Abstract
There are significant variations in practice regarding the use of sleep studies in children with symptoms of sleep disordered breathing (SDB) prior to adenotonsillectomy. Current UK guidance recommends the selective use of sleep studies to confirm a diagnosis of obstructive sleep apnoea (OSA) when there is diagnostic uncertainty, in children with comorbidities, or to assess perioperative risk when severe OSA is suspected. We have developed a novel paediatric sleep service over the past decade based on the routine use of multi-channel sleep studies (MCSS) before adenotonsillectomy. We present the results of a prospective evaluation assessing the impact of our service on treatment outcomes.
We conducted a prospective service evaluation of 49 children with SDB seen between July 2021 and August 2022. We used medical records and a sleep study database to determine treatment outcomes. Otolaryngologists completed a questionnaire before each multi-channel sleep study to help evaluate the impact of sleep study findings on surgical decision making.
Questionnaire responses before MCSS showed that clinicians thought 66 % of children were ‘likely’, ‘very likely’ or ‘definitely’ would require surgery but only 54 % of children underwent surgery following their sleep study. We estimate that the use of MCSS was associated with a 21 % reduction in children undergoing surgery in this small sample.
We conclude that our use of MCSS facilitates conservative management, allowing a significant reduction in the number of children with SDB undergoing surgery, but further validation of MCSS against polysomnography is required.
Highlights
-
•
Sleep disordered breathing is often diagnosed by clinical evaluation before surgery.
-
•
Routine sleep studies to confirm SDB are advised in some guidance but not in the UK.
-
•
Reports suggest a limited multi-channel study may detect SDB with high sensitivity.
-
•
Our routine use of MCSS to confirm SDB reduced the children having surgery by 21 %.
Abbreviations
- AHI
Apnoea-hypopnoea index
- BMI
Body mass index
- BTS
British Thoracic Society
- CCG
Clinical commissioning group
- CI
Confidence interval
- CPAP
Continuous positive airway pressure
- CRSS
Cardiorespiratory sleep study/studies
- ECG
Electrocardiogram
- EEG
Electroencephalogram
- ENT
Ear, nose and throat
- HDU
High dependency unit
- MCSS
Multi-channel sleep studies
- NHS
National health service
- ODI3
3 % oxygen desaturation index
- ODI4
4 % oxygen desaturation index
- OSA
Obstructive sleep apnoea
- PSG
Polysomnography
- PS
primary snoring
- SDB
Sleep-disordered breathing
- UARS
Upper airway resistance syndrome
1. Introduction
Obstructive sleep apnoea (OSA) is estimated to occur in 1–4% of children and is associated with cognitive and behavioural impairments among other things [1,2]. Adenotonsillectomy is considered the mainstay of treatment although nasal corticosteroids ± leukotriene antagonists have a role in the treatment of some children whilst others, particularly school aged children (5–9 years old), with mild OSA can be safely managed with watchful waiting [3]. The children with symptoms of sleep disordered breathing most likely to benefit from treatment are not easily identified on the history and examination findings alone when judged by the gold standard investigation, polysomnography (PSG) [4,5]. Our secondary care centre has established a multi-channel sleep study (MCSS) service combining the findings of oximetry and video to diagnose SDB in children and we have evaluated the impact of the service on treatment outcomes. The evaluation was conducted in two parts with a retrospective and a prospective review of sleep study findings and treatment outcomes. We report the findings of the prospective evaluation here.
2. Methods
We conducted a prospective evaluation of our sleep study service between July 2021–August 2022. We used anonymised details from patient electronic records or case notes and a sleep study database, maintained for audit and service evaluation purposes, to determine demographics, sleep study findings and treatment outcomes. Otolaryngologists completed a questionnaire to document their likely management decisions based on the history and examination findings prior to requesting a sleep study. Participating surgeons completed the questionnaire in consecutive unselected children attending as new patients with concerns about SDB during the study period. The questionnaire was completed in all cases before the sleep study was performed to allow assessment of the impact of MCSS on surgeons’ decision making. A copy of the questionnaire is attached as an appendix.
