.1186/s40104-021-00565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahima Rahal A., Deb R., Latheef S.K., Abdul Samad H., Tiwari R., Verma A.K., et al. Immunomodulatory and therapeutic potentials of herbal tradition/indigenous and ethnoveterinary medicines. Pak J Biol Sci. 2012;15:754–774. doi: 10.3923/pjbs.2012.754.774. [DOI] [PubMed] [Google Scholar]
- Manuelian C.L., Pitino R., Simoni M., Mavrommatis A., de Marchi M., Righi F., et al. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on livestock mammals' performances, health, and oxidative status: a review of the literature in the last 20 Years. Antioxidants. 2021;10:1461. doi: 10.3390/antiox10091461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdipour Z., Afsharmanesh M. Evaluation of synbiotic and cinnamon (Cinnamomum verum) as antibiotic growth promoter substitutions on growth performance, intestinal microbial populations and blood parameters in Japanese quail. J Livest Sci Technol. 2018;6:1–8. [Google Scholar]
- Miri V.H., Tyagi A.K., Ebrahimi S.H., Mohini M. Effect of cumin (Cuminum cyminum) seed extract on milk fatty acid profile and methane emission in lactating goat. Small Ruminant Res. 2013;113:66–72. [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Comparison of the effect of two phytogenic compounds on growth performance and immune response of broilers. J Appl Anim Res. 2017;45:603–608. [Google Scholar]
- Morsy T.A., Kholif A.E., Matloup O.H., Abu Elella A., Anele U.Y., Caton J.S. Mustard and cumin seeds improve feed utilisation, milk production and milk fatty acids of Damascus goats. J Dairy Res. 2018;85:142–151. doi: 10.1017/S0022029918000043. [DOI] [PubMed] [Google Scholar]
- Murugesan G.R., Syed B., Haldar S., Pender C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front Vet Sci. 2015;2:21. doi: 10.3389/fvets.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq M., Durrani F.R., Imtiaz N., Sadique U., Hafeez A., Akhtar S., et al. Effect of administration of Withania somnifera on some hematological and immunological profile of broiler chicks. Pak Vet J. 2012;32:70–72. [Google Scholar]
- Nazzaro F., Fratianni F., Coppola R. Quorum sensing and phytochemicals. Int J Mol Sci. 2013;14:12607–12619. doi: 10.3390/ijms140612607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto G., Díaz P., Bañón S., Garrido M.D. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes' diet. Meat Sci. 2010;85:82–88. doi: 10.1016/j.meatsci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Nieto G., Estrada M., Jordán M.J., Garrido M.D., Bañón S. Effects in ewe diet of rosemary by-product on lipid oxidation and the eating quality of cooked lamb under retail display conditions. Food Chem. 2011;124:1423–1429. [Google Scholar]
- Nikhade C.T., Deshmukh S.G., Kuralkar S.V., Ratnaparkhi A.R., Chepte S.D., Bankar P.S. Comparative efficacy of different herbal extract on subclinical endometritis in postpartum cows. J Entomol Zool Stud. 2019;7:1422–1426. [Google Scholar]
- Nowland T.L., Stanley D., Kirkwood R.N., Torok V.A., Bajagai Y.S., GaTorok V.A., et al. Maternal supplementation with phytogenic additives influenced the faecal microbiota and reproductive potential in sows. AMB Express. 2021;11:107. doi: 10.1186/s13568-021-01268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan C.A., Pendleton S.J., Crandall P.G., Ricke S.C. Potential of plant essential oils and their components in animal agriculture - in vitro studies on antibacterial mode of action. Front Vet Sci. 2015;2:35. doi: 10.3389/fvets.