Highlights
-
•
PFS and OS differ in response categories defined by RecistTM, but not by RECIST.
-
•
RecistTM can accurately differentiate patients who derive a more pronounced benefit from the treatment among those classified as PR and SD by RECIST.
-
•
TmCR based on RecistTM may be the ultimate short-term goal in terms of extending patient survival.
-
•
RecistTM can identify drug-resistant cases early, allowing prompt intervention, which can extends the imaging-demonstrated time to progression.
-
•
RecistTM is superior to RECIST for classifying short- and long-term patient benefit.
Keywords: Non-small-cell lung cancer, Overall survival, Progression-free survival, RecistTM, RECIST
Abstract
Background
Tyrosine kinase inhibitors (TKIs) are standard first-line treatments for advanced non-small-cell lung cancer (NSCLC) with driver gene mutations. The Response Evaluation Criteria in Solid Tumors (RECIST) are limited in predicting long-term patient benefits. A tumour marker-based evaluation criteria, RecistTM, was used to investigate the potential for assessing targeted-therapy efficacy in lung cancer treatment.
Methods
We retrospectively analysed patients with stage IIIA–IV NSCLC and driver gene mutations, whose baseline tumour marker levels exceeded the pre-treatment cut-off value three-fold and who received TKI-targeted therapy as a first-line treatment. We compared efficacy, progression-free survival (PFS), and overall survival (OS) between RecistTM and RECIST.
Findings
The median PFS and OS differed significantly among treatment-response subgroups based on RecistTM but not RECIST. The predicted 1-, 2-, and 3-year disease-progression risk, according to area under the receiver operating characteristic curve, as well as the 1-, 3-, and 5-year mortality risk, differed significantly between RecistTM and RECIST. The median PFS and OS of tmCR according to RecistTM, was significantly longer than (CR+PR) according to RECIST. Imaging analysis revealed that the ΔPFS was 11.27 and 6.17 months in the intervention and non-intervention groups, respectively, suggesting that earlier intervention could extend patients' PFS.
Interpretation
RecistTM can assess targeted-therapy efficacy in patients with advanced NSCLC and driver gene mutations, along with tumour marker abnormalities. RecistTM surpasses RECIST in predicting short- and long-term patient benefits, and allows the early identification of patients resistant to targeted drugs, enabling prompt intervention and extending the imaging-demonstrated time to progression.
Introduction
Lung cancer has the second highest incidence and the highest mortality rate among malignant tumors worldwide [1]. Data have revealed that approximately 85% of patients with lung cancer are diagnosed with non-small-cell lung cancer (NSCLC), among whom approximately 64% of patients with lung adenocarcinoma have mutations in driver genes, such as genes encoding the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS1 [2,3]. For patients with advanced NSCLC and driver gene mutations, targeted therapy, primarily with tyrosine kinase inhibitors (TKI), has become the standard treatments [4].
The conventional treatment response evaluation criteria, i.e., the Response Evaluation Criteria in Solid Tumors (RECIST), are commonly used to assess treatment efficacy in clinical settings. These criteria categorize the results into four groups, namely complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) by imaging [5]. However, notable limitations arise when using these criteria alone for efficacy evaluations. RECIST precision in evaluating certain lesions, including diffuse pulmonary nodules, pleural effusion, and bone metastasis, is restricted to categorizing PD or CR. Furthermore, since targeted therapies extend survival, the efficacy of the treatment evaluated with RECIST does not effectively predict long-term benefits for patients receiving such treatments. For patients with advanced NSCLC under targeted therapy, CR rates according to RECIST rarely exceed 5%, and PD rates are typically below 10%; therefore, approximately 85% of patients are placed in the PR or SD categories [[6], [7], [8]]. He et al. [9] analyzed 179 patients and observed no significant differences in progression-free survival (PFS) and overall survival (OS) between patients with advanced NSCLC that were treated with chemotherapy who were initially assessed as PR and SD according to RECIST. Blumenthal et al. [10] conducted a meta-analysis of 12 clinical trials of advanced NSCLC (treated with targeted therapy, chemotherapy, etc.) and discovered a strong correlation between the overall response rate (ORR = CR + PR) by RECIST and PFS (R2 = 0.89); however, no correlation was found between ORR and OS (R2 = 0.09). Furthermore, with more patients treated with targeted therapy, the same ORR may reflect different PFS rates. By contrast, ORR and PFS may not be correlated. For example, in clinical studies of second-generation ALK inhibitors in ALK-positive cases resistant to crizotinib, ceritinib, and brigatinib, similar ORRs (50–62%), yet exhibited a significant difference in PFS (ceritinib: 6.9 months; brigatinib: 15.6 months) [11]. Therefore, in an era where targeted therapies prolong patient survival, survival is also influenced by subsequent treatments, rendering traditional RECIST-based efficacy evaluations less predictive of long-term survival [12].
Therefore, alternative evaluation methods have been proposed, including depth of response [13], changes in tumor volume [14], and integration of RECIST at specific time points to predict both short- and long-term benefits for patients [15]. However, owing to the influence of sample sizes, the results of various studies are often contradictory [16].
Besides radiological assessment, assays of tumor markers in the blood, circulating tumor cells (CTCs), and circulating tumor DNA (ctDNA) also pertain in clinical research to determine tumor activity, tumor burden, and therapeutic efficacy at a molecular level [[17], [18], [19], [20]]. However, sophisticated experimental technologies, limited accessibility, and high costs associated with ctDNA and CTC tests prevent their widespread application. By contrast, with strong repeatability, high specificity and accuracy, and ease of quantification when assessing the efficacy and prognosis of tumor therapies, serum tumor marker assays have become routine. Evidence from numerous studies has indicated a strong correlation between changes in serum tumor marker levels and the efficacy of tumor therapy [[21], [22], [23], [24]]. For example, Yanwei et al. [25] observed that, for patients with NSCLC undergoing TKI therapy, those with higher baseline carcinoembryonic antigen (CEA) levels exhibited a significantly higher disease control rate than those with lower CEA levels. Despite these insights, clinical research on tumor markers has been disorganized, and standardized criteria for monitoring therapeutic efficacy have been lacking.
In our previous study, we introduced RecistTM, a set of criteria to assess treatment efficacy based on tumor markers [26]. RecistTM scientific merits lie in its exclusion of patients not suitable for tumor marker-based efficacy assessment (it applies only to those with baseline tumor marker levels exceeding three times the cutoff value), while simultaneously accounting for marker abnormalities due to tumor heterogeneity as the disease progresses. The establishment of these criteria has improved the objectivity of tumor markers in clinical efficacy evaluations and has addressed inconsistency in clinical assessments. Our previous study conducted on solid tumors including NSCLC, confirmed that RecistTM yielded a higher tumor marker-based CR (tmCR) rate than the CR rate by the immune-related Response Evaluation Criteria in Solid Tumors (irRECIST), with a significant difference in survival time among tmCR, tumor marker-based PR (tmPR), and tumor marker-based SD (tmSD) subgroups. Moreover, RecistTM refines the classification of patients categorized as PR by irRECIST, identifying those who derive a more pronounced benefit from treatment.
As patients now experience longer survival owing to targeted therapies and conventional RECIST fails to effectively predict long-term benefits, the objective of this study is to explore whether tumor marker-based RecistTM is superior to RECIST in accurately predicting both short- and long-term benefits for targeted therapies of patients with NSCLC with driver gene mutations.
