Abstract
Purpose
There are a wide variety of intraoperative techniques available in breast surgery to achieve low rates for positive margins of excision. The objective of this systematic review was to determine the pooled diagnostic accuracy of intraoperative breast margin assessment techniques that have been evaluated in clinical practice.
Methods
This study was performed in accordance with PRISMA guidelines. A systematic search of the literature was conducted to identify studies assessing the diagnostic accuracy of intraoperative margin assessment techniques. Only clinical studies with raw diagnostic accuracy data as compared with final permanent section histopathology were included in the meta-analysis. A bivariate model for diagnostic meta-analysis was used to determine overall pooled sensitivity and specificity.
Results
Sixty-one studies were eligible for inclusion in this systematic review and meta-analysis. Cytology demonstrated the best diagnostic accuracy, with pooled sensitivity of 0.92 (95 % CI 0.77–0.98) and a pooled specificity of 0.95 (95 % CI 0.90–0.97). The findings also indicate good diagnostic accuracy for optical spectroscopy, with a pooled sensitivity of 0.86 (95 % CI 0.76–0.93) and a pooled specificity of 0.92 (95 % CI 0.82–0.97).
Conclusion
Pooled data indicate that optical spectroscopy, cytology and frozen section have the greatest diagnostic accuracy of currently available intraoperative margin assessment techniques. However, long turnaround time for results and their resource intensive nature has prevented widespread adoption of these methods. The aim of emerging technologies is to compete with the diagnostic accuracy of these established techniques, while improving speed and usability.
Keywords: Breast conserving surgery, Margin, Breast cancer, Breast surgery
Highlights
-
•
An estimated 20 % of patients who undergo breast conserving surgery will require re-operation for positive or close margins.
-
•
Intraoperative margin assessment techniques identify positive margins during primary surgery, avoiding a second operation.
-
•
Available techniques differ in terms of diagnostic accuracy, turnaround times, cost and practicality.
-
•
Cytology, frozen section & optical spectroscopy demonstrated the best diagnostic accuracy of currently available techniques.
Abbreviations
- CDP
cancer diagnostic probe
- CRR
cavity re-excision rate
- CTS
‘click-to-sense’ assay
- CYT
cytology
- FN
false negative
- FP
false positive
- FS
frozen section
- IBTR
ipsilateral breast tumour recurrence
- IOMA
intraoperative margin assessment
- IOMRI
intraoperative MRI
- IOUS
intraoperative ultrasound
- MCT
micro-CT
- MP
MarginProbe
- NPPV
negative predictive value
- OPT
optical spectroscopy
- PMR
positive margin rate
- PPV
positive predictive value
- REIMS
rapid evaporative ionisation mass spectrometry
- ROC
receiver operating characteristic
- ROR
re-operation rate
- SR
specimen radiography
- TAT
turnaround time
- TN
true negative
- TP
true positive
- WBI
whole breast irradiation
1. Introduction
Breast cancer is the most common cancer in women worldwide [1]. Most breast cancer patients present with early-stage disease, making them suitable candidates for breast-conserving surgery (BCS) [2]. However, an estimated 20 % of patients who undergo BCS require an additional operation for positive or close margins [[3], [4], [5]]. Positive margins are associated with significantly higher local recurrence rates [6,7]. Therefore, achieving adequate margins of excision is a crucial component of breast cancer surgery. Re-operation for positive margins not only has physical consequences, such as delayed adjuvant therapy and impaired cosmetic outcome, but also has psychological and economic repercussions. Given the high rates of re-excision following BCS, there has been significant research in the development of an accurate intraoperative margin assessment (IOMA) method. The purpose of IOMA tools is to identify positive margins during the primary surgery, facilitating further excision during the procedure and thus avoiding a second operation. Breast surgeons have numerous intraoperative techniques available to them, however, there is great variety in the evidence and practicality of these. Currently established IOMA techniques include pathological techniques such as frozen section (FS) and cytology (CYT); and imaging techniques such as specimen radiography (SR) and intraoperative ultrasound (IOUS). To address specific limitations associated with these methods, innovative IOMA tools have emerged; such as optical spectroscopy (OPT), micro-CT (MCT) and MarginProbe (MP). In recent years, there has been extensive research in the development and validation of these novel IOMA techniques for BCS. These emerging technologies aim to challenge the diagnostic accuracy of the currently established IOMA techniques, while improving speed, cost and practicality. This systematic review and meta-analysis aims to evaluate the pooled diagnostic accuracy of IOMA methods, both established and novel, that have been investigated in clinical practice.
2. Methods
This systematic review and meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Local institutional ethical approval was not required as all data used in this analysis were obtained from a previously published resource. All authors contributed to formulating the study protocol and it was then registered with the International Prospective Register of Systematic Reviews (PROSPERO Registration ID: CRD42022375035).
2.1. Search strategy
An electronic search was performed of the PubMed Medline, EMBASE, Cochrane and Scopus databases on November 10, 2022 for relevant studies that would be suitable for inclusion in this study. This search was per-formed by two independent reviewers (GPD & CH), using a pre-determined search strategy. The search was performed of all fields and included the search terms: (‘breast cancer’) AND (‘intraoperative’) AND (‘margin’) linked using the Boolean operator ‘AND.’ All study designs were included. Duplicate studies were manually removed. All titles and abstracts were initially screened, and studies deemed appropriate had their full texts reviewed. These studies were reviewed to ensure inclusion criteria were met for the primary outcome, with discordances in opinion arbitrated through consultation with a third author (GRD).
2.2. Inclusion criteria
Studies that reported margin assessment data from 1 or more intraoperative margin assessment technique used during breast surgery for invasive or in situ breast cancer were eligible. Only studies that contained sensitivity and specificity data compared with permanent section histopathology or in whom sensitivity and specificity data could be calculated from the raw data were included. Only studies written in English were included. Included studies were not restricted based on year of publication.
2.3. Exclusion criteria
Studies that did not report sensitivity and/or specificity data as compared with permanent section histopathology were excluded, however, data regarding positive predictive values (PPVs), negative predictive values (NPVs) and overall accuracy were not mandatory (these were calculated from the raw data where possible). Studies not written in English were excluded. Abstracts, conference articles, case studies, reviews and meta-analysis were excluded.
2.4. Data extraction and quality assessment
Two independent reviewers (GPD and CMH) extracted the following data using a pre-defined electronic spreadsheet: (1) the first author; (2) year of publication; (3) study design; (4) number of patients or samples; (5) mean age of patients; (6) diagnostic accuracy raw data—false negative (FN), false positive (FP), true negative (TN), true positive (TP); (7) percentages of sensitivity, specificity, PPV, NPV, diagnostic accuracy; (8) cavity re-excision rates (CRRs); (9) positive margin rates (PMRs); (10) re-operation rates (RORs); and (11) turn-around time for results. Quality assessment was performed using the QUADAS-2 tool (Supplementary Figs. 8 and 9), designed for evaluating risk of bias in diagnostic accuracy studies [8].
2.5. Statistical analysis
Stata version 17 (StataCorp College Station, Texas, USA), particularly the metandi command and metadta, were used for all statistical analyses [9,10]. The number of true positives, false positives, true negatives and false negatives and type of technique were extracted from each study. The number of true positives, false positives, true negatives and false negatives and type of technique were extracted from each study. The bivariate random effects model was applied to estimate summary estimates of sensitivity and specificity and their corresponding 95 % confidence intervals for each technique type. This approach was applied as it preserved the two-dimensional nature of the original data and took into account both study size and heterogeneity beyond chance between studies [11]. Sensitivity referred to the proportion of positive margins correctly classified as positive. Specificity was the proportion negative margins correctly classified negative.
Individual and summary estimates of sensitivity and specificity for the studies investigating each technique were plotted in a receiver operating characteristic (ROC) graph, plotting the rules sensitivity (true positive) on the y axis against 1-specificity (false negative) on the x axis. The 95 % confidence region and 95 % prediction region around the pooled estimates were included to illustrate the precision with which the pooled values were estimated (confidence ellipse around the mean value) and to illustrate the amount of between study variation (prediction ellipse).
Heterogeneity was evaluated visually using the summary ROC plots and statistically by using the variance of logit transformed sensitivity and specificity, with smaller values indicating less heterogeneity among studies. We performed meta-analysis for techniques SR, OPT, CYT, IOUS, FS MCT and MP. However, we acknowledge that there were a very small number of studies in relation to MCT and MP, thus results should be interpreted with caution.
