Abstract
Long-term management of inflammatory skin diseases is challenging because of side effects from repeated use of systemic treatments or topical corticosteroids. This study sought to identify the mechanisms and developmental therapeutics for these diseases using genetic models and pharmacological approaches. We found that mice overexpressing SMAD7 in keratinocytes but not mice overexpressing the N-terminal domain of SMAD7 (i.e., N-SMAD7) were resistant to imiquimod-induced T helper 1/17- and T helper 2-type inflammation. We generated a Tat-PYC-SMAD7 (truncated SMAD7 protein encompassing C-terminal SMAD7 and PY motif fused with cell-penetrating Tat peptide). Topically applied Tat-PYC-SMAD7 to inflamed skin entered cells upon contact and attenuated imiquimod-, 2,4-dinitrofluorobenzene-, and tape-stripping-induced inflammation. RNA-sequencing analyses of mouse skin exposed to these insults showed that in addition to inhibiting TGFβ/NF-κB, SMAD7 blunted IL-22/signal transducer and activator of transcription 3 activation and associated pathogenesis, which is due to SMAD7 transcriptionally upregulating IL-22 antagonist IL-22RA2. Mechanistically, SMAD7 facilitated nuclear translocation and DNA binding of C/EBPβ to IL22RA2 promoter for IL22RA2 transactivation. Consistent with the observations in mice mentioned earlier, transcript levels of IL22RA2 were increased in human atopic dermatitis and psoriasis lesions with clinical remission. Our study identified the anti-inflammation functional domain of SMAD7 and suggests the mechanism and feasibility for developing SMAD7-based biologics as a topical therapy for skin inflammatory disorders.
INTRODUCTION
Inflammatory skin diseases, such as psoriasis and atopic dermatitis (AD), affect a large patient population worldwide (Takeshita et al., 2017a). These conditions often relapse, with persistence of inflammatory skin symptoms and a high risk of comorbidities (Takeshita et al., 2017b). AD and psoriasis are strongly related to poor QOL and high medical and societal burdens, highlighting the need for effective treatments (Chovatiya and Silverberg, 2019). Multiple systemic biologics, which target pathways associated with these diseases, have shown notable treatment efficacy (Beck et al., 2014). However, owing to systemic side effects and long-term therapy resistance caused by development of antidrug antibodies, clinical implementation of biologics has been restricted to patients with moderate-to-severe large, spread-out lesions (Aranez and Ambrus, 2020; Munera-Campos and Carrascosa, 2020). For patients with localized mild-to-moderate lesions, topical corticosteroids (TCSs) remain the most frequently applied drugs (Eichenfield et al., 2014). Owing to the broad, nonspecific effects of TCS, cutaneous and systemic adverse effects frequently occur with long-term or repeated use of TCS. Even mildly potent TCS could cause adverse effects when treating delicate skin areas, for example, facial skin. In this context, developing more effective therapies that can treat focal lesions through topical application remains an unmet medical need. Hence, identifying druggable mechanisms of action is essential for therapeutic development.
We previously established transgenic mice that express a Smad7 transgene under the control of keratin 5 promoter (i.e., K5.Smad7) (He et al., 2002). SMAD7 is a dual inhibitor for TGFβ1 and NF-κβ signaling (Hong et al., 2007; Nakao et al., 1997). K5.Smad7 transgene completely blocked psoriasis-like skin phenotype in transgenic mice overexpressing TGFβ1 in keratinocytes (i.e., K5.TGFβ1). However, because SMAD7 is a known TGFβ inhibitor (Nakao et al., 1997), it remains to be determined whether the effect of SMAD7 is broadly applicable to skin inflammatory disorders. Moreover, whereas NF-κB signaling is considered a therapeutic target for T helper (Th) 1/Th17- and Th2-type inflammation (Yu et al., 2020), the role of TGFβ1 in type II immune responses has been controversial (Bossé and Rola-Pleszczynski, 2007; Han et al., 2012). It is unclear whether anti-TGFβ and anti-NF-κB effects of SMAD7 would function synergistically or antagonistically in certain types of inflammation. In this study, we employed mouse models, combined with pharmacological approaches, to evaluate the functions of SMAD7 under three different inflammatory conditions and explored whether SMAD7 or its derivative has therapeutic effects on inflammatory skin conditions. Our findings revealed the therapeutic effects of SMAD7 on psoriasis-and dermatitis-associated inflammation, and the critical functional domain of SMAD7 fused with a cell-permeable peptide delivered a therapeutic effect through topical application. In addition to anti-TGFβ and -NF-κB, we identified a molecular mechanism in which SMAD7 transcriptionally upregulates IL-22-binding protein, IL-22RA2, to inhibit IL-22/signal transducer and activator of transcription (STAT) 3 activation. Our study also provides evidence for using SMAD7-based topical drugs to treat lesional skin inflammation.