Methods
Collection of clinical data
Data were obtained from patients with advanced NSCLC and driver gene mutations (EGFR, ALK, ROS1, RET, CMET, and HER2), who received TKI-targeted therapy as the first-line treatment, from January 1, 2012, to January 1, 2022, at the Army Medical Center of PLA. The targeted-therapy drugs included EGFR-TKIs (gefitinib, erlotinib, afatinib, osimertinib, almonertinib, furmonertinib, and others), ALK-TKIs (including crizotinib, ceritinib, alectinib, and others), ROS1-TKIs (crizotinib, and others), RET-TKIs (pralsetinib, and others), Cmet-TKIs (savolitinib, and others), and HER-2-TKIs (pyrotinib, and others). The key parameters analyzed included patient information, such as age and sex, stage of the disease, mutated driver gene(s), treatment regimen, imaging data, and tumor marker levels. Patients were included in the study according to the following main criteria: (1) Diagnosis with advanced NSCLC (stages IIIA–IV). (2) The presence of driver gene mutations and corresponding targeted therapy as first-line treatment, which included therapy for sensitive EGFR mutations, ALK rearrangements, ROS1 rearrangements, RET rearrangements, Cmet exon 14-skipping mutations, and HER-2 exon 20 mutations. (3) Levels of any primary tumor markers elevated to over three-fold above normal before treatment. This included CEA > 15 ng/mL, CA-199 > 105 U/mL, CA-125 > 105 U/mL, CA-153 > 105 U/mL, NSE > 60 ng/mL, SCCAg > 7.5 ng/mL, and CYFRA21-1 > 21 ng/mL. After treatment, the dynamics of tumor markers were monitored at least once within three months and at least three times before disease progression. (4) Presence of measurable lesions before treatment and imaging evaluations received at least once within three months post-treatment and at least twice before disease progression.
We excluded patients who lacked sufficient imaging or tumor marker data to determine optimal therapeutic efficacy and progression, had baseline tumor marker levels below the threshold of three times the normal limit, or had incomplete follow-up data.
This study was approved by the Ethics Committee of the PLA Army Medical Center, under the Ratification NO: 2022(262). And all patients were exempted from written informed consent forms according to national legislation and the institutional requirements.
Definitions
OS was defined as the interval from the start of treatment to death from any cause. PFS was defined as the interval from the start of treatment to disease progression, as assessed by RECIST, or to death from any cause before disease progression. Tumor marker-related progression-free survival (tmPFS) was defined as the interval from the start of treatment to disease progression, as assessed by RecistTM, or to death from any cause before disease progression. △PFS was defined as the difference between PFS and tmPFS, that is, PFS - tmPFS. ORR was defined as the percentage of patients who achieved complete or partial remission according to RECIST or RecistTM.
RECIST and RecistTM
RECIST, based on radiographic imaging, classifies clinical efficacy into four categories: CR, PR, SD, and PD [5]. RecistTM classifies clinical efficacy according to tumor marker test results into four categories: tmCR, tmPR, tmSD, and tmPD.
Considering that RecistTM was originally intended for patients undergoing immunotherapy, and given the phenomenon of pseudoprogression in these patients, disease progression assessment (using conventional irRECIST or our RecistTM) requires confirmation after 3–4 weeks. To align with the specificities of the efficacy assessment of targeted therapies, adjustments were made to the original assessment of tmPD within RecistTM [26]. These adjustments included eliminating the requirement for a confirmation of tmPD assessment after 3–4 weeks and establishing new criteria for patients initially assessed as tmCR, but later reassessed as tmPD (Table 1).
Table 1.
Response evaluation criteria in solid tumors based on tumor markers (RecistTM version 1.1)*.
| Primary markers | Secondary markers | Markers with normal baseline levels | Other conditions that need to be met | |
|---|---|---|---|---|
| tmCR | Decreased to normal | Decreased to normal | Normal | All the above conditions were met and maintained for ≥ 6 weeks. |
| tmPR | Decreased ≥30% | Decreased, or increased ≤ 20% | ≤ 1.5 times the cutoff value | All the above conditions were met and maintained for ≥ 6 weeks. |
| tmSD | Did not meet the criteria for tmPR, tmCR, or tmPD | Maintained for ≥ 6 weeks. | ||
| tmPD | Increased ≥ 30% | Increased ≥ 50% | ≥ 2 times the cutoff value | Meeting any of the conditions. |
*(1) Primary tumor markers were identified as those with an increase exceeding three-times the cutoff value, while secondary markers were selected for those within the three-fold threshold. Only one marker was designated as primary; in cases where multiple markers met this criterion, the one with better tumor specificity or a more substantial fold-increase was selected as the primary marker.
(2) Initial efficacy assessment: For patients with a baseline primary marker absolute value of < 20, the threshold for recognizing an increase or decrease in the primary marker was set at 50% instead of 30%.
Efficacy reassessment: For patients initially classified as tmCR, subsequent assessments for disease progression required that the primary or secondary marker increased by > 50% relative to the cutoff value; an increase of > 80% above the cutoff was required for markers with cutoff values < 10 to be considered indicative of disease progression.
(3) With differences in testing methods at different hospitals, the cutoff values for tumor markers could differ; thus, if the test results were in question, a repeat test could be conducted when necessary.
Abbreviation:
tmCR, Tumor marker-related completed response; tmPR, Tumor marker-related partial response; tmSD, Tumor marker-related stable disease; tmPD, Tumor marker-related progressive disease. RecistTM, Response evaluation criteria in solid tumors based on tumor markers.
Statistical analysis
Statistical analyzes were conducted mainly using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). McNemar's test was used to compare differences in efficacy assessment between RECIST and RecistTM. Cohen's Kappa coefficient was used to evaluate the consistency between RECIST and RecistTM, with Kappa < 0.4 indicating poor consistency, 0.4 ≤ Kappa < 0.75 indicating moderate consistency, and Kappa ≥ 0.75 indicating good consistency. Kaplan–Meier analysis with log-rank tests, and Cox regression analysis were performed to assess and compare the differences in median PFS and OS between the two criteria. R (version 4.1.0) was used to plot the receiver operating characteristic (ROC) curves that predict disease progression, as well as the time-dependent ROC curves that predict mortality according to RECIST and RecistTM. Delong's test was conducted to compare the differences in the areas under the ROC curves (AUC) between the two criteria. The ΔPFS for the intervention group and the non-intervention group were expressed as median and interquartile ranges [M (P25, P75)], and the Mann–Whitney test was used to compare the differences in ΔPFS between the two groups. For all tests, P < 0.05 was considered statistically significant.