2.1. Inclusion criteria
All children (<18 years old) attending the Ear Nose and Throat (ENT) clinics and assessed by otolaryngologists for suspected SDB were eligible for inclusion, including those with co-morbidities such as a craniofacial syndrome, a neurodevelopmental disorder or obesity.
2.2. Exclusion criteria
Children who were managed with a clinical evaluation alone and who did not undergo a sleep study were excluded. We excluded 4 children whose sleep study had <4 h artefact-free oximetry or a technical fault causing failure of the video recording.
2.3. Sleep studies
Multi-channel sleep studies were performed with Stowood Scientific Instruments VISI-3 sleep systems incorporating ECG, video, sound, movement, pulse transit time and oximetry data. Video was recorded with a Sony EVI-D90P infra-red camera. The VISI-3 sleep system uses Masimo technology to obtain oximetry data with 2–4 s averaging times. Oximetry was measured with probes on a finger or toe. The following oximetry indices were recorded: mean saturation, minimum saturation, dip index defined as > 4 % drop (i.e. 5 % or greater) in baseline saturation/hour and lasting for >5 but <180 s. Abnormal oximetry was defined as shown in Table 1:
Table 1.
Oximetry risk criteria for OSA.
| Normal | Inconclusive | Abnormal, low risk | Abnormal, high risk | ||
|---|---|---|---|---|---|
| Baseline | ≥94 % | 94 % | <94 % | <94 % | |
| And | Or | Or | Or | ||
| Dip Index (>4 % dip from baseline) | <4/hour | ≥4/hour | <4/hour | ≥4/hour | ≥4/hour |
| And | And | And | And | And | |
| Minimum saturation | >90 % | >90 % | 80–90 % | 80–90 % | <80 % |
Sleep study categories were determined by a clinician (MY) using oximetry, video and sound criteria as listed below. Our sleep reporting methods are described in more detail in an accompanying article with findings from the retrospective study [6]. The oximetry, heart rate, sound and movement traces were used to identify sections of video that needed closer inspection. The video was assessed for evidence of obstructive episodes (defined below). Following assessment of the oximetry data, sleep study montage and video, the reporting clinician assigned one of the following five categories: normal; primary snoring; upper airway resistance syndrome; obstructive sleep apnoea or abnormal other.
2.4. Sleep study category definitions
-
⁃
Normal: No snoring or obstructed breathing evident on video + normal or inconclusive oximetry (Table 1)
-
⁃
Primary snoring: Snoring but <3 obstructive episodes seen on video + normal or inconclusive oximetry
-
⁃
Upper airway resistance syndrome: Video and sound evidence of 3 or more discrete periods of obstructed breathing, associated arousals + normal or inconclusive oximetry
-
⁃
Obstructive sleep apnoea: Video and sound evidence of obstructed breathing, associated arousals + abnormal oximetry
-
⁃
Abnormal other: Abnormal oximetry findings without any associated video evidence of snoring or obstructed breathing.
2.5. Other definitions
-
⁃
‘Obstructed breathing’ - video and sound recordings document a brief pause in snoring but continued chest wall movement, followed by a gasp or other airway opening noise (i.e. a click or grunt). A period of obstructed breathing was considered to have occurred if the airway opening noise was accompanied by an arousal, indicating that a degree of increased respiratory effort was needed to overcome an obstructed airway. Only obstructive episodes with an associated arousal were counted.
-
⁃
An arousal was identified on video if movement of any body part was evident immediately after an obstructive event.
2.6. Treatment outcomes
Children were assessed to have one of the following treatment outcomes: watchful waiting or no treatment; medical therapy (nasal corticosteroids/leukotriene antagonists) or surgery (adenotonsillectomy, adenoidectomy, tonsillectomy).
2.7. Ethical considerations
We approached the Research and Innovation department at the hospital Trust in advance of data collection. Ethical approval was deemed to not be required for this service evaluation.
2.8. Data analysis
Data analysis was performed using MS Excel. Data were excluded if fewer than 4 h of artefact-free oximetry was recorded, and then sorted by outcome or diagnosis. Descriptive statistics were performed.
