Abstract
Background
Steroidal contraceptive use has been associated with changes in bone mineral density in women. Whether such changes increase the risk of fractures later in life is not clear. Osteoporosis is a major public health concern. Age‐related decline in bone mass increases the risk of fracture, especially of the spine, hip, and wrist. Concern about bone health influences the recommendation and use of these effective contraceptives globally.
Objectives
Our aim was to evaluate the effect of using hormonal contraceptives before menopause on the risk of fracture in women.
Search methods
Through April 2014, we searched for studies of fracture or bone health and hormonal contraceptives in MEDLINE, POPLINE, CENTRAL, EMBASE, and LILACS, as well as ClinicalTrials.gov and ICTRP. We examined reference lists of relevant articles for other trials. For the initial review, we wrote to investigators to find additional trials.
Selection criteria
Randomized controlled trials (RCTs) were considered if they examined fractures, bone mineral density (BMD), or bone turnover markers in women with hormonal contraceptive use prior to menopause. Eligible interventions included comparisons of a hormonal contraceptive with a placebo or with another hormonal contraceptive that differed in terms of drug, dosage, or regimen. They also included providing a supplement to one group.
Data collection and analysis
We assessed all titles and abstracts identified through the literature searches. Mean differences were computed using the inverse variance approach. For dichotomous outcomes, the Mantel‐Haenszel odds ratio (OR) was calculated. Both included the 95% confidence interval (CI) and used a fixed‐effect model. Due to differing interventions, no trials could be combined for meta‐analysis. We applied principles from GRADE to assess the evidence quality and address confidence in the effect estimates. In addition, a sensitivity analysis included trials that provided sufficient data for this review and evidence of at least moderate quality.
Main results
We found 19 RCTs that met our eligibility criteria. Eleven trials compared different combined oral contraceptives (COCs) or regimens of COCs; five examined an injectable versus another injectable, implant, or IUD; two studied implants, and one compared the transdermal patch versus the vaginal ring. No trial had fracture as an outcome. BMD was measured in 17 studies and 12 trials assessed biochemical markers of bone turnover. Depot medroxyprogesterone acetate (DMPA) was associated with decreased bone mineral density (BMD). The placebo‐controlled trials showed BMD increases for DMPA plus estrogen supplement and decreases for DMPA plus placebo supplement. COCs did not appear to negatively affect BMD, and some formulations had more positive effects than others. However, no COC trial was placebo‐controlled. Where studies showed differences between groups in bone turnover markers, the results were generally consistent with those for BMD. For implants, the single‐rod etonogestrel group showed a greater BMD decrease versus the two‐rod levonorgestrel group but results were not consistent across all implant comparisons.
The sensitivity analysis included 11 trials providing evidence of moderate or high quality. Four trials involving DMPA showed some positive effects of an estrogen supplement on BMD, a negative effect of DMPA‐subcutaneous on lumbar spine BMD, and a negative effect of DMPA on a bone formation marker. Of the three COC trials, one had a BMD decrease for the group with gestodene plus EE 15 μg. Another indicated less bone resorption in the group with gestodene plus EE 30 μg versus EE 20 μg.
Authors' conclusions
Whether steroidal contraceptives influence fracture risk cannot be determined from existing information. The evidence quality was considered moderate overall, largely due to the trials of DMPA, implants, and the patch versus ring. The COC evidence varied in quality but was low overall. Many trials had small numbers of participants and some had large losses. Health care providers and women should consider the costs and benefits of these effective contraceptives. For example, injectable contraceptives and implants provide effective, long‐term birth control yet do not involve a daily regimen. Progestin‐only contraceptives are considered appropriate for women who should avoid estrogen due to medical conditions.
Keywords: Female; Humans; Bone Density; Bone Density/drug effects; Bone Remodeling; Bone Remodeling/drug effects; Contraceptives, Oral, Hormonal; Contraceptives, Oral, Hormonal/adverse effects; Contraceptives, Oral, Hormonal/pharmacology; Estrogens; Estrogens/pharmacology; Fractures, Bone; Fractures, Bone/chemically induced; Medroxyprogesterone Acetate; Medroxyprogesterone Acetate/adverse effects; Medroxyprogesterone Acetate/pharmacology; Premenopause; Progestins; Progestins/pharmacology; Randomized Controlled Trials as Topic
Plain language summary
Hormonal contraceptives and bone health in women
Hormonal contraceptives have been related to bone changes in women. Whether such changes lead to more bone fractures later in life is not clear. However, bone health is a major public health concern. Bone density declines with age, and the change increases the risk of fracture. Due to concern about bone health, health care providers may not suggest hormonal contraceptives and women may not want to use them.
Through April 2014, we did computer searches for studies of birth control methods containing hormones and risk of fractures. Outcomes could also be bone mineral density or markers of bone changes. Birth control pills included types with both estrogen and progestin. Also included were implants and injectables with only progestin. We wrote to researchers to find other trials. We included randomized trials in any language that had at least three treatment cycles. The studies had to compare two types of birth control or one type of birth control or a supplement with a placebo or 'dummy' method.
We found 19 trials. Fifteen studies compared one birth control method with another hormone method. Two trials used a placebo or 'dummy.' One compared a hormone method to a method without hormones. None had fractures as an outcome and most looked at bone density. Birth control methods with both estrogen and progestin did not appear to affect bone health. However, 'depo,' which is injected and has only progestin, was related to lower bone density. The two depo trials with placebos showed increased bone density when some estrogen was given to women on depo. Bone density decreased in women who got a 'dummy' with the depo. Whether this decrease is important to the woman's health is not known. For implants, an etonogestrel implant with one rod showed a greater decrease in bone density than a two‐rod levonorgestrel implant. However, other implants studied did not show the same pattern.
The studies had data of moderate quality. Whether hormonal contraceptives affect fracture risk cannot be judged from current information. These contraceptive methods work well for birth control. Health‐care providers and women should think about the costs and benefits. For instance, injectable use can occur without a partner's knowledge, and is simpler than taking pills every day. Also, progestin‐only methods are suggested for some women with health problems who should avoid estrogen.
Background
Description of the condition
Steroidal contraceptives, particularly injectable contraceptives and combined oral contraceptives (COCs), have been associated with changes in bone mineral density in women. Whether such changes increase the risk of fractures later in life is not clear. However, osteoporosis is a major public health concern. Age‐related decline in bone mass increases the risk of fracture, especially of the spine, hip, and wrist (Howe 2011; Rachner 2011). The costs of osteoporosis‐related fractures can be substantial for the individual due to disability and to society for health and social care (Howe 2011). Concern about bone health influences the recommendation and use of these effective contraceptives globally.
Skeletal fragility results from suboptimal formation of bone mass and strength, as well as excess bone resorption (NIH 2000; Raisz 2005). Bone loss during contraceptive use may be temporary like that which occurs during pregnancy or breastfeeding (Gourlay 2004; ACOG 2008). Risk of future fractures after contraceptive use depends on whether the bone mass is restored.
Description of the intervention
Depot medroxyprogesterone acetate (DMPA)
DMPA is an effective contraceptive and the most widely‐used injectable (Bartz 2011). Data from developing countries showed median failure rates of 2.4% for injectables versus 10.3% for condoms and 6.5% for pills (Cleland 2004). First‐year failure rates for DMPA in the USA have been estimated at 0.2% for perfect use and 6% for typical use (Trussell 2011). If injectable use were limited due to concerns about effects on bone health, women might switch to less effective methods or use nothing, which could lead to increased pregnancy rates.
Of the injectable contraceptives, DMPA has attracted the most attention regarding bone health. DMPA may reduce bone mineral density (BMD), which is a potential concern for younger women who have not yet achieved peak bone mass. Early research indicated more bone loss among women who used DMPA before 20 years of age and those who used it for longer periods (Cundy 1998; Scholes 1999). More recently, two case‐control studies reported increased fracture risk for longer current use of DMPA (Vestergaard 2006; Meier 2010), although past users had little evidence of increased risk (Meier 2010).
In the US, the Food and Drug Administration requires a warning on DMPA labeling (FDA 2004; FDA 2011). It refers to BMD loss among DMPA users, especially younger women. The warning is based on limited evidence and may limit long‐term use (Kaunitz 2011). Major health organizations have recommended not restricting DMPA use among women 18 to 45 years old (WHO 2006; ACOG 2008; Guilbert 2009). In guidance about medical eligibility criteria for contraceptive use, DMPA is category 1 (no restriction) for women aged 18 to 45 years (CDC 2010; WHO 2009). For women outside that age range, DMPA is category 2, meaning the advantages generally outweigh the theoretical or proven risks.
Oral contraceptives (OCs)
OCs are the most commonly used reversible method in more developed countries (UN 2011). Failure rates for oral contraceptives in the USA (combined and progestin‐only) are estimated at 0.3% for perfect use and 9% for typical use in the first year (Trussell 2011).
Few associations have been noted between OC use and fracture risk in observational studies (Lopez 2012). A cohort study found OC ever‐users had increased risk for all fractures (Cooper 1993). However, a case‐control study, with later data from a subset, reported no association except for those with 10 years or more since use (Memon 2011). Another case‐control study reported increased risk, but only for those who had 10 or more prescriptions (Meier 2010). A cohort study of postmenopausal women found no increased fracture risk for OC use after excluding women with prior fracture (Barad 2005). Two other studies found little evidence of association between OC use and fracture risk. A cohort study noted increased risk for subgroups, such as those with longer use or specific intervals since use (Vessey 1998). A case‐control study reported increased risk for any fracture only among young women with less than average use (Vestergaard 2006).
COCs may have little effect on BMD among healthy adult women. Prospective studies have indicated that ultra‐low dose COCs, containing 20 μg ethinyl estradiol, may affect bone development in young women (Cromer 2003). On the other hand, COCs with 30 to 40 μg ethinyl estradiol may have no negative effect and may even protect against bone loss, at least among women 30 years of age or more (Cromer 2003). Evidence from studies of varying designs indicates that BMD may be affected by COC use in adolescent and young women but not in adult premenopausal or postmenopausal women (Martins 2006; Herrmann 2010; Warholm 2012). However, COC use may have a negative effect on bone turnover markers, although the clinical significance of such change is unclear (Herrmann 2010).
Intrauterine device (IUD) or system (IUS)
For the levonorgestrel IUS, no mechanism is apparent that might affect bone health (Mansour 2012). However, a case‐control study reported reduced fracture risk for ever‐use of the hormonal IUD and longer use of that IUD (Vestergaard 2006).
Why it is important to do this review
Hormonal contraceptives are among the most effective and most widely‐used contraceptives. Concern about fractures may limit the use of these effective contraceptives. Women might switch to less effective methods or use nothing, potentially leading to increased rates of unintended pregnancy. The question about an association between steroidal contraceptives and fractures is important to examine systematically with the available evidence. Since our initial review in 2006, we also examined evidence of actual fracture risk in observational studies of hormonal contraceptives (Lopez 2012). In this update, we further examine the effect of using steroidal contraceptives before menopause on general bone health, based on evidence from randomized controlled trials.
Objectives
Our aim was to evaluate the effect of using hormonal contraceptives before menopause on the risk of fracture in women.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized controlled trials (RCTs) if they examined fractures, bone mineral density, or bone turnover in women who used hormonal contraceptives prior to menopause.
Studies were excluded if hormones were provided for treatment of a specific condition or if the study focused on women with a certain condition, such as endometriosis, polycystic ovary disease, or hirsutism. Also excluded were studies that provided hormone replacement therapy to postmenopausal women.
Types of participants
We included women in the identified trials who were randomly assigned to study groups.
Types of interventions
Interventions included comparisons of a hormonal contraceptive with a placebo or with another hormonal contraceptive that differed in terms of drug, dosage, or regimen. Interventions also included the provision of a supplement, for example, another hormone or a vitamin or mineral preparation, to one group.
We excluded interventions involving exercise, which appears to interact with hormonal contraceptives to affect bone health.
Types of outcome measures
Primary outcomes
The primary outcome was fractures occurring after baseline, particularly fractures of the spine, hip, and wrist.
Secondary outcomes
Bone mineral density, which could have been measured, e.g., at the femur, lumbar spine or whole body;
-
Biochemical markers of bone turnover (Vasikaran 2011a; Vasikaran 2011b), e.g.,
bone formation ‐ serum osteocalcin, alkaline phosphatase, and type I procollagen;
bone resorption ‐ serum calcium and C‐telopeptide; urinary pyridinoline and N‐telopeptides.
Search methods for identification of studies
Electronic searches
Through April 2014, we searched the computerized databases MEDLINE, POPLINE, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, and LILACS for studies of fracture or bone health and hormonal contraceptives. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The strategies are given in Appendix 1. Previous search strategies can be found in Appendix 2.
Searching other resources
We examined reference lists of relevant articles for other trials. For the initial review, we wrote to known investigators for information about other published or unpublished trials not discovered in our search.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches with no language limitation.
Data extraction and management
Two authors independently abstracted the data. Data were entered into RevMan, and a second author verified accuracy. Any discrepancies were resolved by discussion.
Assessment of risk of bias in included studies
Studies were examined for methodological quality, according to the principles recommended in Higgins 2011. Factors considered were study design, method for generating the randomization sequence, allocation concealment, blinding, and losses to follow up and early discontinuation. We also examined the methods used for assessing the outcomes.
Assessment of heterogeneity
None of the trials examined the same types of interventions. Therefore, we did not combine any trials in a meta‐analysis.
Data synthesis
For continuous variables, the mean difference (MD) was computed with 95% confidence interval (CI) using a fixed‐effect model. RevMan uses the inverse variance approach. For dichotomous outcomes, the Mantel‐Haenszel odds ratio (OR) with 95% CI was calculated using a fixed‐effect model.
We applied principles from GRADE to assess the evidence quality and address confidence in the effect estimates (Balshem 2011). When a meta‐analysis is not viable due to varied interventions, a 'Summary of findings' table is not feasible. Therefore, we did not conduct a formal GRADE assessment with an evidence profile and 'Summary of findings' table (Guyatt 2011).
For the 2011 update, we added an assessment of evidence quality using the GRADE approach (Higgins 2011). This assessment was based on the quality of evidence from the individual studies. In 2014, we refined the criteria used, based on our subsequent experience with other reviews. Evidence quality could be high, moderate, low, or very low. We considered the evidence from RCTs to be high quality initially, then downgraded for each of the following: a) randomization sequence generation and allocation concealment: no information on either, or one was inadequate; b) lack of blinding; c) follow up was 12 months or less for BMD measures only; d) losses were greater than 20% for the primary analysis.
Sensitivity analysis
In 2014, we added a sensitivity analysis. This included trials that provided sufficient data and evidence of moderate or high quality.
Results
Description of studies
Results of the search
The 2014 search produced 54 unduplicated citations from the main databases. In addition, we found seven unduplicated trials through ClinicalTrials.gov and ICTRP. Three new trials were added, including one that had been 'ongoing' in the previous update (Cibula 2012; Gai 2012; Sordal 2012). In addition new ongoing trial was added (Bonny 2013). An earlier ongoing trial is still awaiting classification due to lack of a report (Teva 2013).
We identified 19 randomized controlled trials that met the criteria for inclusion. Bone density was measured in 17 trials; the other two assessed biochemical markers of bone turnover (Paoletti 2000; Rad 2011). Twelve studies assessed BMD as well as biochemical markers. None had fracture as an outcome. Of the 17 trials that examined BMD, 14 measured the lumbar spine using dual‐energy X‐ray absorptiometry (DEXA), although the measurement site varied somewhat. The other three studies with BMD used computed tomography for the lumbar spine (Endrikat 2004), DEXA for the arm (Bahamondes 2006), and single photon absorptiometry for the arm (Naessen 1995).
Included studies
Most of the studies were 12 to 24 months in duration and two were 36 months long (Endrikat 2004; Kaunitz 2009). One trial was limited to six months (Naessen 1995). A crossover trial had the participants switch COCs at 9 months for a total duration of 18 months (Cibula 2012). Three studies focused on adolescents (Cibula 2012; Cromer 2005; Gai 2012).
The types and formulations of hormonal contraceptives varied. Eleven trials compared different COCs or regimens of COCs:
Two studied desogestrel‐containing COCs (Berenson 2001; Gai 2012).
Four examined levonorgestrel preparations as the investigational drug or the comparator (Endrikat 2004; Hartard 2006; Rad 2011; Sordal 2012).
Three examined gestodene preparations (Paoletti 2000; Nappi 2003; Cibula 2012).
Two examined drospirenone‐containing COCs (Nappi 2005; Gargano 2008).
In addition, five trials examined an injectable versus another injectable, implant, or IUD (Naessen 1995; Von Kesseru 2000; Cromer 2005; Cundy 2003; Kaunitz 2009), two compared two implants each (Di 1999; Bahamondes 2006), and one studied the transdermal patch versus the vaginal ring (Massaro 2010).
Risk of bias in included studies
Allocation
Study design and reporting varied in quality across these trials.
Randomization information was as follows:
Two trials had interactive voice‐response systems, based on computer‐generated random lists (Kaunitz 2009; Rad 2011).
Most used a random numbers table or a computer for random‐number sequence generation (Bahamondes 2006; Berenson 2001; Cibula 2012; Cromer 2005; Cundy 2003; Massaro 2010; Naessen 1995; Nappi 2003; Nappi 2005; Paoletti 2000; Von Kesseru 2000). Cromer 2005 mentioned block randomization techniques but did not specify the block size.
Gai 2012 reported randomized by 'drawing lots' without further explanation.
Five studies did not provide the method for sequence generation (Di 1999; Endrikat 2004; Gargano 2008; Hartard 2006; Sordal 2012).
Allocation concealment was unclear in many studies and not mentioned in others. As noted above, two had an interactive voice‐response system (Kaunitz 2009; Rad 2011). Bahamondes 2006 reported having sealed envelopes prepared at the WHO, and Cromer 2005 communicated that they used serially‐numbered opaque envelopes. Naessen 1995 used sealed envelopes, and Nappi 2005 reported the sequence was concealed until treatment was assigned. Two trials did not have any concealment and 11 trials had insufficient or no information.
Blinding
Some blinding was used in six trials: double‐blind (Cromer 2005; Cundy 2003; Endrikat 2004); investigators and providers (Kaunitz 2009); laboratory personnel (Massaro 2010); and participants (Berenson 2001).
Six trials were open‐label (Hartard 2006; Nappi 2005; Paoletti 2000; Rad 2011; Sordal 2012; Von Kesseru 2000).
Information on blinding was not available from seven studies (Bahamondes 2006; Cibula 2012; Di 1999; Gai 2012; Gargano 2008; Naessen 1995; Nappi 2003).
