Abstract
To construct Thiobacillus ferrooxidans mutants by marker exchange mutagenesis, a genetic transfer system is required. The transfer of broad-host-range plasmids belonging to the incompatibility groups IncQ (pKT240 and pJRD215), IncP (pJB3Km1), and IncW (pUFR034) from Escherichia coli to two private T. ferrooxidans strains (BRGM1 and Tf-49) and to two collection strains (ATCC 33020 and ATCC 19859) by conjugation was analyzed. To knock out the T. ferrooxidans recA gene, a mobilizable suicide plasmid carrying the ATCC 33020 recA gene disrupted by a kanamycin resistance gene was transferred from E. coli to T. ferrooxidans ATCC 33020 by conjugation under the best conditions determined. The two kanamycin-resistant clones, which have retained the kanamycin-resistant phenotype after growth for several generations in nonselective medium, were shown to have the kanamycin resistance gene inserted within the recA gene, indicating that the recA::Ω-Km mutated allele was transferred from the suicide plasmid to the chromosome by homologous recombination. These mutants exhibited a slightly reduced growth rate and an increased sensitivity to UV and γ irradiation compared to the wild-type strain. However, the T. ferrooxidans recA mutants are less sensitive to these physical DNA-damaging agents than the recA mutants described in other bacterial species, suggesting that RecA plays a minor role in DNA repair in T. ferrooxidans.
Thiobacillus ferrooxidans is an acidophilic chemolithoautotrophic bacterium that obtains its energy from oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+) or of reduced sulfur compounds to sulfuric acid. Its widespread application in mineral leaching and metal remediation has made it an attractive microorganism to study. Considerable progress has been made in studying the biochemistry and molecular biology of T. ferrooxidans in recent years (27, 33). However, the absence of genetic tools has impaired the understanding of the physiology of this microorganism. The study of mutants in which a protein of interest is no longer synthesized can help to establish its function, but there have been no reports of the construction of T. ferrooxidans mutants. The construction by marker exchange mutagenesis of null mutants would be possible if a reliable genetic transfer system between Escherichia coli and T. ferrooxidans were available.
Introduction of plasmids into T. ferrooxidans by electrotransformation (17) and conjugation (23) has been reported. The plasmids electroporated by Kusano et al. (17) consisted of the T. ferrooxidans mer operon, determining resistance to mercury ions, cloned either into the broad-host-range plasmid pKT240 (IncQ group) or into a cryptic T. ferrooxidans natural plasmid carrying the pUC18 vector. Of the 30 independent T. ferrooxidans private strains tested, only one (Y4-3) gave transformants. The efficiency of electrotransformation was low (120 to 200 mercury-resistant colonies per μg of plasmid DNA). On the other hand, Peng et al. (23) reported the genetic transfer of broad-host-range IncP plasmids (RP4, R68.45, RP1::Tn501, and pUB307) by conjugation and the mobilization of a broad-host-range IncQ plasmid (pJRD215) with the aid of an RP4 plasmid to seven T. ferrooxidans private strains. Kanamycin resistance was used as the selection marker. The physiological states of both the donor and the recipient, and the mating time, have been shown to be important. The apparent transfer frequency of the large self-transmissible IncP plasmids was 10−5 to 10−7, depending on the plasmid, and the apparent mobilization frequency of the IncQ pJRD215 plasmid was about 10−5.
The RecA protein plays an essential role in homologous genetic recombination, DNA repair, induction of the SOS response, and initiation of stable DNA replication (29). The RecA protein is thought to be ubiquitous in eubacteria and is among the most conserved proteins across bacterial organisms (15). The recA gene from the T. ferrooxidans ATCC 33020 strain has been cloned (14, 25), sequenced (26), and expressed in E. coli (14, 25, 26). Both recombinase activity and SOS response were restored in an E. coli recA mutant by the T. ferrooxidans recA gene (25, 26), showing that RecA has similar activities in T. ferrooxidans and E. coli.
In this paper, the influence of different factors on the transfer frequency of IncQ plasmids from E. coli to T. ferrooxidans ATCC 33020 has been analyzed. This study was extended to three other T. ferrooxidans strains (ATCC 19859, BRGM1, and Tf-49) and to IncP and IncW plasmids. The feasibility of a marker exchange mutagenesis program has been tested in the ATCC 33020 strain with the construction of a recA mutant.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacteria and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| T. ferrooxidans | ||
| ATCC 33020 | Isolated from a uranium mine in Japan | ATCCa |
| ATCC 19859 | Isolated from acid copper leaching water in Canada | ATCC |
| Tf-49 | Isolated from a coal mine in Yunqin (China) | 24 |
| BRGM1 | Isolated twice on DOP medium from the BRGM strain | 8 |
| E. coli | ||
| HB101 | Δ(gpt-proA)62 leuB6 supE44 ara-14 galK2 lacY1 Δ(mcr-mrr) rpsL20 xyl-5 mtl-1 recA13 | |
| MC1061 | araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL hsdR2(rK− mK+) mcrA mcrB1 | |
| MOS Blue | endA1 hsdR17(rK− mK+) supE44 thi1 gyrA46 recA1 lac/F′ (lacIqZΔM15 proAB Tn10) | |
| S17-1 | recA pro hsdR (RP4-2 Tc::Mu Km::Tn7) | 31 |
| CGSC7330 | pro-81::Tn10 rph-1 | E. coli Genetic Stock Center |
| Plasmids | ||
| pNG23 | recA-recX-alaS region cloned in the HindIII site of pUC19; identical to pNG22 (14) with the insert in the opposite orientation | |
| pHP45Ω-Km | pHP45 plasmid carrying the Ω-Km cassette | 13 |
| pUC18mob | pUC18 plasmid in which an Sau3A fragment carrying the mob region of the RP4 plasmid has been inserted in the BamHI site | 31 |
| RP4 | Apr Tcr Kmr IncP Tra+ | 10 |
| pKT240 | Apr Kmr (Tn903) IncQ Mob+ | 4 |
| pJRD215 | Smr Kmr (Tn5) IncQ Mob+ | 11 |
| pJB3Km1 | Apr Kmr (Tn903) IncP Mob+ | 7 |
| pUFR034 | Kmr (Tn903) IncW Mob+ | 12 |
ATCC, American Type Culture Collection.