3. Results
3.1. Literature search
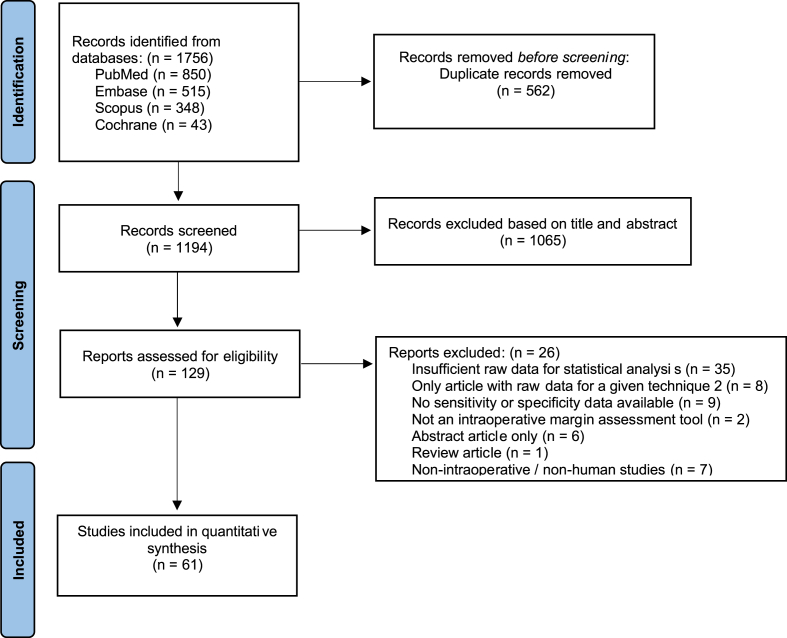
The systematic search strategy identified a total of 1756 studies, of which 562 duplicate studies were manually removed. The remaining 1194 titles and abstracts were screened for relevance, of which 129 studies had their full texts assessed for eligibility. Raw diagnostic accuracy data were unavailable in 35 papers, but were available in 69 papers. To enable meta-analysis, at least 2 studies were required per IOMA group, therefore 8 studies were excluded, as they were the only study for the given technique. Four studies contributed data to 2 IOMA techniques [[12], [13], [14], [15]]. This resulted in a total of 61 studies included for the final analysis, of 7 IOMA techniques (Fig. 1). Quality assessment was performed for each study using the QUADAS-2 tool (Supplementary Figs. 8 and 9).
Fig. 1.
PRISMA flow diagram.
3.2. Study characteristics
Overall, 61 studies were included, and all of these contained sensitivity and specificity percentage data, as well as sufficient raw data to enable meta-analysis. Results are detailed for the 61 studies included in the meta-analysis in Table 1. Forty papers were prospective studies and 21 were retrospective. The studies were published between 1990 and 2022. Mean or median age was available in 39 studies and ranged between 44.9 and 66 years. Distances defined for positive margins varied from 1 mm to 5 mm, with a mode of 2 mm. CRRs were performed within the same operation and PMRs and RORs were performed at an additional operation. Turnaround time for results, when reported, are also listed in Table 1. Reported or calculable percentage sensitivity, specificity, PPV, NPV and overall diagnostic accuracy for each study are listed in Table 2.
Table 1.
Characteristics of studies included in meta-analysis.
| Tech | Author | Year | Study design | Pt | Res | Mar | Ind | M Dist | Age | CRR | PMR | ROR | Time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | Lin et al. [45] | 2020 | Retrospective | 202 | 205 | 1 | 5 | 12.7 | 18 | 1 | |||
| Bathla et al. [46] | 2011 | Retrospective | 99 | 102 | 1 | 1 | 58.6 | 28.4 | 17.6 | 14.7 | |||
| Baù et al. [47] | 2020 | Prospective | 18 | 18 | |||||||||
| Chagpar et al. [48] | 2015 | Prospective | 90 | 1 | 1 | 60 | 28.9 | 30 | 10 | ||||
| Chand et al. [49] | 2019 | Prospective | 30 | 30 | 180 | 2 | 1 | 55.67 | 30 | 0 | |||
| Ciccarelli et al. [50] | 2007 | Retrospective | 102 | 2 | 2 | 31.4 | 22.5 | 20 | |||||
| Coombs et al. [51] | 2006 | Retrospective | 101 | 52 | 1 | 5 | 58.2 | 19.7 | 9.3 | ||||
| Funk et al. [52] | 2019 | Retrospective | 470 | 470 | 2820 | 1 | 1 | 60.2 | 61.9 | 7.6 | 21.7 | ||
| Graham et al. [53] | 1994 | Prospective | 119 | 2 | 1 | ||||||||
| Hisada et al. [54] | 2016 | Retrospective | 174 | 54.7 | 13.8 | 20 | |||||||
| Kulkarni et al. [55] | 2021 | Prospective | 118 | 708 | 1 | 0 | 62 | 3.4 | |||||
| McCormick et al. [56] | 2004 | Retrospective | 93 | 1 | 18 | 5 | 15 | ||||||
| Miller et al. [57] | 2016 | Prospective RCT | 36 | 1 | 59 | ||||||||
| Park et al. [58] | 2019 | Retrospective | 99 | 594 | 1 | 2 | 60.2 | 6 | 10.1 | ||||
| Pop et al. [12] | 2018 | Prospective | 83 | 1 | 2 | 30 | 9 | ||||||
| Prueksadee et al. [59] | 2009 | Retrospective | 12 | 1 | 2 | 59.3 | 50 | 25 | |||||
| Saarela et al. [60] | 2001 | Prospective | 64 | 66 | 2 | 0 | 55 | 74.2 | 16.7 | ||||
| Schaefgen et al. [61] | 2021 | Retrospective | 174 | 174 | 1044 | 1 | 1 | 51.4 | 54.6 | 9.8 | 9.2 | ||
| Stachs et al. [62] | 2022 | Prospective RCT | 117 | 1,4 | 1–2 | 61.2 | 35 | 29.1 | 27.4 | ||||
| Weber et al. [13] | 2008 | Retrospective | 35 | 1 | 1 | 57.5 | 42.9 | 37.1 | |||||
| OPT | Brown et al. [63] | 2010 | Prospective | 57 | 55 | 1 | 2 | 20 | |||||
| Keller et al. [64] | 2010 | Prospective | 40 | 179 | 3 | 1 | |||||||
| Nguyen et al. [65] | 2009 | Prospective | 20 | 20 | 210 | 1 | 2 | 66 | |||||
| Schmidt et al. [66] | 2019 | Prospective | 50 | 185 | 1,4 | 0 | 61 | 14 | 14 | ||||
| Zhu et al. [67] | 2021 | Prospective | 41 | 3 | |||||||||
| MP | Hoffman et al. [68] | 2022 | Prospective | 48 | 51 | 302 | 1 | 1 | 64 | ||||
| Karni et al. [69] | 2007 | Prospective | 57 | 57 | 314 | 1 | 1 | 15.8 | 38.6 | 7 | |||
| LeeVan et al. [70] | 2020 | Prospective | 60 | 360 | 1 | 1 | 63.5 | 30 | 13.3 | 6.6 | |||
| MCT | McClatchy et al. [71] | 2018 | Prospective | 32 | 32 | 1 | 0 | ||||||
| Qiu et al. [35] | 2018 | Prospective | 30 | 30 | 1 | 0 | 62 | 13.5 | |||||
| Tang et al. [36] | 2013 | Prospective | 6 | 25 | 1 | 2 | 55 | 10 | |||||
| IOUS | Kumar et al. [15] | 2021 | Prospective | 62 | |||||||||
| Londero et al. [72] | 2010 | Prospective | 46 | 184 | 1 | 2 | 53 | 3-6. | |||||
| Mesurolle et al. [73] | 2006 | Retrospective | 81 | 1 | 2 | 59.1 | 17.4 | 3-6. | |||||
| Moschetta et al. [74] | 2015 | Prospective | 132 | 1 | 2 | 51 | |||||||
| Perera et al. [75] | 2020 | Prospective | 95 | 99 | 384 | 2 | 0 | 5.3 | 9.5 | 2.1 | 3 | ||
| Pop et al. [12] | 2018 | Prospective | 83 | 1 | 2 | 30 | 9 | ||||||
| Ramos et al. [76] | 2013 | Prospective | 223 | 225 | 1 | 2 | 59.5 | 45.7 | 4 | ||||