Incomplete outcome data
Losses were high in several trials, but largely due to method discontinuation or missing data. Losses greater than 20% threaten trial validity (Strauss 2005).
In Von Kesseru 2000, loss of participants or missing data for BMD at 12 months was 48% and 79% in the two intervention groups. At 24 months, the figures were 70% and 84%.
Berenson 2001 losses were attributed to discontinuation or failure to obtain a bone scan within the required window: at 12 months, 62% and 68% for the two intervention groups; at 24 months, 71% and 54%.
In Endrikat 2004, loss was 52% at 36 months with 61% loss for bone data.
Cundy 2003 had a 29% loss due to early discontinuation.
In Cromer 2005, 24% withdrew by 12 months and 43% withdrew by 24 months. This does not include those without assessments due to early study closure.
Three trials had high overall losses: Kaunitz 2009 (39%); Rad 2011 (29%); Sordal 2012 (41%); losses to follow up were under 20%.
Effects of interventions
Progestin‐only methods
Six trials examined methods containing only the hormone progestin, including two trials of implants and four that examined the studied DMPA 150 mg.
Implants
Di 1999 examined the six‐capsule Norplant versus a similar domestic implant (manufactured in China). BMD at Ward's triangle was higher among Norplant users than domestic implant users at 12 months (mean difference (MD) 0.07; 95% CI 0.00 to 0.14, Analysis 1.4). Both types of implants had six capsules with the same amount of levonorgestrel. The groups did not differ significantly for BMD at the other locations, nor for serum and urinary measures.
In Bahamondes 2006, the implants studied were a single‐rod etonogestrel‐releasing implant and a two silicone rod levonorgestrel‐releasing implant. By 18 months, the etonogestrel‐implant group had a greater percent decrease in BMD at the midshaft ulna than the two‐rod levonorgestrel group (MD ‐0.39; 95% CI ‐0.56 to ‐0.22, Analysis 2.2) and at the distal radius (MD ‐1.00; 95% CI ‐1.09 to ‐0.91, Analysis 2.4). A secondary paper reported on BMD at 36 months, but the losses to follow up by that time were large and the groups were not significantly different for BMD at the distal radius.
1.4. Analysis.
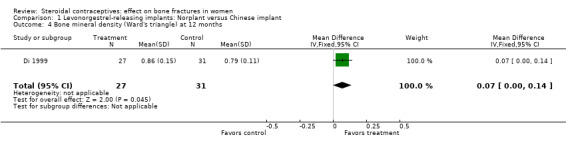
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 4 Bone mineral density (Ward's triangle) at 12 months.
2.2. Analysis.
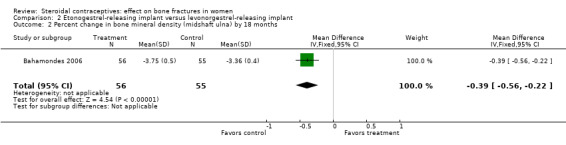
Comparison 2 Etonogestrel‐releasing implant versus levonorgestrel‐releasing implant, Outcome 2 Percent change in bone mineral density (midshaft ulna) by 18 months.
2.4. Analysis.
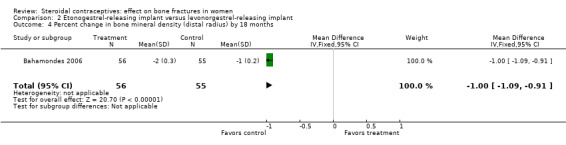
Comparison 2 Etonogestrel‐releasing implant versus levonorgestrel‐releasing implant, Outcome 4 Percent change in bone mineral density (distal radius) by 18 months.
Injectable DMPA 150 mg
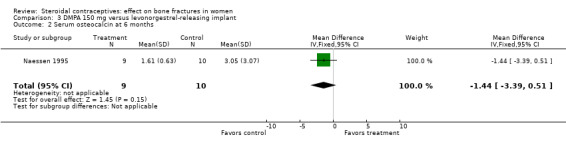
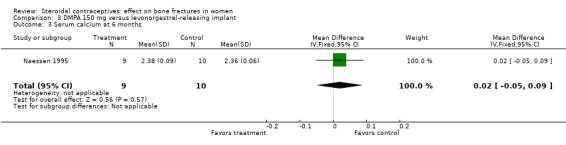
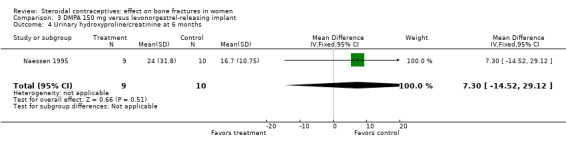
Naessen 1995 randomized women to either DMPA 150 mg every 12 weeks or the levonorgestrel implant (Norplant). The DMPA group had a lower mean for alkaline phosphatase, a marker of bone formation, than the implant group at six months (MD ‐0.65; 95% CI ‐1.21 to ‐0.09, Analysis 3.1). The groups did not differ significantly for serum osteocalcin and calcium and for urinary hydroxyproline/creatinine. BMD data were shown in a figure rather than a table. By six months, BMD at the forearm reportedly increased in the levonorgestrel implant group (reported P = 0.006) and decreased insignificantly in the DMPA group. The group difference was reportedly significant at the proximal (reported P = 0.025) but not the distal forearm.
Two trials examined estrogen supplement versus a placebo for women on DMPA. All participants had an injection of DMPA 150 mg every 12 weeks.
In Cromer 2005, one DMPA group received monthly injections of estradiol cypionate (E2C) 5 mg whereas the other received the placebo supplement of 5 mL normal saline solution. Bone mineral apparent density (BMAD) was used to correct for variation in bone (see Characteristics of included studies). At 12 months, the groups with the estrogen supplement had increases while the placebo‐supplement group had decreases for spine BMD (MD 2.90; 95% CI 1.80 to 4.00, Analysis 4.1), spine BMAD (MD 2.70; 95% CI 1.60 to 3.80, Analysis 4.2), and femoral neck BMD (MD 3.20; 95% CI 1.36 to 5.04, Analysis 4.3). The groups were not significantly different for femoral neck BMAD (Analysis 4.4). At 24 months, the same trend was seen: spine BMD (MD 4.60; 95% CI 2.87 to 6.33, Analysis 4.5), spine BMAD (MD 4.90; 95% CI 3.11 to 6.69, Analysis 4.6), femoral neck BMD (MD 9.80; 95% CI 4.96 to 14.64, Analysis 4.7), and femoral neck BMAD (MD 7.10; 95% CI 0.50 to 13.70, Analysis 4.8). The trial was stopped early due to the differences reaching the predetermined significance level (P < 0.001).
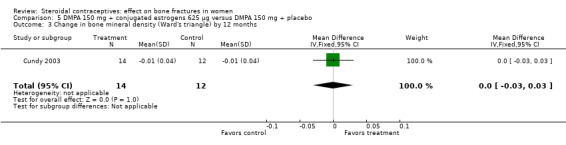
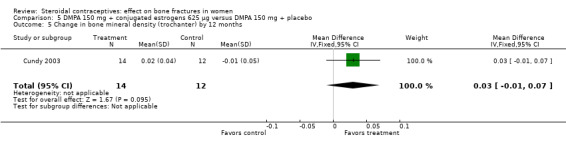
Cundy 2003 randomized DMPA users to daily intake of conjugated estrogens 62.5 μg or to a placebo supplement. BMD was measured at the lumbar spine, femoral neck, Ward's triangle, trochanter, and total body. For lumbar spine BMD, the group with the estrogen supplement had a small increase and the placebo‐supplement group had a small decrease by 12 months (MD 0.02; 95% CI 0.00 to 0.04, Analysis 5.1) and by 24 months (MD 0.04; 95% CI 0.02 to 0.06, Analysis 5.2). More than a fourth of the participants discontinued early. No significant changes were reportedly apparent in plasma calcium, phosphate, or alkaline phosphatase activity or in urinary N‐telopeptides/creatinine.
In Kaunitz 2009, intramuscular DMPA 150 mg/mL (DMPA‐IM) was compared with subcutaneous DMPA 104 mg/0.65 mL (DMPA‐SC). The groups did not differ significantly in the proportions with a 5% or greater decrease in total hip BMD at one, two, or three years. For lumbar spine BMD, more of the DMPA‐SC group had a 5% or greater decrease by year 3 (OR 2.11; 95% CI 1.00 to 4.45, Analysis 6.6). Losses due to discontinuation were high.
3.1. Analysis.
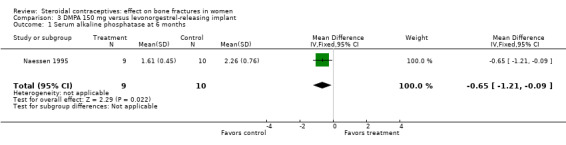
Comparison 3 DMPA 150 mg versus levonorgestrel‐releasing implant, Outcome 1 Serum alkaline phosphatase at 6 months.
4.1. Analysis.
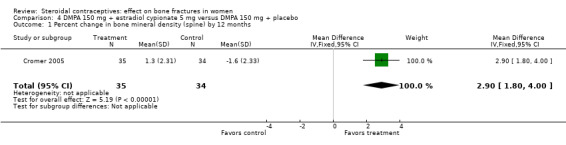
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 1 Percent change in bone mineral density (spine) by 12 months.
4.2. Analysis.
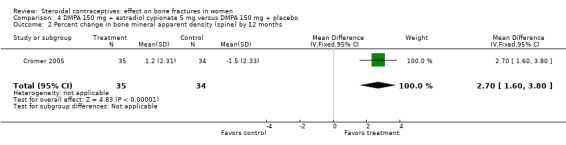
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 2 Percent change in bone mineral apparent density (spine) by 12 months.
4.3. Analysis.
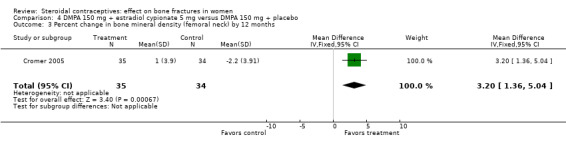
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 3 Percent change in bone mineral density (femoral neck) by 12 months.
4.4. Analysis.
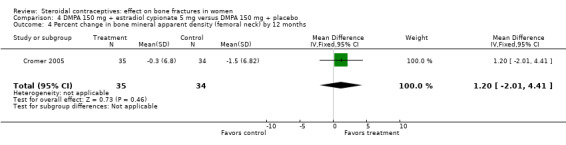
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 4 Percent change in bone mineral apparent density (femoral neck) by 12 months.
4.5. Analysis.
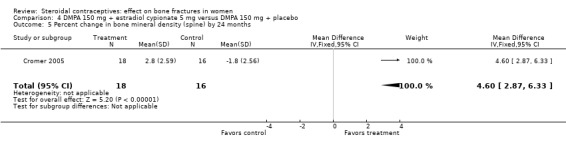
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 5 Percent change in bone mineral density (spine) by 24 months.
4.6. Analysis.
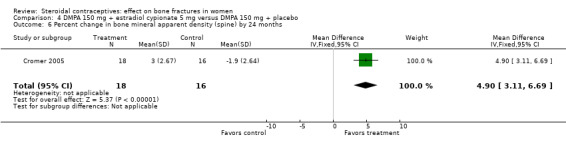
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 6 Percent change in bone mineral apparent density (spine) by 24 months.
4.7. Analysis.
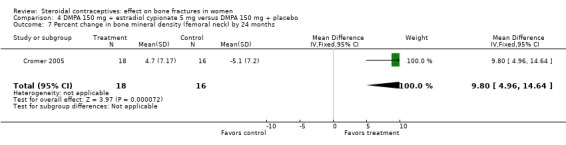
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 7 Percent change in bone mineral density (femoral neck) by 24 months.
4.8. Analysis.
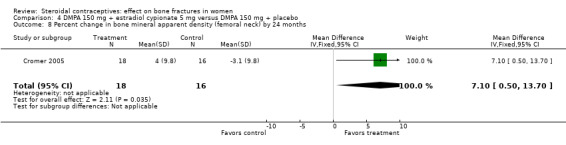
Comparison 4 DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo, Outcome 8 Percent change in bone mineral apparent density (femoral neck) by 24 months.
5.1. Analysis.
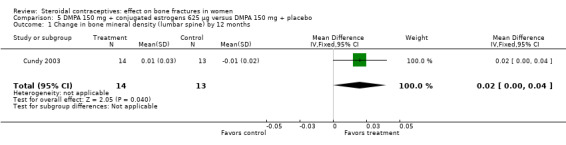
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 1 Change in bone mineral density (lumbar spine) by 12 months.
5.2. Analysis.
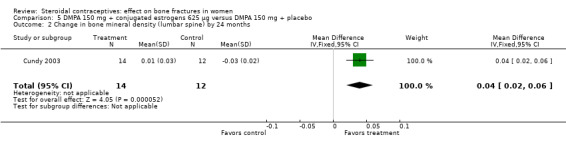
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 2 Change in bone mineral density (lumbar spine) by 24 months.
6.6. Analysis.
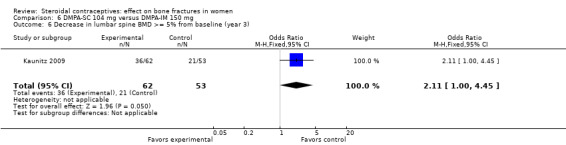
Comparison 6 DMPA‐SC 104 mg versus DMPA‐IM 150 mg, Outcome 6 Decrease in lumbar spine BMD >= 5% from baseline (year 3).
Combination contraceptives
These 13 trials included 11 that compared combined oral contraceptives, as well as one of a combination injectable versus a non‐hormonal IUD and one of the transdermal patch versus the vaginal ring.
Oral contraceptives
Two compared desogestrel‐containing COCs versus other COCs:
Berenson 2001 randomized women to norethindrone 1 mg plus ethinyl estradiol (EE) 35 μg or to desogestrel 150 μg plus EE 30 μg. The norethindrone group had a significantly greater increase in BMD at the lumbar spine at 12 months than the desogestrel group (MD 1.83; 95% CI 0.42 to 3.24, Analysis 7.1). By 24 months, both groups had decreases from baseline but they were not significantly different. However, only about one‐third of the original participants remained at 12 months.
Gai 2012 also used desogestrel 150 μg plus EE 30 μg, but the comparison was cyproterone acetate (CPA) 2 mg plus EE 35 μg. The group with the desogestrel‐containing COC did not differ significantly from the CPA group for the BMD measures of lumbar spine or femoral neck at 12 or 24 months (Analysis 8.1 to Analysis 8.4).
7.1. Analysis.
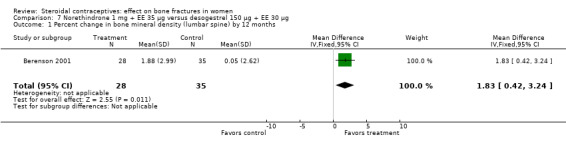
Comparison 7 Norethindrone 1 mg + EE 35 µg versus desogestrel 150 µg + EE 30 µg, Outcome 1 Percent change in bone mineral density (lumbar spine) by 12 months.
8.1. Analysis.
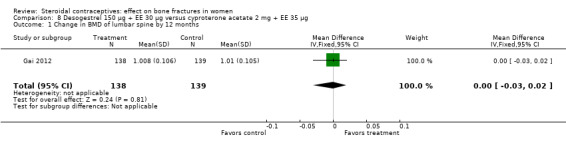
Comparison 8 Desogestrel 150 µg + EE 30 µg versus cyproterone acetate 2 mg + EE 35 µg, Outcome 1 Change in BMD of lumbar spine by 12 months.
8.4. Analysis.
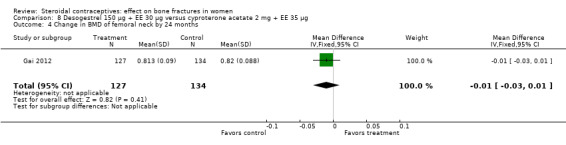
Comparison 8 Desogestrel 150 µg + EE 30 µg versus cyproterone acetate 2 mg + EE 35 µg, Outcome 4 Change in BMD of femoral neck by 24 months.
Four trials used preparations containing levonorgestrel, either as the investigational drug or the comparator:
Endrikat 2004 compared levonorgestrel 100 μg plus 20 EE μg versus levonorgestrel 150 μg plus EE 30 μg. The two groups did not differ significantly in their slight decreases in BMD at 36 months (Analysis 9.1 to Analysis 9.4). Serum alkaline phosphatase increased and N‐telopeptides decreased, but change did not differ significantly between the groups. More than half of the participants were lost to follow up.
Hartard 2006 examined levonorgestrel 100 μg plus 20 EE μg versus desogestrel 150 μg plus EE 20 μg. By 12 months, the desogestrel group lost more areal BMD at the lumbar spine than the levonorgestrel group, but the difference was small (MD 1.41; 95% CI ‐0.11 to 2.93, Analysis 10.1). The desogestrel group had a greater decrease in serum alkaline phosphatase (MD 15.31; 95% CI 3.91 to 26.71, Analysis 10.3). The groups did not differ significantly in change in areal BMD at the femoral neck or in serum osteocalcin or C‐telopeptides (Analysis 10.2; Analysis 10.4; Analysis 10.5).
Rad 2011 compared a continuous regimen of levonorgestrel 90 μg plus EE 20 μg versus a cyclic regimen of levonorgestrel 100 μg plus EE 20 μg. The report provided standard errors and did not contain cell sizes, so we could not analyze any data. Reportedly, changes in osteocalcin and C‐telopeptides were not significantly different between the groups by cycle 13.
For Sordal 2012, the COC of interest was nomegestrol 2.5 mg plus [17ß] estradiol 1.5 mg (NOMAC‐E2), and the comparison was levonorgestrel 150 μg plus EE 30 μg. By cycle 26, the groups did not differ significantly for change in z‐score of the lumbar spine (Analysis 11.1) or femoral neck (Analysis 11.2).
9.1. Analysis.
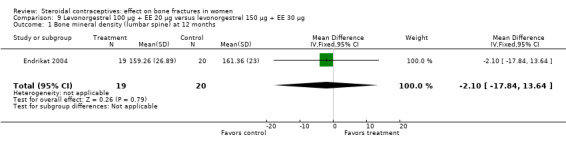
Comparison 9 Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 Bone mineral density (lumbar spine) at 12 months.
9.4. Analysis.
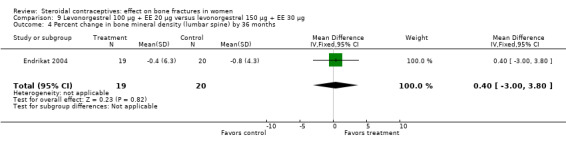
Comparison 9 Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 4 Percent change in bone mineral density (lumbar spine) by 36 months.
