Abstract
To determine the changes in surveillance category by adding a polygenic risk score based on 311 breast cancer (BC)-associated variants (PRS311), questionnaire-based risk factors and breast density on personalized BC risk in unaffected women from Dutch CHEK2 c.1100delC families.
In total, 117 unaffected women (58 heterozygotes and 59 non-carriers) from CHEK2 families were included. Blood-derived DNA samples were genotyped with the GSAMDv3-array to determine PRS311. Lifetime BC risk was calculated in CanRisk, which uses data from the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA). Women, were categorized into three surveillance groups.
The surveillance advice was reclassified in 20 (34.5%) heterozygotes and 21 (35.6%) non-carriers after adding PRS311. Including questionnaire-based risk factors resulted in an additional change in 11 (20.0%) heterozygotes and 8 (15.1%) non-carriers; and a sub-analysis showed that adding breast density on top shifted another 9 (23.1%) heterozygotes and 5 (27.8%) non-carriers. Overall, the majority of heterozygotes were reclassified to a less intensive surveillance, while non-carriers would require intensified surveillance.
The addition of PRS311, questionnaire-based risk factors and breast density to family history resulted in a more personalized BC surveillance advice in CHEK2-families, which may lead to more efficient use of surveillance.
Keywords: CHEK2 c.1100delC, Breast cancer, Risk prediction
Highlights
-
•
Lifetime breast cancer risk in unaffected CHEK2 heterozygotes and familial non-carriers was calculated using CanRisk.
-
•
Risks ranged from 22.1 to 51.7% in heterozygotes and 10.7–31.0% in non-carriers based on family history alone.
-
•
Adding PRS311 caused the largest shift in risk prediction, followed by breast density and questionnaire-based risk factors.
-
•
Risk stratifications were similar among distant relatives, not supporting modified cascade screening in CHEK2 families.
1. Introduction
The pathogenic germline CHEK2 c.1100delC variant is the most prevalent breast cancer (BC) predisposition variant in the Netherlands. It is present in about 1% of the general Dutch population, increasing to 2.5% in unselected BC cases and up to 5% in familial BC cases [1,2]. Depending on family history (FH), the CHEK2 variant confers a lifetime BC risk (LTBCR, defined as the risk of developing BC from age 20 to 80) of 20–55% [[1], [2], [3], [4], [5], [6], [7]], compared to the population-based BC risk of 14% [8]. As a result, relatives of the index case are eligible for genetic counselling. However, over time, discussions have been ongoing to what extent relatives should be eligible for genetic counselling in families with moderate-risk pathogenic variants. Currently, in the Netherlands, only female first and second degree relatives are eligible for CHEK2 testing (modified cascade screening) [9]. Based on personal genetic status and FH, an age-specific breast surveillance program is advised [10].
However, there are other known BC risk modifiers, which are mostly currently not yet taken into account during counselling. A more personalized BC risk estimation and surveillance advice would be preferable, especially for young unaffected women. Several comprehensive BC risk prediction models have been developed over the last decade, including the Breast and Ovarian analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) [11], which has been incorporated into the CanRisk prediction tool [12,13]. This model has recently been validated in Dutch women from the general population [14], and includes information on FH [15], genetic testing results, the polygenic risk score (PRS) [16], BMI [17], alcohol consumption [18,19], hormonal factors [20,21], and breast density (BD) [15].
While evidence on the clinical utility of incorporating the PRS in risk prediction of CHEK2 carriers is still lacking, several studies have shown the added improvement of risk stratification [11,16,[22], [23], [24], [25]]. Firstly, risk effects conferred by CHEK2 and 77 common variants acted multiplicatively [16] and more recently, two studies including variant status, individual clinical variables and PRS showed meaningful shifts in LTBCR in CHEK2 carriers regardless of a family history of BC [24,25]. In the familial cancer setting, one study on BC risk prediction of non-BRCA1/2 Dutch families showed that the addition of PRS313 changed clinical management in 58% of the CHEK2 carriers [26]. However, a recent study found linkage between two SNPs located on the 22nd chromosome (chr22:29203724:C/T and chr22:29551872:A/G) and the CHEK2 c.1100delC variant, and therefore using PRS313 would result in an overestimation of BC risk in heterozygotes. It is advised to exclude these two SNPs from the PRS and use the PRS311 for CHEK2 families [27].
Within this study, we aimed to investigate the benefit of incorporating PRS311, BD and questionnaire-based risk factors (QRFs: including anthropometric information, lifestyle factors, and hormonal factors) in the CanRisk tool one-by-one by evaluating the effect on individual BC risk prediction and changes in screening surveillance advice for unaffected women from CHEK2 families. Adding this next to FH and CHEK2 status is expected to result in a more accurate and personalized risk prediction and consequently, more appropriate surveillance advice. We evaluated the impact of using the PRS311 versus the PRS313 next to FH and CHEK2 status on BC risk stratification. In addition, we compared risk stratification for first-, second-, and third-degree relatives, contributing to the ongoing discussion on cascade screening for these families.