Results
Baseline data
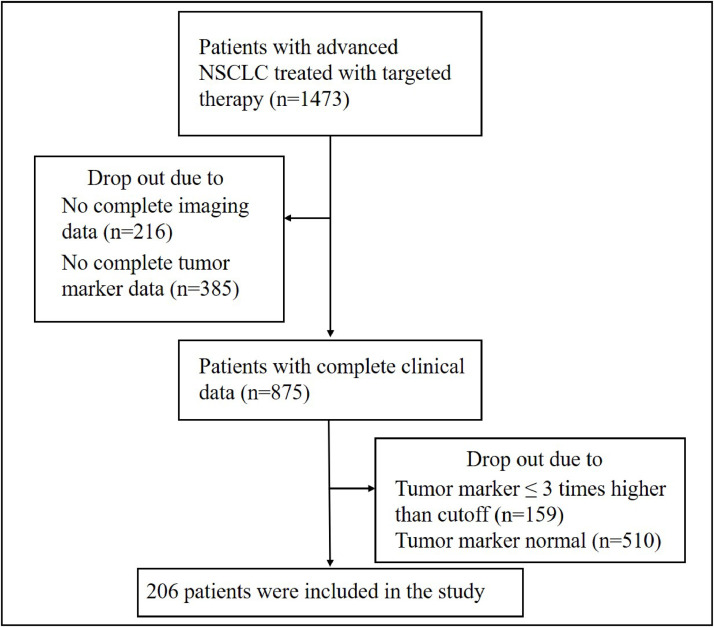
All included tumors were cases of lung adenocarcinoma, and all patients were those who received targeted therapy as first-line treatment. Of 1,473 patients with lung cancer who received targeted therapy as first-line treatment, 875 with comprehensive clinical data were selected for analysis. Of these, 206 patients (23.5%) exhibited serum tumor marker levels that exceeded three times the established cutoff value for their first test (Fig. 1). The median age of the patients was 59 years (range: 23–90 years), with genetic mutations identified in EGFR, ALK, HER2, CMET, and ROS1. The primary tumor markers used for efficacy assessment according to RecistTM included CEA, CA125, CA199, and CA153, with CEA being the most common marker, accounting for 91.3% of the cases. See Table 2 for details.
Fig. 1.
Flow diagram of this study. NSCLC, non-small-cell lung cancer.
Table 2.
Patients’ characteristics.
| N (%) | ||
|---|---|---|
| Age (years) | < 65 | 146 (70.9%) |
| ≥ 65 | 60 (29.1%) | |
| Sex | Female | 124 (60.2%) |
| Male | 82 (39.8%) | |
| Stage | IIIA–IIIB | 11 (5.3%) |
| IV | 195 (94.7%) | |
| Metastatic site | Bone | 111 (53.9%) |
| Lung | 55 (26.7%) | |
| Brain | 39 (18.9%) | |
| Pleura | 35 (17%) | |
| Liver | 26 (12.6%) | |
| Adrenals | 11 (5.3%) | |
| Spleen | 4 (1.9%) | |
| Pericardium | 4 (1.9%) | |
| Others | 7 (3.4%) | |
| Mutation_type | EGFR | 183 (88.8%) |
| ALK | 17 (8.2%) | |
| HER2 | 1 (0.5%) | |
| CMET | 2 (1.0%) | |
| ROS1 | 3 (1.5%) | |
| Combination therapy | No | 91 (44.2%) |
| Chemotherapy | 93 (45.1%) | |
| Anti-angiogenic therapy | 55 (26.7%) | |
| Radiotherapy | 15 (7.3%) | |
| Targeted drugs | Gefitinib | 104 (50.5%) |
| Erotinib | 22 (10.7%) | |
| Icotinib | 16 (7.8%) | |
| Osimertinib | 18 (8.7%) | |
| Almonertinib | 6 (2.9%) | |
| Afatinib | 15 (7.3%) | |
| Alectinib | 9 (4.3%) | |
| Cozotinib | 13 (6.3%) | |
| Pyrotinib | 1 (0.5%) | |
| Dacomitinib | 2 (1.0%) | |
| Markers | CEA | 188 (91.3%) |
| CA125 | 9 (4.3%) | |
| CA199 | 6 (2.9%) | |
| CA153 | 3 (1.5%) | |
Abbreviation:
EGFR, the epidermal growth factor receptor. ALK, anaplastic lymphoma kinase. ROS1, ROS proto-oncogene 1, receptor tyrosine kinase. RET, rearranged during transfection, CMET, C-Mesenchymal-epithelia transition factor, and HER2, human epidermal growth 2; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; CA153, carbohydrate antigen 153.
Comparison of efficacy assessment results between the evaluation criteria
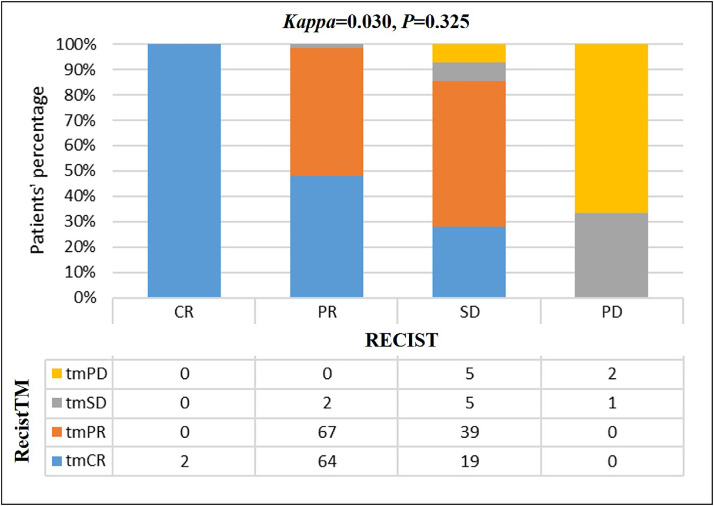
Both sets of criteria (RecistTM and RECIST) were used to assess the efficacy of treatment in all patients. The efficacy evaluation assessed by RecistTM yielded the following results: tmCR: 85 (41.3%), tmPR: 106 (51.4%), tmSD: 8 (3.9%), tmPD: 7 (3.4%), with an ORR of 92.7%. Using RECIST, the efficacy evaluations yielded the following results: CR: 2 (1%), PR: 133 (64.5%), SD: 68 (33%), PD: 3 (1.5%), with an ORR of 65.5%. A significant difference was observed between the efficacy results obtained from the two sets of criteria (χ2= 64.295, P < 0.001). Of the 133 patients classified as PR by RECIST, 64 (48.1%) were classified as tmCR by RecistTM. Of the 68 patients classified as SD by RECIST, 58 (85.3%) were classified as tmCR (n = 19, 27.9%) or tmPR (n = 39, 57.4%) by RecistTM. The consistency between the criteria was 36.9%, and the Kappa test indicated that the two sets of criteria demonstrated poor consistency (Kappa= 0.030, P= 0.325; Fig. 2).
Fig. 2.
Consistency test between RecistTM and RECIST (n = 206). Efficacy assessment of targeted therapy with RECIST and RecistTM. The consistency between the criteria was 36.9%. The Kappa test indicated poor consistency between the criteria. RECIST, Response Evaluation Criteria in Solid Tumors; RecistTM, Response Evaluation Criteria in Solid Tumors based on Tumor Markers; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; tmCR, tumor marker-related complete response; tmPR, tumor marker-related partial response; tmSD, tumor marker-related stable disease; tmPD, tumor marker-related progressive disease.