RESULTS
K5.Smad7 mice were resistant to imiquimod-induced psoriasis-like dermatitis
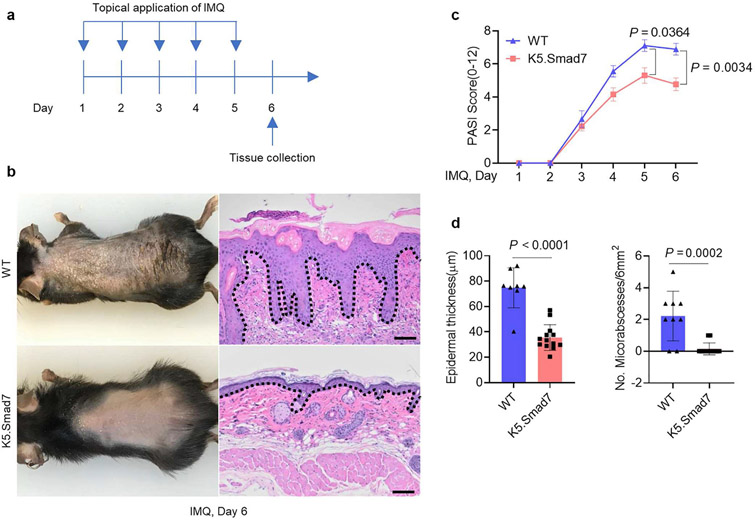
We previously developed K5.Smad7 mice maintained on a C57BL/6J strain background (He et al., 2002). We applied imiquimod (IMQ) on the skin of both K5.Smad7 transgenic and wildtype (WT) littermates to induce psoriasis-like lesions (Figure 1a). SMAD7 expression in K5.Smad7 skin was about 2 ~ 3 fold higher than that in WT skin on day 6 of IMQ application (Supplementary Figure S1a). Correspondingly, severe psoriatic lesions were observed on IMQ-treated WT skin, whereas only mild lesions were observed on IMQ-treated K5.Smad7 skin (Figure 1b). PASI scoring showed that K5.Smad7 mice had significantly attenuated psoriasis severity compared with WT mice (Figure 1c). Consistently, histological analysis revealed reduced epidermal thickness and fewer Munro microabscesses in IMQ-treated K5.Smad7 skin (Figure 1d), indicating that K5.Smad7 mice are resistant to IMQ-induced psoriatic inflammation. Because IL-17 and IL-23 signaling are involved in the pathogenesis of IMQ-induced psoriasis (van der Fits et al., 2009), we performed RNA-sequencing analysis comparing K5.Smad7 IMQ-treated skin with WT IMQ-treated skin, revealing significant inhibition of IL-17 signaling in K5.Smad7-transgenic skin (Supplementary Figure S1b and c), but we did not find a direct suppression of IL-23 signaling by SMAD7 overexpression (data not shown).
Figure 1. K5.Smad7 mice are resistant to IMQ-induced psoriasis-like skin dermatitis.
(a) Experimental design for IMQ-induced psoriatic lesions in K5.Smad7 and WT skin. (b) Representative gross images and corresponding H&E skin sections of IMQ-induced lesions on experimental day 6 from WT and K5.Smad7 mice. (c) PASI scores of IMQ-induced skin phenotypes of the WT littermate and K5.Smad7 mice on days 1–6 during the IMQ application. (d) The average epidermal thickness and the number of microabscesses in H&E. Dotted lines: epidermal-dermal boundary. Bars = 100 μm. Skin samples from WT (n = 8) and K5.Smad7 (n = 13) mice were analyzed using two-way ANOVA or two-tailed unpaired t-test for statistics. Data are representative of at least three independent experiments with 3–6 samples per group. Data represent mean ± SEM. IMQ, imiquimod; WT, wild type.
To evaluate the functional domains of SMAD7 in skin inflammation, we developed K5.N-Smad7-transgenic mice that express human N-terminal SMAD7 (1-258 amino acid) in keratinocytes under the control of keratin 5 promoter (Supplementary Figure S2a). K5.N-Smad7 mice did not develop spontaneous skin phenotypes and exhibited IMQ-induced lesions similar to those of WT littermates (Supplementary Figure S2b-g), suggesting that anti-inflammatory effect of SMAD7 could be attributed to the C-terminus of SMAD7.