| FS | Caruso et al. [77] | 2011 | Retrospective | 50 | 52 | 1 | 2 | 10 | 10 | 20 | |||
| Ikeda et al. [78] | 1997 | Retrospective | 54 | 56 | 1 | 0 | 44.9 | 35.7 | 12.5 | 10.7 | |||
| Jorns et al. [79] | 2014 | Prospective | 46 | 5 | 2 | 57.4 | 23.9 | 39.1 | 19.6 | 22 | |||
| Kikuyama et al. [80] | 2015 | Prospective | 220 | 763 | 1 | 51.3 | |||||||
| Kim et al. [81] | 2016 | Retrospective | 25 | 29 | 4 | 1 | 53 | 12 | 12 | 0 | |||
| Ko et al. [82] | 2017 | Prospective | 509 | 3 | 0 | 50 | 12.6 | 7.2 | 6.3 | ||||
| Kumar et al. [15] | 2021 | Prospective | 62 | 25.8 | 0 | ||||||||
| Mahadevappa et al. [14] | 2017 | Prospective | 62 | 3 | |||||||||
| Noguchi et al. [83] | 1995 | Prospective | 95 | 100 | 1 | 35 | 24 | ||||||
| Nowikiewicz et al. [84] | 2019 | Retrospective | 505 | 1 | 58.7 | 14.3 | 15 | ||||||
| Olson et al. [85] | 2007 | Retrospective | 290 | 292 | 1404 | 1 | 57.2 | 24.1 | 11.4 | 25 | |||
| Osako et al. [86] | 2015 | Retrospective | 1029 | 1327 | 1 | 5 | 30.3 | 30.3 | 0.1 | 50 | |||
| Rusby et al. [87] | 2008 | Prospective | 115 | 557 | 1 | 5 | 49.5 | 4.4 | 7 | 2.6 | 20 | ||
| Weber et al. [13] | 2008 | Retrospective | 80 | 1 | 1 | 59.6 | 22.5 | 12.5 | |||||
| CYT | Bakhshandeh et al. [88] | 2007 | Retrospective | 100 | 510 | 1 | 20 | ||||||
| Blair et al. [89] | 2007 | Prospective | 20 | 20 | 120 | 1 | |||||||
| Cox et al. [90] | 1991 | Prospective | 111 | 111 | 1 | 58.4 | 15 | ||||||
| Creager et al. [91] | 2002 | Retrospective | 137 | 141 | 758 | 1 | 2 | 58 | 20 | ||||
| D'Halluin et al. [92] | 2009 | Prospective | 396 | 400 | 1 | 2 | 58.6 | 38.3 | 13.3 | 10 | |||
| Ku et al. [93] | 1991 | Prospective | 87 | 1 | 15 | ||||||||
| Mahadevappa et al. [14] | 2017 | Prospective | 62 | 3 | |||||||||
| Muttalib et al. [94] | 2004 | Prospective | 26 | 27 | 1 | 1 | 22.2 | 22.5 | |||||
| Sumiyoshi et al. [95] | 2010 | Prospective | 160 | 1 | 58.1 | ||||||||
| Tamanuki et al. [96] | 2020 | Retrospective | 522 | 1 | 0 | 62 | |||||||
| Tohnosu et al. [97] | 1998 | Prospective | 50 | 200 | 1 | 5 | 52.9 | ||||||
| Valdes et al. [98] | 2007 | Prospective | 12 | 72 | 6 | 23 | 33.3 | 15 | |||||
| Valdes et al. [99] | 2007 | Prospective | 30 | 68 | 5 | 15 |
Tech, technique; SR, Specimen Radiography; OPT, Optical Spectroscopy; CYT, Cytology; IOUS, Intraoperative Ultrasound; FS, Frozen Section; MP, Margin Probe; MCT, Micro Computerised Topography; Pt, number of patients; Res, number of resections/specimens; Mar, number of margins; Ind, indication (1: BCS for BC; 2: BCS for impalpable BC; 3: BCS or mastectomy for BC; 4: BCS for DCIS; 5: Re-excision of BC after positive margins; 6: BCS for ILC); M Dist, positive margin distance in mm; CRR, cavity re-excision rate; PMR, positive margin rate; ROR, re-operation rate.
Table 2.
Raw diagnostic accuracy data of studies included in meta-analysis.
| Tech | Author | TP | FP | TN | FN | Total | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | Lin et al. [45] | 24 | 10 | 158 | 13 | 205 | 64.9 | 94.1 | 70.6 | 92.4 | 88.8 |
| Bathla et al. [46] | 24 | 5 | 56 | 102 | 187 | 58.5 | 91.8 | 82.8 | 76.7 | 78.4 | |
| Baù et al. [47] | 2 | 0 | 15 | 1 | 18 | 66.7 | 100 | 100 | 93.8 | 94.4 | |
| Chagpar et al. [48] | 12 | 12 | 44 | 90 | 158 | 41.2 | 78.6 | 53.9 | 68.8 | 64.4 | |
| Chand et al. [49] | 4 | 2 | 22 | 2 | 30 | 66.7 | 91.7 | 66.7 | 91.7 | 86.7 | |
| Ciccarelli et al. [50] | 25 | 9 | 55 | 102 | 191 | 65.8 | 85.9 | 73.5 | 80.9 | 41.8 | |
| Coombs et al. [51] | 12 | 4 | 25 | 52 | 93 | 52.2 | 86.2 | 75 | 69.4 | 39.8 | |
| Funk et al. [52] | 114 | 331 | 2179 | 196 | 2820 | 36.8 | 86.8 | 25.6 | 91.8 | 81.3 | |
| Graham et al. [53] | 62 | 1 | 18 | 119 | 200 | 62 | 95 | 98 | 32 | 67.2 | |
| Hisada et al. [54] | 6 | 6 | 106 | 23 | 141 | 20.7 | 94.6 | 50 | 82.2 | 79.4 | |
| Kulkarni et al. [55] | 23 | 123 | 538 | 24 | 708 | 48.9 | 81.4 | 15.8 | 95.7 | 79.2 | |
| McCormick et al. [56] | 6 | 10 | 72 | 93 | 181 | 54.6 | 87.8 | 37.5 | 93.5 | 83.9 | |
| Miller et al. [57] | 2 | 2 | 16 | 2 | 22 | 50 | 88.9 | 50 | 88.9 | 81.8 | |
| Park et al. [58] | 14 | 61 | 24 | 0 | 99 | 100 | 28.2 | 18.7 | 100 | 38.4 | |
| Pop et al. [12] | 4 | 11 | 63 | 5 | 83 | 44.4 | 85.1 | 26.7 | 92.7 | 80.7 | |
| Prueksadee et al. [59] | 3 | 3 | 1 | 12 | 19 | 37.5 | 25 | 50 | 16.7 | 33.3 | |
| Saarela et al. [60] | 9 | 8 | 31 | 66 | 114 | 33 | 79 | 53 | 63 | 61 | |
| Schaefgen et al. [61] | 13 | 62 | 87 | 12 | 174 | 52 | 58.4 | 17.3 | 87.9 | 57.5 | |
| Stachs et al. [62] | 34 | 16 | 67 | 0 | 117 | 70 | 56.7 | 54.7 | 71.7 | 62.4 | |
| Weber et al. [13] | 12 | 6 | 9 | 35 | 62 | 60 | 60 | 66.7 | 52.9 | 60 | |
| OPT | Brown et al. [63] | 27 | 7 | 14 | 7 | 55 | 79.4 | 66.7 | 79.4 | 66.7 | 74.6 |
| Keller et al. [64] | 29 | 6 | 139 | 5 | 179 | 85.3 | 95.9 | 82.9 | 96.5 | 94 | |
| Nguyen et al. [65] | 9 | 2 | 9 | 0 | 20 | 100 | 81.8 | 81.8 | 100 | 90 | |
| Schmidt et al. [66] | 11 | 1 | 32 | 6 | 50 | 64.7 | 97 | 91.7 | 84.2 | 86 | |
| Zhu et al. [67] | 222 | 26 | 454 | 18 | 720 | 92.5 | 94.6 | 89.5 | 96.2 | 93.9 | |
| MP | Hoffman et al. [68] | 3 | 97 | 192 | 10 | 302 | 23.1 | 66.4 | 3 | 95.1 | 64.6 |