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olawuwo O.S., Famuyide I.M., McGaw L.J. Antibacterial and antibiofilm activity of selected medicinal plant leaf extracts against pathogens implicated in poultry diseases. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.820304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski S., Flees J., Greene E.S., Ashley D., Lee S.O., Yang F.L., et al. Effects of phytogenic additives on meat quality traits in broiler chickens1. J Anim Sci. 2018;96:3757–3767. doi: 10.1093/jas/sky238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orengo J., Buendía A., Ruiz-Ibáñez M., Madrid J., Del Río L., Catalá-Gregori P., et al. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet Parasitol. 2012;185:158–163. doi: 10.1016/j.vetpar.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Osemwegie O., Nwonuma C., Oluyori A., Abraham P., Akanbi A., Opaleke D., et al. In vitro and in vivo lead acetate poison abatement study of Garcinia kola Heckel. J Taibah Univ Sci. 2017;11:883–894. [Google Scholar]
- Oubihi A., Ouryemchi I., Nounah I., Tarfaoui K., Guessous Z. Chemical composition, antibacterial and antifungal activities of Thymus leptobotrys Murb essential oil. Adv Tradit Med. 2020;20:673–679. [Google Scholar]
- Pan J.J., He S.Y., Shao J.Y., Li N., Gong Y.Q., Gong X.C. Critical pharmaceutical process identification considering chemical composition, biological activity, and batch-to-batch consistency: a case study of notoginseng total saponins. Chin Herb Med. 2020;12:29–35. doi: 10.1016/j.chmed.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande G.S.J., Scheie A.A., Benneche T., Wille M., Defoirdt T. Quorum-sensing disrupting compounds protect larvae of the giant freshwater prawn Macrobrachium rosenbegii from Vibrio harveyi infection. Aquaculture. 2013;407:121–124. [Google Scholar]
- Pant M., Ambwani T., Umapathi V. Antiviral activity of Ashwagandha extract on infectious bursal disease virus replication. Indian J Sci Technol. 2012;5:2750–2751. [Google Scholar]
- Paraskeuas V.V., Mountzouris K.C. Modulation of broiler gut microbiota and gene expression of Toll-like receptors and tight junction proteins by diet type and inclusion of phytogenics. Poult Sci. 2019;98:2220–2230. doi: 10.3382/ps/pey588. [DOI] [PubMed] [Google Scholar]
- Park J.H., Kim I.H. Effects of a protease and essential oils on growth performance, blood cell profiles, nutrient retention, ileal microbiota, excreta gas emission, and breast meat quality in broiler chicks. Poult Sci. 2018;97:2854–2860. doi: 10.3382/ps/pey151. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Halkes S.B.A., Mes J.J., Landman W.J.M. In vivo screening of four phytochemicals/extracts and a fungal immunomodulatory protein against an Eimeria acervulina infection in broilers. Vet Q. 2013;33:132–138. doi: 10.1080/01652176.2013.844378. [DOI] [PubMed] [Google Scholar]
- Perricone V., Comi M., Giromini C., Rebucci R., Agazzi A., Savoini G., et al. Green tea and pomegranate extract administered during critical moments of the production cycle improves blood antiradical activity and alters cecal microbial ecology of broiler chickens. Animals. 2020;10:785. doi: 10.3390/ani10050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal P., Veeraselvam M., Nahak A.K. Herbal treatment in animal reproduction. Int J Bio-resour Stress Manag. 2013;4:460–467. [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Lillehoj H.S., Bravo D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult Sci. 2019;98:3443–3449. doi: 10.3382/ps/pey472. [DOI] [PubMed] [Google Scholar]