3. Results
Questionnaire responses (n = 49):
-
⁃
33/49 (68 %) had a “strong history of OSA” that met CCG criteria* for surgery
-
⁃
33/49 (68 %) of parents thought their child probably needed surgery
-
⁃
Clinicians thought that 32/49 (66 %) were ‘likely’, ‘very likely’ or ‘definitely’ required surgery
-
⁃
4 children did not have sleep studies and were managed using clinical evaluation
-
⁃
45 children underwent sleep studies
-
⁃
Data from 4 studies were excluded for technical reasons
-
⁃
Data from 41 studies are included in analysis
-
⁃
29/41 (71 %) had a “strong history of OSA” that met CCG criteria for surgery
-
⁃
29/41 (71 %) thought their child probably needed surgery
-
⁃
Clinicians thought that 28/41 (68%) were ‘likely’, ‘very likely’ or ‘definitely’ required surgery
*CCG criteria that need to be met before funding for adenotonsillectomy for sleep apnoea syndrome is approved include one or more of the following: positive sleep study; significant impact on quality of life demonstrated; or strong clinical history suggestive of sleep apnoea
Table 2 lists the sleep study findings and demographics.
-
⁃
2 children had a BMI >2.5 SDS
-
⁃
1 child had cerebral palsy (dystonic quadriplegia)
-
⁃
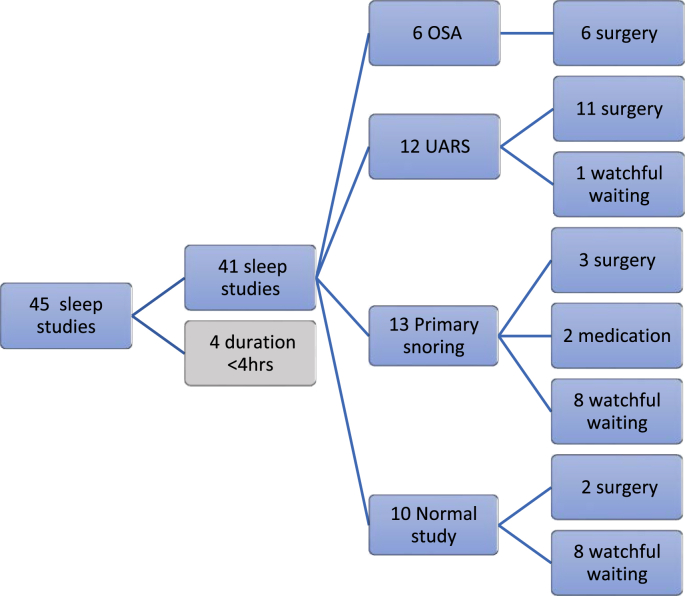
18/41 (44 %) of children had a positive sleep study - OSA/UARS
-
⁃
22/41 (54 %) of children underwent surgery
-
⁃
There was a 21 % reduction in the number of children having surgery compared to the questionnaire estimate prior to a sleep study - 28/41 (68 %)
Table 2.
Sleep study findings and demographics.