2. Materials and methods
2.1. Study cohort
We used retrospective cohort data from women participating in the nation-wide Hereditary Breast and Ovarian cancer study Netherlands (Hebon) study. The selected women were counselled at Erasmus MC or AVL-NKI hospital for the CHEK2 c.1100delC variant. All women had signed informed consent. The Hebon study has been approved by the Medical Ethics Committee of the Erasmus MC and the Institutional Review Board (IRB) of the AVL-NKI.
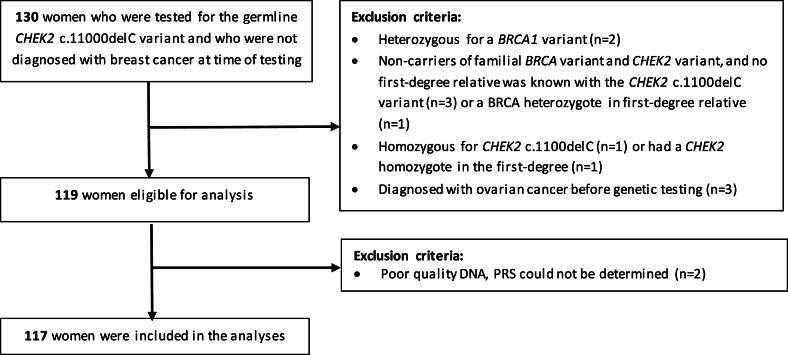
We selected 130 women from 93 CHEK2 families who were unaffected at the time of testing and of whom a DNA sample was available for PRS testing. After excluding six women where a gBRCA1 PV was found in themselves or their family, two CHEK2 homozygotes and three women diagnosed with ovarian cancer before testing, 119 women were eligible for this study. After genotyping, two women were excluded as the PRS could not be determined, resulting in a total of 117 women (58 heterozygotes and 59 non-carriers) included in the analyses (Fig. 1).
Fig. 1.
flowchart of women excluded from this study.
2.2. Data collection
2.2.1. Questionnaire-based risk factors
Women participating in the Hebon study were asked to complete a detailed questionnaire containing information on risk factors, such as lifestyle factors (alcohol consumption, smoking, physical activity, BMI), hormonal factors (age at menarche and menopause, parity, exogenous hormone use), medical information (e.g. cancer diagnosis, treatment) and preventive strategies (e.g. prophylactic mastectomy). In this study, we will refer to BMI, alcohol consumption and hormonal factors as QRFs. For ten women (four heterozygotes, six non-carriers), no information on any of the addressed QRFs was available.
2.2.2. Pedigrees and medical information
Pedigrees were collected from all participating women. For the LTBCR calculations, defined as the risk of developing BC from age 20 to 80, FH included all known first-degree to third-degree relatives of genotyped individuals. In addition, missing information from the questionnaire, breast surveillance recommendations given after genetic testing, and BD information (ACR BI-RADS score, category A-D) were extracted from the medical records.
2.2.3. Genotyping
The SNP array platform used was a customized version of the Illumina BeadChip GSA MD, v3 (Illumina GSA Arrays “Infinium iSelect 24 × 1 HTS Custom BeadChip Kit” – version GOALL). This array was used to determine the 313 BC PRS SNPs, but also the CHEK2 c.1100delC variant. Samples were processed using the manufacturer's recommended protocol. 111 of the PRS313 variants that could not be directly typed using this array were extracted after imputation against the 1000 genomes reference panel using Minimac [28]. PRS313 was standardized to the mean and SD from the population controls included in the total data set from the BCAC study [29], which were −0.424 and 0.611 respectively. PRS311 was calculated using a mean of −0,096 and SD of 0.609 [27]. The CHEK2 c.1100delC variant status measured by GSA was compared to the result with the variant status that was retrieved from diagnostic DNA laboratories.
2.3. Statistical analysis
We tested for statistical differences (P-value <0.05) of characteristics between heterozygotes and non-carriers using an ANOVA test for continuous data and Chi-square tests for categorical data. Analyses were performed with STATA (version 17.0) and R (version 4.2.2).
2.3.1. Cumulative risk score calculation
The LTBCR was calculated in the CanRisk tool [version 2.0]. Women were categorized in three risk groups according to the Dutch guidelines [10] by considering risk at general population level if LTBCR was under 20%, moderate risk if LTBCR was between 20% and 30%, and high risk when LTBCR was above 30%. Also, we evaluated if women would reach a LTBCR of above 50%, as this threshold is often used in unaffected heterozygote carriers to determine eligibility for preventive surgery. In the LTBCR calculations, we included risk factors stepwise, starting with FH retrieved from the pedigree and the CHEK2 status. Risk estimation using these two variables was considered the reference, since this is currently used in clinical practice. Our main interest was to address the effect of adding information regarding the PRS311, QRFs and BD by considering three different approaches: (1) adding PRS311, as this information could be retrieved from the same blood sample and does not require any more information; (2) adding information that could be gathered directly from the individual during genetic testing and counselling, including PRS311 and QRFs; and (3) adding all available information, including BD, which was available in our study for 40 heterozygotes and 18 non-carriers. Reasons for missing BD information included (1) not being able to retrieve information from the surveillance hospitals indicated in the questionnaire (n = 13); (2) being younger than 35 years at time of data collection and therefore no candidate for mammographic surveillance (n = 11); (3) eligible for participating in the Dutch population-based screening program, where we could not retrieve BD information at time of data collection (n = 14); and (4) no information on whether women were under BC surveillance or the location of BC surveillance (n = 21).