- Placha I., Takacova J., Ryzner M., Cobanova K., Laukova A., Strompfova V., et al. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci. 2014;55:105–114. doi: 10.1080/00071668.2013.873772. [DOI] [PubMed] [Google Scholar]
- Pop L.M., Varga E., Coroian M., Nedisan M.E., Mircean V., Dumitrache M.O., et al. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasit Vectors. 2019;12:343. doi: 10.1186/s13071-019-3595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid Z., Mirani Z.A., Zehra S., Gilani S.M.H., Ashraf A., Azhar A., et al. Enhanced modulation of gut microbial dynamics affecting body weight in birds triggered by natural growth promoters administered in conventional feed. Saudi J Biol Sci. 2020;27:2747–2755. doi: 10.1016/j.sjbs.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Morales E., Rossi G., Cattin M., Jones E., Braganca R., Newbold C.J. The effect of an isoflavonid-rich liquorice extract on fermentation, methanogenesis and the microbiome in the rumen simulation technique. FEMS Microbiol Ecol. 2018;94 doi: 10.1093/femsec/fiy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranucci D., Beghelli D., Trabalza-Marinucci M., Branciari R., Forte C., Olivieri O., et al. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 2014;100:319–326. doi: 10.1016/j.meatsci.2014.09.149. [DOI] [PubMed] [Google Scholar]
- Rashidi N., Khatibjoo A., Taherpour K., Akbari-Gharaei M., Shirzadi H. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B1. Poult Sci. 2020;99:5896–5906. doi: 10.1016/j.psj.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redoy M.R.A., Shuvo A.A.S., Cheng L., Al-Mamun M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal. 2020;14:2433–2441. doi: 10.1017/S1751731120001196. [DOI] [PubMed] [Google Scholar]
- Reyes-Camacho D., Vinyeta E., Pérez J.F., Aumiller T., Criado L., Palade L.M., et al. Phytogenic actives supplemented in hyperprolific sows: effects on maternal transfer of phytogenic compounds, colostrum and milk features, performance and antioxidant status of sows and their offspring, and piglet intestinal gene expression. J Anim Sci. 2020;98 doi: 10.1093/jas/skz390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B., Toschi A., Piva A., Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr Res Rev. 2020;33:218–234. doi: 10.1017/S0954422420000013. [DOI] [PubMed] [Google Scholar]
- Ros-Santaella J.L., Pintus E. Plant extracts as alternative additives for sperm preservation. Antioxidants. 2021;10:772. doi: 10.3390/antiox10050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Arain M.A., Naveed M., Alagawany M., El-Hack M.E., Bhutto Z.A., et al. Yucca schidigera can mitigate ammonia emissions from manure and promote poultry health and production. Environ Sci Pollut Res Int. 2018;25:35027–35033. doi: 10.1007/s11356-018-3546-1. [DOI] [PubMed] [Google Scholar]
- Salini R., Pandian S.K. Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens. Pathog Dis. 2015;73 doi: 10.1093/femspd/ftv038. [DOI] [PubMed] [Google Scholar]
- Samanta A.K., Gali J.M., Dutta T.K., Rajkhowa T.K., Mandal G.P., Patra A.K. Effect of dietary phytobiotic mixture on growth performance, nutrient utilization, and immunity in weaned piglets. Trop Anim Health Prod. 2021;53:459. doi: 10.1007/s11250-021-02910-0. [DOI] [PubMed] [Google Scholar]
- Saxena M.J., Ravikanth K., Thakur A., Maini S. Role of herbals in reproductive health management and improving fertility in livestock. https://www.ayurvet.com Retrieved June 25, 2019, from Ayurvet.