10.1. Analysis.
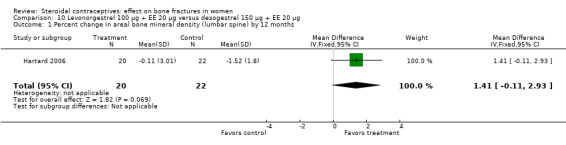
Comparison 10 Levonorgestrel 100 µg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 1 Percent change in areal bone mineral density (lumbar spine) by 12 months.
10.3. Analysis.
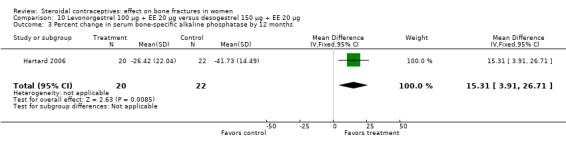
Comparison 10 Levonorgestrel 100 µg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 3 Percent change in serum bone‐specific alkaline phosphatase by 12 months.
10.2. Analysis.
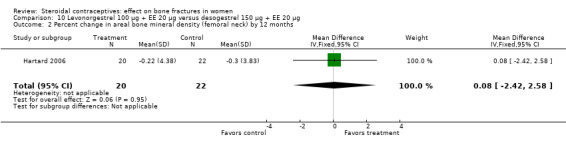
Comparison 10 Levonorgestrel 100 µg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 2 Percent change in areal bone mineral density (femoral neck) by 12 months.
10.4. Analysis.
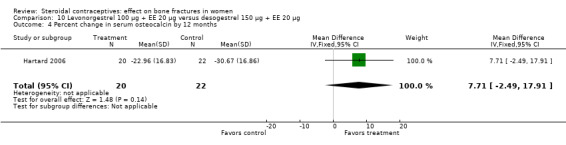
Comparison 10 Levonorgestrel 100 µg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 4 Percent change in serum osteocalcin by 12 months.
10.5. Analysis.
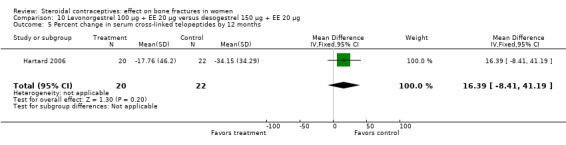
Comparison 10 Levonorgestrel 100 µg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 5 Percent change in serum cross‐linked telopeptides by 12 months.
11.1. Analysis.
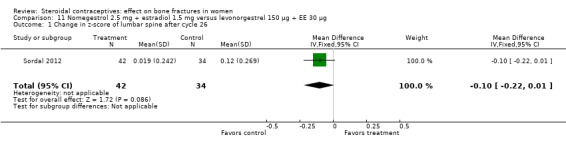
Comparison 11 Nomegestrol 2.5 mg + estradiol 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 Change in z‐score of lumbar spine after cycle 26.
11.2. Analysis.
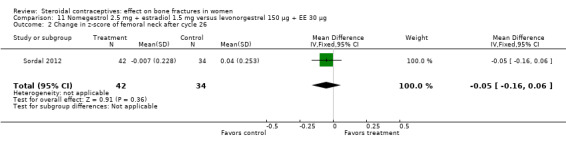
Comparison 11 Nomegestrol 2.5 mg + estradiol 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 2 Change in z‐score of femoral neck after cycle 26.
Gestodene‐containing COCs were the focus of three trials:
Paoletti 2000 randomized women to gestodene 75 μg plus EE 20 μg or gestodene 75 μg plus EE 30 μg. At 12 months, urinary deoxypyridinoline was lower in the EE 30 μg group than in the EE 20 μg group (MD 1.20; 95% CI 0.37 to 2.03, Analysis 12.2). The study groups were not significantly different for serum osteocalcin and urinary pyridinoline.
Nappi 2003 studied gestodene 75 μg plus EE 20 μg versus gestodene 60 μg plus EE 15 μg. The results were presented in figures without absolute values. The investigators reported no significant difference at 12 months between or within the groups in BMD at the lumbar spine or in serum osteocalcin. The study groups reportedly had significant declines in urinary pyridinoline and deoxypyridinoline by 6 and 12 months (reported P < 0.05), but the groups did not differ significantly.
The crossover study of Cibula 2012 compared gestodene 75 μg plus EE 30 μg versus gestodene 60 μg plus EE 15 μg. The participants were switched to the other formulation at nine months; study duration was 18 months. Measures included BMD of the lumbar spine, femur, and distal radius, as well as serum type I procollagen (PINP) and type I collagen cross‐linked C‐telopeptide (ßCTX1). The report included results of the full analysis of variance model (ANOVA) for lumbar BMD, PINP, and ßCTX1 (Table 1). Reportedly, dose was significantly associated with change in lumbar BMD (reported F‐ratio = 4.6; reported P value = 0.037). The COC containing EE 30 μg showed an increase while the COC containing EE 15 μg showed a decrease. Dose was also reportedly associated with a difference in PINP (reported F‐ratio = 8.3; reported P value = 0.005), but the text and figure were inconsistent regarding the direction of change. For ßCTX1, no significant difference was reported.
12.2. Analysis.
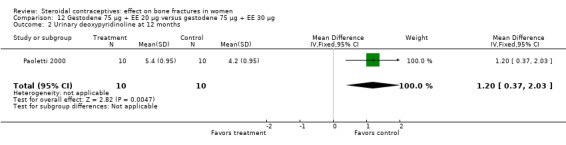
Comparison 12 Gestodene 75 µg + EE 20 µg versus gestodene 75 µg + EE 30 µg, Outcome 2 Urinary deoxypyridinoline at 12 months.
3. Outcomes by 18 months (Cibula 2012).
| Outcome at 18 months (crossover at 9 months)1 | Variable2 | Reported F‐ratio | Reported P value |
| Change in lumbar spine BMD (g/cm2) | dose | 4.6 | 0.037 |
| Change in propeptide of type I procollagen (μg/l) | dose | 8.3 | 0.005 |
| Change in cross‐linked telopeptide (μg/l) | dose | 0.7 | 0.397 |
1From full ANOVA model for crossover design; reportedly adjusted for intraindividual variability, sequence of treatment, period of trial, initial value of outcome measure, age, and smoking. Report did not mention ANOVA for the other BMD measures. 2Gestodene 75 μg plus EE 30 μg versus gestodene 60 μg plus EE 15 μg; participants were switched to the other formulation at 9 months
Drospirenone‐containing COCs were examined in two trials:
Nappi 2005 examined drospirenone 3 mg plus EE 30 μg versus gestodene 75 μg plus EE 30 μg. Bone mineral density at the lumbar spine did not differ significantly between the two groups at 12 months (Analysis 13.1). Data for biochemical markers were presented in figures without absolute numbers. Reportedly, the groups did not differ significantly for urinary pyridinoline or deoxypyridinoline but both groups decreased significantly. Serum calcium reportedly increased significantly in the drospirenone‐COC group and was significantly different from that gestodene‐COC group. Reportedly, other changes in serum and urinary calcium were not significant. The investigators also reported that serum osteocalcin did not change significantly; within‐group changes were not mentioned.
Gargano 2008 compared drospirenone 3 mg plus EE 30 μg versus drospirenone 3 mg plus EE 20 μg. BMD at the lumbar spine did not differ significantly between the two groups at 12 months (Analysis 14.1). Biochemical measures were shown in figures without absolute numbers. The investigators reported the study groups were not significantly different at 12 months for urinary pyridinoline and deoxypyridinoline but that both groups decreased significantly. They also reported that no significant difference between groups for serum or urinary calcium or for serum osteocalcin. Reportedly, both groups had significant increases in serum calcium but changes within group were not significant for the other measures.
13.1. Analysis.
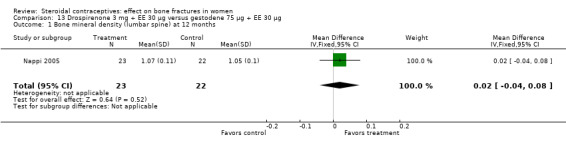
Comparison 13 Drospirenone 3 mg + EE 30 µg versus gestodene 75 µg + EE 30 µg, Outcome 1 Bone mineral density (lumbar spine) at 12 months.
14.1. Analysis.
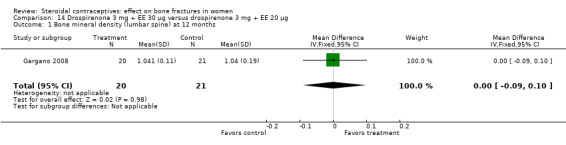
Comparison 14 Drospirenone 3 mg + EE 30 µg versus drospirenone 3 mg + EE 20 µg, Outcome 1 Bone mineral density (lumbar spine) at 12 months.
Injectable versus non‐hormonal IUD
Von Kesseru 2000 compared monthly injections of norethisterone enanthate 50 mg plus estradiol valerate (E2V) 5 mg versus the Nova‐T IUD. Bone density did not differ significantly between the injectable group and the IUD group at 24 months (Analysis 16.1, Analysis 16.2). The trial focused on serum lipid patterns; bone density was of secondary interest. Only half the women were assigned to have bone density measures, and many did not have outcome data. Changes in BMD were modest but positive. However, the analyzed groups were so small due to high losses that the results may not be meaningful.
16.1. Analysis.
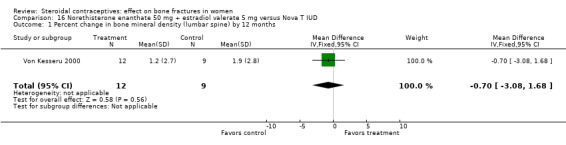
Comparison 16 Norethisterone enanthate 50 mg + estradiol valerate 5 mg versus Nova T IUD, Outcome 1 Percent change in bone mineral density (lumbar spine) by 12 months.
16.2. Analysis.
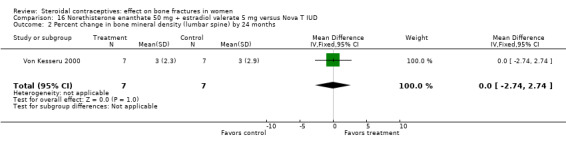
Comparison 16 Norethisterone enanthate 50 mg + estradiol valerate 5 mg versus Nova T IUD, Outcome 2 Percent change in bone mineral density (lumbar spine) by 24 months.
Patch versus ring
Massaro 2010 compared the contraceptive patch delivering norelgestromin 150 μg plus EE 20 μg daily versus the vaginal ring releasing etonogestrel 120 μg plus EE 15 μg daily. At 12 months, the study groups did not differ significantly in spinal BMD, urinary pyridinoline, urinary deoxypyridinoline, and serum osteocalcin (Analysis 15.1 to Analysis 15.4). Spinal BMD did not change much from baseline, while the biochemical markers generally had changes that were positive for bone health.
15.1. Analysis.
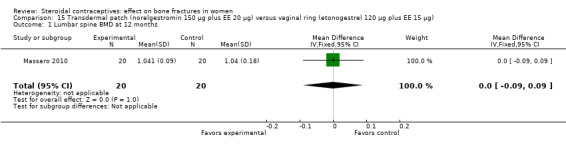
Comparison 15 Transdermal patch (norelgestromin 150 µg plus EE 20 µg) versus vaginal ring (etonogestrel 120 µg plus EE 15 µg), Outcome 1 Lumbar spine BMD at 12 months.
15.4. Analysis.
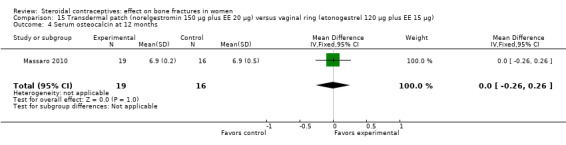
Comparison 15 Transdermal patch (norelgestromin 150 µg plus EE 20 µg) versus vaginal ring (etonogestrel 120 µg plus EE 15 µg), Outcome 4 Serum osteocalcin at 12 months.
Discussion
Summary of main results
We summarized the results by contraceptive method and composition (Table 2). Two studies of DMPA plus a supplement were placebo‐controlled, and one study compared a combination injectable to a non‐hormonal IUD. Since the estrogen preparations and routes of administration differed for the DMPA trials, we did not conduct a meta‐analysis. Nonetheless, the two trials showed BMD increases for the women who received DMPA plus estrogen supplement and decreases for those who had DMPA plus placebo supplement. In the combination injectable study, BMD changes were modest but the losses were too high for results to be informative.
4. Summary of outcome data.
| Study | Comparison groups | Bone mineral density: # differences/ # measures1 | Biochemical markers: # differences/ # measures |
| Progestin‐only contraceptives | |||
| Implants | |||
| Di 1999 | levonorgestrel 6‐rod (standard) vs levonorgestrel 6‐rod (domestic) |
1/4 | 0/4 |
| Bahamondes 2006 | etonogestrel 1‐rod vs levonorgestrel 2‐rod |
2/5 | ‐‐‐ |
| Injectable DMPA | |||
| Naessen 1995 | DMPA vs levonorgestrel 6‐rod implant |
insufficient data | 1/4 |
| Cromer 2005 | DMPA + E2C vs DMPA + placebo |
7/8 | ‐‐‐ |
| Cundy 2003 | DMPA + conjugated estrogens 62.5 μg vs DMPA + placebo |
2/10 | ‐‐‐ |
| Kaunitz 2009 | DMPA subcutaneous vs DMPA intramuscular |
1/6 | ‐‐‐ |
| Combination contraceptives | |||
| Oral contraceptives | |||
| Berenson 2001 | norethindrone 1 mg + EE 35 μg vs desogestrel 150 μg + EE 30 μg |
1/2 | ‐‐‐ |
| Gai 2012 | desogestrel 150 μg + EE 30 μg vs cyproterone acetate 2 mg + EE 35 μg |
0/4 | ‐‐‐ |
| Endrikat 2004 | levonorgestrel 100 μg + EE 20 μg vs levonorgestrel 150 μg + EE 30 μg |
0/4 | 0/2 |
| Hartard 2006 | levonorgestrel 100 μg + EE 20 μg vs desogestrel 150 μg + EE 20 μg |
0/2 | 1/3 |
| Rad 2011 | levonorgestrel 90 μg + EE 20 μg (continuous) vs levonorgestrel 100 μg + EE 20 μg (cyclic) |
‐‐‐ | insufficient data |
| Sordal 2012 | nomegestrol 2.5 mg + 17ß estradiol 1.5 mg vs levonorgestrel 150 μg + EE 30 μg |
0/2 | ‐‐‐ |
| Paoletti 2000 | gestodene 75 μg + EE 20 μg vs gestodene 75 μg + EE 30 μg |
‐‐‐ | 1/3 |
| Nappi 2003 | gestodene 75 μg + EE 20 μg vs gestodene 60 μg + EE 15 μg |
insufficient data | insufficient data |
| Cibula 2012 | gestodene 75 μg + EE 30 μg vs gestodene 60 μg + EE 15 μg |
1/1 | insufficient data |
| Nappi 2005 | drospirenone 3 mg + EE 30 μg vs gestodene 75 μg + EE 30 μg |
0/1 | insufficient data |
| Gargano 2008 | drospirenone 3 mg + EE 30 vs drospirenone 3 mg + EE 20 |
0/1 | insufficient data |
| Injectable | |||
| Von Kesseru 2000 | norethisterone enanthate + E2V vs Nova‐T IUD |
0/2 | ‐‐‐ |
| Patch versus ring | |||
| Massaro 2010 | transdermal patch vs vaginal ring |
0/1 | 0/3 |
1Number of significant differences between study group divided by number of outcome measures (e.g., lumbar spine, femoral neck).
Most trials compared two different hormonal contraceptives. Combination oral contraceptives did not appear to negatively affect bone density, and some formulations had more positive effects than others. However, none were placebo‐controlled. Where trials showed differences between groups in biochemical markers of bone formation, the results were generally consistent with those for bone mineral density. For the progestin‐only implants, two trials studied different implants, used different sites for measuring BMD, and had varying durations. One study showed a greater decrease in BMD for the etonogestrel‐implant group than the two‐rod levonorgestrel group.
Sensitivity analysis
We assessed the quality of the evidence, as discussed below (Table 19). Our sensitivity analysis had 11 trials that provided sufficient data for the outcome and evidence of moderate or high quality (Table 20). These included the four DMPA studies, the two implant trials, the combination injectable and patch versus study, but only 3 of the 11 COC trials. The results were similar to those for the review overall. Of the four DMPA‐IM trials, two showed a positive effect of an estrogen supplement on BMD, one had a negative effect of DMPA‐SC on lumbar spine BMD, and the fourth indicated a negative effect of DMPA‐IM on a bone formation marker. The two implant studies each had one significant difference in BMD out of several measures. Of the three COC trials, two examined BMD, of which one showed a decrease for the group with gestodene plus EE 15 μg. Of the two that used biochemical measures, one indicated less bone resorption in the group with gestodene plus EE 30 μg versus EE 20 μg. No significant differences were noted in the trials of the combination injectable or the patch versus ring.