Media and growth conditions.
The E. coli growth medium was Luria-Bertani medium (21) except for conjugation experiments (see below). The composition of the T. ferrooxidans 9K liquid medium has been reported previously (5). The 2:2 and DOP solid media are described in references 24 and 19, respectively.
Conjugation.
Initial conjugation experiments were performed according to the method of Peng et al. (23). The conjugation experiments were optimized as presented in Results. The modified mating procedure was as follows. The E. coli donor strains were grown at 37°C until late exponential growth phase in 2:2 basal salt medium (2:2 liquid medium pH 5.2 to 5.4 without an energy source) supplemented with 0.5% (wt/vol) yeast extract and one antibiotic selective for the plasmid that they contained. The T. ferrooxidans recipient strains were usually cultured in 9K sulfur liquid medium (pH 3.5) at 30°C for 5 days to stationary phase. The cells were collected by centrifugation. T. ferrooxidans cells were washed three times with 2:2 basal salt medium to remove the sulfur precipitates. For matings, donor and recipient cells were combined in a 1:2 ratio. From this cell suspension (approximately 2 × 109 cells per ml), 0.1 ml was spotted on 2:2 solid medium (0.6% agar) supplemented with 0.05% (wt/vol) yeast extract, 0.5 × 10−4 M diaminopimelic acid, and 0.05% (wt/vol) Na2S2O3. After 3 days of incubation at 30°C, the cells were harvested and suspended in 1.5 ml of 2:2 basal salt medium. The number of viable recipient bacteria was obtained by plating on 2:2 or DOP solid medium. Transconjugants were selected at 30°C on DOP solid medium containing 200 μg of kanamycin/ml, with recipient bacteria being counterselected by kanamycin and donor bacteria by pH and the absence of a carbon source in the selective medium. The plates were incubated at 30°C for 10 to 15 days. The frequencies of plasmid transfer are expressed as the “apparent transfer frequency,” that is, the number of transconjugants scored on selective medium per recipient colony scored on nonselective medium after the mating period.
Stability analysis.
A single colony of T. ferrooxidans conjugant was grown to late exponential phase in 9K ferrous iron liquid medium without antibiotic, diluted 103-fold in fresh 9K ferrous iron medium, and grown again to late exponential phase. The last two steps were repeated three times. An aliquot was taken at the beginning of each new culture, diluted, and plated on 2:2 solid medium with or without kanamycin. The plasmid stability was calculated as the ratio between the number of colonies observed in the presence and absence of antibiotic.
Construction of a recA mutant of T. ferrooxidans ATCC 33020.
The pNG23 plasmid, carrying the ATCC 33020 recA and alaS genes cloned into pUC19 (Table 1), was digested with HindIII and religated to delete the alaS gene. The resulting plasmid, pUC19recA, replicates in E. coli but not in T. ferrooxidans. The blunt-ended HindIII fragment carrying the Kmr gene from the pHP45Ω-Km plasmid (13) was inserted into the SmaI site of the pUC19recA plasmid. The resulting plasmid, pUC19recA::Ω-Km, carries the recA gene disrupted by a 3.3-kb fragment as shown by restriction analyses, PCR, Southern hybridizations, and sequence determination. This fragment consists of the Ω-Km interposon, as expected, but also of an additional 1.3-kb fragment which corresponds to an internal region of the Ω-Km HindIII fragment. The 1.6-kb BamHI fragment from pUC18mob, corresponding to the mobilization region of the RP4 plasmid, was cloned into the ScaI site of the ampicillin resistance gene of the pUC19recA::Ω-Km plasmid. This plasmid, called pUC19recA::Ω-Kmmob, was transformed into E. coli S17-1 with kanamycin resistance selection. The mobilization ability of pUC19recA::Ω-Kmmob was checked by transferring it by conjugation from E. coli S17-1 to E. coli CGSC7330; the selection was for tetracycline and kanamycin resistance.
The pUC19recA::Ω-Kmmob plasmid was then transferred from E. coli S17-1 to T. ferrooxidans ATCC 33020 under the conditions given above except that the mating time was extended to 5 days. The selective medium was the 2:2 medium containing 200 μg of kanamycin/ml. The kanamycin plates were incubated at 30°C for 4 weeks.
Growth curves of the wild-type and recA ATCC 33020 strains.
To determine the growth rate of the wild-type and recA mutant strains, liquid media were inoculated with fresh cultures of ATCC 33020 and recA mutant 5 and shaken at 30°C until the cultures reached the stationary phase. Samples were removed every day, diluted, and plated on solid 2:2 medium. The number of CFU was plotted against the incubation time.
UV and γ irradiation.
ATCC 33020 and recA mutant derivatives were grown to mid-exponential phase in 9K liquid medium with ferrous iron as an energy source. Aliquots (50 μl) of 100 to 10−6 dilutions were spread on 2:2 or DOP solid medium. For UV irradiation, the plates were exposed to UV light (254 nm) at a dose rate of about 1.5 J/m2/s for 5 to 25 s. For γ irradiation, the plates were irradiated with 60Co at a dose rate of 147 and 35 Gy/min. The plates were incubated for at least 2 weeks at 30°C. Survival was determined as the ratio of CFU per milliliter after irradiation to CFU per milliliter before irradiation.
DNA manipulations.
General techniques were performed according to standard procedures (3) or the manufacturers' recommendations. Ultrapure plasmid DNA was obtained using the Wizard DNA purification system from Promega. T. ferrooxidans genomic DNA was prepared as previously described (5).
PCR.