- Sayrafi R., Mirzakhani N., Mobaseri R. Effects of turmeric (Curcuma longa) and vitamin E on histopathological lesions induced in bursa of Fabricius of broiler chicks by salinomycin. Vet Res Forum. 2017;8:231–236. [PMC free article] [PubMed] [Google Scholar]
- Seidavi A.R., Tavakoli M., Asroosh F., Scanes C.G., Abd El Hack M.E., Naiel M.A., et al. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ Sci Pollut Res Int. 2022;29:5006–5031. doi: 10.1007/s11356-021-17401-w. [DOI] [PubMed] [Google Scholar]
- Shan C.H., Guo J.J., Sun X.S., Li N., Yang X.Y., Gao Y.H., et al. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J Anim Sci. 2018;96:4444–4457. doi: 10.1093/jas/sky270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front Vet Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry M., Charcosset C., Fessi H., Greige-Gerges H. Essential oils encapsulated in liposomes: a review. J Liposome Res. 2013;23:268–275. doi: 10.3109/08982104.2013.819888. [DOI] [PubMed] [Google Scholar]
- Shirzadegan K., Falahpour P. The physicochemical properties and antioxidative potential of raw thigh meat from broilers fed a dietary medicinal herb extract mixture. Open Vet J. 2014;4:69–77. [PMC free article] [PubMed] [Google Scholar]
- Silva Júnior C.D., Martins C.C.S., Dias F.T.F., Sitanaka N.Y., Ferracioli L.B., Moraes J.E., et al. The use of an alternative feed additive, containing benzoic acid, thymol, eugenol, and piperine, improved growth performance, nutrient and energy digestibility, and gut health in weaned piglets. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.K., Kumar A., Tiwari D.P. Effects of dietary supplementation of black cumin, garlic and turmeric on the production performance and egg quality of White Leghorn hens. Anim Nutr Feed Technol. 2019;19:361–370. [Google Scholar]
- Sobeh M., Hassan S.A., El Raey M.A., Khalil W.A., Hassan M.A.E., Wink M. Polyphenolics from Albizia harveyi exhibit antioxidant activities and counteract oxidative damage and ultra-structural changes of cryopreserved bull semen. Molecules. 2017;22:1993. doi: 10.3390/molecules22111993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaib M., Butt M.S., Shabbir M.A., Shahid M. Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with α-tocopherol enriched diets. Lipids Health Dis. 2015;14:61. doi: 10.1186/s12944-015-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Sridhar M., Suganthi R.U., Thammiaha V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J Anim Physiol Anim Nutr. 2015;99:1094–1104. doi: 10.1111/jpn.12260. [DOI] [PubMed] [Google Scholar]
- Sun K., Liu H., Fan H., Liu T., Zheng C. Research progress on the application of feed additives in ruminal methane emission reduction: a review. PeerJ. 2021;9 doi: 10.7717/peerj.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Hashem N.M., Abdelnour S.A., Taha A.E., Ohran H., Khafaga A.F., et al. Effects of phytogenic feed additives on the reproductive performance of animals. Saudi J Biol Sci. 2021;28:5816–5822. doi: 10.1016/j.sjbs.2021.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha H., Abdelnour S., Alagawany M. Growth performance, biochemical, cytological and molecular aspects of rabbits exposed to lead toxicity. J Anim Physiol Anim Nutr. 2019;103:747–755. doi: 10.1111/jpn.13073. [DOI] [PubMed] [Google Scholar]
- Talukdar D.J., Shyam J., Talukdar P., Ahmed K., Gogoi R. Documentation of traditional herbal medicines for reproductive disorders of livestock in Kamrup district of Assam. Int J Agric Sci Res. 2015;5:221–228. [Google Scholar]
- Téllez-López M.A., Mora-Tovar G., Ceniceros-Méndez I.M., García-Lujan C., Puente-Valenzuela K.O., Vega-Menchaca M.C., et al. Evaluation of the chelating effect of methanolic extract of Coriandrum sativum and its fractions on wistar rats poisoned with lead acetate. Afr J Tradit Complement Altern Med. 2017;14:92–102. doi: 10.21010/ajtcam.v14i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlabela C.P., Cai W.B., Gxasheka M., Tyasi T.L., Jiang H. Roles of carbohydrates extracted from Flammulina velutipes mushroom as nutraceuticals in the development of monogastric animals: a review. Int J Agric Res Rev. 2015;3:406–410. [Google Scholar]