1. Evidence quality.
| Study | Comparison groups | Inadequate randomization and allocation concealment | No blinding | Follow up <= 12 months (only BMD) | Losses > 20% | Quality of evidence1 |
| Injectable (versus implant, injectable, or IUD) | ||||||
| Naessen 1995 | DMPA vs LNG 6‐rod implant |
‐‐‐ | unclear | ‐‐‐ | ‐‐‐ | high |
| Cromer 2005 | DMPA + E2C vs DMPA + placebo |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | moderate |
| Cundy 2003 | DMPA + estrogen vs DMPA + placebo |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | moderate |
| Kaunitz 2009 | DMPA‐subcutaneous vs DMPA‐intramuscular |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | moderate |
| Evidence quality from 4 DMPA trials | moderate | |||||
| Implants | ||||||
| Di 1999 | levonorgestrel 6‐rod (standard) vs levonorgestrel 6‐rod (domestic) |
‐1 | unclear | ‐‐‐ | ‐‐‐ | moderate |
| Bahamondes 2006 | etonogestrel 1‐rod vs levonorgestrel 2‐rod |
‐‐‐ | unclear | ‐‐‐ | ‐‐‐ | high |
| Evidence quality from 2 implant trials | moderate to high | |||||
| Combined oral contraceptives | ||||||
| Berenson 2001 | norethindrone 1 mg + EE 35 μg vs desogestrel 150 μg + EE 30 μg |
‐1 | ‐‐‐ | ‐1 | ‐1 | very low |
| Gai 2012 | desogestrel 150 μg + EE 30 μg vs cyproterone acetate 2 mg + EE 35 μg |
‐1 | unclear | ‐‐‐ | ‐‐‐ | moderate |
| Endrikat 2004 | levonorgestrel 100 μg + EE 20 μg vs levonorgestrel 150 μg + EE 30 μg |
‐1 | ‐‐‐ | ‐‐‐ | ‐1 | low |
| Hartard 2006 | levonorgestrel 100 μg + EE 20 μg vs desogestrel 150 μg + EE 20 μg |
‐1 | ‐1 | ‐‐‐ | ‐‐‐ | low |
| Rad 2011 | levonorgestrel 90 μg + EE 20 μg (continuous) vs levonorgestrel 100 μg + EE 20 μg (cyclic) |
‐‐‐ | ‐1 | ‐‐‐ | ‐1 | low |
| Sordal 2012 | nomegestrol 2.5 mg + 17ß estradiol 1.5 mg vs levonorgestrel 150 μg + EE 30 μg |
‐1 | ‐1 | ‐‐‐ | ‐1 | very low |
| Paoletti 2000 | gestodene 75 μg + EE 20 μg vs gestodene 75 μg + EE 30 μg |
‐‐‐ | ‐1 | ‐‐‐ | ‐‐‐ | moderate |
| Nappi 2003 | gestodene 75 μg + EE 20 μg vs gestodene 60 μg + EE 15 μg |
‐‐‐ | unclear | ‐‐‐ | ‐‐‐ | high |
| Cibula 2012 | gestodene 75 μg + EE 30 μg vs gestodene 60 μg + EE 15 μg |
‐‐‐ | unclear | ‐‐‐ | ‐‐‐ | high |
| Nappi 2005 | drospirenone 3 mg + EE 30 μg vs gestodene 75 μg + EE 30 μg |
‐‐‐ | ‐1 | ‐1 | ‐‐‐ | low |
| Gargano 2008 | drospirenone 3 mg + EE 30 vs drospirenone 3 mg + EE 20 |
‐1 | unclear | ‐1 | ‐‐‐ | low |
| Evidence quality from 11 COC studies | low | |||||
| Injectable or patch versus ring | ||||||
| Von Kesseru 2000 | norethisterone enanthate + E2V vs Nova‐T IUD |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | moderate |
| Massaro 2010 | transdermal patch vs vaginal ring |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | high |
| Evidence quality from 2 trials of injectable or patch versus ring | moderate to high | |||||
| Overall evidence quality from 19 trials | moderate | |||||
1Grade levels were high, moderate, low, or very low. RCTs were downgraded one level for each of the following: a) randomization sequence generation and allocation concealment: no information on either, or one was inadequate; b) no blinding; c) follow up <= 12 months for BMD only; d) losses >= 20% for primary analysis.
2. Sensitivity analysis.
| Study1 | Comparison groups | Fracture | Bone mineral density2 | Biochemical markers2 |
| Injectable DMPA | ||||
| Naessen 1995 | DMPA vs levonorgestrel 6‐rod implant |
‐‐‐ | ‐‐‐ | 1/4 |
| Cromer 2005 | DMPA + E2C vs DMPA + placebo supplement |
‐‐‐ | 7/8 | ‐‐‐ |
| Cundy 2003 | DMPA + estrogen vs DMPA + placebo supplement |
‐‐‐ | 2/10 | ‐‐‐ |
| Kaunitz 2009 | DMPA subcutaneous vs DMPA intramuscular |
‐‐‐ | 1/6 | ‐‐‐ |
| Implants | ||||
| Di 1999 | levonorgestrel 6‐rod (standard) vs levonorgestrel 6‐rod (domestic) |
‐‐‐ | 1/4 | 0/4 |
| Bahamondes 2006 | etonogestrel 1‐rod vs levonorgestrel 2‐rod |
‐‐‐ | 2/5 | ‐‐‐ |
| Combined oral contraceptives | ||||
| Gai 2012 | desogestrel 150 μg + EE 30 μg vs cyproterone acetate 2 mg + EE 35 μg |
‐‐‐ | 0/4 | ‐‐‐ |
| Paoletti 2000 | gestodene 75 μg + EE 20 μg vs gestodene 75 μg + EE 30 μg |
‐‐‐ | ‐‐‐ | 1/3 |
| Cibula 2012 | gestodene 75 μg + EE 30 μg vs gestodene 60 μg + EE 15 μg |
‐‐‐ | 1/1 | ‐‐‐ |
| Combination injectable or patch versus ring | ||||
| Von Kesseru 2000 | norethisterone enanthate + E2V vs Nova‐T IUD |
‐‐‐ | 0/2 | ‐‐‐ |
| Massaro 2010 | transdermal patch vs vaginal ring |
‐‐‐ | 0/1 | 0/3 |
Overall completeness and applicability of evidence
None of the studies included fracture as an outcome. Longer follow up would be needed for meaningful assessment of fracture. Since fragility fractures are rare in young people, fracture is not usually an outcome in studies of premenopausal bone health (Gourlay 2004). Researchers and clinicians may have to rely on bone mineral density and biochemical measures of bone health. BMD correlates with fracture but is not a valid surrogate endpoint for fracture (Grimes 2010). BMD is considered useful in screening for osteoporosis in postmenopausal women. However, its validity in assessing risk is still unclear for premenopausal women, including those using steroidal contraceptives (Nappi 2012). International organizations have recommended using bone turnover markers rather than BMD for monitoring treatment of osteoporosis (Vasikaran 2011a). In this review, markers of bone formation included serum alkaline phosphatase, osteocalcin, and type I procollagen; bone resorption indicators included serum calcium and C‐telopeptide as well as urinary pyridinoline and N‐telopeptides. Recommended reference markers are serum procollagen type I N propeptide, s‐PINP (bone formation); and serum C‐terminal cross‐linking telopeptide of type I collagen, s‐CTX (bone resorption) (Vasikaran 2011a; Vasikaran 2011b). Decreases in these markers correlate better than others with reduction in fracture risk. However, the relationship of hormonal contraceptive use and bone health is less well understood (Herrmann 2010).
Methodological differences limited the analysis and interpretation of the data. Due to the differing interventions studied, no trials were combined for meta‐analysis. Studies compared COCs with different formulations, two types of implants, injectables with other methods, and the skin patch with the vaginal ring. Such differences in treatments limit the conclusions about any one contraceptive method.
Of 19 studies, only 3 were limited to adolescents (Cromer 2005; Cibula 2012; Gai 2012); a fourth focused on young women (Hartard 2006). Three of those four trials showed some differences between study groups in BMD or bone turnover markers, compared to 7 of the 15 studies with older women. Adolescents are rapidly acquiring bone mass (Cibula 2012; Nappi 2012). Whether COCs affect the development of peak bone mass is unclear, though COCs with less than 30 μg EE may be a concern (Nappi 2012). DMPA may affect BMD in adolescents, but some studies have indicated a return to baseline after discontinuation (Kaunitz 2011; Nappi 2012). More studies focused on adolescents and younger women would be useful. However, placebo‐controlled trials in contraception are limited for ethical reasons, especially among a population at high risk for unplanned pregnancy.
Quality of the evidence
As noted earlier, we refined our criteria to identify the specific risk of bias issues in 2014 (Assessment of risk of bias in included studies). For this review, the evidence quality was considered moderate overall (Table 19). The DMPA studies provided moderate quality evidence. The quality of the COC evidence varied widely, but was considered low overall. The quality was moderate to high for the studies of implants, combination injectable, and patch versus ring.
Limitations of these studies include incomplete or no description of sequence generation and allocation concealment, lack of blinding, and high losses. Some reports did not have sufficient data for assessment; outcomes were presented in tables without absolute numbers. For losses, we could not always distinguish between losses to follow up or discontinuation. Therefore, we included all losses in our assessment. About half of the trials had high losses, so the results may not represent randomized comparisons. As noted earlier, losses greater than 20% can bias the results and threaten trial validity (Strauss 2005). At least three trials lost more than half the participants and some had differential losses between the comparison groups.
Agreements and disagreements with other studies or reviews
A review of observational studies did not indicate an overall association between OC use and fracture risk, except for some increased risk among specific user subgroups (Lopez 2012). As noted earlier, COCs may have little effect on BMD among healthy adult women (Herrmann 2010; Warholm 2012). BMD in adolescent and young women may be affected by the use of COCs with lower estrogen doses, i.e., 20 μg (Nappi 2012). COC use may have a negative effect on bone turnover markers, although the clinical significance of such change is unclear (Herrmann 2010).
Of progestin‐only methods, DMPA has been associated with decreased bone mineral density (Nappi 2012). However, no published RCT has linked DMPA use with fracture later in life. A review of observational studies had two case‐control studies in the sensitivity analysis that examined DMPA (Lopez 2012). One of the studies reported increased fracture risk for DMPA ever‐use, more than four years of use, and women over 50 years of age. The other noted increased risk for any past DMPA use and for current use of 3 or more prescriptions. A loss of BMD during adolescence may be recovered after discontinuing DMPA (Kaunitz 2011; Nappi 2012). The changes may be transient like those occurring during pregnancy or lactation (ACOG 2008).
Authors' conclusions
Implications for practice.
Whether steroidal contraceptives influence fracture risk cannot be determined from existing information. Combination contraceptives do not appear to negatively affect bone mineral density or bone turnover markers. Of progestin‐only methods, DMPA may alter bone mineral density. Whether DMPA affects fracture risk cannot be determined, as no randomized trial assessed fracture. Health care providers and women should consider the costs and benefits of these effective contraceptives. The advantages of DMPA outweigh concerns about fracture risk for adolescents and for women over 45 years of age (WHO 2009). Injectable contraceptives may be appropriate for women who want long‐term birth control without a daily regimen. Other candidates for progestin‐only contraceptives are women with contraindications to estrogen use.
Implications for research.
Additional trials of estrogen supplementation with progestin‐only contraceptives would provide more evidence regarding any effect on bone health. Many trials had limitations for interpretation, including small numbers of participants and large losses. Stronger evidence is needed to make recommendations for clinical practice. Trials of longer duration could provide information on whether there was any reversal of earlier decreases in BMD. Studies could focus on adolescents, who have not yet reached peak bone mass, and on perimenopausal women, who may be losing bone mass. Results of such trials could help determine if these two groups are at greater risk of adverse outcomes due to the effects of progestin.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2014 | New search has been performed | Search updated. |
| 25 March 2014 | New citation required but conclusions have not changed | Three new trials included (Cibula 2012; Gai 2012; Sordal 2012); one ongoing trial added (Bonny 2013); one study excluded (Berenson 2012). |
| 27 January 2014 | Amended | Criteria revised for assessing evidence quality (Data synthesis; Table 19). Sensitivity analysis added (Table 20). |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 8 June 2011 | New search has been performed | Searches were updated for MEDLINE, POPLINE, and CENTRAL |
| 21 April 2011 | New citation required but conclusions have not changed | Three new trials were included (Kaunitz 2009; Massaro 2010; Rad 2011). Other studies were excluded or added as Ongoing studies. |
| 29 March 2011 | New search has been performed | Searches were updated |
| 18 December 2008 | New citation required but conclusions have not changed | Added: two new trials (Gargano 2008; Hartard 2006), follow‐up data from earlier trial (Bahamondes 2006), and an ongoing trial (Schering‐Plough 2011a). An additional trial was excluded (Teichmann 2009a). |
| 24 November 2008 | New search has been performed | Searches updated in Nov and Dec 2008 |
| 15 April 2008 | Amended | Converted to new review format. |
| 28 June 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
From FHI 360, Carol Manion assisted with the literature searches and Florence Carayon helped check data extraction for the 2014 update.
The authors sincerely thank the investigators who responded to requests for design information and data.
Appendices
Appendix 1. Search 2014
MEDLINE via PubMed (01 Jan 2011 to 07 May 2014)
(contraceptive agents, female OR ((steroid OR steroids OR steroidal) AND contracept*) OR ortho evra OR "ortho evra" OR "norelgestromin" OR (contraceptive devices, female and ring) OR NuvaRing OR cyclofem OR lunell* OR mesigyna OR cycloprovera OR (medroxyprogesterone 17‐acetate AND (contracept* OR inject* OR depo OR depot)) OR depot medroxyprogesterone OR depo medroxyprogesterone OR depot medroxyprogesterone OR depomedroxyprogesterone OR dmpa OR "net en" OR norethisterone enanthate OR norplant OR uniplant OR jadelle OR implanon OR ((levonorgestrel OR etonogestrel) AND implant) OR (levonorgestrel AND intrauterine device*) OR mirena OR ((progestational hormones OR progestin) AND contracept* AND (oral OR pill* OR tablet*))) AND (bone density OR fracture* OR osteoporosis OR "bone mass" OR "bone mineral density" OR "bone density" OR "bone turnover" OR "bone mineral content" OR "bone loss" OR "bone resorption") NOT hormone replacement therapy AND (Clinical Trial[ptyp])
POPLINE (2011 to 25 Nov 2013)
All fields: bone AND (fracture OR density OR turnover OR mineral OR mass OR loss OR resorption)
Keyword: contraceptive agents, female OR contraceptive methods
CENTRAL (01 Jan 2011 to 25 Nov 2013)
contracept* AND (fracture* OR bone) [in Title, Abstract, or Keywords] NOT exercise OR postmenopaus* OR hypothalamic OR hirsutism [in Record Title]
EMBASE (2011 to 21 Nov 2013)
contraceptive* OR 'contraception'/exp AND ('bone'/exp AND 'density'/exp OR 'fracture'/exp) NOT 'postmenopause'/exp AND ([controlled clinical trial]/lim OR [randomized controlled trial]/lim) AND [humans]/lim
LILACS (01 Jan 2011 to 16 Dec 2013)
contraceptive agents, female or contraception or contraceptives or contraceptive [Words] and bone or bones or fracture or fractures [Words]
ClinicalTrials.gov (01 Jan 2011 to 15 May 2014)
Study type: Interventional studies Condition: NOT (polycystic OR cancer OR endometriosis OR menopause) Intervention: contraceptive OR contraception Outcome measures: fracture OR bone Gender: studies with female participants
ICTRP (01 Jan 2011 to 15 May 2014)
Title: fracture OR bone Intervention: contraception OR contraceptive
Appendix 2. Previous searches
2011 update
MEDLINE via PubMed (Sep 2009 to 08 Jun 2011)
(contraceptive agents, female OR ((steroid OR steroids OR steroidal) AND contracept*) OR ortho evra OR "ortho evra" OR "norelgestromin" OR (contraceptive devices, female and ring) OR NuvaRing OR cyclofem OR lunell* OR mesigyna OR cycloprovera OR (medroxyprogesterone 17‐acetate AND (contracept* OR inject* OR depo OR depot)) OR depot medroxyprogesterone OR depo medroxyprogesterone OR depot medroxyprogesterone OR depomedroxyprogesterone OR dmpa OR "net en" OR norethisterone enanthate OR norplant OR uniplant OR jadelle OR implanon OR ((levonorgestrel OR etonogestrel) AND implant) OR (levonorgestrel AND intrauterine devices) OR mirena OR ((progestational hormones OR progestin) AND contracept* AND (oral OR pill* OR tablet*))) AND (bone density OR fracture* OR osteoporosis OR "bone mass" OR "bone mineral density" OR "bone density" OR "bone turnover" OR "bone mineral content" OR "bone loss" OR bone resorption) NOT hormone replacement therapy
POPLINE (2008 to 08 Jun 2011)
(Contraceptive Agents Female/depo provera/dmpa/medroxyprogesterone/(steroid* & contracept*) /orthoevra/ortho evra /norelgestromin/(contraceptive devices, female and ring)/ NuvaRing /cyclofem /lunelle/ mesigyna/ cycloprovera/ (medroxyprogesterone 17‐acetate & (contracept* /inject*/depo/depot))/ depot medroxyprogesterone/ depo medroxyprogesterone/ depot medroxyprogesterone/depo medroxyprogesterone/dmpa/ net en/ norethisterone‐enantate/norplant/uniplant/jadelle/implanon/((levonorgestrel/ etonogestrel) & implant)/(levonorgestrel & intrauterine devices)/mirena /((progestational hormones/progestin) & contracept* & (oral/pill*/tablet*)))& (bone/fracture*& bone density/fracture*/osteoporosis/bone mass/ bone mineral density/ bone density/bone turnover/bone mineral content/bone loss/bone resorption) !(hormone replacement therapy/HRT)
CENTRAL (2008 to 08 Jun 2011)
contracept* AND (fracture* OR bone) [in Title, Abstract, or Keywords] NOT exercise OR postmenopaus* OR hypothalamic OR hirsutism [in Record Title]
LILACS (to 29 Mar 2011)
contraceptive agents, female or contraception or contraceptives or contraceptive [Words] and bone or bones or fracture or fractures [Words]
ClinicalTrials.gov (to 29 Mar 2011)
Condition: NOT (polycystic OR cancer OR endometriosis OR menopause) Intervention: contraceptive OR contraception Outcome measures: fracture OR bone Gender: studies with female participants
ICTRP (to 29 Mar 2011)
Title: fracture OR bone Intervention: contraception OR contraceptive
Initial review (2006) and 2009 update
The strategies were as listed above for 2011 update. In addition, EMBASE was searched using the strategy shown below.
EMBASE
(contraceptive agent or steroid? (w)contracept?) AND bone density or fracture? NOT (hormone substitution or estrogen therapy)
Data and analyses
Comparison 1. Levonorgestrel‐releasing implants: Norplant versus Chinese implant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density (L2 to L4) at 12 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.02, 0.10] |
| 2 Bone mineral density (femoral neck) at 12 months | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 3 Bone mineral density (trochanter) at 12 months | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 4 Bone mineral density (Ward's triangle) at 12 months | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.00, 0.14] |
| 5 Serum osteocalcin at 12 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.58, 0.90] |
| 6 Serum alkaline phosphatase at 12 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.94 [‐13.29, 17.17] |
| 7 Urine hydroxyproline/creatinine at 12 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐2.86, 6.26] |
| 8 Urine calcium/creatinine at 12 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.12 [‐44.01, 3.77] |
1.1. Analysis.
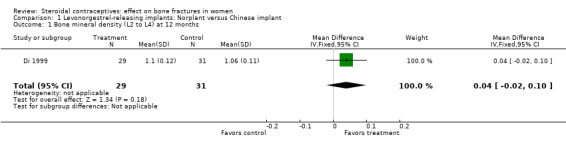
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 1 Bone mineral density (L2 to L4) at 12 months.