The 475-bp fragment from the rusticyanin gene of T. ferrooxidans was amplified with the oligonucleotides RUSNM (5′-GGCACGCTGGATTCCACATGGAAAGAGGCG-3′) and RCX (5′-CCACTCGAGCCTTGACAATGATTTTACCAAACATACC-3′). The presence of E. coli cells was detected by the amplification of a 396- and a 570-bp fragment of the regulatory region of the operon encoding the major nitrate reductase of E. coli (6) with the oligonucleotide pairs S1 (5′-CACGGTTGGTATTGAGAAGC-3′) plus S2 (5′-CGCCGGATTTCATTAAGAGC-3′) and S4 (5′-GCCTGCTTAAAGCTTTTCGC-3′) plus 64G (5′-TCCCCATCACTCTTGATCGTTATC-3′). The oligonucleotides KMTN5 (5′-CGATGCGCTGCGAATCGG-3′) and AKMTN5 (5′-GCAGCTGTGCTCGACGTTG-3′) were used to amplify a 531-bp fragment of the kanamycin resistance gene from Tn5 (pJRD215), and the oligonucleotides KM1 (5′-AAGATCCTGGTATCGGTCTGC-3′) and KM2 (5′-AACATGGCAAAGGTAGCG-3′) were used to amplify a 524-bp fragment of the kanamycin resistance gene from Tn903 (pKT240, pJB3Km1, and pUFR034). The presence of the ampicillin resistance gene of the pKT240 and pJB3Km1 plasmids was tested for by amplification of a 633-bp fragment with the oligonucleotides AAMP (5′-CCGTGTCGCCCTTATTCCC-3′) and AMP3 (5′-TGGTCCTGCAACTTTATCCGCC-3′). To amplify the region of the ATCC 33020 recA gene which overlaps the SmaI site where the Ω-Km interposon was inserted, the oligonucleotides RECA2 (5′-CGATGACGATGAGGTCC-3′) and RECA3 (5′-AAGGATGGTTACCCCTCG-3′) were chosen. The insertion junctions between the recA gene and the Ω-Km cassette were obtained by amplification of the DNA from Kmr clone 5 between oligonucleotides hybridizing on one side of the recA SmaI site, in which the Ω-Km cassette had been inserted RECA4 (5′-CGGCTCGCTGGGTCTGG-3′) or ARECA5 (5′-CTGACAACTGGCTATGGC-3′), and an oligonucleotide corresponding to the end of the Ω-Km cassette, CKMTN5 (5′-GGAGTGGGGAGGCACGATGG-3′).
Sequence.
The sequence of the PCR fragments RecA4-CKMTN5 and ARECA5-CKMTN5, corresponding to the junctions of the Ω-Km cassette insertion inside the recA gene (see above), were determined with the Thermo Sequenase II dye terminator cycle-sequencing premix kit from Amersham. The DNA sequences were compiled and analyzed through the World Wide Web Netscape facilities.
Southern hybridization.
Genomic DNAs of strain ATCC 33020 and the kanamycin-resistant derivatives were digested with KpnI and EcoRV restriction endonucleases, electrophoresed on agarose gel, and transferred by capillary blotting to positively charged Hybond-N membranes (Roche Biochemicals). The kanamycin resistance gene and recA probes were obtained by incorporation of alkali-labile DIG-dUTP (Roche Biochemicals) during PCR elongation with the oligonucleotides KMTN5 and AKMTN5 (see above) on one hand and the oligonucleotides RECA3 and RECA2 (see above), which bracket the recA SmaI site where the Ω-Km interposon was inserted, on the other hand. The hybridization was carried out under stringent conditions as recommended by the manufacturer.
RESULTS
Conjugative transfer of the IncP RP4 plasmid and mobilization of IncQ plasmids from E. coli to T. ferrooxidans.
Although conjugation between E. coli and private T. ferrooxidans strains has been described (23), there is no report yet of this genetic transfer technique using collection strains of T. ferrooxidans. We first focused our study on the ATCC 33020 strain, because we have cloned several genes from this strain (1, 2, 5, 14), particularly the recA gene (14). Conjugation experiments between E. coli HB101 or MOS blue strains carrying the conjugative plasmid RP4 and T. ferrooxidans ATCC 33020 were carried out according to the method previously described by Peng et al. (23). Kanamycin-resistant clones were selected; T. ferrooxidans ATCC 33020 is sensitive to 200 μg of this antibiotic/ml on solid medium. The apparent transfer frequency obtained was lower than 10−8.
Mobilization by RP4 of the broad-host-range IncQ plasmids pKT240 and pJRD215, which have been shown to replicate in T. ferrooxidans (17, 23) and which carry kanamycin resistance genes from different origins (Tn903 and Tn5), was tested. Three- and two-partner conjugations were performed. In the first experiment, two E. coli strains were used: one (HB101) carries the helper plasmid (RP4), and the second (MC1061) carries the mobilizable plasmid (pKT240 or pJRD215); in the second experiment, only one E. coli strain was used, with the donor strain carrying the helper plasmid RP4 integrated within the chromosome (S17-1) and the mobilizable plasmid (pKT240 or pJRD215). In all the cases, the apparent transfer frequency to ATCC 33020 was approximately 10−7. The mobilization of plasmid pJRD215 from E. coli S17-1 to the ATCC 19859 strain was also tested. In that case, the apparent transfer frequency was higher (10−4).
Determination of optimal conditions for the mobilization of pJRD215 from E. coli S17-1 to T. ferrooxidans ATCC 33020.