Correlation between different criteria for efficacy assessment and patient PFS (tmPFS)
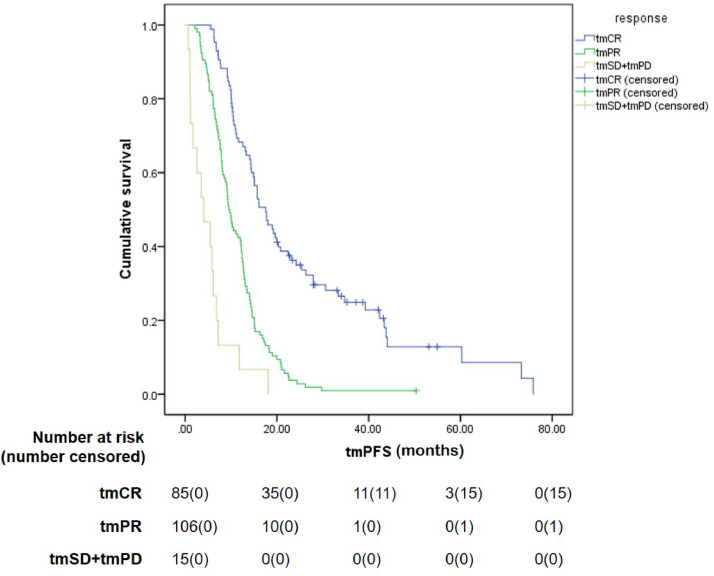
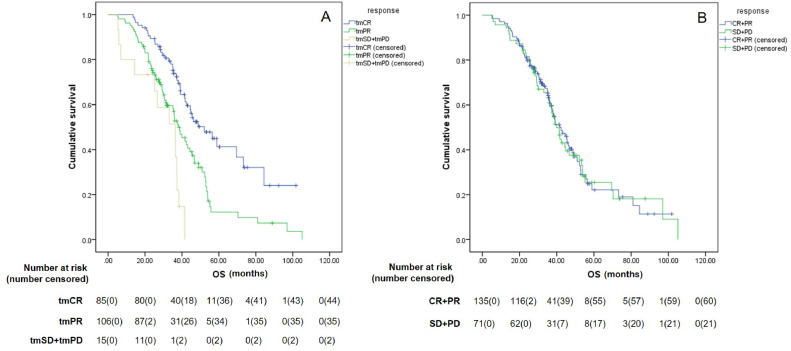
The median duration of follow-up was 49.2 months. At the time of submission, disease progression had been radiographically confirmed in 187 patients, whereas tumor markers indicated progression in 190 patients. The treatment efficacy was evaluated for all patients, using both criteria sets (RecistTM and RECIST), and revealed different PFS rates for these different criteria sets. The median tmPFS obtained with RecistTM was 12.0 months (95% CI: 10.4–13.6), whereas the median PFS obtained with RECIST was 16.1 months (95% CI: 14.4–17.9), with a significant difference in PFS between the two sets of criteria (χ2= 9.431, P = 0.002). When evaluating the correlation between efficacy assessment and patient survival, we pooled the tmSD (n = 8) and tmPD (n = 7) categories under RecistTM for analysis, owing to their small numbers. Similarly, for RECIST, cases of CR (n= 2) and PD (n = 3) were also few; thus, CR was combined with PR, and SD with PD before analysis. The results revealed that, according to RecistTM, the median tmPFS for tmCR, tmPR, and tmSD+tmPD was 17.6 months (95% CI: 14.0–21.1), 9.5 months (95% CI: 8.4–10.7), and 4.1 months (95% CI: 0.4–7.7), respectively. Among these, a significant difference in median tmPFS was observed between tmCR and tmPR (χ2= 45.853, P < 0.001), as well as between tmPR and tmSD+tmPD (χ2 = 19.032, P < 0.001) (Fig. 3).
Fig. 3.
Comparison of tumor marker-based progression-free survival (tmPFS) among different efficacy categories by RecistTM. RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers. tmCR, tumor marker-related complete response; tmPR, tumor marker-related partial response; tmSD, tumor marker-related stable disease; tmPD, tumor marker-related progressive disease.
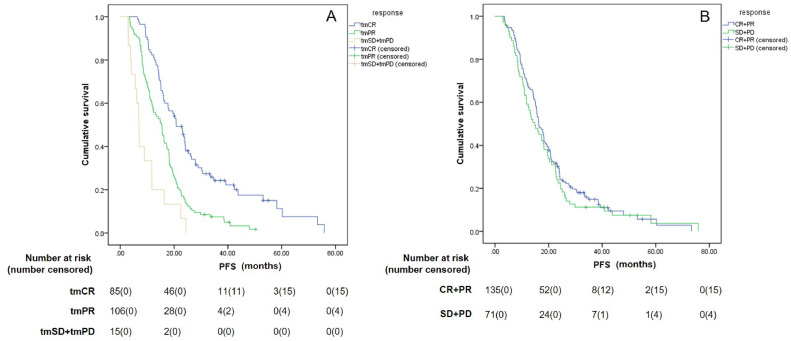
When using the PFS as determined with RECIST as a reference, the median PFS for tmCR, tmPR, and tmSD+tmPD subgroups, defined according to RecistTM, were 20.8 months (95% CI: 16.8–24.9), 15.2 months (95% CI: 12.1-18.4), and 6.9 months (95% CI: 5.7–8.2), respectively. The median PFS of tmCR patients was significantly longer than tmPR patients (χ2= 22.548, P < 0.001), and the median PFS of tmPR patients was significantly longer than tmSD+tmPD patients (χ2= 9.333, P = 0.002). However, according to RECIST, the median PFS for (CR+PR) and (SD+PD) were 16.3 months (95% CI: 14.5–18.1) and 14.8 months (95% CI: 10.5–19.1), respectively, with no significant differences between the two groups (χ2= 0.709, P = 0.400). Furthermore, the median PFS of tmCR patients was significantly longer than patients (CR+PR), as defined by RECIST (χ2= 6.564, P = 0.010) (Fig. 4).
Fig. 4.
Differences in progression-free survival (PFS) among different efficacy categories by RecistTM (A) and RECIST (B). RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers; RECIST, Response Evaluation Criteria in Solid Tumors. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; tmCR, tumor marker-related complete response; tmPR, tumor marker-related partial response; tmSD, tumor marker-related stable disease; tmPD, tumor marker-related progressive disease.
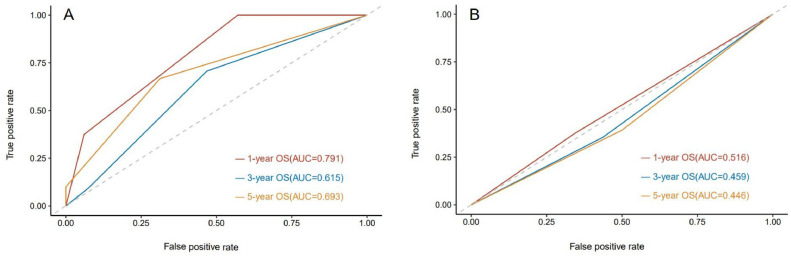
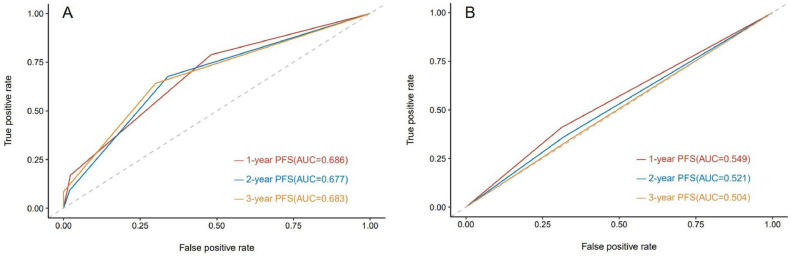
To evaluate the efficacy of the two criteria in predicting patient survival, ROC curves of the efficacy of treatment, evaluated by the two criteria relative to PFS were plotted. The results revealed that the AUC for predicting the risk of disease progression at 1 year, 2 years, and 3 years with RecistTM was 0.686 (95% CI: 0.619–0.753), 0.677 (95% CI: 0.603–0.752), and 0.683 (95% CI: 0.584–0.783), respectively; whereas, according to RECIST, the AUC was 0.549 (95% CI: 0.479–0.618), 0.521 (95% CI: 0.446–0.596), and 0.504 (95% CI: 0.394–0.615), respectively (Fig. 8). When comparing the AUCs to predict the risk of disease progression at 1 year, 2 years, and 3 years, a significant difference was observed between the criteria sets (P = 0.003, 0.001, 0.022), further indicating that RecistTM was superior to RECIST in predicting PFS (Fig. 5).