1.2. Analysis.
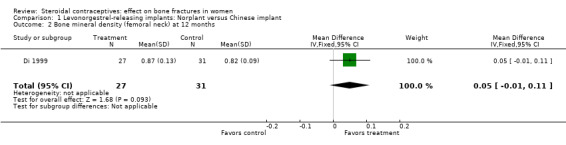
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 2 Bone mineral density (femoral neck) at 12 months.
1.3. Analysis.
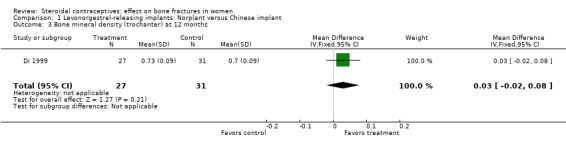
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 3 Bone mineral density (trochanter) at 12 months.
1.5. Analysis.
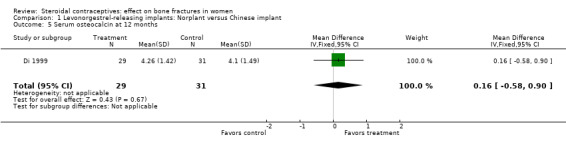
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 5 Serum osteocalcin at 12 months.
1.6. Analysis.
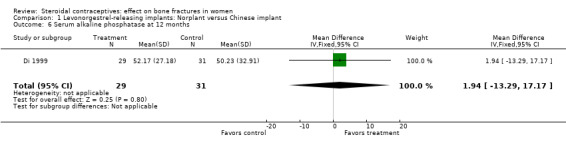
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 6 Serum alkaline phosphatase at 12 months.
1.7. Analysis.
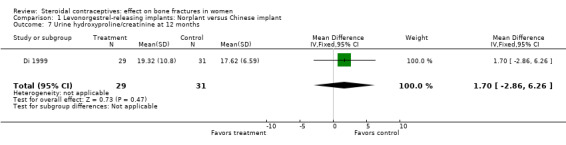
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 7 Urine hydroxyproline/creatinine at 12 months.
1.8. Analysis.
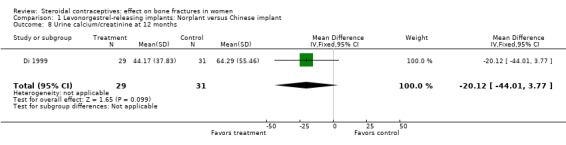
Comparison 1 Levonorgestrel‐releasing implants: Norplant versus Chinese implant, Outcome 8 Urine calcium/creatinine at 12 months.
Comparison 2. Etonogestrel‐releasing implant versus levonorgestrel‐releasing implant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density (midshaft ulna) at 18 months | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 2 Percent change in bone mineral density (midshaft ulna) by 18 months | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.56, ‐0.22] |
| 3 Bone mineral density (distal radius) at 18 months | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 4 Percent change in bone mineral density (distal radius) by 18 months | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.09, ‐0.91] |
| 5 Bone mineral density (distal radius) at 36 months | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
2.1. Analysis.
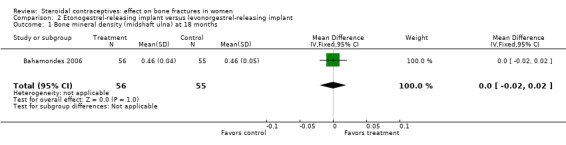
Comparison 2 Etonogestrel‐releasing implant versus levonorgestrel‐releasing implant, Outcome 1 Bone mineral density (midshaft ulna) at 18 months.
2.3. Analysis.
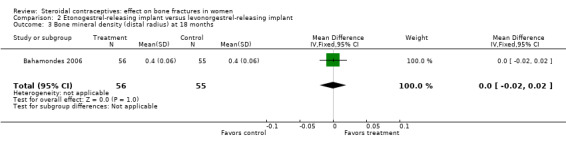
Comparison 2 Etonogestrel‐releasing implant versus levonorgestrel‐releasing implant, Outcome 3 Bone mineral density (distal radius) at 18 months.
2.5. Analysis.
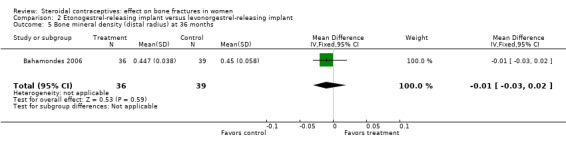
Comparison 2 Etonogestrel‐releasing implant versus levonorgestrel‐releasing implant, Outcome 5 Bone mineral density (distal radius) at 36 months.
Comparison 3. DMPA 150 mg versus levonorgestrel‐releasing implant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum alkaline phosphatase at 6 months | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.21, ‐0.09] |
| 2 Serum osteocalcin at 6 months | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.44 [‐3.39, 0.51] |
| 3 Serum calcium at 6 months | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 4 Urinary hydroxyproline/creatinine at 6 months | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 7.30 [‐14.52, 29.12] |
3.2. Analysis.
Comparison 3 DMPA 150 mg versus levonorgestrel‐releasing implant, Outcome 2 Serum osteocalcin at 6 months.
3.3. Analysis.
Comparison 3 DMPA 150 mg versus levonorgestrel‐releasing implant, Outcome 3 Serum calcium at 6 months.
3.4. Analysis.
Comparison 3 DMPA 150 mg versus levonorgestrel‐releasing implant, Outcome 4 Urinary hydroxyproline/creatinine at 6 months.
Comparison 4. DMPA 150 mg + estradiol cypionate 5 mg versus DMPA 150 mg + placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent change in bone mineral density (spine) by 12 months | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [1.80, 4.00] |
| 2 Percent change in bone mineral apparent density (spine) by 12 months | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [1.60, 3.80] |
| 3 Percent change in bone mineral density (femoral neck) by 12 months | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 3.2 [1.36, 5.04] |
| 4 Percent change in bone mineral apparent density (femoral neck) by 12 months | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [‐2.01, 4.41] |
| 5 Percent change in bone mineral density (spine) by 24 months | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 4.6 [2.87, 6.33] |
| 6 Percent change in bone mineral apparent density (spine) by 24 months | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 4.9 [3.11, 6.69] |
| 7 Percent change in bone mineral density (femoral neck) by 24 months | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 9.8 [4.96, 14.64] |
| 8 Percent change in bone mineral apparent density (femoral neck) by 24 months | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 7.10 [0.50, 13.70] |
Comparison 5. DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in bone mineral density (lumbar spine) by 12 months | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [0.00, 0.04] |
| 2 Change in bone mineral density (lumbar spine) by 24 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.06] |
| 3 Change in bone mineral density (Ward's triangle) by 12 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 4 Change in bone mineral density (Ward's triangle) by 24 months | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.00, 0.08] |
| 5 Change in bone mineral density (trochanter) by 12 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 6 Change in bone mineral density (trochanter) by 24 months | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 7 Change in bone mineral density (femoral neck) by 12 months | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8 Change in bone mineral density (femoral neck) by 24 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 9 Change in bone mineral density (total body) by12 months | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10 Change in bone mineral density (total body) by 24 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
5.3. Analysis.
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 3 Change in bone mineral density (Ward's triangle) by 12 months.
5.4. Analysis.
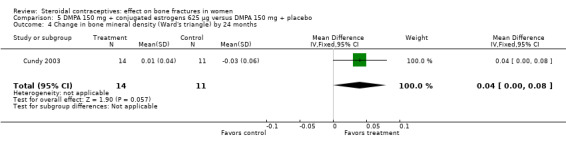
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 4 Change in bone mineral density (Ward's triangle) by 24 months.
5.5. Analysis.
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 5 Change in bone mineral density (trochanter) by 12 months.
5.6. Analysis.
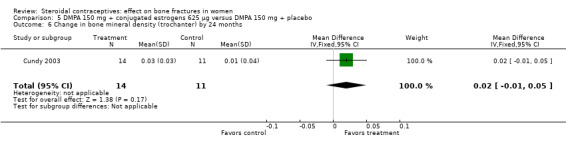
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 6 Change in bone mineral density (trochanter) by 24 months.
5.7. Analysis.
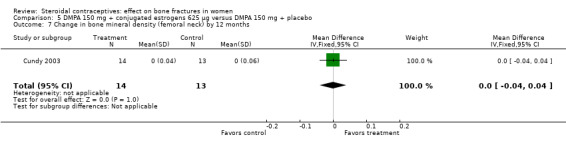
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 7 Change in bone mineral density (femoral neck) by 12 months.
5.8. Analysis.
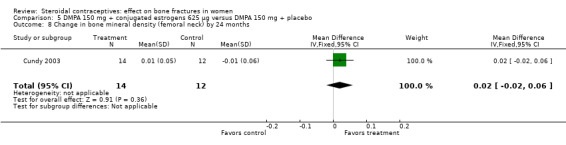
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 8 Change in bone mineral density (femoral neck) by 24 months.
5.9. Analysis.
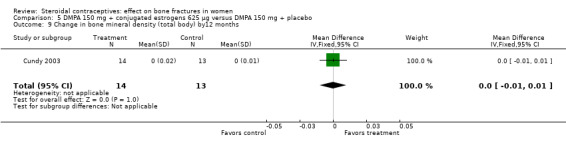
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 9 Change in bone mineral density (total body) by12 months.
5.10. Analysis.
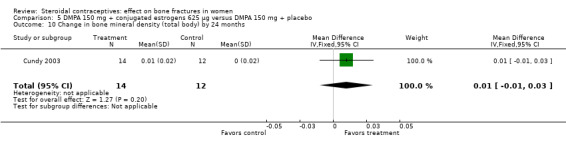
Comparison 5 DMPA 150 mg + conjugated estrogens 625 µg versus DMPA 150 mg + placebo, Outcome 10 Change in bone mineral density (total body) by 24 months.
Comparison 6. DMPA‐SC 104 mg versus DMPA‐IM 150 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decrease in total hip BMD >= 5% from baseline (year 1) | 1 | 328 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 2 Decrease in total hip BMD >= 5% from baseline (year 2) | 1 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.40] |
| 3 Decrease in total hip BMD >= 5% from baseline (year 3) | 1 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.56, 2.41] |
| 4 Decrease in lumbar spine BMD >= 5% from baseline (year 1) | 1 | 328 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.32] |
| 5 Decrease in lumbar spine BMD >= 5% from baseline (year 2) | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 1.01] |
| 6 Decrease in lumbar spine BMD >= 5% from baseline (year 3) | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.00, 4.45] |
6.1. Analysis.
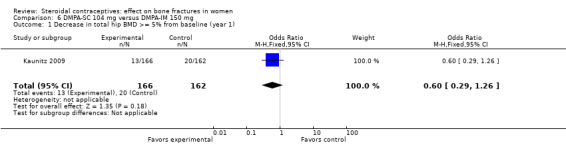
Comparison 6 DMPA‐SC 104 mg versus DMPA‐IM 150 mg, Outcome 1 Decrease in total hip BMD >= 5% from baseline (year 1).
6.2. Analysis.
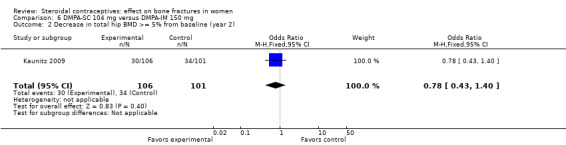
Comparison 6 DMPA‐SC 104 mg versus DMPA‐IM 150 mg, Outcome 2 Decrease in total hip BMD >= 5% from baseline (year 2).
6.3. Analysis.
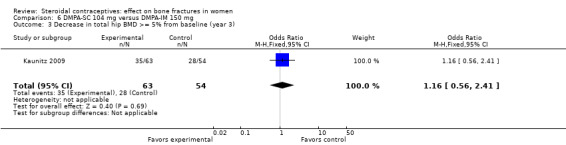
Comparison 6 DMPA‐SC 104 mg versus DMPA‐IM 150 mg, Outcome 3 Decrease in total hip BMD >= 5% from baseline (year 3).
6.4. Analysis.
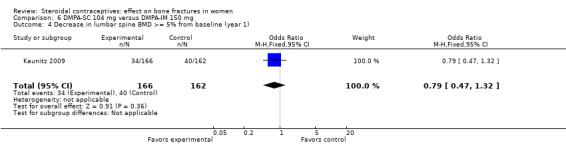
Comparison 6 DMPA‐SC 104 mg versus DMPA‐IM 150 mg, Outcome 4 Decrease in lumbar spine BMD >= 5% from baseline (year 1).
6.5. Analysis.
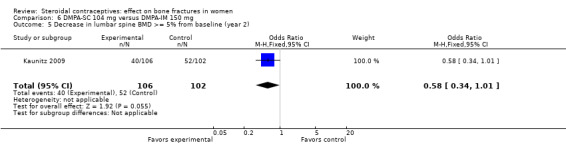
Comparison 6 DMPA‐SC 104 mg versus DMPA‐IM 150 mg, Outcome 5 Decrease in lumbar spine BMD >= 5% from baseline (year 2).
Comparison 7. Norethindrone 1 mg + EE 35 µg versus desogestrel 150 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent change in bone mineral density (lumbar spine) by 12 months | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [0.42, 3.24] |
| 2 Percent change in bone mineral density (lumbar spine) by 24 months | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐1.24, 2.92] |
7.2. Analysis.
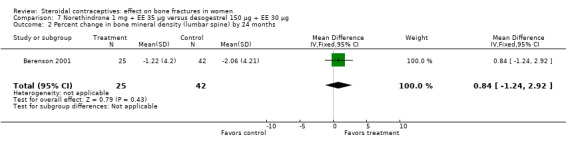
Comparison 7 Norethindrone 1 mg + EE 35 µg versus desogestrel 150 µg + EE 30 µg, Outcome 2 Percent change in bone mineral density (lumbar spine) by 24 months.
Comparison 8. Desogestrel 150 µg + EE 30 µg versus cyproterone acetate 2 mg + EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in BMD of lumbar spine by 12 months | 1 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 2 Change in BMD of lumbar spine by 24 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 3 Change in BMD of femoral neck by 12 months | 1 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 4 Change in BMD of femoral neck by 24 months | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
8.2. Analysis.
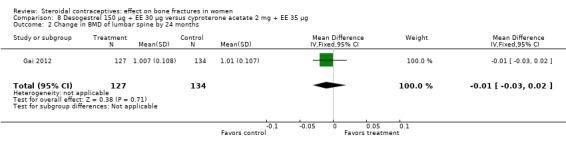
Comparison 8 Desogestrel 150 µg + EE 30 µg versus cyproterone acetate 2 mg + EE 35 µg, Outcome 2 Change in BMD of lumbar spine by 24 months.
8.3. Analysis.
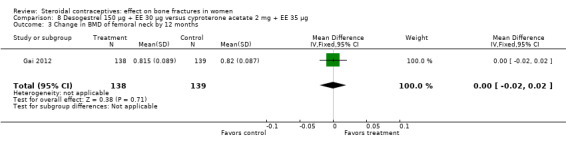
Comparison 8 Desogestrel 150 µg + EE 30 µg versus cyproterone acetate 2 mg + EE 35 µg, Outcome 3 Change in BMD of femoral neck by 12 months.
Comparison 9. Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density (lumbar spine) at 12 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐17.84, 13.64] |
| 2 Bone mineral density (lumbar spine) at 24 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐18.29, 15.09] |
| 3 Bone mineral density (lumbar spine) at 36 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.53 [‐17.27, 10.21] |
| 4 Percent change in bone mineral density (lumbar spine) by 36 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐3.00, 3.80] |
| 5 Percent change in serum alkaline phosphatase (36 months) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐57.80 [‐160.03, 44.43] |
| 6 Percent change in cross‐linked N‐telopeptides (36 months) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐7.70 [‐31.90, 16.50] |
9.2. Analysis.
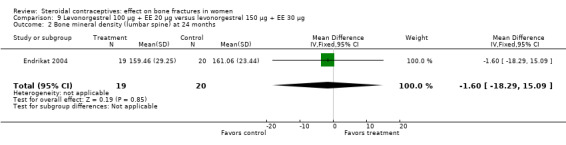
Comparison 9 Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 2 Bone mineral density (lumbar spine) at 24 months.
9.3. Analysis.
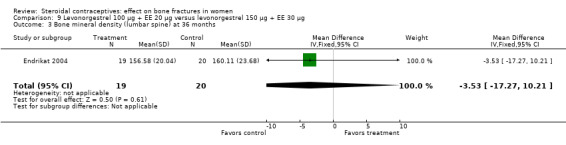
Comparison 9 Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 3 Bone mineral density (lumbar spine) at 36 months.
9.5. Analysis.
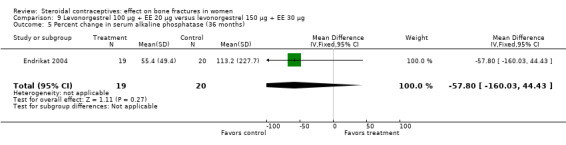
Comparison 9 Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 5 Percent change in serum alkaline phosphatase (36 months).
9.6. Analysis.
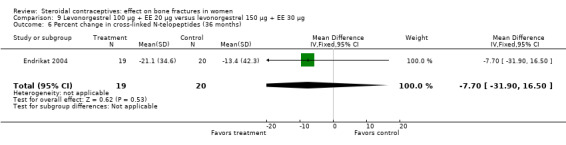
Comparison 9 Levonorgestrel 100 µg + EE 20 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 6 Percent change in cross‐linked N‐telopeptides (36 months).
Comparison 10. Levonorgestrel 100 µg + EE 20 µg versus desogestrel 150 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent change in areal bone mineral density (lumbar spine) by 12 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [‐0.11, 2.93] |
| 2 Percent change in areal bone mineral density (femoral neck) by 12 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐2.42, 2.58] |
| 3 Percent change in serum bone‐specific alkaline phosphatase by 12 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 15.31 [3.91, 26.71] |
| 4 Percent change in serum osteocalcin by 12 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 7.71 [‐2.49, 17.91] |
| 5 Percent change in serum cross‐linked telopeptides by 12 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 16.39 [‐8.41, 41.19] |
Comparison 11. Nomegestrol 2.5 mg + estradiol 1.5 mg versus levonorgestrel 150 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in z‐score of lumbar spine after cycle 26 | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.22, 0.01] |
| 2 Change in z‐score of femoral neck after cycle 26 | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.06] |
Comparison 12. Gestodene 75 µg + EE 20 µg versus gestodene 75 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urinary pyridinoline at 12 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.65, 2.05] |
| 2 Urinary deoxypyridinoline at 12 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.37, 2.03] |
| 3 Serum osteocalcin at 12 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐1.87, 5.07] |
12.1. Analysis.
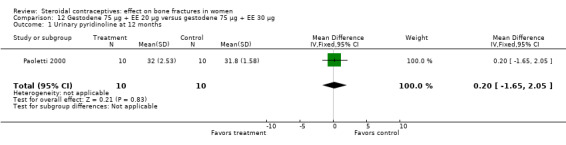
Comparison 12 Gestodene 75 µg + EE 20 µg versus gestodene 75 µg + EE 30 µg, Outcome 1 Urinary pyridinoline at 12 months.