3. Results
3.1. Characteristics of the study population
Table 1 shows the demographic characteristics and risk factors of heterozygotes and non-carriers. Heterozygotes and non-carriers were similar in terms of all established risk factors, except for the use of hormone replacement therapy, which was more common in non-carriers (9.6% in non-carriers and 0% in carriers; P-value = 0.02). Overall, over 70% of the women were a first-degree relative of the index case. Furthermore, the CHEK2 status as determined by the GSA was always coincident with the CHEK2 status as determined in the diagnostic setting.
Table 1.
Characteristics of all women (heterozygotes and non-carriers of the familial CHEK2 c.1100delC variant) included in this study.
| Number | Heterozygotes |
Non-carriers |
||
|---|---|---|---|---|
| 58 | 59 | P-value | ||
| Center, n (%) | AVL | 22 (37.9) | 26 (44.1) | 0.50 |
| Erasmus MC | 36 (62.1) | 33 (55.9) | ||
| Birth cohort, n (%) | <1960 | 4 (6.9) | 6 (10.1) | 0.69 |
| 1960–1970 | 16 (27.6) | 18 (30.5) | ||
| 1970–1980 | 22 (37.9) | 17 (28.8) | ||
| 1980–1990 | 11 (19.0) | 15 (25.4) | ||
| >1990 | 5 (8.6) | 3 (5.1) | ||
| Distant relative from the index in pedigree, n (%) | First-degree | 42 (72.4) | 42 (71.2) | 0.98 |
| Second-degree | 10 (17.2) | 11 (18.6) | ||
| Third-degree | 6 (10.3) | 6 (10.2) | ||
| Age at time of genetic testing | Mean ± SD | 43.7 ± 10.2 | 45.9 ± 11.3 | 0.27 |
| RISK FACTORS | ||||
| Height, cm | Mean ± SD | 172.7 ± 6.0 | 171.0 ± 5.4 | 0.15 |
| Unknown | 8 | 12 | ||
| Alcohol use, grams per day | None, n (%) | 25 (48.1) | 26 (50.0) | 1.00 |
| Mean ± SD | 83.7 ± 57.5 | 96.6 ± 65.5 | 0.48 | |
| Unknown | 9 | 7 | ||
| Age menarche, years | Mean ± SD | 13.0 ± 1.5 | 12.9 ± 1.3 | 0.67 |
| Unknown | 8 | 8 | ||
| Age menopause, years | Premenopausal, n (%) | 34 (65.4) | 28 (56.0) | 0.33 |
| Mean ± SD | 46.6 ± 9.0 | 46.3 ± 7.6 | 0.89 | |
| Unknown | 7 | 9 | ||
| Number of children, n (%) | 0 | 8 (15.4) | 9 (17.7) | 0.96 |
| 1 | 5 (9.6) | 6 (11.8) | ||
| 2 | 26 (50.0) | 25 (49.0) | ||
| >2 | 13 (25.0) | 11 (21.6) | ||
| Unknown | 6 | 8 | ||
| Age at first childbirth, years | Mean ± SD | 31.6 ± 4.4 | 30.6 ± 3.8 | 0.25 |
| No children | 8 | 9 | ||
| Unknown | 8 | 5 | ||
| Use of oral contraception, n (%) | Never | 2 (3.9) | 1 (2.0) | 0.57 |
| Ever | 50 (96.2) | 50 (98.0) | ||
| Unknown | 6 | 8 | ||
| Use hormone replacement therapy, n (%) | Never | 52 (100.0) | 46 (90.4) | 0.02 |
| Ever | – | 5 (9.6) | ||
| Unknown | 6 | 7 | ||
| Body mass index, kg/m2 | Mean ± SD | 25.5 ± 4.9 | 26.2 ± 4.0 | 0.45 |
| Unknown | 9 | 12 | ||
| Standardized PRS313, Z-score | Mean | 0.479 | 0.444 | 0.84 |
| SD | 1.002 | 0.851 | ||
| Raw PRS313 | Mean | −0.132 | −0.153 | 0.84 |
| SD | 0.612 | 0.520 | ||
| Standardized PRS311, Z-score | Mean | 0.197 | 0.456 | 0.13 |
| SD | 0.988 | 0.848 | ||
| Raw PRS311 | Mean | 0.028 | 0.186 | 0.13 |
| SD | 0.601 | 0.516 | ||
| Breast density, n (%) | BIRADS A | 2 (5.0) | 1 (5.6) | 0.87 |
| BIRADS B | 15 (37.5) | 8 (44.4) | ||
| BIRADS C | 15 (37.5) | 7 (38.9) | ||
| BIRADS D | 8 (20.0) | 2 (11.1) | ||
| Unknown | 18 | 41 | ||
PRS313 = polygenic breast cancer risk score based on 313 variants; PRS311 = alternative polygenic risk score based on 311 BC-associated variants. *Percentages may not add up to 100% due to rounding.