- Torki M., Mohebbifar A., Mohammadi H. Effects of supplementing hen diet with Lavandula angustifolia and/or Mentha spicata essential oils on production performance, egg quality and blood variables of laying hens. Vet Med Sci. 2020;7:184–193. doi: 10.1002/vms3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S. Phytogenics to manage reproductive disorders in ruminants. Int Anim Health J. 2015;2:50–53. [Google Scholar]
- Tsirtsikos P., Fegeros K., Kominakis A., Balaskas C., Mountzouris K.C. Modulation of intestinal mucin composition and mucosal morphology by dietary phytogenic inclusion level in broilers. Animal. 2012;6:1049–1057. doi: 10.1017/S1751731111002680. [DOI] [PubMed] [Google Scholar]
- Upadhyaya I., Upadhyay A., Kollanoor-Johny A., Baskaran S.A., Mooyottu S., Darre M.J., et al. Rapid inactivation of Salmonella Enteritidis on shell eggs by plant-derived antimicrobials. Poult Sci. 2013;92:3228–3235. doi: 10.3382/ps.2013-03126. [DOI] [PubMed] [Google Scholar]
- Upadhyaya I., Upadhyay A., Kollanoor-Johny A., Mooyottu S., Baskaran S.A., Yin H.B., et al. In-feed supplementation of trans-cinnamaldehyde reduces layer-chicken egg-borne transmission of Salmonella enterica serovar Enteritidis. Appl Environ Microbiol. 2015;81:2985–2994. doi: 10.1128/AEM.03809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Maele L., Heyndrickx M., Maes D., De Pauw N., Mahu M., Verlinden M., et al. In vitro susceptibility of Brachyspira hyodysenteriae to organic acids and essential oil components. J Vet Med Sci. 2016;78:325–328. doi: 10.1292/jvms.15-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena N., Arora S., Singh R.R., Katara A., Rastogi S., Rawat A.K. Effect of Asparagus racemosus (shatavari) extract on physicochemical and functional properties of milk and its interaction with milk proteins. J Food Sci Technol. 2015;52:1176–1181. doi: 10.1007/s13197-013-1073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden M., Pasmans F., Mahu M., Vande Maele L., De Pauw N., Yang Z., et al. In vitro sensitivity of poultry Brachyspira intermedia isolates to essential oil components and in vivo reduction of Brachyspira intermedia in rearing pullets with cinnamaldehyde feed supplementation. Poult Sci. 2013;92:1202–1207. doi: 10.3382/ps.2012-02690. [DOI] [PubMed] [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Wanapat M., Chanthakhoun V., Phesatcha K., Kang S. Influence of mangosteen peel powder as a source of plant secondary compounds on rumen microorganisms, volatile fatty acids, methane and microbial protein synthesis in swamp buffaloes. Livest Sci. 2014;162:126–133. [Google Scholar]
- Wang G.C., Han L.L., Wang J., Lang W.N., Pan C.Y., Li Y.F. Effects of allicin on lipid metabolism and antioxidant activity in chickens. J Northeast Agric Univ (Engl Ed) 2014;21:46–49. [Google Scholar]
- Wang H.F., Yang W.R., Wang Y.X., Yang Z.B., Cui Y.H. The study on the effects of Chinese herbal mixtures on growth, activity of post-ruminal digestive enzymes and serum antioxidant status of beef cattle. Agric Sci China. 2011;10:448–455. [Google Scholar]
- Wang J., Lyu W., Zhang W., Chen Y., Luo F., Wang Y., et al. Discovery of natural products capable of inducing porcine host defense peptide gene expression using cell-based high throughput screening. J Anim Sci Biotechnol. 2021;12:14. doi: 10.1186/s40104-020-00536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.P., Kim I.H. Effect of caprylic acid and Yucca schidigera extract on production performance, egg quality, blood characteristics, and excreta microflora in laying hens. Br Poult Sci. 2011;52:711–717. doi: 10.1080/00071668.2011.635638. [DOI] [PubMed] [Google Scholar]
- Wang L., Han X., Zheng X., Zhou Y., Hou H., Chen W., et al. Ginsenoside 20(S)-Rg3 upregulates tumor suppressor VHL gene expression by suppressing DNMT3A-Mediated promoter methylation in ovarian cancer cells. J South Med Univ. 2021;41:100–106. doi: 10.12122/j.issn.1673-4254.2021.01.14. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hou Y., Yi D., Ding B., Zhao D., Wang Z., et al. Beneficial roles of dietary oleum cinnamon in alleviating intestinal injury. Front Biosci. 2015;20:814–828. doi: 10.2741/4339. [DOI] [PubMed] [Google Scholar]