As shown above, the apparent transfer frequency obtained by the protocol of Peng et al. (23) for T. ferrooxidans ATCC 33020 was lower than 10−8 in the case of conjugative plasmids and about 10−7 in the case of mobilizable plasmids. We hypothesized that these low frequencies were due to the completely different growth conditions of the donor and recipient cells. Indeed, E. coli is a neutrophile, while T. ferrooxidans is an extreme acidophile; E. coli is a heterotroph, while T. ferrooxidans is a strict chemolithoautotroph. Furthermore, E. coli is a fast-growing microorganism (20-min generation time in Luria-Bertani medium) while T. ferrooxidans is a slow-growing microorganism (9-h generation time in 9K medium supplemented with ferrous iron). Transfer of genetic material by conjugation requires cell-to-cell contacts and energy for both the donor and the recipient cells. Accordingly, we sought growth media and mating medium that could minimize differences in growth conditions, thereby avoiding possible stress during mating. Rawlings et al. (28) have studied the effect of mixing Luria agar with inorganic agar medium (pH 4) containing tetrathionate on E. coli and T. ferrooxidans ATCC 33020 growth and on the mating efficiency between E. coli cells. They have noticed that E. coli was able to grow on all media tested except 100% inorganic agar but that the mating efficiency between E. coli cells dropped off as the percentage of inorganic agar increased. T. ferrooxidans was unable to grow in the presence of even a low concentration of Luria medium but remained viable. Because these results suggest that E. coli adapts more easily to inorganic conditions than T. ferrooxidans to organic conditions, the E. coli growth medium and the mating medium used were based on the inorganic medium described by Peng et al. (24).
The effect of adaptation of the donor and recipient cells to the mating medium on the transfer frequency was analyzed first. When E. coli S17-1 (pJRD215) was grown in 2:2 medium supplemented with 0.5% yeast extract instead of in Luria-Bertani medium, the apparent transfer frequency increased slightly (Table 2). The influences of the basal salt medium and of the energy source present in the T. ferrooxidans growth medium were also tested. As can be seen in Table 2, the best result was obtained with the 9K medium and with a sulfur compound (S0 or thiosulfate) rather than ferrous iron as an energy source.
TABLE 2.
Effect of the E. coli and T. ferrooxidans growth media, mating medium, and donor-to-recipient ratio on apparent transfer frequency
| Parameter | Value | Apparent transfer frequency |
|---|---|---|
| E. coli growth medium | Luria-Bertani | 0.7 × 10−6 |
| 2:2 (pH 5.2–5.4) + 0.5% yeast extract | 2 × 10−6 | |
| T. ferrooxidans growth medium | 9K + FeSO4 | 1 × 10−6 |
| 9K + S0 | 3.2 × 10−5 | |
| 2:2 + Na2O3S2 + FeSO4 | 2.6 × 10−6 | |
| Mating medium | ||
| pH | 4.3 | 1 × 10−7 |
| 4.6 | 1.8 × 10−6 | |
| 4.8 | 2 × 10−6 | |
| 5 | 2.2 × 10−6 | |
| 5.2 | 2 × 10−6 | |
| 5.6 | 1 × 10−6 | |
| 6 | 1 × 10−7 | |
| Na2O3S2 (%) | 0 | 5.3 × 10−6 |
| 0.05 | 6.9 × 10−6 | |
| 0.1 | 2.8 × 10−6 | |
| 0.2 | 2 × 10−6 | |
| DAP (M) | 0 | 0.6 × 10−6 |
| 0.25 × 10−4 | 6 × 10−6 | |
| 0.5 × 10−4 | 8 × 10−6 | |
| 10−4 | 4 × 10−6 | |
| 2 × 10−4 | 2 × 10−6 | |
| E. coli/T. ferrooxidans ratio | 1/4 | 4.1 × 10−6 |
| 1/3 | 7 × 10−6 | |
| 1/2 | 1.2 × 10−5 | |
| 1/1 | 2 × 10−6 | |
| 2/1 | 1.4 × 10−6 | |
| 4/1 | 6 × 10−7 |
Different components of the mating medium were then analyzed. The effect of the pH ranging from 4.3 to 6.0 is given in Table 2. The apparent transfer frequency dropped quickly when the pH was lower than 4.6 or higher than 5.2. The concentration of thiosulfate in the mating medium was analyzed because this compound is an energy source for T. ferrooxidans but could be an inhibitor of E. coli when the concentration is too high. The highest apparent transfer frequency was obtained with a concentration of 0.05%. At higher concentrations, the frequency decreased. As already observed (23), the conjugation occurred even in the absence of an energy source for T. ferrooxidans. We have shown previously that diaminopimelic acid (DAP), a cell wall component, increases not only the growth rate of ATCC 33020 but also the viable cell numbers on 2:2 solid medium (19). Different concentrations of DAP in the mating medium were tested. At a concentration of 0.5 × 10−4 M, a significant increase in the apparent transfer frequency was obtained (Table 2).
We also tested different donor-to-recipient cell ratios, which is known to be important for conjugational transfer. As observed in Table 2, the apparent transfer frequency can vary from 6 × 10−7 for a 4/1 ratio to 1.2 × 10−5 for a 1/2 ratio.
By combining all the factors described above, that is, (i) by growing E. coli in 2:2 liquid medium with 0.5% yeast extract and growing ATCC 33020 in 9K liquid medium supplemented with sulfur as an energy source; (ii) by using the 2:2 solid medium with a pH of 4.8, 0.05% thiosulfate, and 0.5 × 10−4 M DAP as a mating medium; and (iii) by using a donor-to-recipient cell ratio of 1/2, the apparent transfer frequency of pJRD215 from E. coli S17-1 to ATCC 33020 can be increased 102- to 103-fold.
Mobilization of pUFR034 (IncW) and pJB3Km1 (IncP) plasmids from E. coli S17-1 to T. ferrooxidans ATCC 33020.
Genetic transfer to T. ferrooxidans ATCC 33020 was also tested with the mobilizable plasmids from the IncW (pUFR034) and IncP (pJB3Km1) incompatibility groups. Under the optimized conditions described above, the apparent transfer frequency of pJB3Km1 and pUFR034 was about 10 times lower than the frequency obtained with the IncQ plasmids (Table 3).
TABLE 3.