12.3. Analysis.
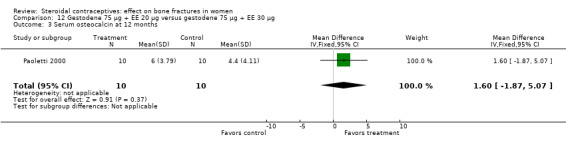
Comparison 12 Gestodene 75 µg + EE 20 µg versus gestodene 75 µg + EE 30 µg, Outcome 3 Serum osteocalcin at 12 months.
Comparison 13. Drospirenone 3 mg + EE 30 µg versus gestodene 75 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density (lumbar spine) at 12 months | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
Comparison 14. Drospirenone 3 mg + EE 30 µg versus drospirenone 3 mg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density (lumbar spine) at 12 months | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.09, 0.10] |
Comparison 15. Transdermal patch (norelgestromin 150 µg plus EE 20 µg) versus vaginal ring (etonogestrel 120 µg plus EE 15 µg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lumbar spine BMD at 12 months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 2 Urinary pyridinoline at 12 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.65, 1.05] |
| 3 Urinary deoxypyridinoline at 12 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.24, 0.44] |
| 4 Serum osteocalcin at 12 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
15.2. Analysis.
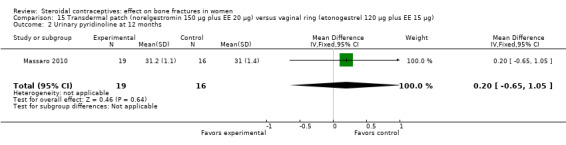
Comparison 15 Transdermal patch (norelgestromin 150 µg plus EE 20 µg) versus vaginal ring (etonogestrel 120 µg plus EE 15 µg), Outcome 2 Urinary pyridinoline at 12 months.
15.3. Analysis.
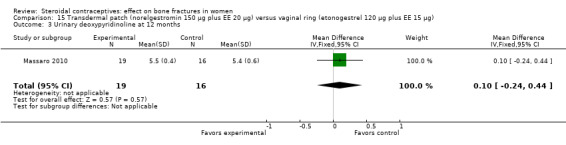
Comparison 15 Transdermal patch (norelgestromin 150 µg plus EE 20 µg) versus vaginal ring (etonogestrel 120 µg plus EE 15 µg), Outcome 3 Urinary deoxypyridinoline at 12 months.
Comparison 16. Norethisterone enanthate 50 mg + estradiol valerate 5 mg versus Nova T IUD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent change in bone mineral density (lumbar spine) by 12 months | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐3.08, 1.68] |
| 2 Percent change in bone mineral density (lumbar spine) by 24 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.74, 2.74] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bahamondes 2006.
| Methods | Randomized controlled trial; enrollment from Aug 2003 to Jul 2004. Sample size calculation based on assuming a BMD change of 0.014 g/cm2 by 18 months; 48 women per group would provide 80% power to detect difference of 0.008 (presumably between groups). | |
| Participants | 111 women, 19 to 43 years, requesting implant for contraception. Women were a subset of a larger study by UNDP/UNFPA/World Bank/WHO. Inclusion criteria: not pregnant or lactating within 12 months of enrollment. Exclusion criteria: women with chronic diseases, such as diabetes mellitus, chronic renal failure, hyper/hypothyroidism, hyper/hypoparathyroidism, hepatitis, cancer or pituitary disease. Also excluded were women who used calcium or Vitamin D supplements, anticonvulsants, any corticosteroids, thiazide diuretics or drugs for thyroid disease. | |
| Interventions | 1) Single‐rod etonogestrel‐releasing implant (Implanon, NV Organon, Oss, Netherlands) (N=56) versus 2) Two silicone rod levonorgestrel‐releasing implant (Jadelle; Schering Oy, Turku, Finland) (N=55). Insertions performed in first 5 days of cycle; no wash‐out period for those with previous contraception. [9 women had amenorrhea at insertion due to DMPA use.] Duration: 18 months |
|
| Outcomes | Bone mineral density (BMD) at midshaft of ulna (mainly cortical bone) and at distal radius (mainly trabecular bone). Measures done in non‐dominant arm with DEXA. Measures taken at baseline and 18 months. Later report (Monteiro‐Dantas, 2007) provided 36‐month data for BMD at distal radius (also ultra‐distal radius, not available in earlier report and not used in this review). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization system |
| Allocation concealment (selection bias) | Low risk | Allocation concealment with sealed envelopes prepared at WHO |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses: 18 months, all participants included in analysis (primary paper); 36 months (secondary paper), loss was 32% (36% Implanon and 29% Jadelle). |
Berenson 2001.
| Methods | Randomized controlled trial in USA; recruitment from May 1996 to Jan 1999. | |
| Participants | 179 women, 18 to 33 years, who had undergone baseline bone scan as part of larger contraceptive study. Inclusion criteria: due to funding (US Department of Defense), women had to meet criteria for entry into armed forces (high school graduate or equivalency diploma, no felony arrests, within 36% ideal body weight for height, free of medical conditions or physical disabilities that would affect completion of military training). Exclusion criteria: currently pregnant or breastfeeding, had injection for contraception in past 6 months, took OCs in past month, or had medical contraindication to hormonal contraception. | |
| Interventions | 1) Ethinyl estradiol 35 μg plus norethindrone 1 mg OC (Ortho Novum 1/35; Ortho Pharmaceutical Corporation, New Brunswick, NJ) (N=87) versus 2) Ethinyl estradiol 30 μg plus desogestrel 150 μg OC (Mircette; Organon Corporation, Oss, Netherlands) (N=92). Data were reported for 28 and 35 women at 12 months. |
|
| Outcomes | Bone mineral density of lumbar spine (L1 to L4) with DEXA at baseline and at 12 months. Baseline scans performed within 2 months of initiation; follow‐up scans were done 10 to 14 months after baseline. Berenson 2004 reported on scans at 24 months for those who obtained a second scan. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used |
| Allocation concealment (selection bias) | High risk | No allocation concealment, according to communication with investigator. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Providers not blinded to treatment; package labeling was removed for participants. Pills were referred to as "red" or "green." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses due to discontinuation of contraceptive method or failure to have bone scan (at all or within required window): at 12 months, 68% for norethindrone‐containing pill and 62% for desogestrel‐containing pill; at 24 months, 71% and 54%, respectively. |
Cibula 2012.
| Methods | Randomized, crossover trial; investigators from Czech Republic. No a priori sample size calculation. Reportedly had post hoc power analysis; no detail provided. |
|
| Participants | 56 adolescent females who requested hormonal contraception at adolescent gynecology unit. Inclusion criteria: age 15 to 19.5 years, BMI 20 to 27 kg/m2, regular menstrual cycle. Exclusion criteria: recent or past COC use; systemic or chronic diseases including endocrine disorders; using medication that could influence bone metabolism or reliability of hormonal contraception (e.g. corticosteroids, antiepileptics, thyroid hormones); drug use or smoking > ten cigarettes per day; daily dietary calcium intake < population average (< 600 mg/day); endurance physical activity; immobilization or invalidity; COC contraindication. |
|
| Interventions | 1) Gestodene 75 μg plus EE 30 μg versus 2) Gestodene 60 μg plus EE 15 μg Groups switched COCs after 9 months of use; total study duration was 18 months. No 'washout' period between study periods, reportedly for ethical reasons, i.e., high risk of pregnancy among adolescents. |
|
| Outcomes | BMD at lumbar spine L1 to L4, total proximal femur, femoral neck and distal radius, total body mineral content. Reported median and quartiles for percent changes from baseline. Serum propeptide of type I procollagen (PINP, bone formation marker). Serum type I collagen cross‐linked C‐telopeptide (ßCTX1, bone resorption marker). Percent changes in biochemical outcomes shown in figures without absolute values. |
|
| Notes | Control group of nonusers did not want hormonal contraception (not included here). Unable to obtain further data from investigator regarding outcomes, e.g., means and SD. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (STATA software). |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss by 9 months: group 1, EE 15 μg 3/28 = 10.7%; group 2, EE 30 μg 3/28 = 10.7%; Loss by 18 months (after crossover): group 1, EE 30 μg 6/28 = 21.4%; group 2, 5/28 = 17.9%; total loss 19.6%. |
Cromer 2005.
| Methods | Randomized controlled trial in the USA; 4 health clinics in large metropolitan setting Enrollment from May 2000 to Dec 2002. No sample size calculation provided |
|
| Participants | 123 adolescent females. Inclusion criteria: adolescent girls, age 12 to 18 years, who were seeking contraception and selected DMPA. Exclusion criteria: use of DMPA, pregnancy or abortion in past 6 months; use of OCs in past 3 months, chronic medical condition or treatment that may affect bone, or need for confidentiality in contraception. | |
| Interventions | All participants: DMPA as 150 mg deep intramuscular injection every 12 weeks. 1) Monthly intramuscular injections of 5 mg estradiol cypionate (supplement) (N=65) versus 2) 5 mL normal saline solution (placebo) (N=58). Study duration: 24 months |
|
| Outcomes | Bone mineral density obtained at L1 to L4 lumbar vertebrae, total hip (left), femoral neck, trochanter, and Ward's triangle; DEXA was used.
Bone mineral apparent density (BMAD) was used to correct for variation in bone: BMAD = BMC(L1 to L4) / Ap3/2, where BMC = scanned bone mineral content and Ap = projected area; BMAD = BMC(femoral neck) / Ap2(femoral neck). Outcome means were adjusted for baseline body weight and bone mineral density. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by recruitment site. 'Blocked randomization techniques.' |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded: study subjects, technicians conducting the dual energy x‐ray absorptiometry scans, and clinicians providing health care to the participants Nurse providing injections was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The trial was stopped early due to interim analysis showing the differences between the groups reached the pre‐determined significance level of < 0.001. Estrogen supplementation was offered to participants who had the placebo; exercise and diet counseling was offered to all active participants. Many participants had not had 12 or 24 months of observation (N=24 and N=33, respectively). In addition, 30 young women withdrew by 12 months (30/123 = 24%) and 53 withdrew by 24 months (53/123 = 43%). |
Cundy 2003.
| Methods | Randomized controlled trial in Auckland, New Zealand. Analysis by intention to treat. | |
| Participants | 38 women recruited from family planning clinics. Inclusion criteria: age <= 45 years, long‐term users (>= 2 years) of DMPA, areal BMD (from lumbar spine) <= 1.20 g/cm2 (young adult average). Exclusion criteria: known metabolic bone disease, taking drugs (other than DMPA) that can affect bone density, FSH > 20 U/liter. | |
| Interventions | All participants: DMPA 150 mg injection every 12 weeks. 1) Conjugated estrogens 62.5 μg (Premarin; Wyeth‐Ayerst, Collegeville, PA) (N=19) versus 2) Placebo (N=19), taken orally each day for 2 years. Duration: 24 months |
|
| Outcomes | Areal BMD by DEXA at lumbar spine (L2 to L4), femur, and total body; lumbar spine BMD was the primary outcome. Results were reported for plasma calcium, phosphate, total alkaline phosphatase activity; fasting urine N‐telopeptide/creatinine ratio. | |
| Notes | Author provided data for all BMD measures (article had graphs) as well alkaline phosphatase (AP) and N‐telopeptide NT. However, AP and NT had missing data at baseline, and the distributions appeared to be skewed, so those data were not analyzed in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to the investigator, randomization was balanced by blocks of 10; within a block, subject was "allocated a treatment number." Random numbers above the median were assigned to treatment and those below were assigned to placebo. |
| Allocation concealment (selection bias) | Low risk | According to communication with the investigator, allocation concealment was accomplished with serially‐numbered opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27 women completed 18 months or more (29% loss to early discontinuation from study) |
Di 1999.
| Methods | Randomized controlled trial in Beijing. | |
| Participants | 61 women, aged 25 to 40 years, who sought contraception counseling at Ob‐Gyn Hospital. Inclusion criteria: regularly menstruating, no OC use in past 3 months or Norplant removed >=6 months prior, no medications or diseases known to interfere with bone metabolism. | |
| Interventions | 1) Norplant implant (Leiras Pharmaceuticals, Turku, Finland) (N=30) versus 2) Domestic implant (Capsulae Levonorgestreli Silasticus; Yalujiang Medical Factory, Dandong, Laioning Province, China) (N=31). Both types had 6 capsules, each with 36 mg levonorgestrel for a maximum 216 mg levonorgestrel. Study duration 1 year |
|
| Outcomes | BMD of lumbar spine (L2 to L4) and proximal femur via DEXA. Serum osteocalcin (BGP) and alkaline phosphatase (ALP); urine hydroxyproline/creatinine and urine calcium/creatinine. | |
| Notes | Attempts to reach the investigator were unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomly divided into two groups |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One implant removed at subject's request and data excluded from analysis. |
Endrikat 2004.
| Methods | Randomized controlled trial in Germany Analysis was per protocol. | |
| Participants | 100 healthy women at one center. Inclusion criteria: current users of COCs for > 2 years, but no EE >= 50 μg for more than 6 months; started COCs after 16th birthday. Exclusion criteria: contraindications for COC use; smoking >15 cigarettes for women < 30 years and any smoking for women > 30 years; use of DMPA in previous 6 months, use of other sex hormones during treatment, coexisting diseases (unspecified), diagnostically unclassified genital bleeding, and history of migraine with menstruation. | |
| Interventions | 1) 20 μg ethinyl estradiol (EE) plus 100 μg levonorgestrel OC (20/100; Schering AG, Berlin, Germany) versus 2) 30 μg ethinyl estradiol with 150 μg levonorgestrel OC (30/150; Schering AG). First tablet taken on first day of withdrawal bleeding with no wash‐out period. 36 consecutive 28‐day cycles. Study duration was 3 years |
|
| Outcomes | Trabecular BMD of lumbar spine (L1 to L3) using computed tomography; alkaline phosphatase and N‐telopeptides. | |
| Notes | Attempts to reach the investigator were unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 88 took at least one dose; authors' analysis only included the 48 who provided data at baseline and cycle 36 (52% loss). Loss to follow up was 15% overall (15/100). Bone density data provided for the 39 with data at all time points (61% loss). |
Gai 2012.
| Methods | Randomized trial; unspecified location (protocols approved in Shandong, China). No sample size calculation provided. |
|
| Participants | 300 women from 16 to 18 years old, attending family planning clinics and requesting birth control. Inclusion criteria: regular menses, non‐hormonal contraception, no breastfeeding or delivery for at least 6 months, no pregnancy; did not take calcium, vitamin D, or bone‐affecting medication. Exclusion criteria: chronic disease, such as diabetes mellitus, renal dysfunction, thyroid and parathyroid diseases, hepatitis or pituitary diseases. |
|
| Interventions | 1) Desogestrel (DSG) 150 μg plus EE 30 μg versus 2) Cyproterone acetate (CPA) 2 mg plus EE 35 μg Duration: 2 years |
|
| Outcomes | BMD measured at lumbar spine (L 2 to L4) and femoral neck by DEXA. | |
| Notes | A third group was not randomized; women did not want to use hormonal method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By 'drawing lots' |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss by 12 months: DSG+EE 12/150 = 8%; CPA+EE 11/150 = 7.3%. Loss by 24 months: DSG+EE 23/150 = 15.3%; CPA+EE 16/150 = 10.7%. |
Gargano 2008.
| Methods | Randomized trial in Italy No sample size calculation provided. |
|
| Participants | 44 women, 21 to 34 years old, presenting at clinic. Inclusion criteria: age of menarche 12 to 14 years, ovulation during pretreatment cycle, BMI > unclear (report had error in lower cutoff of '2') < 25, normal menstrual cycles, normal diet without high or low caloric intake. Exclusion criteria: confirmed or suspected pregnancy, pregnancy or breastfeeding in past year, liver disease, vascular or metabolic disorder, disorder of bone metabolism, treatment with drugs that affect bone metabolism or drugs that interfere with contraceptive steroids, other contraindication for COCs. | |
| Interventions | 1) Drospirenone 3 mg plus EE 30 μg versus 2) Drospirenone 3 mg plus EE 20 μg Duration of 12 months |
|
| Outcomes | BMD of lumbar spine (L1 to L4) by DEXA. Bone turnover markers: serum and urinary calcium, urinary pyridinoline and deoxypyridinoline (resorption); serum osteocalcin (formation). Results were presented in figures, without absolute values, for urinary pyridinoline and deoxypyridinoline and for serum osteocalcin. | |
| Notes | 'Controls' were not randomized, and therefore were excluded from analysis in this review; they did not request contraception. Unable to obtain from investigator further information on methodology or additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses: 7% (3/44) included 1 excluded for protocol violation (EE 30 μg group) and 2 early discontinuations (EE 20 μg group). |
Hartard 2006.
| Methods | Randomized trial in Munich, Germany No mention of sample size calculation or power. |
|
| Participants | 52 women, 18 to 24 years old, recruited via advertisements and mass mailings. Inclusion criteria: discontinued OCs for at least 2 months; spontaneous menstrual cycle during run‐in period. Exclusion criteria: BMI > 30, history of smoking (>20 cigarettes/day), alcohol consumption (>20 g/day), exercise > 1 hour/week in past, age of menarche > 15 years, long‐term use of OC (> 50% of time since menarche), previous menstrual disorders, past or current pregnancy, hypertension (> 140/90 mmHg), abnormal values for clinical chemistry, current or past medication with drugs that influence bone metabolism, use of contraceptive implants in past 6 months, diseases that affect bone (except well‐controlled Type I diabetes mellitus or well‐controlled hypothyroidism) | |
| Interventions | 1) Levonorgestrel 100 μg plus EE 20 μg (N=24) versus 2) Desogestrel 150 μg plus EE 20 μg (N=28); Duration of 13 cycles |
|
| Outcomes | BMD (spine L2 to L4 and femoral neck) by DXA. Serum bone‐specific alkaline phosphatase; intact osteocalcin; serum C‐terminal cross‐linked telopeptides. |
|
| Notes | 'Controls' were not randomized, and therefore were excluded from the analysis in this review; they did not choose hormonal contraception. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses due to discontinuation (e.g., adverse events, personal reasons): 19%; desogestrel group, 21%; levonorgestrel group, 17%. |
Kaunitz 2009.