Fig. 8.
ROC curves predicting 1-year, 3-year, and 5-year overall survival (OS) by RecistTM (A) and RECIST (B). ROC, receiver operating characteristic curve; AUC, area under the curve. RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers. RECIST, Response Evaluation Criteria in Solid Tumors.
Fig. 5.
ROC curves predicting the risk of disease progression at 1 year, 2 years, and 3 years by RecistTM (A) and RECIST (B). ROC, receiver operating characteristic. AUC, areas under the curves. RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers. RECIST, Response Evaluation Criteria in Solid Tumors. PFS, progression-free survival.
These findings indicate that efficacy assessments using conventional RECIST do not effectively predict the disease-progression timeline, whereas RecistTM provides a more effective prediction of the patient's disease-progression.
Correlation between different criteria for efficacy assessment and OS in patients
At the time of writing, the study had recorded 125 deaths, 71 survivors, and no loss of follow-up. The median OS (mOS) was 41.4 months (95% CI: 37.0–45.8).
The results revealed that, according to RecistTM, mOS for tmCR, tmPR, and tmSD+tmPD were 52.1 (95% CI: 38.8–65.4), 38.0 (95% CI: 32.7–43.3), and 36.3 (95% CI: 19.1–53.5) months, respectively, whereas a significant difference in mOS was observed between tmCR and tmPR (χ2 = 12.587, P < 0.001), as well as between tmPR and tmSD+tmPD (χ2 = 5.971, P = 0.015). According to RECIST, the mOS for (CR+PR) and (SD+PD) was 41.7 months (95% CI: 35.4–48.0) and 40.0 months (95% CI: 35.6–44.5), respectively, without significant differences between the two groups (χ2= 0.005, P = 0.942). Furthermore, a significant difference in mOS was also observed between tmCR and CR+PR (χ2= 5.230, P= 0.022) (Fig. 6).
Fig. 6.
Differences in overall survival (OS) among different efficacy categories by RecistTM (A) and RECIST (B). RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers. RECIST, Response Evaluation Criteria in Solid Tumors. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; tmCR, tumor marker-related complete response; tmPR, tumor marker-related partial response; tmSD, tumor marker-related stable disease; tmPD, tumor marker-related progressive disease.
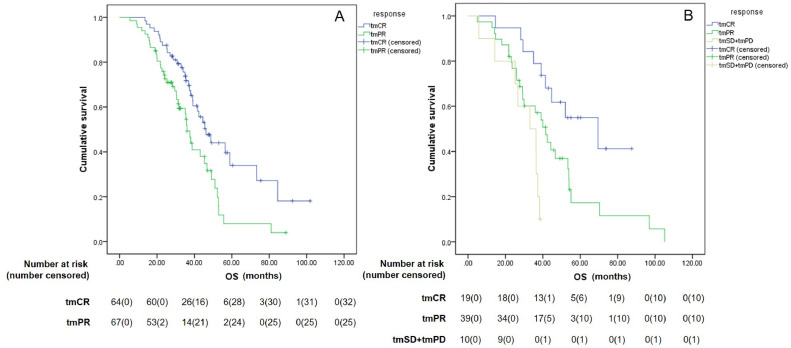
As efficacy assessments by RECIST predominantly categorized patients as PR (n = 133) and SD (n = 68), a re-evaluation was conducted for these patients using RecistTM. The results showed that, for patients classified as PR by RECIST, the mOS for those reclassified as tmCR (n = 64) and tmPR (n = 67) with RecistTM was 46.2 months (95% CI: 38.3–54.0) and 36.0 months (95% CI: 32.8–39.1), respectively, with a significant difference between the two groups (χ2 = 7.890, P = 0.005). For patients classified as SD by RECIST, the mOS for those reclassified as tmCR (n = 19) and tmPR (n = 39) by using RecistTM was 69.5 months (95% CI: 34.3–104.7) and 41.6 months (95% CI: 33.3–49.9), respectively, with a significant difference between the two groups (χ2 = 4.839, P = 0.028). The mOS for those classified as tmSD+tmPD (n = 10) by RecistTM was 33.1 months (95% CI: 18.1–48.1), which was significantly lower than that for those classified as tmPR (χ2 = 4.424, P = 0.035) (Fig. 7). These results indicate that RecistTM can accurately differentiate patients who derive a more pronounced benefit from the treatment among those classified as PR and SD by RECIST.
Fig. 7.
Differences in overall survival (OS) between patients categorized by RecistTM, who were categorized as partial response (PR) (n = 131, A) and stable disease (SD) (n = 68, B) by RECIST. RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers. RECIST, Response Evaluation Criteria in Solid Tumors. tmCR, tumor marker-related complete response; tmPR, tumor marker-related partial response; tmSD, tumor marker-related stable disease; tmPD, tumor marker-related progressive disease.
To evaluate the efficacy of the two sets of criteria for predicting patient survival, we plotted the ROC curves of the efficacy relative to OS as evaluated by the two criteria. The results revealed that the AUC for predicting the risk of death at 1 year, 3 years, and 5 years with RecistTM was 0.791 (95% CI: 0.691–0.892), 0.615 (95% CI: 0.538–0.692), and 0.693 (95% CI: 0.580–0.806), respectively; whereas according to RECIST, the AUC was 0.516 (95% CI: 0.344–0.687), 0.459 (95% CI: 0.384–0.534), and 0.446 (95% CI: 0.315–0.577), respectively. When comparing the AUCs to predict the risk of death at 1 year, 3 years, and 5 years, a significant difference was observed between the criteria (P = 0.001, 0.002, 0.007), further indicating that RecistTM was also superior in predicting OS than RECIST (Fig. 8).
Correlation between early intervention according to RecistTM and disease progression in patients
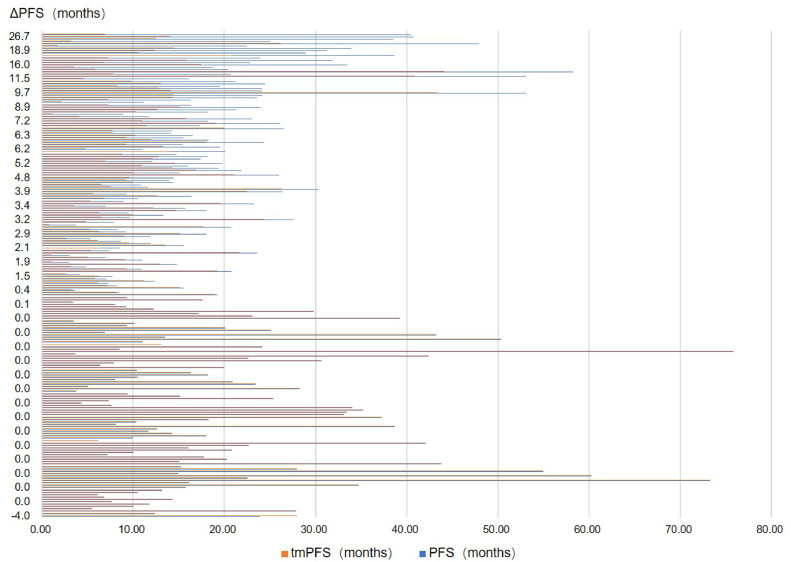
The above results indicated a significant difference between tmPFS and PFS when using different evaluation criteria. Specifically, tmPFS by RecistTM was significantly less, by 4.1 months, as compared to PFS by RECIST. In most all cases, the duration of tmPFS by RecistTM did not exceed that of PFS by RECIST (except for one case involving a patient with a CA153 tumor marker of which levels remained within normal limits until the patient's death, despite disease progression shown on subsequent imaging. This could be owing to the limited correlation between CA153 and lung cancer). For the difference between PFS and tmPFS (△PFS), 123 patients showed a △PFS of < 3 months, 33 patients had a △PFS between 3 and 6 months, and 50 patients had a △PFS of > 6 months (Fig. 9).