3.2. Stepwise addition of risk factors and BC risk distribution
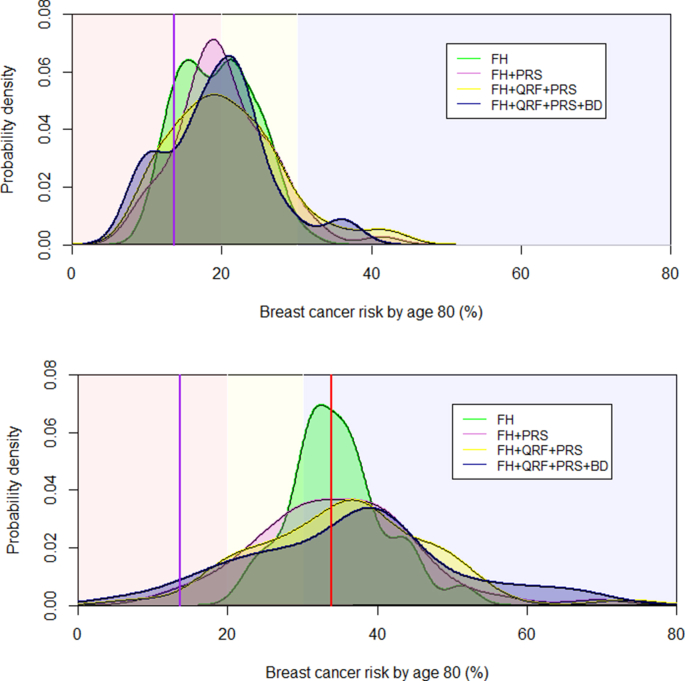
Fig. 2 shows the LTBCR distribution predicted in CanRisk for heterozygotes and non-carriers, separately. The curves represent the distribution of LTBCR that was calculated. The background of the graphs is shaded indicating the Dutch risk categories for women with a FH of BC [10]. The estimated LTBCR based on FH and CHEK2 status (green line labelled: FH) ranged from 22.1% to 51.7% in heterozygotes, and from 10.7% to 31.0% in non-carriers. In addition, the distribution of the estimated LTBCR became wider meaning that we obtained better risk stratification when incorporating the PRS (pink line labelled: FH + PRS). The addition of QRFs provided an additional improvement in risk distribution (yellow line labelled: FH + QRF + PRS). Based on 40 heterozygotes and 18 non-carriers with BD information, the curves show that the variation in risk is largest when including all available information in the model (purple line labelled: FH + QRF + PRS + BD), especially in the heterozygotes.
Fig. 2.
Distribution of life-time breast cancer risk in CHEK2 c.1100delC heterozygotes (A) and non-carriers (B) calculated using BOADICEA version 6 with stepwise inclusion of risk factors. The background of the graphs are shaded indicating the Dutch lifetime breast cancer risk categories for women [10]: red = population risk (≤20%); yellow = moderate risk (>20% and <30%); blue = high risk (≥30%). The vertical purple line indicates the average life-time breast cancer risks for the Dutch population the vertical red line that for CHEK2 c.1100delC heterozygotes. FH = family history; QRF = questionnaire-based risk factors; PRS = polygenic risk score based on 311 variants; BD = breast density based on BIRADS.
3.3. Stepwise addition of risk factors and reclassification of risk category
We compared the LTBCR calculation that is mostly used in current clinical practice (including FH and CHEK2 status) with a second calculation adding PRS311 and a third calculation including all information that could be gathered during genetic testing and counselling (also including PRS311 and QRFs) (Table 2). The majority, but not all, of the non-carriers would have been categorized at population risk level, while most of heterozygotes would have been classified as high risk. Adding PRS311 resulted in a reclassification of risk category for 34.5% of the heterozygotes and 35.6% of the non-carriers compared to the calculation including only FH. Of those who changed risk category, 85.0% of the heterozygotes would be recommended less intensive surveillance, while this was true for 47.6% of the non-carriers. Moreover, adding QRFs resulted in a shift in 34.5% of the heterozygotes and 45.3% of the non-carriers compared to the calculation only including FH. The addition of QRFs to the calculation based on FH and PRS311 contributed to a shift in 20.0% of the heterozygotes and 15.1% of the non-carriers.
Table 2.