| Karni et al. [69] | 30 | 88 | 184 | 12 | 314 | 71.4 | 67.7 | 25.4 | 93.9 | 68.2 | |
| LeeVan et al. [70] | 17 | 32 | 10 | 1 | 60 | 94.4 | 23.8 | 34.7 | 90.9 | 45 | |
| MCT | McClatchy et al. [71] | 3 | 9 | 18 | 2 | 32 | 60 | 66.7 | 25 | 90 | 65.6 |
| Qiu et al. [35] | 5 | 0 | 20 | 4 | 29 | 55.6 | 100 | 100 | 83.3 | 86.2 | |
| Tang et al. [36] | 5 | 1 | 18 | 1 | 25 | 83.3 | 94.7 | 83.3 | 94.7 | 92 | |
| IOUS | Kumar et al. [15] | 16 | 0 | 46 | 0 | 62 | 100 | 100 | 100 | 100 | 100 |
| Londero et al. [72] | 8 | 24 | 132 | 20 | 184 | 28.6 | 84.6 | 25 | 86.8 | 76.1 | |
| Mesurolle et al. [73] | 30 | 8 | 33 | 10 | 81 | 75 | 80.5 | 79 | 76.7 | 77.8 | |
| Moschetta et al. [74] | 16 | 6 | 90 | 20 | 132 | 44.4 | 93.8 | 72.7 | 81.8 | 80.3 | |
| Perera et al. [75] | 5 | 26 | 349 | 4 | 384 | 55.6 | 93.1 | 16.1 | 98.9 | 92.2 | |
| Pop et al. [12] | 8 | 25 | 49 | 1 | 83 | 88.9 | 66.2 | 24.2 | 98 | 68.7 | |
| Ramos et al. [76] | 24 | 79 | 116 | 6 | 225 | 80 | 59.5 | 95.1 | 23.3 | 62.2 | |
| FS | Caruso et al. [77] | 5 | 3 | 44 | 1 | 53 | 83 | 93 | 62 | 97 | 94 |
| Ikeda et al. [78] | 17 | 4 | 34 | 1 | 56 | 94.4 | 89.5 | 81 | 97.1 | 91.1 | |
| Jorns et al. [79] | 12 | 0 | 28 | 6 | 46 | 66.7 | 100 | 100 | 82.4 | 87 | |
| Kikuyama et al. [80] | 287 | 18 | 440 | 18 | 763 | 94.1 | 96.1 | 94.1 | 96.1 | 95.3 | |
| Kim et al. [81] | 3 | 1 | 23 | 2 | 29 | 60 | 95.8 | 75 | 92 | 89.7 | |
| Ko et al. [82] | 120 | 1 | 338 | 24 | 483 | 83.3 | 99.7 | 99.2 | 93.4 | 94.8 | |
| Kumar et al. [15] | 10 | 0 | 46 | 6 | 62 | 62.5 | 100 | 100 | 88.5 | 90.3 | |
| Mahadevappa et al. [14] | 33 | 1 | 28 | 0 | 62 | 100 | 96.6 | 97.1 | 100 | 98.4 | |
| Noguchi et al. [83] | 23 | 12 | 64 | 1 | 100 | 95.8 | 84.2 | 65.7 | 98.5 | 87 | |
| Nowikiewicz et al. [84] | 4 | 0 | 429 | 72 | 505 | 5.3 | 100 | 100 | 85.6 | 85.7 | |
| Olson et al. [85] | 57 | 5 | 1228 | 21 | 1311 | 73.1 | 99.6 | 91.9 | 98.3 | 98 | |
| Osako et al. [86] | 259 | 53 | 955 | 60 | 1327 | 81.2 | 94.7 | 83 | 94.1 | 91.5 | |
| Rusby et al. [87] | 39 | 15 | 495 | 8 | 557 | 83 | 97 | 72.2 | 98.4 | 96 | |
| Weber et al. [13] | 32 | 5 | 35 | 8 | 80 | 80 | 87.5 | 86.5 | 81.4 | 83.8 | |
| CYT | Bakhshandeh et al. [88] | 30 | 7 | 472 | 1 | 510 | 97 | 99 | 81.1 | 99.8 | 98.4 |
| Blair et al. [89] | 3 | 0 | 115 | 1 | 119 | 75 | 100 | 100 | 99.1 | 99.2 | |
| Cox et al. [90] | 22 | 3 | 86 | 0 | 111 | 100 | 96.6 | 88 | 100 | 97.3 | |
| Creager et al. [91] | 12 | 18 | 104 | 3 | 137 | 80 | 85.3 | 40 | 97.2 | 85 | |
| D'Halluin et al. [92] | 71 | 26 | 304 | 9 | 410 | 88.6 | 92.2 | 73.6 | 97 | 91.5 | |
| Ku et al. [93] | 17 | 2 | 68 | 0 | 87 | 100 | 97.1 | 89.5 | 100 | 97.7 | |
| Mahadevappa et al. [14] | 33 | 1 | 27 | 0 | 61 | 100 | 96.4 | 97.1 | 100 | 98.4 | |
| Muttalib et al. [94] | 6 | 6 | 15 | 0 | 27 | 100 | 71.4 | 50 | 100 | 77.8 | |
| Sumiyoshi et al. [95] | 14 | 4 | 136 | 6 | 160 | 70 | 97.1 | 77.8 | 95.8 | 93.8 | |
| Tamanuki et al. [96] | 78 | 58 | 375 | 11 | 522 | 87.6 | 86.6 | 57.4 | 97.2 | 86.8 | |
| Tohnosu et al. [97] | 27 | 16 | 156 | 1 | 200 | 96.4 | 90.7 | 62.8 | 99.4 | 91.5 | |
| Valdes et al. [98] | 1 | 1 | 59 | 11 | 72 | 8.3 | 98.3 | 50 | 84.3 | 83.3 | |
| Valdes et al. [99] | 3 | 11 | 53 | 1 | 68 | 75 | 82.8 | 21.4 | 98.2 | 82.4 |
Tech, technique; SR, Specimen Radiography; OPT, Optical Spectroscopy; CYT, Cytology; IOUS, Intraoperative Ultrasound; FS, Frozen Section; MP, Margin Probe; MCT, Micro Computerised Topography; TP, true positive; FP, false positive; TN, true negative; FN, false negative; PPV, positive predictive value; NPV, negative predictive value; Accuracy, diagnostic accuracy.
3.3. Meta-analysis
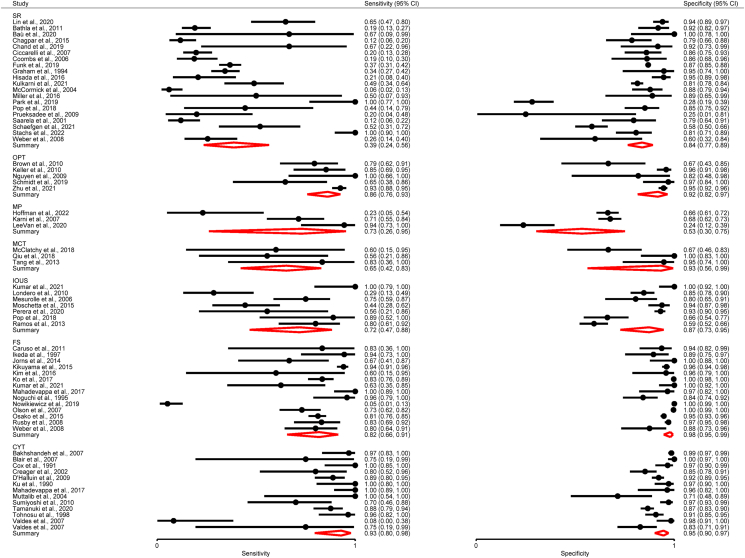
The pooled sensitivity, specificity and the respective variance of the logit transformed sensitivity and specificity for each technique type in the meta-analysis are displayed in Table 3. The forest plot can be seen in Fig. 2.
Table 3.
Meta-analysis: summary estimates of sensitivity and specificity for all included studies for each IOMA technique type.
| Technique | No. of studies (patients/margins) | Sensitivity (95 % CI) | Variance Logit Sensitivity (95 % CI) | Specificity (95 % CI) | Variance Logit Specificity (95 % CI) |
|---|---|---|---|---|---|
| SR | 20 (5622) | 0.39 (0.24–0.56) | 2.32 (1.02–5.27) | 0.84 (0.77–0.89) | 0.89 (0.40–1.94) |
| OPT | 5 (1024) | 0.86 (0.76–0.93) | 0.34 (0.04–3.13) | 0.92 (0.82–0.97) | 0.78 (0.13–4.71) |
| CYT | 13 (2484) | 0.92 (0.77–0.98) | 3.68 (1.11–12.26) | 0.95 (0.90–0.97) | 1.21 (0.43–3.42) |
| IOUS | 7 (1151) | 0.72 (0.47–0.88) | 1.58 (0.33–7.62) | 0.87 (0.73–0.95) | 1.36 (0.31–5.87) |
| FS | 14 (5434) | 0.82 (0.66–0.91) | 2.30 (0.96–5.54) | 0.98 (0.95–0.99) | 2.84 (0.91–8.80) |
| MPa | 3 (165) | 0.73 (0.26–0.95) | 2.68 | 0.53 (0.30–0.75) | 0.67 |
| MCTa | 3 (68) | 0.65 (0.42–0.83) | 0 | 0.93 (0.56–0.99) | 2.52 |
Only three studies and thus results should be interpreted with caution.
Fig. 2.
Pooled meta-analysis forest plot for each IOMA technique, displaying sensitivity and specificity data for all studies included and the pooled estimate.