Fig. 9.
Distribution of the difference in progression-free survival (△PFS) by RecistTM and RECIST. RecistTM, Response Evaluation Criteria in Solid Tumors Based on Tumor Markers. RECIST, Response Evaluation Criteria in Solid Tumors.
A subset of patients (n = 83) with △PFS ≥ 3 months were subjected to further subgroup analysis to determine the effect of clinical intervention before imaging-demonstrated progression, i.e., the subset was divided into intervention and non-intervention groups (Table 3). Analysis revealed 35 cases in the intervention group and 48 in the non-intervention group; the intervention group had a △PFS of 11.3 (6.2, 17.2) months, and the non-intervention group had a △PFS of 6.2 (4.6, 7.9) months. The difference was statistically significant (Z = 3.790, P < 0.001). This finding suggested that early clinical intervention for patients with marker-based progression but lacking radiographic evidence can significantly prolong their imaging-based PFS.
Table 3.
Baseline data of intervention and non-intervention groups after tumor marker-indicated progression.
| Intervention after tmPD (n = 35) | No intervention after tmPD (n = 48) | χ[2] | P | ||
|---|---|---|---|---|---|
| Age (years) | <65 | 27 (77.14%) | 34 (70.83%) | 0.414 | 0.520 |
| ≥65 | 8 (22.86) | 14 (29.17%) | |||
| Sex | Female | 21 (60%) | 30 (62.5%) | 0.053 | 0.817 |
| Male | 14 (40%) | 18 (37.5%) | |||
| Stage | IIIA–IIIB | 2 (5.71%) | 1 (2.08%) | 0.766 | 0.381 |
| IV | 33 (94.29%) | 47 (97.92%) | |||
| Mutation type | EGFR | 31 (88.57%) | 43 (89.58%) | 2.163 | 0.539 |
| ALK | 3 (8.57%) | 2 (4.17%) | |||
| CMET | 1 (2.86%) | 1 (2.08%) | |||
| ROS1 | 0 (0) | 2 (4.17%) | |||
| First-line Targeted drugs | Gefitinib | 18 (51.44%) | 28 (58.34%) | 5.609 | 0.691 |
| Erlotinib | 3 (8.57%) | 5 (10.42%) | |||
| Icotinib | 3 (8.57%) | 5 (10.42%) | |||
| Osimertinib | 1 (2.86%) | 3 (6.25%) | |||
| Almonertinib | 2 (5.71%) | 1 (2.08%) | |||
| Afatinib | 3 (8.57%) | 1 (2.08%) | |||
| Alectinib | 2 (5.71%) | 1 (2.08%) | |||
| Crizotinib | 2 (5.71%) | 4 (8.33%) | |||
| Dacomitinib | 1 (2.86%) | 0 (0) | |||
| Second-line drugs after tmPD | Osimertinib | 6 (17.14%) | - | - | - |
| Chemotherapy | 11 (31.44%) | - | |||
| Chemotherapy+ Anti-angiogenic therapy | 6 (17.14%) | - | |||
| Anti-angiogenic therapy | 10(28.57%) | - | |||
| Other TKIs | 2 (5.71%) | - |
Abbreviation:
EGFR, the epidermal growth factor receptor. ALK, anaplastic lymphoma kinase. ROS1. ROS proto-oncogene 1, receptor tyrosine kinase. CMET, C-Mesenchymal-epithelia transition factor. tmPD, Tumor marker-related progressive disease.
Discussion
In this era of targeted therapies for cancer, survival is also influenced by subsequent treatments. Thus, traditional RECIST-based evaluation of treatment efficacy is not suitable for long-term survival benefit analysis. Here, using RecistTM to classify treatment responses, differences in predicted PFS and OS could be identified, which could not be identified when using RECIST. The predicted 1-year, 2-year, and 3-year disease-progression risk, and 1-year, 3-year, and 5-year death risk according to area under the receiver operating characteristic curve comparisons, differed significantly between RecistTM and RECIST (all P < 0.05). Furthermore, the use of RecistTM made it possible to identify patients resistant to targeted drugs early, enabling prompt intervention.
Numerous studies have reported the prevalence of abnormal tumor markers in patients with driver gene mutations. This is particularly the case for those with EGFR mutations, where the positivity rate of serum CEA is often close to 70% or even higher. For example, Gao et al. [27] observed a 69.5% (48/69) positivity rate for CEA, and a 36.2% (25/69) rate for CYFRA21-1 among patients with EGFR mutations. Yanwei et al. [25] reported that, in patients with EGFR mutations, the positive rate of CEA was 72.5% (145/200), CYFRA21-1 was 55% (110/200), and CA125 was 44.5% (89/200). For patients with ALK rearrangements, Numata et al. reported a 36.3% (41/113) positivity rate for CEA [28]. Wang et al. [29] identified a 52.8% (28/53) positivity rate for CEA, 44.2% (19/43) for CYFRA21-1, and 73.5% (36/49) for CA199. Therefore, RecistTM, which we previously developed to assess immunotherapy efficacy, may be more applicable for evaluating the efficacy of targeted treatment in patients with driver gene mutations [26]. However, the principal inclusion criteria for RecistTM require tumor markers to exceed the normal level three times, yet our study's screening results indicated that only about a quarter of patients satisfied this requirement.
Studies have shown that changes in tumor marker levels during targeted therapy are closely related to PFS [17,30]. For example, Chen et al. [31] analyzed 184 patients with EGFR mutations or ALK rearrangements receiving targeted therapy. They developed a composite score, 4-TMpc, incorporating four tumor markers (CEA, CA125, CA199, CA153). The study concluded that patients with a significant decrease in 4-TMpc within 7–14 days had extended PFS. Our study also found a strong correlation between changes in tumor markers and PFS and OS of the patient. The application of RecistTM for assessing treatment efficacy was shown to predict PFS and OS effectively. Specifically, the AUC values for predicting disease progression at 1 year, 2 years, and 3 years, as well as OS, were significantly higher than those obtained when using RECIST. Therefore, RecistTM exhibits a stronger correlation with PFS and OS than does RECIST, and the former provides a more predictive accuracy for disease progression and mortality. Furthermore, our findings once again demonstrated that conventional RECIST, besides the very few CR and PD cases, classified the vast majority (97.5%) of patients as PR and SD, with no significant differences in PFS or OS between these two groups. Thus, this study further confirmed that conventional RECIST fails to predict short- and long-term patient benefits. However, patients who were classified as CR and PR by RecistTM after initial designation as PR and SD by RECIST displayed a significant difference in survival. This suggests that the RecistTM criteria we established are not only relevant for identifying patients who derive substantial benefit from immunotherapy [26], but also apply to those undergoing targeted therapy.