Apparent transfer frequency of pJRD215, pJB3Km1, and pUFR034 to T. ferrooxidans ATCC 33020, ATCC 19859, BRGM1, and Tf-49
| Recipient | Transfer frequency of:
|
||
|---|---|---|---|
| pJRD215 (IncQ) | pJB3Km1 (IncP) | pUFR034 (IncW) | |
| ATCC33020 | 4.4 × 10−5 | 1.2 × 10−6 | 5 × 10−6 |
| ATCC19859 | 1.4 × 10−3 | 6.2 × 10−7 | 1 × 10−6 |
| BRGM1 | 2.5 × 10−3 | 1.3 × 10−5 | 2.2 × 10−6 |
| Tf-49 | 2 × 10−4 | 2.7 × 10−6 | 6.8 × 10−6 |
Mobilization of IncQ, IncW, and IncP plasmids from E. coli S17-1 to T. ferrooxidans ATCC 19859, BRGM1, and Tf-49.
Three other T. ferrooxidans strains were tested as recipients: the two private strains BRGM1 and Tf-49, the latter being one of the strains tested for conjugation by Peng et al. (23), and the ATCC 19859 collection strain, from which several genes have been characterized. The results obtained under the optimized conditions described above are presented in Table 3. The IncP, IncQ, and IncW plasmids tested were all transferred to the four T. ferrooxidans strains tested. The apparent transfer frequency obtained depended on the T. ferrooxidans recipient strain and on the plasmid incompatibility group. More particularly, for all the strains tested, the apparent transfer frequency was highest with the IncQ plasmid.
Transconjugant analyses.
For each conjugation experiment performed, eight kanamycin-resistant (Kmr) clones were analyzed to establish that they were true T. ferrooxidans transconjugants.
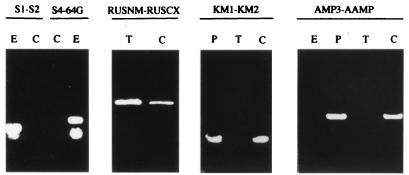
No contaminating E. coli donor cells were detected by PCR with two oligonucleotide pairs hybridizing to E. coli but not to T. ferrooxidans genomic DNA (Fig. 1). On the other hand, a fragment internal to the rusticyanin-encoding gene, which does not exist in E. coli, was obtained in all cases, indicating that the Kmr clones were indeed T. ferrooxidans cells (Fig. 1).
FIG. 1.
PCR analyses of E. coli cells (E), T. ferrooxidans/pKT240 transconjugant cells (C), T. ferrooxidans cells (T), and pKT240 plasmid (P) with oligonucleotides hybridizing to the E. coli genomic DNA (S1 and S2, S4 and 64G), to the T. ferrooxidans genomic DNA (RUSNM and RUSCX), to the kanamycin resistance gene (KM1 and KM2), and to the ampicillin resistance gene (AMP3 and AAMP) of pKT240.
The presence of the kanamycin resistance gene in Kmr clones was checked by PCR with two oligonucleotides hybridizing to the kanamycin resistance gene of Tn5 in the case of pJRD215 plasmids or Tn903 in the case of the pKT240, pUFR034, and pJB3Km1 plasmids (Fig. 1). In the same way, the presence of the ampicillin resistance gene of the pKT240 and pJB3Km1 plasmids was checked by PCR (Fig. 1).
The plasmids purified from the Kmr clones had the expected restriction sites (data not shown). Furthermore, after transformation of E. coli HB101 with these plasmid preparations, Kmr clones were obtained.
All these results show unambiguously that the mobilizable plasmids had been introduced successfully into T. ferrooxidans by conjugation and were not integrated into the chromosome.
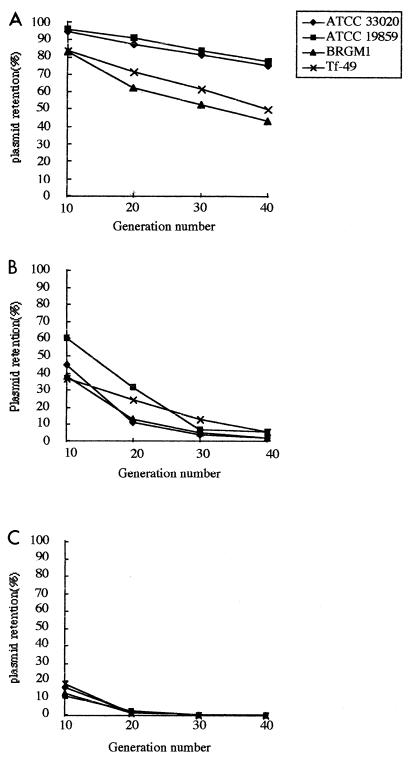
Stability analysis.
The stabilities of the different plasmids tested in the four T. ferrooxidans strains studied were analyzed as described in Materials and Methods. The IncQ plasmid pJRD215 was stable, especially in the two collection strains ATCC 19859 and ATCC 33020, with more than 70% retention after 40 generations without antibiotic selection (Fig. 2A). On the other hand, the plasmid pUFR034 (IncW) (Fig. 2B) and, more particularly, the pJB3Km1 (IncP) (Fig. 2C) plasmids, were unstable: after 40 generations, fewer than 10% of the clones retained pUFR034 (IncW) and all the clones had lost pJFB3Km1 (IncP).
FIG. 2.
Stability analysis of pJRD215 (IncQ) (A), pUFR034 (IncW) (B), and pJB3Km1 (IncP) (C) in T. ferrooxidans ATCC 33020, ATCC 19859, BRGM1, and Tf-49 strains.
Construction of a T. ferrooxidans ATCC 33020 recA mutant.
To test whether conjugation can be used successfully as a genetic transfer technique to construct null mutants by reverse genetics, we chose the recA gene as a model because (i) construction of recA mutants by marker exchange mutagenesis has already been described in several microorganisms (22); (ii) since the RecA protein from the ATCC 33020 strain has the classical RecA biochemical activities (25, 26), a T. ferrooxidans recA mutant would be expected to have the same properties as the bacterial recA null mutants already characterized; (iii) the recA gene has been shown to be independently transcribed from the downstream essential alaS gene encoding alanyl tRNA synthetase (14), and consequently, recA insertional inactivation will not have a detrimental polar effect on alaS expression; and (iv) a T. ferrooxidans recA mutant is required for further genetic studies to stably maintain recombinant plasmids.