- Wang M., Huang H., Hu Y., Liu Y., Zeng X., Zhuang Y., et al. Effects of dietary supplementation with herbal extract mixture on growth performance, organ weight and intestinal morphology in weaning piglets. J Anim Physiol Anim Nutr. 2020;104:1462–1470. doi: 10.1111/jpn.13422. [DOI] [PubMed] [Google Scholar]
- Wang Q.Y., Zhong H., Chen F.Y., Zhang M.Y., Cai J.Y., Zhong J.H. A preliminary study on epigenetic regulation of Acanthopanax senticosus in leukemia cell lines. Exp Hematol. 2016;44:466–473. doi: 10.1016/j.exphem.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Wang S.H., Lin P.Y., Chiu Y.C., Huang J.S., Kuo Y.T., Wu J.C., et al. Curcumin-mediated HDAC inhibition suppresses the DNA damage response and contributes to increased DNA damage sensitivity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.P., Wang W.J., Tan Z.L., Liu G.W., Zhou C.F., Yin M.J. Effect of traditional Chinese medicine compounds on rumen fermentation, methanogenesis and microbial flora in vitro. Anim Nutr. 2019;5:185–190. doi: 10.1016/j.aninu.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhang J. Curcumin: bioavailability, antioxidant function and application in broiler chickens production. Chin J Anim Nutr. 2014;26:3101–3107. [Google Scholar]
- Wilkinson A.W., Gozani O. Cancer epigenetics: reading the future of leukaemia. Nature. 2017;543:186–188. doi: 10.1038/nature21894. [DOI] [PubMed] [Google Scholar]
- Williams C.A., Lamprecht E.D. Some commonly fed herbs and other functional foods in equine nutrition: a review. Vet J. 2008;178:21–31. doi: 10.1016/j.tvjl.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wu B., Yao X., Nie X., Xu R. Epigenetic reactivation of RANK in glioblastoma cells by curcumin: involvement of STAT3 inhibition. DNA Cell Biol. 2013;32:292–297. doi: 10.1089/dna.2013.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Zhang Q., Yi D., Wu T., Chen H., Guo S., et al. Quantitative proteomic analysis reveals antiviral and anti-inflammatory effects of puerarin in piglets infected with porcine epidemic diarrhea virus. Front Immunol. 2020;11:169. doi: 10.3389/fimmu.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Yuan D., Tan B., Wang J., Liu Y., Tan B. The role of Nrf2 signaling pathway in Eucommia ulmoides flavones regulating oxidative stress in the intestine of piglets. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/9719618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Bai S.P., Zhang K.Y., Ding X.M., Wang J.P., Zeng Q.F., et al. Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult Sci. 2019;98:4838–4847. doi: 10.3382/ps/pez219. [DOI] [PubMed] [Google Scholar]
- Xu B.Y., Qin W.X., Xu Y.Z., Yang W., Chen Y., Huang J., et al. Dietary quercetin supplementation attenuates diarrhea and intestinal damage by regulating gut microbiota in weanling piglets. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/6221012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yadav S., Teng P.Y., Souza Dos Santos S.T., Gould R.L., Craig S.W., Lorraine Fuller A., et al. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult Sci. 2020;99:5936–5945. doi: 10.1016/j.psj.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Kim I.H. Effect of eugenol and cinnamaldehyde on the growth performance, nutrient digestibility, blood characteristics, fecal microbial shedding and fecal noxious gas content in growing pigs. Asian-Australas J Anim Sci. 2012;25:1178–1183. doi: 10.5713/ajas.2012.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Meng Q.W., Kim I.H. The effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics and fecal noxious gas content in growing pigs. Livest Sci. 2011;141:143–147. [Google Scholar]
- Yan L., Meng Q.W., Kim I.H. The effects of dietary Houttuynia cordata and Taraxacum officinale extract powder on growth performance, nutrient digestibility, blood characteristics and meat quality in finishing pigs. Livest Sci. 2011;141:188–193. [Google Scholar]