These findings indicate that CYT seems best in terms of diagnostic accuracy, with pooled sensitivity of 0.92 (95 % CI 0.77–0.98) and a pooled specificity of 0.95 (95 % CI 0.90–0.97). These findings also indicate good diagnostic accuracy for OPT, with a pooled sensitivity of 0.86 (95 % CI 0.76–0.93) and a pooled specificity of 0.92 (95 % CI 0.82–0.97). These results demonstrate limited diagnostic accuracy for SR. However, the results indicate SR is better at ruling out rather than ruling individuals, with a higher pooled specificity (0.84, 95 % CI 0.77–0.89) compared to sensitivity (0.39, 95 % CI 0.24–0.56). These findings show IOUS to be a superior imaging method for IOMA to SR, with both a higher pooled sensitivity (0.72, 95 % CI 0.47–0.88) and specificity (0.87, 95 % CI 0.73–0.95). Meta-analysis of the 14 studies investigating FS demonstrated the highest pooled specificity (0.98, 95 % CI 0.95–0.99), however limited pooled sensitivity was observed (0.82, 95 % CI 0.66–0.91). MP and MCT both demonstrated limited sensitivity and specificity as IOMA tools, with the exception of the high specificity of MCT (0.93, 95 % CI 0.56–0.99), however these results must be interpreted with due to the limited number of studies available for meta-analysis for each method.
Individual and summary estimates of sensitivity and specificity for all of the studies included in the meta-analysis, the 95 % confidence region and 95 % prediction region are presented for SR, OPT, CYT, IOUS and FS in the summary ROC graphs (Supplementary Figs. 1–5). For SR (Supplementary Fig. 1), OPT (Supplementary Fig. 2) and IOUS (Supplementary Fig. 4), the 95 % confidence regions were broad, reducing the precision of studies in the pooled estimate. The 95 % prediction regions (amount of variation between studies) were also very wide suggesting heterogeneity between studies. This may be, at least in part, explained by the fact that both patient numbers and margin numbers were pooled together in this analysis.
For CYT (Supplementary Fig. 3) and FS (Supplementary Fig. 5) the 95 % confidence region was narrower, and although the 95 % prediction region were narrower compared to the other techniques, they still indicate heterogeneity between studies. The results for MP and MCT are also presented (Supplementary Figs. 6 and 7) and as seen in these plots the results are unreliable.
4. Discussion
Breast conserving surgery (BCS) now constitutes the mainstay of treatment, being favoured increasingly over mastectomy [16,17]. However, between 16 and 23.1 % of women treated with BCS undergo re-operation due to incomplete excision or inadequate tumour margins [[18], [19], [20]], with re-operation being associated with undesirable consequences such as delay in adjuvant treatments, inferior cosmetic outcome and most notably; increased risk of local and distant disease recurrence [[21], [22], [23]]. Timely and accurate intraoperative margin assessment (IOMA) may provide a means of reducing re-operation rates which would have a significant impact both with regards to improving patient outcomes and optimising healthcare system productivity and cost-effectiveness [24]. Significant reduction in healthcare costs and re-operation rates have already been demonstrated by IOMA use in some centres [25].
Although the significance of positive tumour margins is widely understood, the definition of negative margins varies significantly within the literature. The studies included in this meta-analysis ranged in definition from ‘no ink on tumour’ to a 5 mm tumour free margin. This disparity has been reflected in the changing guidelines, with most guidelines now recommending “no ink on tumour” as the standard margin for invasive cancer treated with BCS followed by whole breast irradiation (WBI) [7,26]. However, for DCIS the guidelines recommend a 2 mm tumour free margin when treated with BCS and WBI [27]. These guidelines were updated based on results of meta-analyses, which showed a twofold increase in ipsilateral breast tumour recurrence (IBTR) with positive margins in invasive cancer and DCIS (“ink on tumour” and <2 mm, respectively) [28,29].
The present meta-analysis analysed the diagnostic accuracy of a range available IOMA techniques. Many of the techniques analysed showed promising capacity in accurately identifying positive margins. Of those analysed, histopathological means of margin assessment demonstrated superior capabilities in terms of diagnostic accuracy, namely CYT (pooled sensitivity 0.92, pooled specificity 0.95) and FS (pooled sensitivity 0.82, pooled specificity 0.98). Although the diagnostic accuracy demonstrated in both cases is impressive, it must be evaluated within the context of the time and resources required. CYT and FS may add an additional 15 and 30 min respectively to time under anaesthesia [30], and is demanding with regards to requiring timely availability of histopathologists sufficiently experienced in cytopathological assessment in particular. It is likely the resource-intensive nature of these pathological techniques, combined with slow turnaround times, surgical workflow disruptions and considerable financial costs that have prevented them being adopted routinely in clinical practice.
Optical spectroscopy (OPT) is a novel IOMA method that demonstrated impressive diagnostic accuracy (pooled sensitivity 0.86, pooled specificity 0.92) and has promising advantages. It is significantly less demanding from a time and resource perspective [31], with assessment time reported as between 10 and 90 s to obtain an adequate spectroscopic margin profile [32]. Therefore, OPT offers sensitive IOMA within a favourable timeframe, minimising disruption in surgical workflow. However, making real-time surgical decisions based off this spectroscopic profile requires a highly trained and validated classifier, requiring significant training. An ongoing clinical trial is investigating whether artificial intelligence can accurately interpret these optical imaging results [33], with the potential of further improving the turnaround time for results and potentially removing the need for surgeons to be trained in their interpretation.
SR is a well-established radiological IOMA technique and, although it is routinely used in many hospitals for IOMA, showed the lowest diagnostic accuracy of all techniques on meta-analysis (pooled sensitivity 0.39, pooled specificity 0.84). However, SR offers many advantages which may explain its widespread adoption in clinical practice including ease of interpretation by the surgeon, minimal disruption to workflow, fast turnaround times and cost-effectiveness. Other radiological IOMA tools such as IOUS are also time-efficient and demonstrated superior diagnostic accuracy on pooled analysis (pooled sensitivity 0.72, pooled specificity 0.87). Other probe-based tools, such as MP, using radio-frequency spectroscopy, have been shown to significantly reduce the ROR [34], although only demonstrating moderate accuracy on meta-analysis (pooled sensitivity 0.73, pooled specificity 0.53). 3D imaging devices for the operating theatre are currently begin evaluated in an attempt to improve IOMA accuracy. MCT is one such device, and although diagnostic accuracy was unimpressive on pooled analysis (pooled sensitivity 0.65, pooled specificity 0.93), the number of patients included in the analysis was small (n = 68) and thus these results should be interpreted with caution. Individual studies have shown promising results with MCT [35,36], however a major disadvantage of this technique is that currently accurate protocols may require up to 14 min for imaging [36]. Intraoperative-MRI (IOMRI) is also being evaluated as a potential IOMA tool, with limited clinical data to date [37,38].
Many novel IOMA tools are currently being developed, with the aim of addressing some limitations of currently established techniques, as well as improving accuracy. Emerging probe-based tools such as the Cancer Diagnostic Probe (CDP) and the “click-to-sense” assay (CTS), using hypoxia glycolysis and acrolein for tumour cell detection, respectively, have shown promising preliminary results (CDP: sensitivity 100 %, specificity 92.3 %; CTS: sensitivity 93.3 %, specificity 98.3 %) [39,40]. Confocal microscopy is another technology which has shown encouraging preliminary results (sensitivity 91–97 %, specificity 86–93 %) [41,42]. Rapid evaporative ionisation mass spectrometry (REIMS) is an interesting technology which may enable an “intelligent knife” to analyse margins for cancer intraoperatively [43], and is currently being investigated in a clinical trial [44].
This study is subject to a number of limitations. As previously mentioned, positive margin definitions of included studies ranged from ‘no ink on tumour’ to a 5 mm tumour free margin. This variance in margin definition may constitute an inherent limitation of this study, similarly the participation criteria varied between studies. Another considerable source of heterogeneity is the fact that some studies reported sensitivity and specificity results by means of resection specimen or margin number as opposed to patient number. As this is a relatively novel area of interest, the number of studies included was small for some IOMA techniques, in particular MCT and MP, and these results should be interpreted with caution. Finally, although diagnostic accuracy is important, re-excision rates are the primary outcome by which these tools will ultimately be measured and remain the most significant in altering clinical practice.