Comparison of risk group categorization between the life-time breast cancer risk calculation including family history and mutation status (FH), the calculation also including PRS311 (FH + PRS), and the calculation including questionnaire-based risk factors and PRS (FH + QRF + PRS).
| Lifetime breast cancer risk | FH |
FH + PRS |
FH + PRS + QRF† |
|||
|---|---|---|---|---|---|---|
| CHEK2 | non-CHEK2 | CHEK2 | non-CHEK2 | CHEK2 | non-CHEK2 | |
| ≤20% | 0 | 30 | 5 | 31 | 6 | 31 |
| 20–30% | 9 | 28 | 15 | 25 | 12 | 22 |
| ≥30% | 49 | 1 | 38 | 3 | 40 | 6 |
| Of whom >50% | 2 | 0 | 3 | 0 | 4 | 0 |
| Total number | 58 | 59 | 58 | 59 | 58 | 59 |
| Shifts in life-time risk category | ||||||
| From FH to other, n (%) | 20 (34.5) | 21 (35.6) | 19 (34.5) | 24 (45.3) | ||
| From FH + PRS to other, n (%) | 11 (20.0) | 8 (15.1) | ||||
| Screening category change compared to FH | ||||||
| Upscaled, n (%) | 3 (15.0) | 11 (52.4) | 4 (21.1) | 14 (58.3) | ||
| Downscaled, n (%) | 17 (85.0) | 10 (47.6) | 15 (78.9) | 10 (41.7) | ||
| Screening category change compared to FH + PRS | ||||||
| Upscaled, n (%) | 5 (45.5) | 5 (62.5) | ||||
| Downscaled, n (%) | 6 (55.5) | 3 (27.5) | ||||
Differences in risk groups are shown for heterozygotes and non-carriers separately. The numbers of shifts might be different than expected from the presented data, as individual changes in risk category are not visible within this Table. † for three heterozygotes and six non-carriers, relevant QRFs were not available, and therefore only data on FH + PRS were used and by defintion they did not change category.
Furthermore, we compared the risk group categorization of women from whom we were able to retrieve BD information (Table 3). Adding the QRFs and BD to the calculations based only on FH and PRS311, resulted in a shift in 20.5% of the heterozygotes (62.5% upscaled), and 37.8% of the non-carriers (40.0% upscaled). The addition of BD alongside FH, PRS311 and QRFs contributed to a shift in 23.1% of the heterozygotes and 27.8% of the non-carriers.
Table 3.
Comparison of risk group categorization between the life-time breast cancer risk calculation including family history and mutation status and PRS311 (FH + PRS), the calculation including questionnaire-based risk factors and PRS (FH + QRF + PRS), and the calculation also including breast density (FH + QRF + PRS + BD) in women from whom breast density information was available.
| Life-time breast cancer risk | FH + PRS |
FH + PRS + QRFa |
FH + PRS + QRF + BD |
|||
|---|---|---|---|---|---|---|
| CHEK2 | non-CHEK2 | CHEK2 | non-CHEK2 | CHEK2 | non-CHEK2 | |
| ≤20% | 2 | 8 | 3 | 8 | 5 | 9 |
| 20–30% | 11 | 10 | 9 | 9 | 8 | 8 |
| ≥30% | 27 | 0 | 28 | 1 | 27 | 1 |
| Of whom >50% | 3 | 0 | 4 | 0 | 6 | 0 |
| Total number | 40 | 18 | 40 | 18 | 40 | 18 |
| Shifts in life-time risk category | ||||||
| From FH + PRS to other, n (%) | 8 (20.5) | 5 (27.8) | 8 (20.5) | 5 (27.8) | ||
| From FH + QRF + PRS to other, n (%)a | 9 (23.1) | 5 (27.8) | ||||
| Screening category change compared to FH + PRS | ||||||
| Upscaled, n (%) * | 4 (50.0) | 3 (60.0) | 5 (62.5) | 2 (40.0) | ||
| Downscaled, n (%)* | 4 (50.0) | 2 (40.0) | 3 (37.5) | 3 (60.0) | ||
| Screening category change compared to FH + PRS + QRF | ||||||
| Upscaled, n (%) * | 3 (33.3) | 2 (40.0) | ||||
| Downscaled, n (%)* | 6 (66.6) | 3 (60.0) | ||||
Differences in risk groups are shown for heterozygotes and non-carriers separately. The numbers of shifts might be different than expected from the presented data, as individual changes in risk category are not visible within this Table.
for one heterozygotes, relevant QRFs were not available, and therefore only data on FH + PRS were used and by definition they did not change category.
Finally, we found that some heterozygotes would exceed the 50% threshold, making them eligible to discuss preventive surgery. In this study, the number of heterozygotes exceeding the 50% threshold increased after more information was included in the model (Table 2: two heterozygotes for FH alone, three when adding PRS311, four when also considering QRFs on top of FH and PRS311 and six after adding BD).