- Yang C.B., Chowdhury M.A.K., Huo Y.Q., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;23:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Tan S., Chen S., Wu Y., Yang Y., Li H., et al. Effects of fermented Yupingfeng on intramuscular fatty acids and ruminal microbiota in Qingyuan black goats. Anim Sci J. 2021;92 doi: 10.1111/asj.13554. [DOI] [PubMed] [Google Scholar]
- Yang Q., Scheie A.A., Benneche T., Wille M., Sorgelos P., Bossier P., et al. Specific quorum sensing-disrupting activity (AQSI) of thiophenones and their therapeutic potential. Sci Rep. 2015;5 doi: 10.1038/srep18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Du E., Yuan J., Gao J., Wang Y.L., Aggrey S.E., et al. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci Rep. 2017;7:7334. doi: 10.1038/s41598-017-07420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Hussain T., Tan B., Liu Y., Ji P., Yin Y. The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoides flavones using diquat-challenged piglet models. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/8140962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S.J., Bae G.S., Park J.H., Song T.H., Choi A., Ryu B.Y., et al. Antioxidant effects of cultured wild ginseng root extracts on the male reproductive function of boars and Guinea pigs. Anim Reprod Sci. 2016;170:51–60. doi: 10.1016/j.anireprosci.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Zhai J.H., Tao L.N., Zhang S.X., Gao H., Zhang Y., Sun J., et al. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother Res. 2020;34:649–659. doi: 10.1002/ptr.6557. [DOI] [PubMed] [Google Scholar]
- Zhang N.Y., Qi M., Zhao L., Zhu M.K., Guo J., Liu J., et al. Curcumin prevents aflatoxin B₁ hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Yang Q., Defoirdt T. Indole decreases the virulence of pathogenic vibrios belonging to the Harveyi clade. J Appl Microbiol. 2022;132:167–176. doi: 10.1111/jam.15227. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Luo H., Liu K., Jia H.N., Chen Y., Wang Z.Z. Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Sci. 2015;105:38–45. doi: 10.1016/j.meatsci.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang H., Mei N., Ma C., Lou Z., Lv W., et al. Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int J Biol Macromol. 2018;107:230–235. doi: 10.1016/j.ijbiomac.2017.08.175. [DOI] [PubMed] [Google Scholar]
- Zhao C., Schieber A., Gänzle M. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations-a review. Food Res Int. 2016;89:39–47. doi: 10.1016/j.foodres.2016.08.042. [DOI] [PubMed] [Google Scholar]
- Zhou L., Zheng H., Tang Y., Yu W., Gong Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol Lett. 2013;35:631–637. doi: 10.1007/s10529-012-1126-x. [DOI] [PubMed] [Google Scholar]
- Zhou R., Wu J., Lang X., Liu L., Casper D.P., Wang C., et al. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J Dairy Sci. 2020;103:2303–2314. doi: 10.3168/jds.2019-16611. [DOI] [PubMed] [Google Scholar]
- Zhou T.X., Zhang Z.F., Kim I.H. Effects of dietary Coptis chinensis herb extract on growth performance, nutrient digestibility, blood characteristics and meat quality in growing-finishing pigs. Asian-Australas J Anim Sci. 2013;26:108–115. doi: 10.5713/ajas.2011.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Yin X., Yu H., Zhao L., Sabour P., Gong J. Involvement of quorum sensing and heat-stable enterotoxin a in cell damage caused by a porcine enterotoxigenic Escherichia coli strain. Infect Immun. 2011;79:1688–1695. doi: 10.1128/IAI.01281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Wu H., Wang X., He J., He S., Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/7591840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Hu X.M., Zhang T., Wei H.K., Zhou Y.F., Zhou Z.X., et al. Effects of dietary oregano essential oil and vitamin E supplementation on meat quality, stress response and intestinal morphology in pigs following transport stress. J Vet Med Sci. 2017;79:328–335. doi: 10.1292/jvms.16-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Xiang Q., Wang J., Wei H., Peng J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest Sci. 2016;192:33–38. [Google Scholar]