| Study outcome | Males | Females | Mean Age (years) | Body Mass Index Kg/m2 (n = 35) |
|---|---|---|---|---|
| Normal (n = 10) | 4 (40 %) | 6 (60 %) | 4 (range 2–6, SD 1.5) | |
| Primary snoring (n = 13) | 5 (38 %) | 8 (62 %) | 6.5 (range 1–15, SD 4.3) | |
| UARS (n = 12) | 5 (42 %) | 7 (58 %) | 4.5 (range 2–7, SD 1.6) | |
| OSA (n = 6) | 3 (50 %) | 3 (50 %) | 2.5 (range 1–4, SD 1.4) | |
| All (n = 41) | 17 (41 %) | 24 (59 %) | 4.7 (range 0–15, SD 3.0) | 17.7 (SD 4.1) |
| Mean study duration | 7.9 h (SD 1.6) | |||
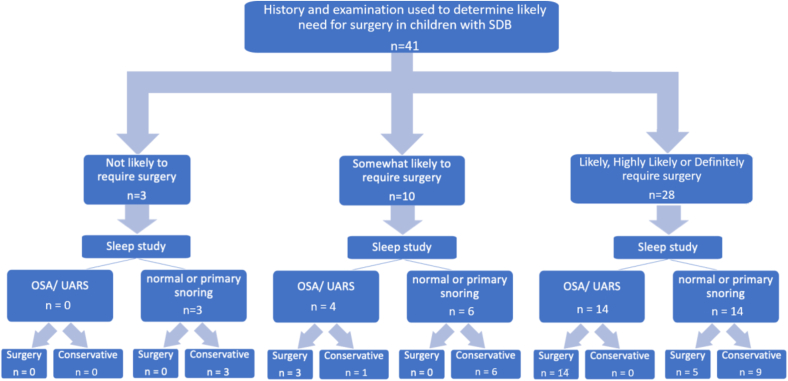
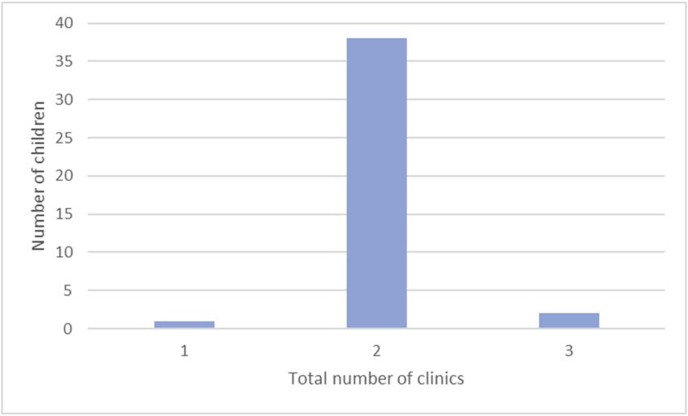
Fig. 1 is a flow chart of the treatment outcomes for children in the prospective study. Fig. 2 is a flow chart demonstrating the impact of the sleep study findings on clinicians’ management decisions. Fig. 3 is a bar chart of the number of appointments attended. The mean total number of appointments in the study were 2.0 (SD 0.3).
Fig. 1.
Flow chart showing treatment outcomes following multi-channel studies.
Fig. 2.
Flow chart showing questionnaire responses and impact of sleep studies on management decisions.
Fig. 3.
Total number of clinic appointments attended.
4. Discussion
Most UK secondary care centres assess children with SDB using oximetry alone whereas tertiary centres usually have the option of using oximetry, cardiorespiratory sleep studies (CRSS) or PSG. Access to PSG in the National Health Service (NHS) is limited and so there is a greater reliance on less sensitive tools to diagnose SDB in children. This was one of the key reasons that the recently published BTS guideline for diagnosing and monitoring paediatric SDB was deemed necessary as the European guidance was felt to be largely inapplicable in the NHS setting [7].
A previous report of our use of MCSS (oximetry plus video) to diagnose SDB in children showed that, compared with using oximetry alone, we could identify twice the number with SDB who might benefit from intervention [8]. As use of MCSS has limited evidence (and so was not evaluated as part of the BTS guidance), we have undertaken this service evaluation to better understand its impact on treatment.
A key strength of the sleep service is the use of two modalities accessible to general paediatricians (non-specialists) which, used together, significantly improve the sensitivity of detecting SDB that may require intervention. We postulate that children with a video diagnosis of UARS have either UARS or mild OSA (PSG defined AHI >1) and evidence from previous work suggests that children with abnormal oximetry (and video confirmation of OSA) would correlate closely with a PSG defined AHI >5 [8,9].
A study by Sivan et al. investigated the diagnostic accuracy of a 30-min sleep video recording in 58 children with symptoms of SDB who also had PSG [10]. Parents were asked to record videos using a defined format (including close-up views of the head and naked trunk), during periods of snoring, laboured breathing, or other breathing patterns of concern. A clinician assessed the videos using a scoring system based on noisy breathing, movements, waking episodes, apnoea, chest retractions and mouth breathing. The authors found that the sleep videos identified SDB with a sensitivity of 0.94 (95 % confidence intervals: 0.81, 0.99] and specificity 0.68 [0.45, 0.86].
