Abstract
Malaria kills more than 600 000 people yearly, mainly children, and eradication is a global priority. Malaria transmission-blocking vaccines are advancing in clinical trials, and strategies for their introduction must be prioritized among stakeholders and the vulnerable populations exposed to the disease.
There is a lot of malaria in Kinango, especially now during the rainy season... Therefore, if that vaccine comes, all of us will feel some relief. And we are really eager for it! (A mother from Kenya, quoted in [1].)
Vaccines confer extensive long-term benefits to society: prevention of disease and premature death, the economic gain from averted health expenses, reduced work absences and improved individual performance (especially with regard to child development), and increased investment in biotechnology research. A malaria vaccine is strongly desired by communities affected by a disease that, in 2017, killed over 600 000 people globally [2], the majority being African children under 5 years of age. Although the complex life cycle and immune-evasion strategies of Plasmodium have made vaccine development an arduous challenge, several candidates are advancing in clinical trials, including transmission-blocking vaccines (TBVs) which uniquely target the mosquito stages of the parasite’s life cycle. Given the recent progress in product and clinical development, increased attention is needed to prepare for the introduction of TBVs in malaria-affected areas, particularly low-resource settings. Here, we briefly highlight some key discussions between communities, researchers, and country stakeholders on the introduction of TBVs in endemic, low-resource communities.
Malaria TBVs: Community Protection by Immunization
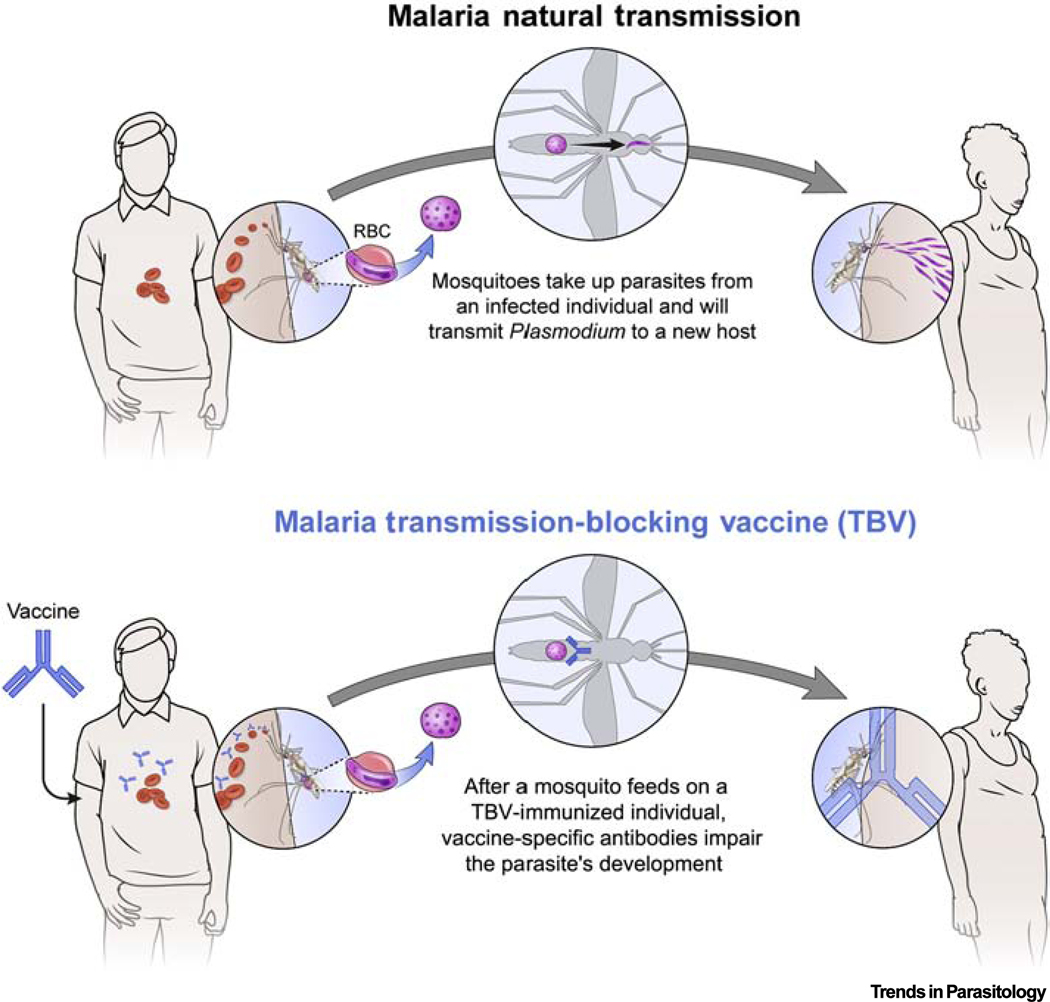
Plasmodium spp. are transmitted by the Anopheles mosquito vector which carries the malaria parasite from an infected human to the next human host. Since this transmission cycle is fundamental for parasite survival, malaria control or elimination may be achieved by interrupting parasite development in the mosquito vector. In pursuit of this strategy, TBVs have been developed to induce (in humans) antibodies against sexual stages of the parasite. Upon uptake of these antibodies by a biting mosquito during a blood meal, the antibodies impair parasite development in the mosquito midgut, reducing potential for further transmission from the mosquito to the next human host (Figure 1). The malaria TBV is sometimes referred to as an ‘altruistic’ vaccine, or vaccine of solidarity, because it requires herd immunity to reduce malaria incidence rates in the community as well as in the vaccine recipient [3]. While TBVs have shown promising safety and functional activity in clinical trials [4,5], introducing this vaccine approach to the community will entail some unique logistical, educational, and ethical challenges.
Figure 1. Mechanism of Action of Transmission-Blocking Vaccines (TBVs) in Malaria-endemic Areas.