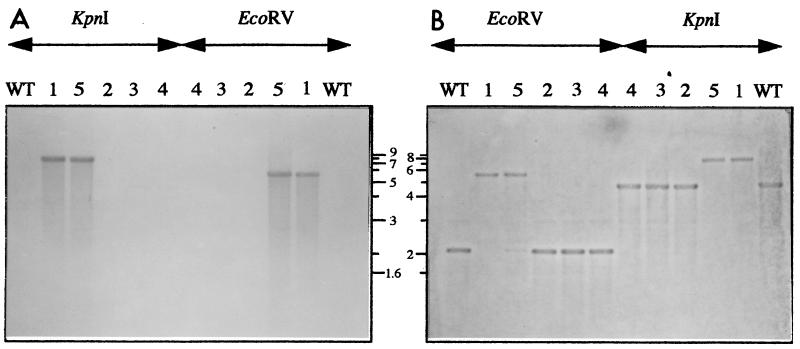
To obtain a T. ferrooxidans recA mutant, a mobilizable suicide plasmid carrying the recA gene disrupted with a cassette carrying the kanamycin resistance gene was constructed (see Materials and Methods). This plasmid (pUC19recA::Ω- Kmmob), which is unable to replicate in T. ferrooxidans, was mobilized from an E. coli S17-1 strain into strain ATCC 33020 by conjugation (see Materials and Methods), and kanamycin-resistant (Kmr) clones were selected. Only five Kmr clones were obtained from two independent conjugation experiments after 4 weeks of incubation at 30°C. These clones were indeed T. ferrooxidans cells carrying the Ω-Km cassette as shown by PCR analyses (see Materials and Methods). Furthermore, no plasmid had been detected by plasmid purification and transformation of E. coli, suggesting that either the plasmid had been integrated into the chromosome by a single-crossover event or that recombination between the suicide plasmid carrying the mutated recA::Ω-Km allele and the chromosome carrying the wild-type recA allele had taken place. To increase the likelihood of this double-crossover event, the five Kmr clones were subcultured twice in liquid medium for several generations without antibiotic. Under these conditions, three clones (no. 2, 3, and 4) lost their resistance to kanamycin, a result confirmed by PCR analysis (data not shown) and Southern blot hybridization with a probe corresponding to an internal fragment of the Kmr gene (Fig. 3A). We conclude that clones 2, 3, and 4 have lost the suicide plasmid. The two other clones (no. 1 and 5) kept the Kmr phenotype after several generations in the absence of kanamycin. The presence of the kanamycin resistance gene was confirmed by PCR analysis (data not shown). On the other hand, the ampicillin resistance gene could not be detected by PCR in these two clones. All these results suggest that a recombination event had taken place at the recA locus.
FIG. 3.
Southern blot analysis of KpnI- and EcoRV-digested DNA isolated from T. ferrooxidans ATCC 33020 (lane WT); Kms clones 2 (lane 2), 3 (lane 3), and 4 (lane 4); and Kmr clones 1 (lane 1) and 5 (lane 5). The blots were probed with a PCR fragment internal to the Km gene (A) and with a PCR fragment internal to the recA gene (B). The numbers between panels A and B indicate the sizes (in kilobases) of some fragments from the molecular size marker from Roche Biochemicals.
Characterization of recA mutants.
To determine if recA is disrupted by the Ω-Km cassette in the putative recA mutants 1 and 5, genomic DNA was purified from these clones and compared by Southern blot hybridizations and PCR analyses to the DNA from the three Kms clones 2, 3, and 4 and to the DNA from the wild-type ATCC 33020 strain.
When an internal fragment of the recA gene was used as the probe, a 4.8-kb KpnI fragment and a 2.1-kb EcoRV fragment were obtained with the wild type and the Kms clones 2, 3, and 4, whereas an 8.1-kb KpnI fragment and a 5.4-kb EcoRV fragment were obtained with the Kmr clones 1 and 5 (Fig. 3B). These results suggest the disruption of the recA gene in Kmr clones 1 and 5. The probe corresponding to an internal fragment of the Kmr gene did not hybridize to the DNA from the wild type or from Kms clones 2, 3, and 4 but did hybridize to the same 8.1-kb KpnI fragment and 5.4-kb EcoRV fragments described above in Kmr clones 1 and 5 (Fig. 3A). Altogether, the Southern hybridization results suggest that the Ω-Km cassette had been inserted within the recA gene in the two Kmr isolates 1 and 5.
PCR analyses and sequencing of the recA region from Kmr clones 1 and 5 confirmed the insertion of the Ω-Km cassette inside the recA gene (data not shown). Kmr clones 1 and 5 will now be referred as recA 1 and recA 5 mutants.
Properties of the T. ferrooxidans recA mutants.
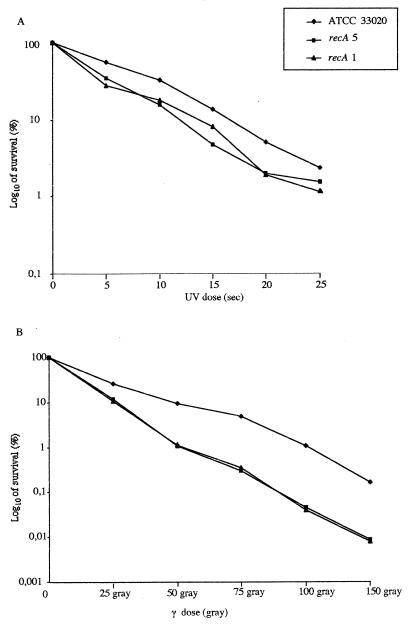
The T. ferrooxidans recA 5 strain showed a slightly reduced growth rate compared to the wild-type strain (data not shown), which is typically observed for recA mutants.
To compare the effectiveness of DNA repair mechanisms in the wild-type strain and recA mutants, we used sensitivity to physical DNA-damaging agents. Sensitivities to irradiation by UV and γ rays are presented in Fig. 4. As expected for recA mutants, recA 1 and recA 5 strains were more sensitive than the parental ATCC 33020 strain to both UV and γ radiations.