Tat-PYC-SMAD7 treatment showed anti-inflammatory effects on both psoriatic and AD-like inflammation
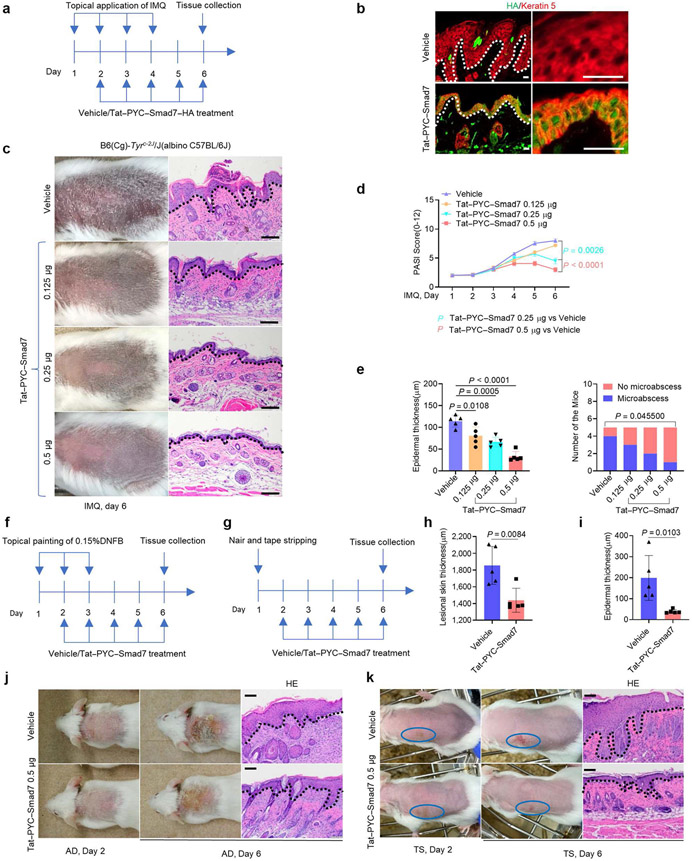
We made a recombinant Tat-PYC-SMAD7-hemagglutinin (HA) protein containing the PY motif and C domain of SMAD7 with a HA tag. In HaCaT keratinocytes, Tat-PYC-SMAD7-HA was detected in the nucleus 5 minutes after treatment, showing a high efficiency of cell penetration (Supplementary Figure S3a). Treatment efficacy of Tat-PYC-SMAD7 was first tested on IMQ-induced psoriatic model in C57BL/6J mice (Figure 2a). Stained sections of IMQ-treated skin show that Tat-PYC-SMAD7-HA was detected primarily in the nucleus of keratinocytes in treated mice (Figure 2b). In contrast to psoriatic phenotypes observed in vehicle-treated mice, Tat-PYC-SMAD7-treated mice developed less severe lesions and exhibited a dose-dependent effect of Tat-PYC-SMAD7 determined by PASI score, epidermal thickness, and microabscess quantification (Figure 2c-e).
Figure 2. Tat-PYC-SMAD7 treatment ameliorates IMQ-, DNFB-, and Tape stripping-induced skin inflammation.
(a, f, g) Experiment design for Tat-PYC-SMAD7 treatment tests on three skin inflammation models. (b) IF staining of HA-tagged Tat-PYC-SMAD7 2 h after topical application of the 0.5 μg protein on day 6 in the skin, with higher-power frames on the right. (c, j, k) Representative gross images and corresponding H&E skin sections of skin lesions. (d) PASI scores of IMQ-induced skin phenotypes. (e) Quantification of average epidermal thickness (left) and the number of mice (right) in c. Quantification of the average thickness (h) for AD skin and (i) for TS skin. Bars = 25 μm for b and 100 μm for the rest images. Data were assessed using two-way or one-way ANOVA or chi-square test. Data are representative of three independent experiments with five samples per group. Dotted lines: epidermal-dermal boundary. Data represent mean ± SEM. AD, atopic dermatitis; HA, hemagglutinin; IF, immunofluorescent; IMQ, imiquimod; TS, tape stripping.
Given the strain-dependent effects of IMQ (Swindell et al., 2017) because C57BL/6 and FVB strains induce Th17 cell differentiation and IL-17-mediated psoriatic inflammation, whereas BALB/c mice preferably exhibit Th2 type inflammation, we also tested the effects of Tat-PYC-SMAD7 on FVB and BALB/C mice induced with IMQ and observed similar therapeutic effects in both strains (Supplementary Figure S3b and c), indicating Tat-PYC-SMAD7 could target both Th1/Th17 and Th2 type inflammation. Therefore, we employed two Th2-dominant atopic inflammatory models-DNFB-induced AD and tape stripping (TS) inflammation-for further evaluation. TS mimics skin barrier defect-associated contact hypersensitivity (Holzmann et al., 2004; Oyoshi et al., 2010; Shimura et al., 2016) and can be viewed as a wound-healing model. We found a robust treatment effect of Tat-PYC-SMAD7 on both AD and TS lesions, evidenced by reduced epidermal thickness (Figure 2f-k). These data reveal the anti-inflammatory effects of Tat-PYC-SMAD7 on both Th1/17-type psoriatic and Th2-type atopic inflammation.