3.4. BC risk stratification using PRS313 versus PRS311
Comparing the model including FH and PRS311 with the model including FH and PRS313 showed that the majority of the women did not change risk category. In total, four heterozygotes (6.8%) and one non-carriers (1.7%) had an overestimation of risk category when PRS313 was used instead of the PRS311 (Supplementary Table 1).
3.5. Risk stratification separated by variant status and distant relatives
Based on FH alone, first-, second-, and third-degree relatives were classified in all risk categories. Adding PRS311 resulted in reclassifications of risk categories within first-degree relatives (in 12/42 heterozygotes and 15/42 non-carriers), second-degree relatives (in 4/10 heterozygotes and 4/11 non-carriers), and third-degree relatives (in 4/6 heterozygotes and 2/6 non-carriers) (Supplementary Table 2).
4. Discussion
This study showed the potential clinical value of adding known risk factors such as FH, CHEK2 status, PRS311, QRFs, and when available, BD to the validated CanRisk tool in personalized BC risk prediction for unaffected heterozygotes and non-carriers of the CHEK2 variant. By incorporating the PRS311 along with QRFs, a better stratification of risk and a potential improvement of clinical management was gained. We identified women who had an LTBCR exceeding 50% and would be eligible for consideration of preventive surgery, which is currently not advised in CHEK2 heterozygotes according to international guidelines [[30], [31], [32]]. Addition of the PRS311 and QRFs compared to FH and CHEK2 status alone resulted in the reclassification of risk category for more than one third of the women. The majority of reallocated heterozygotes would be downscaled (78.9%) and therefore have a later start or lower frequency of screening, whereas most of the non-carriers (58.3%) would begin surveillance earlier.
Incorporating the PRS into the CanRisk tool had the greatest impact on BC risk prediction, which is in line with previous studies [11,16,[23], [24], [25],33]. Here, 34.5% of the heterozygotes and 35.6% of the non-carriers would have been managed differently if the PRS311 had also been included in the model. These percentages are lower than found in another Dutch study, showing that 19 out of the 31 unaffected heterozygotes (58.1%) would receive different screening advice, of whom 6 (31.6%) a more intensive surveillance [26]. This might be explained by the use of the PRS311 versus PRS313, as we showed that the use of PRS311 alongside FH did result in lower BC risk category, especially among heterozygotes. As this is the first study conducted using the PRS311 in CHEK2 families, we expect to find smaller effects than described in previous studies that used the PRS313.
A recent validation of BOADICEA v6 in an independent prospective population-based cohort showed that PRS contributed most to risk stratification followed by BD and finally the QRFs [33], which is in line with our results. Despite the low number of women with information on BD, we found a meaningful extra shift in about one fifth of the women when adding BD on top of the other factors. In addition, although the reported impact of adding QRFs to the model was modest [11,15], their consideration could lead to eventual significant changes in risk prediction, as many are modifiable factors over time. Therefore, special attention should be given while recommending screening for young women under the age of 35 with low PRS and high risk based solely on breast density and nulliparity, as both factors could change over time resulting in a lower BC risk. More research is needed to determine and standardize the best moment for recalculation and the clinical utility of risk-adjusted surveillance recommendations over time.
Currently, discussions in the clinical genetic setting are ongoing on to what extent relatives from CHEK2 families should be tested, as further distant relatives would likely shift more to population-based BC risk. Although the numbers are small, this is not in line with our results. Similar risk stratifications were found in all distant relatives, also after including other risk factors. On the whole, these results do not support the choice for modified cascade screening in CHEK2 families.
This study had some limitations. First, changes in lifestyle, reproductive and hormonal information might have occurred from the moment of completing the questionnaire until entering the data into CanRisk. Changes in the use of oral contraceptives, (number) of births, and use of hormone replacement therapy are subject to change during life. However, it is unlikely that this would have great impact on their surveillance advice, especially for women over 50 years for whom changes in surveillance are limited.
Furthermore, BD was only available for 58 women. While evidence is being gathered regarding benefits of implementing PRS in clinical practice, discussion should continue on the importance of considering all evaluable modifiable information, including BD [34,35] and QRFs when offering surveillance, especially with regard to BD due to its nature as a moderate risk factor.
From a clinical point of view, there is a need for personalized risk stratified BC surveillance instead of surveillance programs, which results in less overdiagnoses and lower costs of surveillance, without affecting the quality-adjusted life-years gained and maintaining reduced BC death [[36], [37], [38], [39]]. We showed that adding more information to the model would result in a more stratified BC risk. However, there are some barriers to overcome before implementing this in clinical practice. The addition of PRS311 had the most impact on stratifying BC risk. Incorporating this in clinical counselling would be wise in nearby future. Second, even though QRFs vary over time, its inclusion did impact management in a substantial 10–20% of women. To reduce the burden for genetic counsellors of (manually) entering this information into the model, a public-facing app (MyCanRisk) is currently being developed, allowing people to provide relevant information including QRFs before counselling. This will provide genetic counsellors the information needed for BC risk prediction.