Based on the findings of this prospective study, we estimate that our use of MCSS reduced the number of children undergoing surgery for SDB by 21 %. This is based on the questionnaire responses indicating that 68 % (28/41) of children undergoing sleep studies were ‘likely’, ‘very likely’ or ‘definitely’ required surgery. If half of the children who were considered ‘somewhat likely’ to need surgery also had it, then we estimate that 80 % of children in this cohort may have been considered for surgery if the decision was based on a clinical evaluation alone. Based on a possible surgery rate of 80 %, the estimated reduction in surgery resulting from the use of MCSS is 33 %.
Use of a tool that detects SDB with high sensitivity increases confidence, in clinicians and parents, that a negative result excludes children with significant disease. In our centre, Otolaryngologists are more confident about recommending conservative management in children with a negative MCSS result which has resulted in a corresponding reduction in rates of adenotonsillectomy. In this service evaluation we demonstrate a potential reduction of 21–33 % in the number of children undergoing surgery after MCSS, compared with the proportion in whom surgery was thought likely to be required after baseline clinical evaluation alone.
4.1. Study limitations and bias
This prospective study is limited by the small sample size which may either underestimate or overestimate the true impact of MCSS on treatment outcomes. We have attempted to address this by conducting a retrospective evaluation in a much larger sample of children which we have reported separately.
Our definition for abnormal oximetry is given in Table 1, where we use the ODI4 (i.e. a desaturation of 5 % or greater from baseline). We acknowledge that in paediatric practice the ODI3 is also frequently used, but we have used an ODI4 of ≥4/hour in our definition see Table 1) [6].
We acknowledge the potential for bias in the selection of children for the prospective study despite the inclusion criteria being consecutive unselected children attending the ENT clinic for a new appointment. Invariably, some children who may have been eligible for selection weren't considered if the clinician running a busy clinic forgot to complete the survey at the time, so it is possible that, by chance, those selected for the study have a higher likelihood of moderate-severe SDB than those not included.
We also acknowledge that, given the survey was evaluating surgical decision-making, it may have had an impact on surgeons’ behaviour and led to them being more circumspect about which children they considered for surgery. We suspect that this could have led to bias in a way that meant surgery was offered to more or fewer children than would have been the case. Given that our centre has a settled practice of managing a significant proportion of children conservatively, there is more experience and confidence within the team about doing this which may also be reflected in the questionnaire responses. It is possible that, in other ENT centres, a higher proportion of children presenting with symptoms of SDB would be considered for surgery than we found in this study.
The questionnaire was intended to be a simple tool to help capture a surgeon's decision about whether they would plan to offer surgery based on the history and examination findings alone and without the influence of the MCSS findings. In our view the questionnaire was effective in achieving its purpose. We also feel that by offering 5 options on a Likert scale we minimised the chance of bias that would be inherent in a ‘Yes’/‘No’ response.
It was of interest to note the concordance between parents and surgeons’ views about the proportion of children that required surgery for OSA. Parental views about whether their child warrants surgery for OSA has been found to be a predictor of those with moderate-severe OSA based on PSG findings [11].
A significant limitation of our findings is that the simplified type of MCSS used, combining oximetry and video to diagnose SDB, has only limited validation against PSG [12]. Further validation work is needed before MCSS can be accepted into regular practice more widely.