FIG. 4.
Survival of strain ATCC 33020 and of the recA mutants after UV (A) and γ (B) irradiation.
DISCUSSION
This paper reports the first construction by marker exchange mutagenesis of a mutant of the extreme acidophilic T. ferrooxidans. Reverse genetics was made possible by significantly improving the conditions for the transfer of plasmids from E. coli to the ATCC 33020 culture collection strain of T. ferrooxidans by conjugation. The apparent transfer frequency was shown to depend on the donor-to-recipient cell ratio and on the physiological state of the donor and of the recipient cells, two factors which are known to be important for conjugational transfer. The conjugation protocol described in this paper was followed to mobilize plasmids from the three incompatibility groups, IncQ, IncP, and IncW, to four T. ferrooxidans strains, BRGM1, Tf-49, ATCC 19859, and ATCC 33020. Significantly, the IncW pUFR034 and, more particularly, the IncP plasmid pJB3Km1 were unstable in the four strains tested. These plasmids may therefore be used as shuttle vectors for a marker exchange mutagenesis program. On the other hand, the IncQ plasmids, which appeared to be stably maintained, may be useful for construction of expression vectors to introduce heterologous or homologous genes into T. ferrooxidans and for construction of operon fusion vectors to study T. ferrooxidans gene expression.
We have demonstrated that conjugation under the conditions described in this study can be used successfully as a genetic transfer technique to construct null mutants by reverse genetics. The knockout of the ATCC 33020 recA gene was confirmed by both molecular and physiological approaches. Evidence of recA gene disruption by the Ω-Km cassette includes the results of Southern hybridizations, PCR analyses, and sequencing on both sides of the Ω-Km cassette. Moreover, as expected for recA mutants (22), the knockout mutants exhibit slightly reduced growth rates and are more sensitive to UV and γ irradiation compared to the parental strain. It is worth mentioning that the T. ferrooxidans recA mutants were not as sensitive to UV and γ irradiation as recA mutants described in other bacterial species. One could then speculate whether another recA gene is present in the T. ferrooxidans ATCC 33020 strain. This is unlikely because only one recA gene has been found by Southern hybridization (this paper and reference 25) and only one recA gene has been cloned by complementation of an E. coli recA mutant (25). Another possibility is that RecA-dependent DNA repair is a minor pathway in T. ferrooxidans compared to other repair mechanisms. In E. coli, the UV and γ irradiation-induced DNA lesions are primarily removed by nucleotide and base excision repair processes, respectively (see reference 30 and references therein). However, if the replication fork encounters a lesion before repair has taken place, replication stalls or collapses. Replication restarts only from recombination intermediates generated by RecA and accessory proteins, such as RecFOR (gap repair) and RecBCD (double-strand break repair) (18; see references 9, 20, and 30 and references therein). Therefore, RecA is absolutely required for DNA repair at the level of the replication fork in E. coli. Because this bacterium is a fast-growing microorganism, RecA plays a key role in the survival of exponentially growing cells exposed to UV or γ irradiation. Accordingly, the basal level of RecA protein is high, and recA gene transcription is increased further by induction of the SOS response (see reference 32 and references therein). Because the T. ferrooxidans generation time is long (9 h at 30°C) but its replication rate is likely similar to that of E. coli (about 1,000 nucleotides per s [16]), it is reasonable to assume that in T. ferrooxidans, prereplicative repair processes (nucleotide and base excision repair) have sufficient time to repair most DNA damage before the arrival of the replication fork. Postreplication RecA-dependent recombinational repair processes would therefore play a minor role. In agreement with this hypothesis, the basal level of RecA protein in the T. ferrooxidans ATCC 33020 strain is low (25) and the recA gene is not induced by DNA damaging agents (26).
The construction of a T. ferrooxidans recA mutant should facilitate future genetic studies of this chemolithoautotrophic acidophilic microorganism by allowing the stable maintenance of plasmids in which homologous or heterologous genes have been cloned. Furthermore, the allelic replacement procedure used to produce the recA mutant should be applicable to the construction of null mutants of the genes encoding proteins whose physiological function have yet to be determined.
ACKNOWLEDGMENTS
We owe special thanks to P. Moreau for fruitful advice and suggestions and also to A. Bengrine for helpful discussions. We thank J. DeMoss for critical reading of the manuscript. We acknowledge J. M. Blatny for the gift of the pJB3Km1 plasmid and the Escherichia coli Genetic Stock Center for strain CGSC7330. We are indebted to M. Kasmaier from C.E.A. (Cadarache, France) who welcomed us for the γ radiation experiments. We are grateful to the Centre de Sequençage de l'ADN (I.B.S.M., L.C.B., Marseille).
Z.L. acknowledges A. Klier (Institut Pasteur, Paris, France) and the support of a CIES grant in the context of an agreement between the University of Shandong (China) and Université Paris 7 (France). This work was partly supported by A.D.E.M.E., B.R.G.M., CO.GE.MA., and by an ACC-SV from the C.N.R.S.