Moreover, the inclusion of BD would be preferred to optimize surveillance recommendations for some women, especially young women who are more likely to have dense breast tissue [15,40]. While BD contributes to large shifts in BC risk categories, we should also keep in mind that BD will decrease over time, e.g. due to (post-)menopausal status, likely resulting in lower BC risk prediction. Therefore, age-dependent risk calculations, or 10-year risk calculations, would be preferable in women with dense breasts and a low risk based on genetic information only.
Overall, this study supports the potential clinical impact of personalized BC risk prediction using CanRisk on individual surveillance advice for women from CHEK2 families as part of clinical genetic counselling. The BC risk stratification observed in distant relatives, may contribute to ongoing discussions on the extent of cascade genetic screening in CHEK2 families. Finally, the pros and cons of scaling up and down surveillance programs need to be carefully weighed before implementing in clinical practice.
Ethics approval
This research was reviewed by the Institutional Review Board at the Antoni van Leeuwenhoek Hospital - Netherlands Cancer Institute (IRBdm19-043). All data were de-identified prior to analysis.
Funding statement
This research was supported by KWF (project 10758).
Author contributions
All authors contributed to study conception, design, data curation and methodology. Formal analysis were conducted by Maartje A.C. Schreurs, Teresa Ramon y Cajal and Jeroen van Rooij. Funding acquisition was done by Muriel A. Adank, Antoinette Hollestelle, Marjanka K. Schmidt and Maartje J Hooning. The first draft of the manuscript was written by Maartje A.C. Schreurs and Teresa Ramon y Cajal, supervised by Muriel A. Adank, Marjanka K. Schmidt and Maartje J. Hooning. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Data availability
The data sets generated and/or analyzed during this study are available to non-commercial parties upon reasonable request from the corresponding author. Only anonymized data will be transferred.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Declaration of competing interest
Authors declare no potential conflict of interest.
Acknowledgements
The authors would like to thank Yvonne Unkel and Kiki Jeanson for their help in the selection and data collection needed for the risk calculations. Also, we would like to thank prof. Douglas F. Easton for his input regarding the PRS311 calculations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103724.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Meijers-Heijboer H., van den Ouweland A., Klijn J., et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31(1):55–59. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 2.Consortium C.B.C.C.-C. CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74(6):1175–1182. doi: 10.1086/421251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weischer M., Bojesen S.E., Ellervik C., Tybjaerg-Hansen A., Nordestgaard B.G. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26(4):542–548. doi: 10.1200/JCO.2007.12.5922. [DOI] [PubMed] [Google Scholar]
- 4.Adank M.A., Verhoef S., Oldenburg R.A., et al. Excess breast cancer risk in first degree relatives of CHEK2*1100delC positive familial breast cancer cases. Eur J Cancer. 2013;49(8):1993–1999. doi: 10.1016/j.ejca.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S.Y., Phelan C.M., Zhang P., et al. Frequency of the CHEK2 1100delC mutation among women with breast cancer: an international study. Cancer Res. 2008;68(7):2154–2157. doi: 10.1158/0008-5472.CAN-07-5187. [DOI] [PubMed] [Google Scholar]
- 6.Weischer M., Bojesen S.E., Tybjaerg-Hansen A., Axelsson C.K., Nordestgaard B.G. Increased risk of breast cancer associated with CHEK2*1100delC. J Clin Oncol. 2007;25(1):57–63. doi: 10.1200/JCO.2005.05.5160. [DOI] [PubMed] [Google Scholar]
- 7.Cybulski C., Wokolorczyk D., Jakubowska A., et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29(28):3747–3752. doi: 10.1200/JCO.2010.34.0778. [DOI] [PubMed] [Google Scholar]
- 8.Netherlands Cancer Registry. 2023. Borstkanker in Nederland, kerncijfers uit de Nederlandse Kankerregistratie.www.iknl.nl/borstkankercijfers [Google Scholar]
- 9.Stichting Opsporting Erfelijke Tumoren. Richtlijnen VKGN-STOET; CHEK2-geassocieerd mammacarcinoom. 2023. https://vkgn.stoet.nl/index.php?article_id=23.
- 10.Richtlijnendatabase, 2024. Borstkanker - Screening buiten het BVO. https://richtlijnendatabase.nl/richtlijn/borstkanker/screening/screening_buiten_het_bvo/screening_buiten_het_bvo.html.