This meta-analysis generated meaningful appraisal of IOMA means with regards to pooled sensitivity and specificity values. Although diagnostic accuracy is of primary importance, the real-world utility and application of each IOMA means is also affected by; capacity for timely inspection and results, ease of result interpretation, requirement for additional personnel/resources for investigation and/or interpretation and financial viability. Due to the global disparity with regards to available resources within the acute hospital setting, the optimal IOMA means may inevitably differ between healthcare systems.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Gavin P. Dowling: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Cian M. Hehir: Writing – review & editing, Formal analysis, Data curation. Gordon R. Daly: Writing – review & editing, Methodology, Data curation. Sandra Hembrecht: Writing – review & editing, Supervision, Data curation. Stephen Keelan: Writing – review & editing, Supervision, Methodology. Katie Giblin: Writing – review & editing, Visualization, Data curation. Maen M. Alrawashdeh: Writing – review & editing, Software, Data curation. Fiona Boland: Writing – review & editing, Visualization, Software, Methodology, Formal analysis. Arnold D.K. Hill: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103749.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alkabban F.M., Ferguson T. StatPearls Publishing LLC.; 2022. Breast cancer. StatPearls. Treasure Island (FL): StatPearls publishing Copyright © 2022. [Google Scholar]
- 2.Nelson J.A., Rubenstein R.N., Haglich K., et al. Analysis of a trend reversal in US lumpectomy rates from 2005 through 2017 using 3 nationwide data sets. JAMA Surgery. 2022;157:702–711. doi: 10.1001/jamasurg.2022.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeevan R., Cromwell D.A., Trivella M., et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. Br Med J. 2012;345 doi: 10.1136/bmj.e4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakedis J.M., Tang A., Savitz A., et al. Economic impact of reducing reexcision rates after breast-conserving surgery in a large, integrated health system. Ann Surg Oncol. 2022;29:6288–6296. doi: 10.1245/s10434-022-12127-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuritzky A., Reyna C., McGuire K.P., et al. Evaluation of 2014 margin guidelines on re-excision and recurrence rates after breast conserving surgery: a multi-institution retrospective study. Breast. 2020;51:29–33. doi: 10.1016/j.breast.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundred J.R., Michael S., Stuart B., et al. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. Br Med J. 2022;378 doi: 10.1136/bmj-2022-070346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran M.S., Schnitt S.J., Giuliano A.E., et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–1515. doi: 10.1200/JCO.2013.53.3935. [DOI] [PubMed] [Google Scholar]
- 8.QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Harbord R. 2008. METANDI: stata module to perform meta-analysis of diagnostic accuracy. [Google Scholar]
- 10.Nyaga V.N., Arbyn M. Metadta: a Stata command for meta-analysis and meta-regression of diagnostic test accuracy data – a tutorial. Arch Publ Health. 2022;80:95. doi: 10.1186/s13690-021-00747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Pop M.M., Cristian S., Hanko-Bauer O., Ghiga D.V., Georgescu R. Obtaining adequate surgical margin status in breast-conservation therapy: intraoperative ultrasound-guided resection versus specimen mammography. Clujul Med. 2018;91:197–202. doi: 10.15386/cjmed-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber W.P., Engelberger S., Viehl C.T., et al. Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J Surg. 2008;32:2599–2606. doi: 10.1007/s00268-008-9757-8. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevappa A., Nisha T.G., Manjunath G.V. Intra-operative diagnosis of breast lesions by imprint cytology and frozen section with histopathological correlation. J Clin Diagn Res. 2017;11:Ec01–ec6. doi: 10.7860/JCDR/2017/24454.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar N., on M., Chintamani C. Intraoperative specimen ultrasonography: is it a reliable tool for margin assessment following breast conservation surgery for breast carcinoma? Cureus. 2021;13 doi: 10.7759/cureus.15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan Ali S., Sp S., N A.K. Rate of breast-conserving surgery vs mastectomy in breast cancer: a tertiary care centre experience from South India. Indian Journal of Surgical Oncology. 2019;10:72–76. doi: 10.1007/s13193-018-0818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crown A., Handy N., Weed C., Laskin R., Rocha F.G., Grumley J. Oncoplastic breast-conserving surgery: can we reduce rates of mastectomy and chemotherapy use in patients with traditional indications for mastectomy? Ann Surg Oncol. 2021;28:2199–2209. doi: 10.1245/s10434-020-09044-x. [DOI] [PubMed] [Google Scholar]
- 18.Schulman A.M., Mirrielees J.A., Leverson G., Landercasper J., Greenberg C., Wilke L.G. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) Mastery SM database following the SSO-ASTRO “no ink on tumor” guidelines. Ann Surg Oncol. 2017;24:52–58. doi: 10.1245/s10434-016-5516-5. [DOI] [PubMed] [Google Scholar]
- 19.Tang S.S.-K., Kaptanis S., Haddow J.B., et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur J Cancer. 2017;84:315–324. doi: 10.1016/j.ejca.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 20.Mamtani A., Zabor E.C., Rosenberger L.H., Stempel M., Gemignani M.L., Morrow M. Was reexcision less frequent for patients with lobular breast cancer after publication of the SSO-ASTRO margin guidelines? Ann Surg Oncol. 2019;26:3856–3862. doi: 10.1245/s10434-019-07751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pleijhuis R.G., Graafland M., de Vries J., Bart J., de Jong J.S., van Dam G.M. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16:2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menes T.S., Tartter P.I., Bleiweiss I., Godbold J.H., Estabrook A., Smith S.R. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol. 2005;12:881–885. doi: 10.1245/ASO.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Kouzminova N.B., Aggarwal S., Aggarwal A., Allo M.D., Lin A.Y. Impact of initial surgical margins and residual cancer upon re-excision on outcome of patients with localized breast cancer. Am J Surg. 2009;198:771–780. doi: 10.1016/j.amjsurg.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Garcia M.T., Mota B.S., Cardoso N., et al. Accuracy of frozen section in intraoperative margin assessment for breast-conserving surgery: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uecker J.M., Bui E.H., Foulkrod K.H., Sabra J.P. Intraoperative assessment of breast cancer specimens decreases cost and number of reoperations. Am Surg. 2011;77:342–344. [PubMed] [Google Scholar]
- 26.Buchholz T.A., Somerfield M.R., Griggs J.J., et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32:1502–1506. doi: 10.1200/JCO.2014.55.1572. [DOI] [PubMed] [Google Scholar]
- 27.Morrow M., Van Zee K.J., Solin L.J., et al. Society of surgical oncology-American Society for Radiation Oncology-American Society of clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;23:3801–3810. doi: 10.1245/s10434-016-5449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah C., Hobbs B.P., Vicini F., et al. The diminishing impact of margin definitions and width on local recurrence rates following breast-conserving therapy for early-stage invasive cancer: a meta-analysis. Ann Surg Oncol. 2020;27:4628–4636. doi: 10.1245/s10434-020-08878-9. [DOI] [PubMed] [Google Scholar]
- 29.Marinovich M.L., Azizi L., Macaskill P., et al. The association of surgical margins and local recurrence in women with ductal carcinoma in situ treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2016;23:3811–3821. doi: 10.1245/s10434-016-5446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esbona K., Li Z., Wilke L.G. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol. 2012;19:3236–3245. doi: 10.1245/s10434-012-2492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boichenko E., Kirsanov D. Optical spectroscopy and chemometrics in intraoperative tumor margin assessment. TrAC, Trends Anal Chem. 2023 [Google Scholar]
- 32.Zúñiga W.C., Jones V., Anderson S.M., et al. Raman spectroscopy for rapid evaluation of surgical margins during breast cancer lumpectomy. Sci Rep. 2019;9 doi: 10.1038/s41598-019-51112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wide Field OCT + AI for Positive Margin Rates in Breast Conservation Surgery. at https://classic.clinicaltrials.gov/show/NCT05113927.).