Currently, achieving a complete pathological response (pCR) is currently our short-term goal in clinical care for early-stage patients undergoing neoadjuvant therapy. However, for those with advanced disease treated with targeted therapy, and given the low CR rate with RECIST, aiming for a radiological CR based on RECIST may be impractical. In our study, a higher tmCR rate of 41.3% was observed with the RecistTM. Furthermore, these patients had the longest PFS and OS compared with those of other treatment outcomes evaluated by RecistTM as well as all outcomes assessed by RECIST. Therefore, pursuing tmCR based on RecistTM may be the short-term goal to extend patient survival. However, more clinical studies are needed to validate this approach.
This study revealed that the median PFS with RECIST was 16.1 months, whereas the median tmPFS with RecistTM was only 12 months, with an average advancement of 4.1 months. In the FLAURA trial, the detection of drug resistance mutations via ctDNA analysis also preceded disease progression, as determined by RECIST, with a median progression of 3.7 months (15.2 months vs 18.9 months) [32]. Furthermore, Zheng et al. [33] revealed a certain relationship between ctDNA and serum CEA levels, showing that a greater reduction in CEA was correlated with a higher early clearance rate of ctDNA. Therefore, our study indicated that tumor markers may reveal resistance to targeted therapy and signal disease progression earlier than imaging techniques. To date, no similar studies have been conducted on whether intervention is delivered in a timely manner after progression indicated by tumor markers and the impact of such intervention on prognosis. Our study demonstrated that timely intervention after tumor marker-based progression could significantly extend the time to progression with radiography, increasing PFS (11.3 vs. 6.2 months). Therefore, relevant interventions should be provided as soon as possible after tumor markers indicate disease progression, to delay the progression observed by radiography. However, this needs to be confirmed by prospective studies.
This study had several limitations. First, the scope of the application was limited. Whereas patients with driver gene mutations are more likely to exhibit abnormalities in tumor markers, only around a quarter of the patients studied had tumor marker levels exceeding three times the cutoff value. Second, certain tumor markers showed weak correlations with NSCLC, such as CA153 in this study, which is theoretically more closely related to breast cancer [[34], [35], [36]]. In this study, this marker did not show an increase after a subsequent imaging-determined progression, suggesting that the type of correlation between the cancer and primary tumor marker used is crucial to the applicability of RecistTM. Third, the study design, being single-center and retrospective, had a relatively low level of evidence. Furthermore, PFS and tmPFS data may have been affected by the different detection timings among patients, and therefore validation through prospective studies is needed. To address this issue, we have recently initiated a relevant registered study (clinicaltrials.gov Identifier: NCT06142058) to examine the correlation between RecistTM and imaging assessments (including positron emission tomography CT) and the correlation between RecistTM and ctDNA levels, to confirm the reliability and advantages of RecistTM in assessing the efficacy of treatment.
In conclusion, this study has shown that the RecistTM criteria is also applicable for assessing the efficacy of targeted therapy in patients with advanced NSCLC and driver gene mutations, along with tumor marker abnormalities. RecistTM exceeded the conventional RECIST criteria to predict substantial treatment benefits in such patients, and RecistTM-based tmCR may be the short-term goal to increase patient survival. In addition, RecistTM allowed early identification of patients with cancer resistant to targeted drugs, allowing prompt intervention, which can extend the imaging-based time to progression for these patients.
Funding statement
This work was supported by the Key projects of Chongqing Health and Family Planning Commission (to Xueqin Yang, grant number 2019ZDXM011) and the Scientific and Technological Innovation Special Project of Army Medical University (to Xueqin Yang, grant number 2022XLC06).
Ethics statement
This study protocol involving human participants was reviewed and approved by the Ethics Committee of Army Medical Center of PLA and the Ratification NO was 2022(262). And all patients were exempt from written informed consent forms in accordance with the national legislation and the institutional requirements.
CRediT authorship contribution statement
Kai Xiong: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Yi Yang: Data curation. Yanan Yang: Data curation, Investigation. Zhengbo Wang: Data curation, Investigation. Yun Liu: Data curation, Investigation. Hong Duo: Data curation, Formal analysis. Xinya Yuan: Data curation, Formal analysis. Yao Xiao: Data curation. He Xiao: Data curation, Formal analysis, Software. Xueqin Yang: Data curation, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2024.101006.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Molina J.R., Yang P., Cassivi S.D., Schild S.E., Adjei A.A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kris M.G., Johnson B.E., Berry L.D., Kwiatkowski D.J., Iafrate A.J., Wistuba I.I., et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J.R., Bharat A., et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J. Natl. Compr. Cancer Netw. 2021;19:254–266. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz L.H., Litière S., de Vries E., Ford R., Gwyther S., Mandrekar S., et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur. J. Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [Epub 2016 May 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuoka M., Wu Y.L., Thongprasert S., Sunpaweravong P., Leong S.S., Sriuranpong V., et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J. Clin. Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [Epub 2011 Jun 13]. [DOI] [PubMed] [Google Scholar]
- 7.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [Epub 2017 Nov 18]. [DOI] [PubMed] [Google Scholar]
- 8.Hida T., Nokihara H., Kondo M., Kim Y.H., Azuma K., Seto T., et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [Epub 2017 May 10] [DOI] [PubMed] [Google Scholar]
- 9.He L., Teng Y., Jin B., Zhao M., Yu P., Hu X., et al. Initial partial response and stable disease according to RECIST indicate similar survival for chemotherapeutical patients with advanced non-small cell lung cancer. BMC Cancer. 2010;10:681. doi: 10.1186/1471-2407-10-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal G.M., Karuri S.W., Zhang H., Zhang L., Khozin S., Kazandjian D., et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J. Clin. Oncol. 2015;33:1008–1014. doi: 10.1200/JCO.2014.59.0489. [Epub 2015 Feb 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reckamp K., Lin H.M., Huang J., Proskorovsky I., Reichmann W., Krotneva S., et al. Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small cell lung cancer. Curr. Med. Res. Opin. 2019;35:569–576. doi: 10.1080/03007995.2018.1520696. [Epub 2018 Oct 5] [DOI] [PubMed] [Google Scholar]
- 12.Morgan R.L., Camidge D.R. Reviewing RECIST in the era of prolonged and targeted therapy. J. Thorac. Oncol. 2018;13:154–164. doi: 10.1016/j.jtho.2017.10.015. [Epub 2017 Nov 4] [DOI] [PubMed] [Google Scholar]