REFERENCES
- 1.Appia-Ayme C, Bengrine A, Cavazza C, Giudici-Orticoni M-T, Bruschi M, Chippaux M, Bonnefoy V. Characterization and expression of the cotranscribed cyc1 and cyc2 genes encoding the cytochrome c4 (c552) and a high molecular weight cytochrome c from Thiobacillus ferrooxidans ATCC 33020. FEMS Microbiol Lett. 1998;167:171–177. doi: 10.1111/j.1574-6968.1998.tb13224.x. [DOI] [PubMed] [Google Scholar]
- 2.Appia-Ayme C, Guiliani N, Ratouchniak J, Bonnefoy V. Characterization of an operon encoding two c-type cytochromes, an aa3-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl Environ Microbiol. 1999;65:4781–4787. doi: 10.1128/aem.65.11.4781-4787.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing; 1992. [Google Scholar]
- 4.Bagdasarian M M, Amann E, Lurz R, Rückert B, Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983;26:273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- 5.Bengrine A, Guiliani N, Appia-Ayme C, Jedlicki E, Holmes D S, Chippaux M, Bonnefoy V. Sequence and expression of the rusticyanin structural gene from Thiobacillus ferrooxidans ATCC 33020 strain. Biochim Biophys Acta. 1998;1443:99–112. doi: 10.1016/s0167-4781(98)00199-7. [DOI] [PubMed] [Google Scholar]
- 6.Blasco F, Iobbi C, Giordano G, Chippaux M, Bonnefoy V. Nitrate reductase of Escherichia coli: completion of the nucleotide sequence of the nar operon and reassessment of the role of the α and β subunits in iron binding and electron transfer. Mol Gen Genet. 1989;218:249–256. doi: 10.1007/BF00331275. [DOI] [PubMed] [Google Scholar]
- 7.Blatny J M, Brautaset T, Winther-Larsen H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collinet-Latil M N, Morin D. Characterization of arsenopyrite oxidizing Thiobacillus. Tolerance to arsenite, arsenate, ferrous and ferric iron. Antonie Leeuwenhoek. 1990;57:237–244. doi: 10.1007/BF00400155. [DOI] [PubMed] [Google Scholar]
- 9.Cox M M. Recombinational DNA repair in bacteria and the RecA protein. Prog Nucleic Acid Res Mol Biol. 1999;63:311–366. doi: 10.1016/s0079-6603(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 10.Datta N, Hedges R W, Shaw E J, Sykes R B, Richmond M H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971;108:1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 12.DeFeyter R, Kado C I, Gabriel D W. Small, stable shuttle vectors for use in Xanthomonas. Gene. 1990;88:65–72. doi: 10.1016/0378-1119(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 13.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 14.Guiliani N, Bengrine A, Borne F, Chippaux M, Bonnefoy V. Alanyl tRNA synthetase gene of extreme acidophilic chemolithotrophic Thiobacillus ferrooxidans is highly homologous to alaS from all living kingdoms but cannot be transcribed from its promoter in Escherichia coli. Microbiology. 1997;143:2179–2187. doi: 10.1099/00221287-143-7-2179. [DOI] [PubMed] [Google Scholar]
- 15.Karlin S, Brocchieri L. Evolutionary conservation of RecA genes in relation to protein structure and function. J Bacteriol. 1996;178:1881–1894. doi: 10.1128/jb.178.7.1881-1894.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornberg A, Baker T A. DNA replication. W. H. New York, N.Y: Freeman and Co.; 1992. [Google Scholar]
- 17.Kusano T, Sugawara K, Inoue C, Takeshima T, Numata M, Shiratori T. Electrotransformation of Thiobacillus ferrooxidans with plasmids containing a mer determinant. J Bacteriol. 1992;174:6617–6623. doi: 10.1128/jb.174.20.6617-6623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Xu L, Sandler S J, Marians K J. Replication fork assembly at recombination intermediates is required for bacterial growth. Proc Natl Acad Sci USA. 1999;96:3552–3555. doi: 10.1073/pnas.96.7.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Borne F, Ratouchniak J, Bonnefoy V. Genetic transfer of IncP, IncQ, IncW plasmids to four Thiobacillus ferrooxidans strains by conjugation. In: Amils R, Ballester A, editors. Biohydrometallurgy and the environment toward the mining of the 21st century. International Biohydrometallurgy Symposium. Amsterdam, The Netherlands: Elsevier; 1999. pp. 39–49. [Google Scholar]
- 20.Lloyd R G, Low K B. Homologous recombination. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 2236–2255. [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 22.Miller R V, Kokjohn T A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- 23.Peng J-B, Yan W-M, Bao X-Z. Plasmid and transposon transfer to Thiobacillus ferrooxidans. J Bacteriol. 1994;176:2892–2897. doi: 10.1128/jb.176.10.2892-2897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng J-B, Yan W-M, Bao X-Z. Solid medium for the genetic manipulation of Thiobacillus ferrooxidans. J Gen Appl Microbiol. 1994;40:243–253. [Google Scholar]
- 25.Ramesar R S, Woods D R, Rawlings D E. Cloning and expression in Escherichia coli of a recA-like gene from the acidophilic autotroph Thiobacillus ferrooxidans. J Gen Microbiol. 1988;134:1141–1146. doi: 10.1099/00221287-134-5-1141. [DOI] [PubMed] [Google Scholar]
- 26.Ramesar R S, Abratt V, Woods D R, Rawlings D E. Nucleotide sequence and expression of a cloned Thiobacillus ferrooxidans recA gene in Escherichia coli. Gene. 1989;78:1–8. doi: 10.1016/0378-1119(89)90308-9. [DOI] [PubMed] [Google Scholar]
- 27.Rawlings D E. The molecular genetics of mesophilic, acidophilic, chemolithotrophic, iron or sulfur-oxidizing microorganisms. In: Amils R, Ballester A, editors. Biohydrometallurgy and the environment toward the mining of the 21st century. International Biohydrometallurgy Symposium. Amsterdam, The Netherlands: Elsevier; 1999. pp. 3–20. [Google Scholar]
- 28.Rawlings D E, Sewcharan R, Woods D R. Characterization of a broad-host-range mobilizable Thiobacillus ferrooxidans plasmid and the construction of Thiobacillus cloning vectors. In: Lawrence R W, Branion R N R, Ebnes H G, editors. Fundamental and applied biohydrometallurgy. Amsterdam, The Netherlands: Elsevier; 1986. pp. 419–427. [Google Scholar]
- 29.Roca A L, Cox M M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25:415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- 30.Rupp W D. DNA repair mechanisms. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 2277–2294. [Google Scholar]
- 31.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 32.Walker G C. The SOS response of Escherichia coli. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1400–1416. [Google Scholar]
- 33.Yamanaka T, Fukumori Y. Molecular aspects of the electron transfer system which participates in the oxidation of ferrous ion by Thiobacillus ferrooxidans. FEMS Microbiol Rev. 1995;17:401–413. doi: 10.1111/j.1574-6976.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]