- 11.Lee A., Mavaddat N., Wilcox A.N., et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708–1718. doi: 10.1038/s41436-018-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer S., Babb de Villiers C., Scheibl F., et al. Evaluating clinician acceptability of the prototype CanRisk tool for predicting risk of breast and ovarian cancer: a multi-methods study. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0229999. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carver T., Hartley S., Lee A., et al. CanRisk tool-A Web Interface for the prediction of breast and ovarian cancer risk and the Likelihood of carrying genetic pathogenic variants. Cancer Epidemiol Biomarkers Prev. 2021;30(3):469–473. doi: 10.1158/1055-9965.EPI-20-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakeman I.M.M., Rodriguez-Girondo M., Lee A., et al. Validation of the BOADICEA model and a 313-variant polygenic risk score for breast cancer risk prediction in a Dutch prospective cohort. Genet Med. 2020;22(11):1803–1811. doi: 10.1038/s41436-020-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson H.D., Zakher B., Cantor A., et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156(9):635–648. doi: 10.1059/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muranen T.A., Greco D., Blomqvist C., et al. Genetic modifiers of CHEK2*1100delC-associated breast cancer risk. Genet Med. 2017;19(5):599–603. doi: 10.1038/gim.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K., Zhang W.N., Dai Z.M., et al. Association between body mass index and breast cancer risk: evidence based on a dose-response meta-analysis. Cancer Manag Res. 2018;10:143–150. doi: 10.2147/CMAR.S144619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W.Y., Rosner B., Hankinson S.E., Colditz G.A., Willett W.C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S.M., Lee I.M., Manson J.E., Cook N.R., Willett W.C., Buring J.E. Alcohol consumption and breast cancer risk in the Women's Health Study. Am J Epidemiol. 2007;165(6):667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative Group on Hormonal Factors in Breast C Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter D.J., Colditz G.A., Hankinson S.E., et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidem Biomar. 2010;19(10):2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakeman I.M.M., Hilbers F.S., Rodriguez-Girondo M., et al. Addition of a 161-SNP polygenic risk score to family history-based risk prediction: impact on clinical management in non-BRCA1/2 breast cancer families. J Med Genet. 2019;56(9):581–589. doi: 10.1136/jmedgenet-2019-106072. [DOI] [PubMed] [Google Scholar]
- 23.Mars N., Widen E., Kerminen S., et al. The role of polygenic risk and susceptibility genes in breast cancer over the course of life. Nat Commun. 2020;11(1):6383. doi: 10.1038/s41467-020-19966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao C., Polley E.C., Hart S.N., et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39(23):2564–2573. doi: 10.1200/JCO.20.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher S., Hughes E., Kurian A.W., et al. Comprehensive breast cancer risk assessment for CHEK2 and ATM pathogenic variant carriers incorporating a polygenic risk score and the tyrer-cuzick model. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakeman I.M.M., Rodriguez-Girondo M.D.M., Lee A., et al. Clinical applicability of the Polygenic Risk Score for breast cancer risk prediction in familial cases. J Med Genet. 2023;60(4):327–336. doi: 10.1136/jmg-2022-108502. [DOI] [PubMed] [Google Scholar]
- 27.Mavaddat N., Ficorella L., Carver T., et al. Incorporating alternative polygenic risk scores into the BOADICEA breast cancer risk prediction model. Cancer Epidem Biomar. 2023;32(3):422–427. doi: 10.1158/1055-9965.EPI-22-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howie B., Fuchsberger C., Stephens M., Marchini J., Abecasis G.R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44(8):955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavaddat N., Michailidou K., Dennis J., et al. Polygenic risk scores for prediction of breast cancer and breast cancer Subtypes. Am J Hum Genet. 2019;104(1):21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly M.B., Pal T., Berry M.P., et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(1):77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 31.Sessa C., Balmana J., Bober S.L., et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34(1):33–47. doi: 10.1016/j.annonc.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Robson M. Management of women with breast cancer and pathogenic variants in genes other than BRCA1 or BRCA2. J Clin Oncol. 2021;39(23):2528–2534. doi: 10.1200/JCO.21.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X., Eriksson M., Czene K., et al. Prospective validation of the BOADICEA multifactorial breast cancer risk prediction model in a large prospective cohort study. J Med Genet. 2022;59(12):1196–1205. doi: 10.1136/jmg-2022-108806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuzick J., Warwick J., Pinney E., et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 35.Azam S., Eriksson M., Sjolander A., et al. Mammographic density change and risk of breast cancer. J Natl Cancer Inst. 2020;112(4):391–399. doi: 10.1093/jnci/djz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pashayan N., Duffy S.W., Chowdhury S., et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104(10):1656–1663. doi: 10.1038/bjc.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pashayan N., Morris S., Gilbert F.J., Pharoah P.D.P. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a life-table model. JAMA Oncol. 2018;4(11):1504–1510. doi: 10.1001/jamaoncol.2018.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schousboe J.T., Kerlikowske K., Loh A., Cummings S.R. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darabi H., Czene K., Zhao W., Liu J., Hall P., Humphreys K. Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. Breast Cancer Res. 2012;14(1):R25. doi: 10.1186/bcr3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Checka C.M., Chun J.E., Schnabel F.R., Lee J., Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. 2012;198(3):W292–W295. doi: 10.2214/AJR.10.6049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during this study are available to non-commercial parties upon reasonable request from the corresponding author. Only anonymized data will be transferred.