TBVs elicit antibodies that are able to neutralize sexual-stage parasites and prevent mosquito infectivity, thereby reducing transmission from one person to the next. TBVs require herd immunity to reduce malaria incidence in the community, including the vaccine recipient. Abbreviation: RBC, red blood cell.
Malaria TBVs May Become a Reality, But How Will Affected Communities Perceive Them?
While malaria cases and deaths declined substantially over a decade of improved global control, progress has stalled since 2015 in Africa and Latin America [6]. Several factors hinder elimination efforts and allow resurgence both globally and in Africa: (i) antimalarial drug resistance; (ii) mosquito resistance to insecticides; (iii) human migrations of vulnerable populations to disease-endemic regions; (iv) political instability, conflict, and war which thwart public health programs; (v) climate change that expands Anopheles vector habitats; and (vi) lack of sustained domestic financing and predictable international funding. Novel strategies must be developed and implemented to reverse this alarming trend.
While malaria vaccine research has been ongoing for decades [7,8], success in late-stage trials was only achieved recently [9]. Following its approval by the European Medical Agency (EMA) for children, the WHO recently recommended implementation trials of RTS,S (Mosquirix®) vaccine in several African countries. Mosquirix® incorporates the Plasmodium falciparum circumsporozoite protein (CSP) formulated in the adjuvant AS01E® [10]. Since Mosquirix® is only partially protective, the vaccine will likely supplement rather than replace existing malaria-control approaches, including antimalarial drugs and bed nets. Thus, continued efforts are needed to develop other types of malaria vaccine, including whole-cell sporozoite vaccines that prevent infection, recombinant vaccines that limit blood-stage parasite growth, and TBVs.
The introduction of TBV poses unique challenges because of its unusual community-based approach to protection. We summarize these challenges into four categories: (i) logistical, (ii) educational, (iii) ethical, and (iv) public policy challenges:
Logistical challenges. A herd-immunity approach requires adequate and potentially high levels of vaccine coverage in the community [3], which means that adults, children, pregnant women, and women of child-bearing potential might all be considered for inclusion in mass immunization campaigns. For children, finding an existing suitable vaccine infrastructure is paramount; for example, the Expanded Program on Immunization (EPI) currently delivers vaccines in early infancy [e.g., diphtheria, pertussis, tetanus (DPT) and polio vaccines], or later in infancy and at school entry [e.g., measles, mumps, rubella (MMR) vaccine]. Effectively incorporating a TBV into the current EPI schedule will require further studies to rule out immunological interference between the newly introduced product and the existing vaccines, and identify which vaccines should be coadministered. Another option is to utilize school-based delivery systems that may provide the human papillomavirus virus (HPV) vaccine to prevent cervical cancer.