| Methods | Randomized controlled trial at 36 sites in USA, 9 sites in Canada, and 3 sites in Brazil; conducted from 2001 to 2004. Sample size of 250 for each group was based on 80% power to detect 2% difference in bone mineral density at 2 years. |
|
| Participants | 535 women aged 18 to 35 years, sexually active, desiring long‐term contraception. Inclusion criteria: regular menstruation in past 3 months, negative urine pregnancy test, and willingness to rely upon DMPA‐SC or DMPA‐IM for contraception for at least 2 years. Exclusion criteria: use of oral contraceptives, implant, or hormonal IUD in past 2 months or DMPA‐IM in past 10 months (contraceptive patches and rings were not in use at the time of study enrollment); lumbar spine or femur BMD T‐score of less than −1.0, history of pathologic or compression fracture; abnormal cervical cytology; undiagnosed abnormal genital bleeding; known or suspected pregnancy; history of breast cancer, thrombotic event, hepatic or renal disease, alcoholism or other drug abuse (in past 5 years); uncontrolled hypertension, active hepatic or renal disease, type 1 diabetes, or poorly controlled type 2 diabetes; and taking anticancer agent aminoglutethimide. Calcium, multivitamins, and other mineral supplements were not required nor prohibited if part of the participants' normal daily regimen. |
|
| Interventions | 1) DMPA 104 mg/0.65 mL by subcutaneous injection every 3 months (DMPA‐SC) versus 2) DMPA 150 mg/mL by intramuscular injection every 3 months (DMPA‐IM) Duration: 2 years; secondary objectives included assessing efficacy, safety, and tolerability over 3 years |
|
| Outcomes | Primary: percent change (baseline to 2 years) in BMD at total hip and lumbar spine; dual X‐ray absorptiometry used. Efficacy endpoint was pregnancy (urine test). Adverse events recorded throughout study. |
|
| Notes | Study was sponsored by Pfizer. Three investigators were not compensated, investigators reportedly retained full editorial control over content of paper; other 2 were Pfizer employees. Report did not specify where the analysis was conducted or by whom. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice‐response system based on computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators and evaluators at each site were blinded. Independent person received the study syringes, maintained the randomization code, and administered the study drug. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participant flow chart provided. Overall losses by 1 year: 39% (38% DMPA‐SC, 40% DMPA‐IM). Loss to follow up by 2 years (1 year not available): 19% overall; 16% DMPA‐SC, 22% DMPA‐IM Efficacy analysis included all participants who received at least one dose of study drug and made at least one visit after receiving study drug. |
Massaro 2010.
| Methods | Randomized controlled trial at university‐based family planning clinic in Italy during 2008. No mention of sample size calculation. |
|
| Participants | 40 healthy women, 23 to 34 years old, requesting contraception. Inclusion criteria: age of menarche 12 to 14 years, demonstrable ovulation during pretreatment cycle, body mass index (BMI) > 20 and < 22 kg/m2, normal menstrual cycles and normal diet. Exclusion criteria: confirmed or suspected pregnancy, pregnancy or breastfeeding in past year, liver disease, vascular or metabolic disorder, disorder of bone metabolism (Paget disease, hyperparathyroidism, renal osteodystrophy) and treatment with drugs that affect bone metabolism (bisphosphonates, sodium fluoride, calcitonin, estroprogestins or anabolic steroids, corticosteroids, calcium or vitamin D, phosphate, thiazide diuretics) or drugs that interfere with contraceptive steroids (barbiturates, antiepileptics, rifampicin, griseofulvin), contraindications for the use of hormonal contraceptives |
|
| Interventions | 1) Contraceptive patch delivering norelgestromin 150 μg plus EE 20 μg daily versus 2) Vaginal ring releasing etonogestrel 120 μg plus EE 15 μg daily Duration: 12 months |
|
| Outcomes | Bone mineral density by DEXA of lumbar spine (L1 to L4) Bone formation: serum osteocalcin; bone resorption: urinary pyridinoline and deoxypyridinoline. |
|
| Notes | Results for bone formation and resorption were presented in figures. On request, the investigator provided means, standard deviations, and Ns for analysis. Study was supported by institutional funds from investigators' university department |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blood and urine samples analyzed in laboratory blinded to treatment group; absorptiometry performed by blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant flow chart provided. Losses due to drop‐outs were 20% in each group; reasons for discontinuation were personal, irregular bleeding, and reaction to patch. No losses to follow up mentioned. |
Naessen 1995.
| Methods | Randomized trial in Uppsala, Sweden. | |
| Participants | 22 women, 20 to 45 years, seeking contraceptive advice at family planning clinic, University Hospital. | |
| Interventions | 1) Levonorgestrel implant (Norplant) versus 2) DMPA 150 mg every 12 weeks Study duration: 6 months |
|
| Outcomes | Bone density in distal forearm by single photon absorptiometry. Bone density results shown in figure; no absolute numbers for variance. Serum calcium, alkaline phosphatase, and osteocalcin; urinary hydroxyproline/creatinine. |
|
| Notes | Investigator was unable to retrieve the BMD means and SD; publication was > 10 years old. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to the investigator, randomization procedure was a 'computer‐based randomization.' |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were opened after inclusion of each subject. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information; interventions differed (injection versus implant) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/22 women completed the 6‐month follow up; bone density measured in 18 (18% loss). |
Nappi 2003.
| Methods | Randomized trial | |
| Participants | 40 healthy women desiring contraception. Inclusion criteria: 22 to 34 years old, age of menarche 12 to 14 years, ovulation in pretreatment cycle, BMI > 20 and < 22, normal menstrual cycles, normal diet without high or low calories. Exclusion criteria: pregnancy or breastfeeding in past year, liver disease, vascular or metabolic disorder, bone disease or disorder, smoking >10 cigarettes/day, migraine with aura, drugs that affect bone metabolism or with steroidal contraceptives, hysterectomy or oophorectomy, other contraindications for COCs. | |
| Interventions | 1) Ethinyl estradiol 20 μg plus gestodene 75 μg OC (FEDRA, Schering; Milan, Italy) (N=20) versus 2) Ethinyl estradiol 15 μg plus gestodene 60 μg OC (ARIANNA, Schering; Milan, Italy) (N=20) Study duration 1 year |
|
| Outcomes | BMD by DEXA at posterior‐anterior lumbar spine (L1 to L4) Serum osteocalcin, urinary pyridinoline and deoxypyridinoline | |
| Notes | Results shown in figures without absolute values. Unable to obtain further information from the investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss: 8%; 3/40 women dropped out or missed the follow‐up visit |
Nappi 2005.
| Methods | Randomized controlled trial in Naples, Italy | |
| Participants | 48 healthy women in family planning clinic. Inclusion criteria: 22 to 34 years old, age of menarche 12 to 14 years, ovulation in pretreatment cycle, normal menstrual cycles, no abnormal dietary requirements. Exclusion criteria: pregnancy or breastfeeding in past year, liver disease, vascular or metabolic disorder, bone disease or disorder, smoke >= 10 cigarettes/day, migraine with aura, drugs that affect bone metabolism, drugs that interfere with steroidal contraceptives, hysterectomy or oophorectomy, other contraindications for COCs. | |
| Interventions | 1) Ethinyl estradiol 30 μg and drospirenone 3 mg OC (Yasmin; Schering, Milan, Italy) (N=24) versus 2) Ethinyl estradiol 30 μg and gestodene 75 μg OC (Ginoden; Schering) (N=24) Study duration 1 year |
|
| Outcomes | BMD by DEXA at posterior‐anterior lumbar spine (L1 to L4). Serum and urinary calcium, serum osteocalcin, urinary pyridinoline and deoxypyridinoline. Results for biochemical measures shown in figures without absolute numbers. | |
| Notes | Unable to obtain further information from investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Sequence was 'concealed both to researchers and patients until treatments were assigned.' |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss: 6%; 3/48 women dropped out. |
Paoletti 2000.
| Methods | Randomized trial | |
| Participants | 20 healthy women desiring contraception. Inclusion criteria: 22 to 30 years old, normal menstrual cycles, age of menarche 12 to 14 years, BMI > 20 and < 22, no bone disease or disorder of bone metabolism, normal diet without low or high calories. | |
| Interventions | 1) Ethinyl estradiol 20 μg plus gestodene 75 μg OC versus 2) ethinyl estradiol 30 μg plus gestodene 75 μg OC. Each group had 10 women, according to communication from investigator. Study duration 12 months |
|
| Outcomes | Serum osteocalcin, urinary pyridinoline and deoxypyridinoline. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study not blinded, according to author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report does not mention loss or discontinuation. Investigator communicated that all participants were included in the final analysis. |
Rad 2011.
| Methods | Randomized multicenter trial conducted 2003‐04; 2 sites in Netherlands and 2 sites in Poland Report is from a sub‐study within larger trial (Teichmann 2009). Sample size of 50 per group based on 90% power to detect a difference of 0.458 mmol/l in total cholesterol values between treatment groups |
|
| Participants | 147 healthy females. Inclusion criteria: 18 to 49 years old, body mass index 16 to 30 kg/m2 (inclusive), regular menstrual cycle, and no contraindications for contraceptive steroids; willing to rely on study medication as only method of contraception. Exclusion criteria: diabetes; smoke <10 cigarettes daily; use of anticoagulant drugs, aspirin, lipid‐lowering drugs, or drugs that would compromise contraceptive effect of COCs |
|
| Interventions | 1) Continuous regimen of COC containing LNG 90 μg plus EE 20 μg versus 2) Cyclic regimen (21 days on, 7 days off) of COC containing LNG 100 μg and EE 20 μg Duration: 1 year |
|
| Outcomes | Bone markers were C‐telopeptide and osteocalcin. Other metabolic measures included carbohydrate, lipid, and hemostatic variables. |
|
| Notes | Data insufficient for analysis: standard errors reported without sample sizes. Unable to obtain additional information from investigator. Study was sponsored by Wyeth (acquired by Pfizer in Oct 2009); 6 investigators received sponsor support for study and analysis and the other 2 were sponsor employees. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice‐recognition system |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐recognition system |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses other than discontinuations: 7% overall; continuous regimen 8% (6/74) and cyclic regimen 5% (4/73) Losses total: 29% overall; continuous regimen 34% (25/74) and cyclic regimen 23% (17/73) Excluded from analysis participants with: a) only baseline values and b) outlying on‐treatment values (mean + 3 SD; determined in blinded fashion). |
Sordal 2012.
| Methods | Randomized trial, conducted in Norway from Sep 2006 to Jun 2009. Sample size based on change in z‐scores of BMD of lumbar spine and femoral neck after 2 years. Based on variability in recent BMD studies, 55 women in each group, with 35% drop‐out rate, would provide 80% power to detect 0.20 to 0.34 difference. |
|
| Participants | 110 healthy women, 20 to 35 years old, from one gynecology center. Inclusion criteria: sexually active women, at risk for pregnancy and not planning to use condoms during treatment; 20 to 35 years of age at screening; body mass index of 17 to 35 kg/m2; good physical and mental health. Exclusion criteria: family history of osteoporotic fracture < age of 70; postgastrectomy; history of eating disorder; endocrine disorder (including controlled diabetes, [para]thyroid disease, Cushing's disease); rheumatoid arthritis; significant scoliosis; laboratory results outside reference range for fasting parathyroid hormone (PTH), fasting calcitonin, prolactin (hyperprolactinemia), fasting cholesterol or triglycerides (above range for age or treatment with lipid lowering drugs); engaging in vigorous exercise such as marathon, competitive swimming, triathlon; smoking > 10 cigarettes per day; > two units of alcohol a day; use of gonadotropin releasing hormone analogues, corticosteroids, thiazide diuretics, thyroid hormone, bisphosphonates, calcium with vitamin D supplementation; treatment after childhood with fluorides; contraindications for contraceptive steroids; abnormal cervical smear at screening; use of injectable hormonal contraception; pregnancy or breastfeeding in past 12 months; use in past 2 months of phenytoin, barbiturates, primidone, carbamazepine, oxcarbazepine, topiramate, felbamate, rifampicin, nelfinavir, ritonavir, griseofulvin, ketoconazole, sex steroids (other than pre‐ and post‐treatment contraceptive method) and herbal remedies containing Hypericum perforatum (St John's Wort); other investigational drug or trial participation in past 2 months. |
|
| Interventions | 1) Nomegestrol 2.5 mg plus [17ß] estradiol 1.5 mg (NOMAC‐E2) (24/4 day regimen) versus 2) Levonorgestrel 150 μg plus ethinyl estradiol 30 μg (LNG/EE) (21/7 day regimen) Duration: 2 years |
|
| Outcomes | Bone mineral density by DEXA: mean change in z‐score of lumbar spine (L2 to L4) and femoral neck. Cell sizes for analysis obtained from ClinicalTrials.gov posting. z‐score = (BMD ‐ mean for age‐matched reference population)/ SD for age‐matched reference population |
|
| Notes | Listed as Schering‐Plough 2011 in the previous update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow up: NOMAC‐E2, 2% (1/56), LNG/EE, 9% (5/54). Total losses: NOMAC‐E2, 23% (13/56); LNG/EE, 41% (22/54). |
Von Kesseru 2000.
| Methods | Randomized controlled trial in Argentina | |
| Participants | 148 healthy women, 38 to 50 years old, requesting contraception in 6 family planning centers. Inclusion criterion: normal menstrual patterns. Exclusion criteria: FSH < 40 IU, total cholesterol > 240 mg/dl, total triglycerides > 250 mg/dl, high‐density lipoprotein cholesterol < 35 mg/dl, low‐density lipoprotein cholesterol > 160 mg/dl. | |
| Interventions | 1) Monthly injections of norethisterone enanthate 50 mg plus estradiol valerate 5 mg (Mesigyna) (N=49). The first injection was given within first 5 days of first treatment cycle. 2) Nova‐T IUD (N=99). IUD was fitted within first 5 days of cycle. Study duration was 24 months |
|
| Outcomes | BMD of lumbar spine with DEXA was a secondary outcome; half the women had bone density measures. Primary study outcome was measurement of lipids. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list used to allocate 1:2. According to the author, allocation was from a randomized table provided by Schering. |
| Allocation concealment (selection bias) | Unclear risk | Co‐author could not provide information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high from the half allocated to bone density measures: for 12‐month change, 48% Mesigyna and 79% IUD; for 24‐month change, 70% and 84%, respectively. |
BMD = bone mineral density DEXA = dual‐energy X‐ray absorptiometry DMPA = depot medroxyprogesterone acetate; Depo‐Provera FSH = follicle stimulating hormone OC = oral contraceptive; COC = combination oral contraceptive
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berenson 2012 | Not RCT; women chose contraceptive method. |
| Carr 2003 | Participants were being treated for either uterine leiomyomata or endometriosis. |
| Gai 2011 | No mention of randomization |
| Gambacciani 2006 | Perimenopausal women recruited from Menopause Clinic. Comparison groups were not clearly reported. |
| Hauser 1970 | Study was identified in CENTRAL search, but bone health was not an outcome. |
| Lattakova 2009 | Not a randomized study. Participants were assigned numbers; even numbers were for the gestodene COC and odd numbers for the drospirenone COC. |
| Pfizer 2008 | Non‐randomized study, according to company report. Information on ClinicalTrials.gov was inconsistent, listing the study as randomized while the description stated the comparison would be a group electing non‐hormonal contraception or abstinence. |
| Pinter 2003 | Study was not RCT, according to correspondence with the investigator. |
| Shuzhi 2000 | English translation of abstract included the word 'randomly'. Article, written in Chinese, had no mention of randomization. In this convenient cohort study, groups were based on willingness to use DMPA or condoms. |
| Teegarden 2005 | Intervention was dietary counseling regarding calcium intake; groups were stratified by OC use or non‐use. |
OC = oral contraceptive
Characteristics of studies awaiting assessment [ordered by study ID]
Teva 2013.
| Methods | Randomized trial |
| Participants | 1363 adolescent females seeking contraception Inclusion criteria: healthy postmenarchal adolescent female 12‐18 years old, non‐pregnant, non‐lactating, regular menstrual cycles, BMI: 18 kg/m² to <30 kg/m², weight < 200 lbs, others as dictated by FDA‐approved protocol Exclusion criteria: contraindication to use of oral contraceptives, history of clinically significant adverse event while taking hormonal contraceptives, use of medication which could significantly interfere with study assessments, others as dictated by FDA‐approved protocol. |
| Interventions | 1) Levonorgestrel 150 μg plus ethinyl estradiol 30 μg and ethinyl estradiol 10 μg versus 2) levonorgestrel 100 μg plus ethinyl estradiol 20 μg and placebo Duration: 13 months |
| Outcomes | Primary: Mean percent change in lumbar spine BMD via dual energy x‐ray absorptiometry (DXA) Secondary: Absolute change in lumbar spine, proximal femur, and total body bone mineral density and bone mineral content (BMC) via DXA scan from baseline to month 6 and to month 12; effects on biochemical markers of bone resorption and bone formation; safety and tolerability of DR‐105 |
| Notes | Completion date: Oct 2012; no report found and no results posted. ClinicalTrials.gov last updated 10 Apr 2013. Listed as Duramed 2011 in previous update. Control group (not seeking contraception) not randomized. |
Characteristics of ongoing studies [ordered by study ID]
Bonny 2013.
| Trial name or title | Drug Exposure and Depot Medroxyprogesterone Acetate (DMPA) in Adolescent Subjects |
| Methods | Randomized controlled trial, open‐label, conducted at a hospital in Columbus, Ohio (USA). |
| Participants | 45 young women, age 12 to 21 years. Inclusion Criteria: 12 to 21 years old, healthy, post‐menarchal, self‐selected to use DMPA, willing to use barrier method of contraception in addition to DMPA. Exclusion criteria:
|
| Interventions | Depot medroxyprogesterone acetate (DMPA) intramuscular every 12 weeks for 12 months: 150 mg versus 104 mg versus 75 mg |
| Outcomes | Change in lumbar spine bone mineral density (BMD); change in total hip and femoral neck BMD. Duration: 48 weeks |
| Starting date | Sep 2011; estimated study completion, Dec 2014. |
| Contact information | Andrea Bonny, MD; Nationwide Children's Hospital |
| Notes | Purpose: to examine effect of different doses of DMPA on weight gain and BMD among adolescents. |
Contributions of authors
LM Lopez conducted the primary data abstraction and developed the review. Through the 2011 update, DA Grimes did the secondary data abstraction and KF Schulz provided statistical oversight. K Curtis contributed to the search methods. For the 2014 update, M Chen reviewed the evidence quality assessment and advised on presentation of statistics. All authors edited the manuscript and helped interpret the results.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
Support for conducting the review and updates at FHI 360 (through 2014)
-
US Agency for International Development, USA.