- 13.McCoach C.E., Blumenthal G.M., Zhang L., Myers A., Tang S., Sridhara R., et al. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann. Oncol. 2017;28:2707–2714. doi: 10.1093/annonc/mdx414. Erratum in: Ann Oncol 2019;30:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X., Zhang Y., Ma Y., Zhou T., Zhang J., Hong S., et al. Optimal tumor shrinkage predicts long-term outcome in advanced nonsmall cell lung cancer (NSCLC) treated with target therapy: result from 3 clinical trials of advanced NSCLC by 1 institution. Medicine. 2016;95:e4176. doi: 10.1097/MD.0000000000004176. (Baltim) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvador-Coloma C., Lorente D., Palanca S., Simarro J., Mancheño N., Sandoval J., et al. Early radiological response as predictor of overall survival in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor mutations. J. Thorac. Dis. 2018;10:1386–1393. doi: 10.21037/jtd.2018.02.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu T.H., Hsiue E.H., Lee J.H., Lin C.C., Liao W.Y., Ho C.C., et al. Best response according to RECIST during first-line EGFR-TKI treatment predicts survival in EGFR mutation-positive non-small-cell lung cancer patients. Clin. Lung Cancer. 2018;19:e361–e372. doi: 10.1016/j.cllc.2018.01.005. [Epub 2018 Feb 1] [DOI] [PubMed] [Google Scholar]
- 17.Chiu C.H., Shih Y.N., Tsai C.M., Liou J.L., Chen Y.M., Perng R.P. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer. 2007;57:213–221. doi: 10.1016/j.lungcan.2007.02.016. [Epub 2007 Apr 20] [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Wu W., Pan X., Li F., Zhu Q., He Z., et al. Serum tumor markers level and their predictive values for solid and micropapillary components in lung adenocarcinoma. Cancer Med. 2022;11:2855–2864. doi: 10.1002/cam4.4645. [Epub 2022 Mar 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbosh C., Birkbak N.J., Wilson G.A., Jamal-Hanjani M., Constantin T., Salari R., et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–451. doi: 10.1038/nature22364. Erratum in: Nature 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquette C.H., Boutros J., Benzaquen J., Ferreira M., Pastre J., Pison C., et al. Circulating tumour cells as a potential biomarker for lung cancer screening: a prospective cohort study. Lancet Respir. Med. 2020;8:709–716. doi: 10.1016/S2213-2600(20)30081-3. Erratum in: Lancet Respir Med 2020;8:e94. [DOI] [PubMed] [Google Scholar]
- 21.Feng L.X., Wang J., Yu Z., Song S.A., Zhai W.X., Dong S.H., et al. Clinical significance of serum EGFR gene mutation and serum tumor markers in predicting tyrosine kinase inhibitor efficacy in lung adenocarcinoma. Clin. Transl. Oncol. 2019;21:1005–1013. doi: 10.1007/s12094-018-02014-6. [Epub 2019 Jan 12] [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K., Hata A., Kaji R., Fujita S., Otoshi T., Fujimoto D., et al. Cytokeratin 19 fragment predicts the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small-cell lung cancer harboring EGFR mutation. J. Thorac. Oncol. 2013;8:892–898. doi: 10.1097/JTO.0b013e31828c3929. [DOI] [PubMed] [Google Scholar]
- 23.Fiala O., Pesek M., Finek J., Benesova L., Minarik M., Bortlicek Z., et al. The role of neuron-specific enolase (NSE) and thymidine kinase (TK) levels in prediction of efficacy of EGFR-TKIs in patients with advanced-stage NSCLC [corrected] Anticancer Res. 2014;34:5193–5198. Erratum in: Anticancer Res. Erratum 2014;34:7485, in: Anticancer Res 2014;34:5193–8. [PubMed] [Google Scholar]
- 24.Suh K.J., Keam B., Kim M., Park Y.S., Kim T.M., Jeon Y.K., et al. Serum neuron-specific enolase levels predict the efficacy of first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring EGFR mutations. Clin. Lung Cancer. 2016;17:245–252. doi: 10.1016/j.cllc.2015.11.012. .e1. [DOI] [PubMed] [Google Scholar]
- 25.Yanwei Z., Bo J., Yuqing L., Rong L., Xueyan Z., Song H., et al. Serum carcinoembryonic antigen levels predicts the efficacy of EGFR-TKI in non-small cell lung cancer harboring EGFR mutations. J. Cancer Res. Ther. 2016;12:254–258. doi: 10.4103/0973-1482.153666. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y., Jiang X., Liu Y., Huang H., Xiong Y., Xiao H., et al. Elevated tumor markers for monitoring tumor response to immunotherapy. EClinicalMedicine. 2022;46 doi: 10.1016/j.eclinm.2022.101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y., Song P., Li H., Jia H., Zhang B. Elevated serum CEA levels are associated with the explosive progression of lung adenocarcinoma harboring EGFR mutations. BMC Cancer. 2017;17:484. doi: 10.1186/s12885-017-3474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Numata T., Endo T., Yanai H., Ota K., Yamamoto Y., Shimizu K., et al. Serum CEA and CYFRA levels in ALK-rearranged NSCLC patients: correlation with distant metastasis. In Vivo. 2020;34:2095–2100. doi: 10.21873/invivo.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Ma P., Ma G., Lv Z., Wu F., Guo M., et al. Value of serum tumor markers for predicting EGFR mutations and positive ALK expression in 1089 Chinese non-small-cell lung cancer patients: a retrospective analysis. Eur. J. Cancer. 2020;124:1–14. doi: 10.1016/j.ejca.2019.10.005. [Epub 2019 Nov 7] [DOI] [PubMed] [Google Scholar]
- 30.Jung M., Kim S.H., Lee Y.J., Hong S., Kang Y.A., Kim S.K., et al. Prognostic and predictive value of CEA and CYFRA 21–1 levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib. Exp. Ther. Med. 2011;2:685–693. doi: 10.3892/etm.2011.273. [Epub 2011 May 12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H.J., Tu C.Y., Huang K.Y., Chien C.R., Hsia T.C. Early serum tumor marker levels after fourteen days of tyrosine kinase inhibitor targeted therapy predicts outcomes in patients with advanced lung adenocarcinoma. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0240736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray J.E., Ahn M.J., Oxnard G.R., Shepherd F.A., Imamura F., Cheng Y., et al. Early clearance of plasma epidermal growth factor receptor mutations as a predictor of outcome on osimertinib in advanced non-small cell lung cancer; exploratory analysis from AURA3 and FLAURA. Clin. Cancer Res. 2023;29:3340–3351. doi: 10.1158/1078-0432.CCR-22-3146. [DOI] [PubMed] [Google Scholar]
- 33.Zheng J., Wang Y., Hu C., Zhu M., Ii J., Lin C., et al. Predictive value of early kinetics of ctDNA combined with cfDNA and serum CEA for EGFR-TKI treatment in advanced non-small cell lung cancer. Thorac. Cancer. 2022;13:3162–3173. doi: 10.1111/1759-7714.14668. [Epub 2022 Oct 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barros A.C., Fry W., Jr, Nazario A.C., Santos M.O., Sato MK. Experience with CA 15.3 as a tumor marker in breast cancer. Eur. J. Surg. Oncol. 1994;20:130–133. PMID: 8181577. [PubMed] [Google Scholar]
- 35.Tampellini M., Berruti A., Bitossi R., Gorzegno G., Alabiso I., Bottini A., et al. Prognostic significance of changes in CA 15–3 serum levels during chemotherapy in metastatic breast cancer patients. Breast Cancer Res. Treat. 2006;98:241–248. doi: 10.1007/s10549-005-9155-y. [Epub 2006 May 3] [DOI] [PubMed] [Google Scholar]
- 36.Hammer J., Track C., Hohenwallner W., Seewald D.H., Zoidl J.P., Wimmer E., et al. 15–3 in der Verlaufskontrolle bei Patientinnen mit Mammakarzinom [MCA and CA 15–3 in the follow-up of patients with breast cancer] Strahlenther. Onkol. 1992;168:102–106. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.