Educational challenges. Understanding the cultural and social context of communities in malaria-endemic areas, as well as their perception of malaria vaccine implementation, will be critical for successful deployment [1,11]. Since broadly-based malaria vaccine licensure and indications have still not been realized, few studies have investigated community perception of immunization as a malaria-prevention strategy (Table 1). The limited inquiries performed in endemic areas of Africa and Latin America suggest that, while few lay people are aware of vaccine progress, communities generally have a positive perception around implementation of malaria vaccines [12–15]. One study assessed parental opinions on TBVs in an Amazonian community where more than 90% of the population has been infected by Plasmodium; 99.3% of mothers expressed willingness to receive such a vaccine, and 90.2% would approve TBV vaccination for their children [12]. However, studies are needed to assess community perception of TBV more widely, and community leaders and country public health stakeholders must understand the potential for TBV to confer community protection and thereby contribute to malaria elimination. We contend that community perceptions and perspectives on implementation of TBVs should be considered when designing public-health programs to prevent malaria, which then can better inform policies to educate communities about TBV. The goals of vaccine administration should be clearly explained to parents to ensure that they make informed decisions to vaccinate. In low-resource settings, parents need additional education on prevention of an infectious disease that primarily affects children. In places where malaria is still attributed to cultural and religious factors, vaccines are often rejected [1]. Thus, education in primary public health strategies becomes essential to mobilize the population and combat the disease. For example, 51.4% of residents in a malaria-endemic community in Peru were not aware of any prospect for malaria vaccines [13], posing a significant challenge for community engagement and vaccine promotion.
Ethical challenges. While the literature onethical issues pertinent to other malaria prevention strategies (i.e., drugs and vector control) have been identified and addressed, ethical aspects of malaria vaccination have sparked only modest discussion and debate, particularly for the novel approach of TBVs. A key ethical consideration for TBVs is the reliance on herd immunity principles, as benefits to vaccination are only derived when a sufficient proportion of the population is vaccinated. Benefits will also presumably be afforded equally to vaccinees (assuming they reside in the target community) and nonvaccinated individuals. This approach is advantageous since protection will be offered to those who cannot be vaccinated based on factors such as age or potential vaccine sensitivity. In this way, vaccinees can contribute to protection of their family and neighbors and reduce the disease burden in the community. Ethical issues surrounding TBVs should also be considered in the context of other available prevention tools (drugs, vector approaches, other components in a multistage malaria vaccine), since elimination campaigns that incorporate TBVs will likely deploy a combination of tools to maximize communitywide malaria protection. In these scenarios, ethical issues pertinent to the other tools will also need to be addressed.
Public policy challenges. Even in a favorable scenario, where endemic communities understand the potential benefits of a TBV and foster initiatives for implementation, country leaders, policy makers, manufacturers, and other public health stakeholders must also be persuaded to adopt this unorthodox approach. In particular, decision-makers must be convinced of the favorable cost-benefit ratio for a vaccine that does not provide individual benefit to the recipient, but instead provides group benefits to a community of recipients. It is necessary to identity domestic and international solutions for the introduction and implementation of TBVs for affected communities in low-resource settings. In addition to public policy, the regulatory science behind TBVs presents another related challenge. Regulatory agencies will need to appreciate the potential clinical benefits of these vaccines, as well as understand their role in designing future policies for TBV introduction in affected countries. Those agencies will need to monitor TBV safety, effectiveness, and possible side effects, as well as oversee ongoing manufacture to ensure continuing safety. In agreement with recent WHO recommendations [6], we believe that malaria elimination requires affected countries to start prioritizing country-driven policies, such as the strategic use of information to raise national awareness of malaria deaths, coordination of country-led responses to epidemics, and domestic financing of control and elimination programs.
Table 1.
Studies Evaluating Acceptability of Malaria Vaccines in Malaria-Endemic Areas
| Community | Type of malaria vaccine | Profile of interviewed people | Acceptability | Refs, year |
|---|---|---|---|---|
| Amazon (Peru) | Malaria TBVa | 18 years old and older | 90.2% of caregivers would give their children a malaria TBV | [12] 2018 |
| South East Nigeria | Malaria vaccines | 21 years old and older | 86.7% of caregivers would give their children a malaria vaccine | [13] 2018 |
| 12 districts in Tanzania | Malaria vaccines | Between 15 and 60 years old | 97.6% of caregivers would give their children a malaria vaccine | [14] 2016 |
| 23 regions in Tanzania | Malaria vaccines | Women, 18 years or older and with children under 11 months old | 94.5 % of caregivers would give their children a malaria vaccine | [15] 2015 |
Only one study, in the Amazon, assessed community perception of malaria TBVs.