- 34.Thill M., Dittmer C., Baumann K., Friedrichs K., Blohmer J.U. MarginProbe®--final results of the German post-market study in breast conserving surgery of ductal carcinoma in situ. Breast. 2014;23:94–96. doi: 10.1016/j.breast.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Qiu S.Q., Dorrius M.D., de Jongh S.J., et al. Micro-computed tomography (micro-CT) for intraoperative surgical margin assessment of breast cancer: a feasibility study in breast conserving surgery. Eur J Surg Oncol. 2018;44:1708–1713. doi: 10.1016/j.ejso.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Tang R., Coopey S.B., Buckley J.M., et al. A pilot study evaluating shaved cavity margins with micro-computed tomography: a novel method for predicting lumpectomy margin status intraoperatively. Breast J. 2013;19:485–489. doi: 10.1111/tbj.12146. [DOI] [PubMed] [Google Scholar]
- 37.Thill M., Szwarcfiter I., Kelling K., et al. Magnetic resonance imaging system for intraoperative margin assessment for DCIS and invasive breast cancer using the ClearSight™ system in breast-conserving surgery—results from a postmarketing study. J Surg Oncol. 2022;125:361–368. doi: 10.1002/jso.26721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa M., Allweis T., Karni T., et al. An intraoperative MRI system for margin assessment in breast conserving surgery: initial results from a novel technique. J Surg Oncol. 2016;114:22–26. doi: 10.1002/jso.24246. [DOI] [PubMed] [Google Scholar]
- 39.Miripour Z.S., Abbasv i F., et al. Human study on cancer diagnostic probe (CDP) for real-time excising of breast positive cavity side margins based on tracing hypoxia glycolysis; checking diagnostic accuracy in non-neoadjuvant cases. Cancer Med. 2022;11:1630–1645. doi: 10.1002/cam4.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubo A., Tanei T., A R.P., et al. Comparison of "click-to-sense" assay with frozen section analysis using simulated surgical margins in breast cancer patients. Eur J Surg Oncol. 2022;48:1520–1526. doi: 10.1016/j.ejso.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Brachtel E.F., Johnson N.B., Huck A.E., et al. Spectrally encoded confocal microscopy for diagnosing breast cancer in excision and margin specimens. Lab Invest. 2016;96:459–467. doi: 10.1038/labinvest.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang T.P., Leff D.R., Shousha S., et al. Imaging breast cancer morphology using probe-based confocal laser endomicroscopy: towards a real-time intraoperative imaging tool for cavity scanning. Breast Cancer Res Treat. 2015;153:299–310. doi: 10.1007/s10549-015-3543-8. [DOI] [PubMed] [Google Scholar]
- 43.St John E.R., Balog J., McKenzie J.S., et al. Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery. Breast Cancer Res. 2017;19:59. doi: 10.1186/s13058-017-0845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Real Time Tissue Characterisation Using Mass Spectrometry REI-EXCISE iKnife Study (REI-EXCISE). ClinicalTrials.gov identifier: NCT03432429. Updated March 31, 2022. Accessed December 20, 2023. https://clinicaltrials.gov/study/NCT03432429.
- 45.Lin C., Wang K.Y., Xu F., et al. The application of intraoperative specimen mammography for margin status assessment in breast-conserving surgery: a single-center retrospective study. Breast J. 2020;26:1871–1873. doi: 10.1111/tbj.13835. [DOI] [PubMed] [Google Scholar]
- 46.Bathla L., Harris A., Davey M., Sharma P., Silva E. High resolution intra-operative two-dimensional specimen mammography and its impact on second operation for re-excision of positive margins at final pathology after breast conservation surgery. Am J Surg. 2011;202:387–394. doi: 10.1016/j.amjsurg.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Baù M.G., Surace A., Gregori G., et al. Vacuum intraoperative specimen mammography: a novel technique. Eur J Obstet Gynecol Reprod Biol. 2020;253:1–6. doi: 10.1016/j.ejogrb.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Chagpar A.B., Butler M., Killelea B.K., Horowitz N.R., Stavris K., Lannin D.R. Does three-dimensional intraoperative specimen imaging reduce the need for re-excision in breast cancer patients? A prospective cohort study. Am J Surg. 2015;210:886–890. doi: 10.1016/j.amjsurg.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Ch J.T., Sharma M.M., Dharmarajan J.P., Nambiar A. Digital breast tomosynthesis as a tool in confirming negative surgical margins in non-palpable breast lesions. Indian Journal of Surgical Oncology. 2019;10:624–628. doi: 10.1007/s13193-019-00956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciccarelli G., Di Virgilio M.R., Menna S., et al. Radiography of the surgical specimen in early stage breast lesions: diagnostic reliability in the analysis of the resection margins. Radiol Med. 2007;112:366–376. doi: 10.1007/s11547-007-0147-3. [DOI] [PubMed] [Google Scholar]
- 51.Coombs N.J., Vassallo P.P., Parker A.J., Yiangou C. Radiological review of specimen radiographs after breast localisation biopsy is not always necessary. Eur J Surg Oncol. 2006;32:516–519. doi: 10.1016/j.ejso.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Funk A., Heil J., Harcos A., et al. Efficacy of intraoperative specimen radiography as margin assessment tool in breast conserving surgery. Breast Cancer Res Treat. 2020;179:425–433. doi: 10.1007/s10549-019-05476-6. [DOI] [PubMed] [Google Scholar]
- 53.Ra G., Mj H., Cj S., et al. The efficacy of specimen radiography in evaluating the surgical margins of impalpable breast carcinoma. AJR American journal of roentgenology. 1994;162:33–36. doi: 10.2214/ajr.162.1.8273685. [DOI] [PubMed] [Google Scholar]
- 54.Hisada T., Sawaki M., Ishiguro J., et al. Impact of intraoperative specimen mammography on margins in breast-conserving surgery. Mol Clin Oncol. 2016;5:269–272. doi: 10.3892/mco.2016.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulkarni S.A., Kulkarni K., Schacht D., et al. High-Resolution full-3D specimen imaging for lumpectomy margin assessment in breast cancer. Ann Surg Oncol. 2021;28:5513–5524. doi: 10.1245/s10434-021-10499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCormick J.T., Keleher A.J., Tikhomirov V.B., Budway R.J., Caushaj P.F. Analysis of the use of specimen mammography in breast conservation therapy. Am J Surg. 2004;188:433–436. doi: 10.1016/j.amjsurg.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 57.Miller C.L., Coopey S.B., Rafferty E., Gadd M., Smith B.L., Specht M.C. Comparison of intra-operative specimen mammography to standard specimen mammography for excision of non-palpable breast lesions: a randomized trial. Breast Cancer Res Treat. 2016;155:513–519. doi: 10.1007/s10549-016-3700-8. [DOI] [PubMed] [Google Scholar]
- 58.Park K.U., Kuerer H.M., Rauch G.M., et al. Digital breast tomosynthesis for intraoperative margin assessment during breast-conserving surgery. Ann Surg Oncol. 2019;26:1720–1728. doi: 10.1245/s10434-019-07226-w. [DOI] [PubMed] [Google Scholar]
- 59.Prueksadee J., Khamapirad T. Margin determination of two-view specimen radiography in breast cancer. Asian Biomed. 2009;3:537–543. [Google Scholar]
- 60.Ao S., Tj R., Km L., et al. Wire-guided excision of non-palpable breast cancer: determinants and correlations between radiologic and histologic margins and residual disease in re-excisions. Breast. 2001;10:28–34. doi: 10.1054/brst.2000.0174. [DOI] [PubMed] [Google Scholar]
- 61.Schaefgen B., Funk A., Sinn H.P., et al. Does conventional specimen radiography after neoadjuvant chemotherapy of breast cancer help to reduce the rate of second surgeries? Breast Cancer Res Treat. 2022;191:589–598. doi: 10.1007/s10549-021-06466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stachs A., Bollmann J., Martin A., et al. Radiopaque tissue transfer and X-ray system versus standard specimen radiography for intraoperative margin assessment in breast-conserving surgery: randomized clinical trial. BJS Open. 2022;6 doi: 10.1093/bjsopen/zrac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown J.Q., Bydlon T.M., Richards L.M., et al. Optical assessment of tumor resection margins in the breast. IEEE J Sel Top Quant Electron. 2010;16:530–544. doi: 10.1109/jstqe.2009.2033257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keller M.D., Majumder S.K., Kelley M.C., et al. Autofluorescence and diffuse reflectance spectroscopy and spectral imaging for breast surgical margin analysis. Laser Surg Med. 2010;42:15–23. doi: 10.1002/lsm.20865. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen F.T., Zysk A.M., Chaney E.J., et al. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009;69:8790–8796. doi: 10.1158/0008-5472.CAN-08-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt H., Connolly C., Jaffer S., et al. Evaluation of surgically excised breast tissue microstructure using wide-field optical coherence tomography. Breast J. 2020;26:917–923. doi: 10.1111/tbj.13663. [DOI] [PubMed] [Google Scholar]