Through 2011: Support for conducting the review and updates at FHI 360. 2014: This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of The Evidence Project, cooperative agreement no. AID‐OAA‐A‐13‐00087. The findings and conclusions are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Declarations of interest
DA Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bahamondes 2006 {published data only}
- Bahamondes L, Monteiro‐Dantas C, Espejo‐Arce X, Dos Santos Fernandes AM, Lui‐Filho JF, Perrotti M, et al. A prospective study of the forearm bone density of users of etonogestrel‐ and levonorgestrel‐releasing contraceptive implants. Human Reproduction 2006;21(2):466‐70. [DOI] [PubMed] [Google Scholar]
- Monteiro‐Dantas C, Espejo‐Arce X, Lui‐Filho JF, Fernandes AM, Montiero I, Bahamondes L. A three‐year longitudinal evaluation of the forearm bone density of users of etonogestrel‐ and levonorgestrel‐releasing contraceptive implants. Reproductive Health 2007;4:11. [DOI: 10.1186/1742-4755-4-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berenson 2001 {published and unpublished data}
- Berenson AB, Breitkopf CR, Grady JJ, Rickert VI, Thomas A. Effects of hormonal contraception on bone mineral density after 24 months of use. Obstetrics and Gynecology 2004;103(5):899‐906. [DOI] [PubMed] [Google Scholar]
- Berenson AB, Radecki CM, Grady JJ, Rickert VI, Thomas A. A prospective, controlled study of the effects of hormonal contraception on bone mineral density. Obstetrics and Gynecology 2001;98(4):576‐82. [DOI] [PubMed] [Google Scholar]
Cibula 2012 {published data only (unpublished sought but not used)}
- Cibula D, Skrenkova J, Hill M, Stepan JJ. Low‐dose estrogen combined oral contraceptives may negatively influence physiological bone mineral density acquisition during adolescence. European Journal of Endocrinology 2012;166(6):1003‐11. [DOI] [PubMed] [Google Scholar]
Cromer 2005 {published data only}
- Cromer BA, Lazebnik R, Rome E, Stager M, Bonny A, Ziegler J, Debanne SM. Double‐blinded randomized controlled trial of estrogen supplementation in adolescent girls who receive depot medroxyprogesterone acetate for contraception. American Journal of Obstetrics and Gynecology 2005;192(1):42‐7. [DOI] [PubMed] [Google Scholar]
Cundy 2003 {published and unpublished data}
- Cundy T, Ames R, Horne A, Clearwater J, Roberts H, Gamble G, et al. A randomized controlled trial of estrogen replacement therapy in long‐term users of depot medroxyprogesterone acetate. Journal of Clinical Endocrinology and Metabolism 2003;88(1):78‐81. [DOI] [PubMed] [Google Scholar]
Di 1999 {published data only (unpublished sought but not used)}
- Di X, Li Y, Zhang C, Jiang J, Gu S. Effects of levonorgestrel‐releasing subdermal contraceptive implants on bone density and bone metabolism. Contraception 1999;60(3):161‐6. [DOI] [PubMed] [Google Scholar]
Endrikat 2004 {published data only (unpublished sought but not used)}
- Endrikat J, Mih E, Dusterberg B, Land K, Gerlinger C, Schmidt W, et al. A 3‐year double‐blind, randomized, controlled study on the influence of two oral contraceptives containing either 20 μg or 30 μg ethinylestradiol in combination with levonorgestrel on bone mineral density. Contraception 2004;69(3):179‐87. [DOI] [PubMed] [Google Scholar]
Gai 2012 {published data only}
- Gai L, Jia Y, Zhang M, Gai P, Wang S, Shi H, et al. Effect of two kinds of different combined oral contraceptives use on bone mineral density in adolescent women. Contraception 2012;86(4):332‐6. [DOI] [PubMed] [Google Scholar]
Gargano 2008 {published data only (unpublished sought but not used)}
- Gargano V, Massaro M, Morra I, Formisano C, Carlo C, Nappi C. Effects of two low‐dose combined oral contraceptives containing drospirenone on bone turnover and bone mineral density in young fertile women: a prospective controlled randomized study. Contraception 2008;78(1):10‐5. [DOI] [PubMed] [Google Scholar]
Hartard 2006 {published data only}
- Hartard M, Kleinmond C, Luppa P, Zelger O, Egger K, Wiseman M, et al. Comparison of the skeletal effects of the progestogens desogestrel and levonorgestrel in oral contraceptive preparations in young women: controlled, open, partly randomized investigation over 13 cycles. Contraception 2006;74(5):367‐75. [DOI] [PubMed] [Google Scholar]
Kaunitz 2009 {published data only}
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2‐year randomized study of contraceptive efficacy and bone mineral density. Contraception 2009;80(1):7‐17. [DOI] [PubMed] [Google Scholar]
Massaro 2010 {published and unpublished data}
- Massaro M, Carlo C, Gargano V, Formisano C, Bifulco G, Nappi C. Effects of the contraceptive patch and the vaginal ring on bone metabolism and bone mineral density: a prospective, controlled, randomized study. Contraception 2010;81(3):209‐14. [DOI] [PubMed] [Google Scholar]
Naessen 1995 {published and unpublished data}
- Naessen T, Olsson SE, Gudmundson J. Differential effects on bone density of progestogen‐only methods for contraception in premenopausal women. Contraception 1995;52(1):35‐9. [DOI] [PubMed] [Google Scholar]
Nappi 2003 {published data only (unpublished sought but not used)}
- Nappi C, Spiezio Sardo A, Acunzo G, Bifulco G, Tommaselli GA, Guida M, et al. Effects of a low‐dose and ultra‐low‐dose combined oral contraceptive use on bone turnover and bone mineral density in young fertile women: a prospective controlled randomized study. Contraception 2003;67(5):355‐9. [DOI] [PubMed] [Google Scholar]
Nappi 2005 {published data only}
- Nappi C, Spiezio Sardo A, Greco E, Tommaselli GA, Giordano E, Guida M. Effects of an oral contraceptive containing drospirenone on bone turnover and bone mineral density. Obstetrics and Gynecology 2005;105(1):53‐60. [DOI] [PubMed] [Google Scholar]
Paoletti 2000 {published and unpublished data}
- Paoletti AM, Orru M, Floris S, Mannias M, Vacca AMB, Ajossa S, et al. Evidence that treatment with monophasic oral contraceptive formulations containing ethinylestradiol plus gestodene reduces bone resorption in young women. Contraception 2000;61(4):259‐63. [DOI] [PubMed] [Google Scholar]
Rad 2011 {published data only (unpublished sought but not used)}
- Rad M, Kluft C, Kam ML, Meijer P, Cohen AF, Grubb GS, et al. Metabolic profile of a continuous versus a cyclic low‐dose combined oral contraceptive after one year of use. European Journal of Contraception and Reproductive Health Care 2011;16(2):85‐94. [DOI] [PubMed] [Google Scholar]
- Teichmann A, Apter D, Emerich J, Greven K, Klasa‐Mazurkiewicz D, Melis GB, et al. Continuous, daily levonorgestrel/ethinyl estradiol vs. 21‐day,cyclic levonorgestrel/ethinyl estradiol: efficacy, safety and bleeding in a randomized, open‐label trial. Contraception 2009;80(6):504‐11. [DOI] [PubMed] [Google Scholar]
- Teichmann AT, Kluft C, Grubb G, Constantine G, Spielmann D. Comparative trial of continuous‐use and 21‐day cyclic levonorgestrel and ethinyl estradiol oral contraceptive. Obstetrics and Gynecology 2006;107:12S. [Google Scholar]
- Wyeth. Study evaluating levonorgestrel and ethinyl estradiol in oral contraception. http://clinicaltrials.gov/ct2/show/NCT00248963 (accessed 29 Mar 2011).
Sordal 2012 {published data only}
- Merck Sharpt, Dohme Corp. Effects on bone mineral density (BMD) of the combined oral contraceptive NOMAC‐E2 compared to a COC containing LNG/EE (292005) (P05765). http://clinicaltrials.gov/ct2/show/NCT00511342 (accessed 02 Jan 2014).
- Sordal T, Grob P, Verhoeven C. Effects on bone mineral density of a monophasic combined oral contraceptive containing nomegestrol acetate/17beta‐estradiol in comparison to levonorgestrel/ethinylestradiol. Acta Obstetricia Gynecologica Scandinavica 2012;91(11):1279‐85. [DOI] [PubMed] [Google Scholar]
Von Kesseru 2000 {published and unpublished data}
- Kesseru E, Etchepareborda JJ, Wikinski R, Beier S. Premenopause contraception with monthly injectable Mesigyna with special emphasis on serum lipid and bone density patterns. Contraception 2000;61(5):317‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berenson 2012 {published data only}
- Berenson AB, Rahman M. Effect of hormonal contraceptives on vitamin B12 level and the association of the latter with bone mineral density. Contraception 2012;86(5):481‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2003 {published data only}
- Carr BR, Breslau NA, Peng N, Adams‐Huet B, Bradshaw KD, Steinkampf MP. Effect of gonadotropin‐releasing hormone agonist and medroxyprogesterone acetate on calcium metabolism: a prospective, randomized, double‐blind, placebo‐controlled, crossover trial. Fertility and Sterility 2003;80:1216‐23. [DOI] [PubMed] [Google Scholar]
Gai 2011 {published data only}
- Gai L, Zhang J, Zhang H, Gai P, Zhou L, Liu Y. The effect of depot medroxyprogesterone acetate (DMPA) on bone mineral density (BMD) and evaluating changes in BMD after discontinuation of DMPA in Chinese women of reproductive age. Contraception 2011;83(3):218‐22. [DOI] [PubMed] [Google Scholar]
Gambacciani 2006 {published data only}
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: Effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas 2006;54:176‐80. [DOI] [PubMed] [Google Scholar]
Hauser 1970 {published data only}
- Hauser GA, Girotti M. Effect of placebo instead of a pause in the taking of ovulation inhibitors [Wirking von placebo statt pause bei ovulationshemmern]. Therapeutische Umschau / Revue Thérapeutique 1970;27:666‐70. [PubMed] [Google Scholar]
Lattakova 2009 {published data only}
- Lattakova M, Borovsky M, Payer J, Killinger Z. Oral contraception usage in relation to bone mineral density and bone turnover in adolescent girls. European Journal of Contraception and Reproductive Health Care 2009;14(3):207‐14. [DOI] [PubMed] [Google Scholar]
Pfizer 2008 {published and unpublished data}
- Pfizer Inc. Dep‐Provera: bone mineral density and total body calcium in adolescent DP150CI users and non hormonal contraception. http://clinicaltrials.gov/ct2/show/NCT00139685 (accessed 29 Mar 2011).
- Pfizer Inc. Evaluation of bone mineral density and total body calcium in adolescent DP150CI users and non hormonal contraceptive users. http://www.clinicalstudyresults.org/documents/company‐study_2143_0.pdf (accessed 29 Mar 2011).
Pinter 2003 {published and unpublished data}
- Pinter B, Kocijancic A, Marc J, Andolsek‐Jeras L, Prezelj J. Vitamin D receptor gene polymorphism and bone metabolism during low‐dose oral contraceptive use in young women. Contraception 2003;67:33‐7. [DOI] [PubMed] [Google Scholar]
Shuzhi 2000 {published data only}
- Shuzhi F, Yuanjiao L, Laiying C, et al. The study on the effect of using depo medroxyprogesterone acetate for contraception on bone metabolism. Acta Academiae Medicinae Hubei 2000;21:330‐2. [Google Scholar]
Teegarden 2005 {published data only}
- Teegarden D, Legowski P, Gunther CW, McCabe GP, Peacock M, Lyle RM. Dietary calcium intake protects women consuming oral contraceptives from spine and hip bone loss. Journal of Clinical Endocrinology & Metabolism 2005;90:5127‐33. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Teva 2013 {published data only}
- Teva Pharmaceutical Industries (Duramed Research). A multicenter study to evaluate the effects of DR‐105 on bone mineral density in adolescent females. http://clinicaltrials.gov/ct2/show/NCT00924560 (accessed 02 Jan 2014).
References to ongoing studies
Bonny 2013 {published data only}
- Bonny A. Drug exposure and depot medroxyprogesterone acetate (DMPA) in adolescent subjects. http://clinicaltrials.gov/ct2/show/NCT01461824 (accessed 21 Nov 2013). [NCT01461824]
Additional references
ACOG 2008
- Committee on Adolescent Health, Committee on Gynecologic Practice. Depot medroxyprogesterone acetate and bone effects. Obstetrics and Gynecology 2008;112:727‐30. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Barad 2005
- Barad D, Kooperberg C, Wactawski‐Wende J, Liu J, Hendrix SL, Watts NB. Prior oral contraception and postmenopausal fracture: a Womens' Health Initiative observational cohort study. Fertility and Sterility 2005;84:374‐83. [DOI] [PubMed] [Google Scholar]
Bartz 2011
- Bartz D, Goldberg AB. Injectable contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:209‐36. [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. http://www.cdc.gov/mmwr/pdf/rr/rr59e0528.pdf (accessed 23 Jan 2012):34.
Cleland 2004
- Cleland J, Ali MM. Reproductive consequences of contraceptive failure in 19 developing countries. Obstetrics and Gynecology 2004;104:314‐20. [DOI] [PubMed] [Google Scholar]
Cooper 1993
- Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women: a prospective study. Bone 1993;14(1):41‐5. [DOI] [PubMed] [Google Scholar]
Cromer 2003
- Cromer BA. Bone mineral density in adolescent and young adult women on injectable or oral contraception. Current Opinion in Obstetrics & Gynecology 2003;15:353‐7. [DOI] [PubMed] [Google Scholar]
Cundy 1998
- Cundy T, Cornish J, Roberts H, Elder H, Reid IR. Spinal bone density in women using depot medroxyprogesterone contraception. Obstetrics and Gynecology 1998;92(4 Pt 1):569‐73. [DOI] [PubMed] [Google Scholar]
FDA 2004
- U.S. Food, Drug Administration. Safety. Depo‐Provera (medroxyprogesterone acetate injectable suspension). http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm (accessed 05 Feb 2014).
FDA 2011
- U.S. Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products. Depo‐Provera. Label and Approval History. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory (accessed 05 Feb 2014).
Gourlay 2004
- Gourlay ML, Brown SA. Clinical considerations in premenopausal osteoporosis. Archives of Internal Medicine 2004;164:603‐14. [DOI] [PubMed] [Google Scholar]
Grimes 2010
- Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women's health research: science, protoscience, and pseudoscience. Fertility and Sterility 2010;93(6):1731‐4. [DOI] [PubMed] [Google Scholar]
Guilbert 2009
- Guilbert ER, Brown JP, Kaunitz AM, Wagner MS, Bérubé J, Charbonneau L, et al. The use of depot‐medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009;79:167‐77. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Herrmann 2010
- Herrmann M, Seibel MJ. The effects of hormonal contraceptives on bone turnover markers and bone health. Clinical Endocrinology 2010;72(5):571‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 30 Mar 2011).
Howe 2011
Kaunitz 2011
- Kaunitz A M, Grimes D A. Removing the black box warning for depot medroxyprogesterone acetate. Contraception 2011;84:212‐3. [DOI] [PubMed] [Google Scholar]
Lopez 2012
- Lopez LM, Chen M, Mullins S, Curtis KM, Helmerhorst FM. Steroidal contraceptives and bone fractures in women: evidence from observational studies. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009849.pub2] [DOI] [PubMed] [Google Scholar]
Mansour 2012
- Mansour D. The benefits and risks of using a levonorgestrel‐releasing intrauterine system for contraception. Contraception 2012;85(3):224‐34. [DOI] [PubMed] [Google Scholar]
Martins 2006
- Martins SL, Curtis KM, Glasier AF. Combined hormonal contraception and bone health: a systematic review. Contraception 2006;73:445‐69. [DOI] [PubMed] [Google Scholar]
Meier 2010
Memon 2011
- Memon S, Iversen L, Hannaford PC. Is the oral contraceptive pill associated with fracture in later life? New evidence from the Royal College of General Practitioners Oral Contraception Study. Contraception 2011;84(1):40‐7. [DOI] [PubMed] [Google Scholar]
Nappi 2012
- Nappi C, Bifulco G, Tommaselli G A, Gargano V, Carlo C. Hormonal contraception and bone metabolism: a systematic review. Contraception 2012;86:606‐21. [DOI] [PubMed] [Google Scholar]
NIH 2000
- Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement 2000 March 27‐29 2000; Vol. 19, issue 1:1‐45. [PubMed]
Rachner 2011
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011/04/01 2011; Vol. 377, issue 9773:1276‐87. [DOI] [PMC free article] [PubMed]
Raisz 2005
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. Journal of Clinical Investigation 2005;115:3318‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholes 1999
- Scholes D, Lacroix AZ, Ott SM, Ichikawa LE, Barlow WE. Bone mineral density in women using depot medroxyprogesterone acetate for contraception. Obstetrics and Gynecology 1999;93:233‐8. [DOI] [PubMed] [Google Scholar]
Strauss 2005
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Trussell 2011
- Trussell J. Contraceptive failure in the United States. Contraception 2011;83:397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2011
- United Nations, Department of Economic and Social Affairs, Population Division. World Contraceptive Use 2011. http://www.un.org/esa/population/publications/contraceptive2011/wallchart_front.pdf (accessed 02 May 2012).
Vasikaran 2011a
- Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, et al. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clinical Chemistry and Laboratory Medicine 2011;49(8):1271‐4. [DOI] [PubMed] [Google Scholar]
Vasikaran 2011b
- Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporosis International 2011;22(2):391‐420. [DOI] [PubMed] [Google Scholar]
Vessey 1998
- Vessey M, Mant J, Painter R. Oral contraception and other factors in relation to hospital referral for fracture. Findings in a large cohort study. Contraception 1998;57:231‐5. [DOI] [PubMed] [Google Scholar]
Vestergaard 2006
- Vestergaard P, Rejnmark L, Mosekilde L. Oral contraceptive use and risk of fracture. Contraception 2006;73:571‐6. [DOI] [PubMed] [Google Scholar]
Warholm 2012
- Warholm L, Petersen K R, Ravn P. Combined oral contraceptives' influence on weight, body composition, height, and bone mineral density in girls younger than 18 years: a systematic review. Eur J Contracept Reprod Health Care 2012;17:245‐53. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. WHO statement on hormonal contraception and bone health. Contraception 2006;73:443‐4. [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. Fourth Edition, 2009. http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/index.html. Geneva: World Health Organization, (accessed 05 Dec 2011):45.