Concluding Remarks
TBV implementation planning should incorporate realistic time considerations. TBV benefits and implementation strategies will need to be understood in advance, since policies and actions must be coordinated among stakeholders at many levels [16]. Even conventional vaccines face a sobering reality: life-saving products such as vaccines against Haemophilus influenzae type B or pneumococcus were implemented in only 15% of low-income countries on average 10 years after licensure [17]. No low-income country introduced any of the vaccines surveyed (hepatitis B, H. influenzae type B, rotavirus, and pneumococcus) within the first 5 years after licensure. Financial sustainability is critical. Unfortunately, ‘where malaria prospers most, human societies have prospered least’ [18]. Although low-income countries are largely dependent on external financial support for vaccine implementation, they have increased their investment in immunization policy. However, this increase is insufficient to financially sustain malaria vaccination. In countries that receive support from Gavi, the Vaccine Alliance (a public–private global health partnership committed to increasing access to immunization in poor countries), the average price paid for vaccines by the government per child in 2015 ranged from $3.80 to $5.09 USD. A recent survey in a malaria-endemic area in Peru showed that 61% of the population interviewed would pay for their own malaria vaccine – but only if the price ranged from $0.36 to $2.00 [12]. Ultimately, since TBVs will most likely be used in extremely low-resource areas, where malaria is holoendemic or where there is intense seasonal transmission, it will almost certainly require full Gavi support.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. We thank J. Patrick Gorres for assistance editing the manuscript.
Footnotes
Disclaimer Statement
R.R. is a full-time employee of the GlaxoSmithKline group of companies.
References
- 1.Ojakaa DI et al. (2011) Community perceptions of malaria and vaccines in the South Coast and Busia regions of Kenya. Malar. J. 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980– 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White MT et al. (2015) Vaccine approaches to malaria control and elimination: Insights from mathematical models. Vaccine 33, 7544–7550 [DOI] [PubMed] [Google Scholar]
- 4.Talaat KR et al. (2016) Safety and immunogenicity of Pfs25-EPA/Alhydrogel(R), a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria naive adults. PLoS One 11, e0163144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagara I. et al. (2018) Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparatorcontrolled, dose-escalation study in healthy Malian adults. Lancet Infect. Dis. 18, 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO (2018) World Malaria Report, WHO [Google Scholar]
- 7.Ananian SA (1945) Experimental data regarding malaria immunization with formol vaccine. Mikrobiologiia 14, 55–58 [PubMed] [Google Scholar]
- 8.Richie TL and Saul A. (2002) Progress and challenges for malaria vaccines. Nature 415, 694–701 [DOI] [PubMed] [Google Scholar]
- 9.Coelho CH et al. (2017) Advances in malaria vaccine development: report from the 2017 malaria vaccine symposium. NPJ Vaccines 2, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The RTS S Clinical Trials Partnership (2014) Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 11, e1001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menaca A. et al. (2014) Factors likely to affect community acceptance of a malaria vaccine in two districts of Ghana: a qualitative study. PLoS One 9, e109707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whit SE. et al. (2018) Acceptability of a herd immunityfocused, transmission-blocking malaria vaccine in malaria-endemic communities in the Peruvian Amazon: an exploratory study. Malar. J. 17, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chukwuocha UM et al. (2018) Awareness, perceptions and intent to comply with the prospective malaria vaccine in parts of South Eastern Nigeria. Malar. J. 17, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mtenga S. et al. (2016) Stakeholders’ opinions and questions regarding the anticipated malaria vaccine in Tanzania. Malar. J. 15, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romore I. et al. (2015) Assessment of parental perception of malaria vaccine in Tanzania. Malar. J. 14, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standaert B. and Rappuoli R. (2017) 3. How comprehensive can we be in the economic assessment of vaccines? J. Mark. Access. Health Policy 5, 1336044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks A. et al. (2012) Implementing new health interventions in developing countries: why do we lose a decade or more? BMC Pub. Health 12, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachs J. and Malaney P. (2002) The economic and social burden of malaria. Nature 415, 680–685 [DOI] [PubMed] [Google Scholar]