5. Conclusion
We have shown that our use of MCSS facilitates conservative management and is associated with a significant reduction in the number of children with symptoms of SDB undergoing surgery. We note that this form of MCSS, is more accessible to non-specialist paediatricians than PSG or CRSS and is therefore more suited to use in secondary care. Further validation of this limited form of MCSS against PSG is required to establish its value and limitations before it can be recommended for use more widely. A larger prospective study is required to assess more accurately the degree to which the routine use of MCSS reduces the need for surgery in children with symptoms of SDB.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Michael Yanney: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Nicola Rowbotham: Writing – review & editing, Formal analysis, Data curation, Conceptualization. Christabella Ng: Writing – review & editing, Methodology, Formal analysis, Data curation. Muhammad Zulkifli: Methodology, Formal analysis, Data curation, Conceptualization. Ahmed Shehata: Data curation. Alagappan Chidambaram: Writing – review & editing, Methodology, Data curation, Conceptualization. Paraskevi Tsirevelou: Writing – review & editing, Methodology, Data curation, Conceptualization. Neil Fergie: Writing – review & editing, Methodology, Data curation, Conceptualization. Pathik Thakkar: Formal analysis, Data curation. Emma Crookes: Methodology, Data curation. Roy Dean: Methodology, Data curation. Andrew Prayle: Writing – review & editing, Methodology, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nicola Rowbotham reports a relationship with Cystic Fibrosis Trust that includes: travel reimbursement. Nicola Rowbotham reports a relationship with European Cystic Fibrosis Society that includes: travel reimbursement. Nicola Rowbotham reports a relationship with British Paediatric Respiratory Society that includes: travel reimbursement. Andrew Prayle reports a relationship with Vertex Pharmaceuticals Incorporated that includes: funding grants and travel reimbursement. Andrew Prayle reports a relationship with Cystic Fibrosis Trust that includes: funding grants. Andrew Prayle reports a relationship with Sir Jules Thorn Charitable Trust that includes: funding grants. Andrew Prayle reports a relationship with Nottingham University Hospitals NHS Trust that includes: funding grants. Andrew Prayle reports a relationship with UKRI Medical Research Council that includes: funding grants. Andrew Prayle reports a relationship with Action for AT that includes: funding grants.
References
- 1.Lumeng J.C., Chervin R.D. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horne R.S.C., Roy B., Walter L.M., Biggs S.N., Tamanyan K., Weichard A., et al. Regional brain tissue changes and associations with disease severity in children with sleep disordered breathing. Sleep. 2018;41:1–10. doi: 10.1093/sleep/zsx203. [DOI] [PubMed] [Google Scholar]
- 3.Marcus C.L., Moore R.H., Rosen C.L., Giordani B., Garetz S.L., Taylor H.G., et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brietzke S.E., Katz E.S., Roberson D.W. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg. 2004;131:827–832. doi: 10.1016/j.otohns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang R.C., Elkins T.P., Keech D., Wauquier A., Hubbard D. Accuracy of clinical evaluation in pediatric obstructive sleep apnea. Otolaryngol Head Neck Surg. 1998;118:69–73. doi: 10.1016/S0194-5998(98)70377-8. [DOI] [PubMed] [Google Scholar]
- 6.Yanney M.P., Rowbotham N.J., Ng C., Zulkifli M., Shehata A., Chidambaram A., et al. Retrospective review of treatment outcomes and costs in children with sleep disordered breathing assessed with multi-channel studies. Sleep Med. 2024 doi: 10.1016/j.sleepx.2024.100115. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans H.J., Gibson N.A., Bennett J. On behalf of the BTS paediatric sleep disorders Guideline Development Group, et al British Thoracic Society guideline for diagnosing and monitoring paediatric sleep-disordered breathing. Thorax. 2023;78:s1–s27. doi: 10.1136/thorax-2022-218938. [DOI] [PubMed] [Google Scholar]
- 8.Yanney M.P., Prayle A.P., Rowbotham N.J., Kurc M., Tilbrook S., Ali N. Observational study of pulse transit time in children with sleep disordered breathing. Front Neurol. 2000;11:316. doi: 10.3389/fneur.2020.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouillette R.T., Morielli A., Leimanis A., Waters K.A., Luciano R., Ducharme F.M. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405–412. doi: 10.1542/peds.105.2.405. [DOI] [PubMed] [Google Scholar]
- 10.Sivan Y., Kornecki A., Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J. 1996;9:2127–2131. doi: 10.1183/09031936.96.09102127. [DOI] [PubMed] [Google Scholar]
- 11.Lavi M., Taumanc R., Greenfeld M., Fishmana G., Wasserzuga O., DeRowe A. Parental concern as an indicator of the severity of Obstructive Sleep Apnea in children. Int J Pediatr Otorhinolaryngol. 2020;136 doi: 10.1016/j.ijporl.2020.110144. [DOI] [PubMed] [Google Scholar]
- 12.van Someren V., Burmester M., Alusi G., Lane R. Are sleep studies worth doing? Arch Dis Child. 2000;83:76–81. doi: 10.1136/adc.83.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]