- 67.Zhu D., Wang J., Marjanovic M., et al. Differentiation of breast tissue types for surgical margin assessment using machine learning and polarization-sensitive optical coherence tomography. Biomed Opt Express. 2021;12:3021–3036. doi: 10.1364/BOE.423026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffman A., Ashkenazi I. The efficiency of MarginProbe in detecting positive resection margins in epithelial breast cancer following breast conserving surgery. Eur J Surg Oncol. 2022;48:1498–1502. doi: 10.1016/j.ejso.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 69.Karni T., Pappo I., bank J., et al. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am J Surg. 2007;194:467–473. doi: 10.1016/j.amjsurg.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 70.LeeVan E., Ho B.T., Seto S., Shen J. Use of MarginProbe as an adjunct to standard operating procedure does not significantly reduce re-excision rates in breast conserving surgery. Breast Cancer Res Treat. 2020;183:145–151. doi: 10.1007/s10549-020-05773-5. [DOI] [PubMed] [Google Scholar]
- 71.McClatchy D.M., 3rd, Zuurbier R.A., Wells W.A., Paulsen K.D., Pogue B.W. Micro-computed tomography enables rapid surgical margin assessment during breast conserving surgery (BCS): correlation of whole BCS micro-CT readings to final histopathology. Breast Cancer Res Treat. 2018;172:587–595. doi: 10.1007/s10549-018-4951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.V L., C Z., M P., A L., R G., M B. Surgical specimen ultrasound: is it able to predict the status of resection margins after breast-conserving surgery? Breast. 2010;19:532–537. doi: 10.1016/j.breast.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 73.B M., E-K M., D H., et al. Sonography of postexcision specimens of nonpalpable breast lesions: value, limitations, and description of a method. AJR American journal of roentgenology. 2006;186:1014–1024. doi: 10.2214/AJR.05.0002. [DOI] [PubMed] [Google Scholar]
- 74.Moschetta M, Telegrafo M, Introna T, Coi L, Rella L, Ranieri V, Cirili A, Stabile Ianora AA, Angelelli G. Role of specimen US for predicting resection margin status in breast conserving therapy. G Chir. 2015;36(5):201–204. doi: 10.11138/gchir/2015.36.5.201. PMID: 26712255; PMCID: PMC4711974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perera N., Bourke A.G. The technique and accuracy of breast specimen ultrasound in achieving clear margins in breast conserving surgery. Journal of Medical Imaging and Radiation Oncology. 2020;64:747–755. doi: 10.1111/1754-9485.13077. [DOI] [PubMed] [Google Scholar]
- 76.Ramos M., Díaz J.C., Ramos T., et al. Ultrasound-guided excision combined with intraoperative assessment of gross macroscopic margins decreases the rate of reoperations for non-palpable invasive breast cancer. Breast. 2013;22:520–524. doi: 10.1016/j.breast.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Caruso F., Ferrara M., Castiglione G., et al. Therapeutic mammaplasties: full local control of breast cancer in one surgical stage with frozen section. Eur J Surg Oncol. 2011;37:871–875. doi: 10.1016/j.ejso.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Ikeda T., Enomoto K., Wada K., et al. Frozen-section-guided breast-conserving surgery: implications of diagnosis by frozen section as a guide to determining the extent of resection. Surg Today. 1997;27:207–212. doi: 10.1007/BF00941646. [DOI] [PubMed] [Google Scholar]
- 79.Jorns J.M., Daignault S., Sabel M.S., Wu A.J. Is intraoperative frozen section analysis of Reexcision specimens of value in preventing Reoperation in breast-conserving therapy? Am J Clin Pathol. 2014;142:601–608. doi: 10.1309/AJCPRSOA2G8RLEXY. [DOI] [PubMed] [Google Scholar]
- 80.Kikuyama M., Akashi-Tanaka S., Hojo T., et al. Utility of intraoperative frozen section examinations of surgical margins: implication of margin-exposed tumor component features on further surgical treatment. Jpn J Clin Oncol. 2015;45:19–25. doi: 10.1093/jjco/hyu158. [DOI] [PubMed] [Google Scholar]
- 81.Kim M.J., Kim C.S., Park Y.S., Choi E.H., Han K.D. The efficacy of intraoperative frozen section analysis during breast-conserving surgery for patients with ductal carcinoma in situ. Breast Cancer Basic Clin Res. 2016;10:205–210. doi: 10.4137/BCBCR.S40868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ko S., Chun Y.K., Kang S.S., Hur M.H. The usefulness of intraoperative circumferential frozen-section analysis of lumpectomy margins in breast-conserving surgery. J Breast Cancer. 2017;20:176–182. doi: 10.4048/jbc.2017.20.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noguchi M., Minami M., Earashi M., et al. Intraoperative histologic assessment of surgical margins and lymph node metastasis in breast-conserving surgery. J Surg Oncol. 1995;60:185–190. doi: 10.1002/jso.2930600309. [DOI] [PubMed] [Google Scholar]
- 84.Nowikiewicz T., Śrutek E., Głowacka-Mrotek I., Tarkowska M., Żyromska A., Zegarski W. Clinical outcomes of an intraoperative surgical margin assessment using the fresh frozen section method in patients with invasive breast cancer undergoing breast-conserving surgery - a single center analysis. Sci Rep. 2019;9 doi: 10.1038/s41598-019-49951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olson T.P., Harter J., Muñoz A., Mahvi D.M., Breslin T. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol. 2007;14:2953–2960. doi: 10.1245/s10434-007-9437-1. [DOI] [PubMed] [Google Scholar]
- 86.Osako T., Nishimura R., Nishiyama Y., et al. Efficacy of intraoperative entire-circumferential frozen section analysis of lumpectomy margins during breast-conserving surgery for breast cancer. Int J Clin Oncol. 2015;20:1093–1101. doi: 10.1007/s10147-015-0827-2. [DOI] [PubMed] [Google Scholar]
- 87.Rusby J.E., Paramanathan N., Laws S.A., Rainsbury R.M. Immediate latissimus dorsi miniflap volume replacement for partial mastectomy: use of intra-operative frozen sections to confirm negative margins. Am J Surg. 2008;196:512–518. doi: 10.1016/j.amjsurg.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 88.Bakhsh eh M., Tutuncuoglu S.O., Fischer G., Masood S. Use of imprint cytology for assessment of surgical margins in lumpectomy specimens of breast cancer patients. Diagn Cytopathol. 2007;35:656–659. doi: 10.1002/dc.20704. [DOI] [PubMed] [Google Scholar]
- 89.Blair S.L., Wang-Rodriguez J., Cortes-Mateos M.J., et al. Enhanced touch preps improve the ease of interpretation of intraoperative breast cancer margins. Am Surg. 2007;73:973–976. [PubMed] [Google Scholar]
- 90.Cox C.E., Ku N.N., Reintgen D.S., Greenberg H.M., Nicosia S.V., Wangensteen S. Touch preparation cytology of breast lumpectomy margins with histologic correlation. Arch Surg. 1991;126:490–493. doi: 10.1001/archsurg.1991.01410280094014. [DOI] [PubMed] [Google Scholar]
- 91.Creager A.J., Shaw J.A., Young P.R., Geisinger K.R. Intraoperative evaluation of lumpectomy margins by imprint cytology with histologic correlation: a community hospital experience. Arch Pathol Lab Med. 2002;126:846–848. doi: 10.5858/2002-126-0846-IEOLMB. [DOI] [PubMed] [Google Scholar]
- 92.D'Halluin F., Tas P., Rouquette S., et al. Intra-operative touch preparation cytology following lumpectomy for breast cancer: a series of 400 procedures. Breast. 2009;18:248–253. doi: 10.1016/j.breast.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Ku N.N., Cox C.E., Reintgen D.S., Greenberg H.M., Nicosia S.V. Cytology of lumpectomy specimens. Acta Cytol. 1991;35:417–421. [PubMed] [Google Scholar]
- 94.Muttalib M., Tisdall M., Scawn R., Shousha S., Cummins R.S., Sinnett H.D. Intra-operative specimen analysis using faxitron microradiography for excision of mammographically suspicious, non-palpable breast lesions. Breast. 2004;13:307–315. doi: 10.1016/j.breast.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 95.Sumiyoshi K., Nohara T., Iwamoto M., et al. Usefulness of intraoperative touch smear cytology in breast-conserving surgery. Exp Ther Med. 2010;1:641–645. doi: 10.3892/etm_00000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tamanuki T., Namura M., Aoyagi T., Shimizu S., Suwa T., Matsuzaki H. Effect of intraoperative imprint cytology followed by frozen section on margin assessment in breast-conserving surgery. Ann Surg Oncol. 2021;28:1338–1346. doi: 10.1245/s10434-020-08955-z. [DOI] [PubMed] [Google Scholar]
- 97.Tohnosu N., Nabeya Y., Matsuda M., et al. Rapid intraoperative scrape cytology assessment of surgical margins in breast conservation surgery. Breast Cancer. 1998;5:165–169. doi: 10.1007/BF02966689. [DOI] [PubMed] [Google Scholar]
- 98.Valdes E.K., Boolbol S.K., Ali I., Feldman S.M., Cohen J.M. Intraoperative touch preparation cytology for margin assessment in breast-conservation surgery: does it work for lobular carcinoma? Ann Surg Oncol. 2007;14:2940–2945. doi: 10.1245/s10434-007-9364-1. [DOI] [PubMed] [Google Scholar]
- 99.Valdes E.K., Boolbol S.K., Cohen J.M., Feldman S.M. Intra-operative touch preparation cytology; does it have a role in re-excision lumpectomy? Ann Surg Oncol. 2007;14:1045–1050. doi: 10.1245/s10434-006-9263-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.