Tat-PYC-SMAD7 suppressed IL-22 signaling in psoriatic and AD-like inflammation
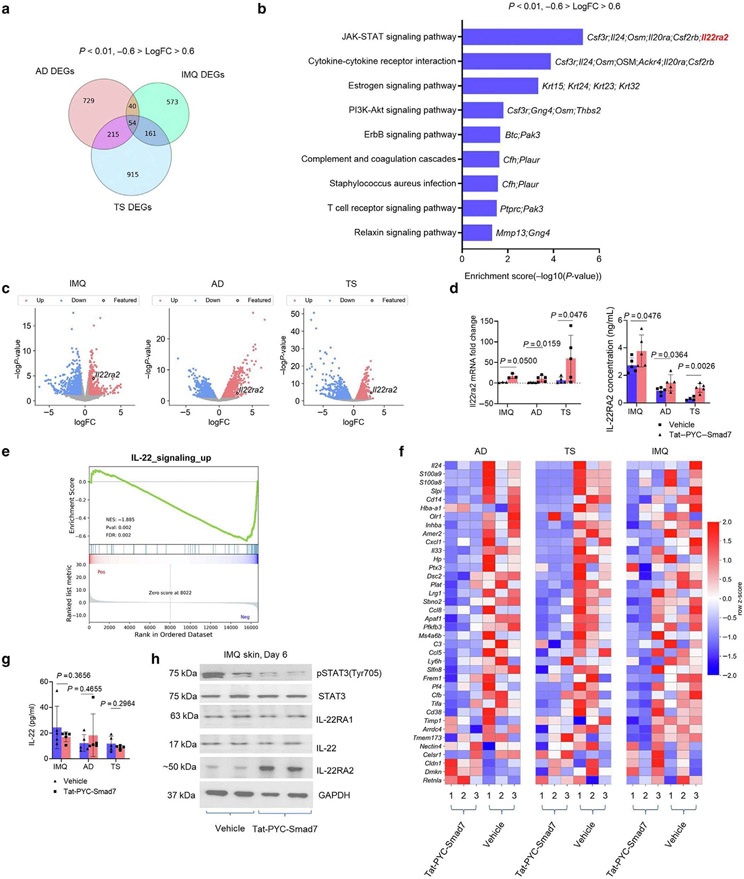
To investigate the mechanism underlying the therapeutic effect of Tat-PYC-SMAD7, RNA sequencing was performed using tissue samples of IMQ, AD, and TS skin from vehicle- and Tat-PYC-SMAD7-treated skin on day 6. Principal components analysis revealed distinct genomic profiles of three inflammatory models (Supplementary Figure S4a). A total of 1,038; 828; and 1,345 differentially expressed genes were identified for AD, IMQ, and TS groups, respectively (P < 0.01, -0.6 > log fold change > 0.6) (Figure 3a). Among all differentially expressed genes, 54 genes were similarly changed in three skin inflammation models with respect to Tat-PYC-SMAD7 treatment (Figure 3a and Supplementary Figure S4b). Gene set enrichment analyses of Kyoto Encyclopedia of Genes and Genomes gene sets using these 54 genes revealed that Jak/STAT signaling was the most highly enriched pathway, in which Il22ra2 was the only upregulated gene (Figure 3b). Upregulation of the Il22ra2 transcript in Tat-PYC-SMAD7-treated samples was consistent in all three models (Figure 3c and Supplementary Figure S4b). Il22ra2 gene encoding IL-22RA2 protein is a soluble, monomeric protein that binds IL-22, thereby blocking IL-22 signaling cascade (Huber et al., 2012). We confirmed a consistent upregulation of Il22ra2 mRNA and IL-22RA2 protein in Tat-PYC-SMAD7-treated skin compared with that in vehicle-treated samples (Figure 3d), suggesting that IL-22RA2 could be a mediator of PYC-SMAD7-derived anti-inflammatory effect. Consistent with previous reports (Li et al., 2019), we found downregulation of TGFβ and NF-κB signaling in Tat-PYC-SMAD7-treated skin by gene set enrichment analysis against Hallmark gene sets and reduced nuclear levels of TGFβ and NF-κB activation markers phosphorylated SMAD2 and NF-κB p50, respectively (Supplementary Figure S4c and d). We also found consistent repression of T-cell-regulated IL-17 signaling in Tat-PYC-SMAD7-treated lesions in all three models (Supplementary Figure S5), but we did not find a direct suppression of IL-23 signaling by Tat-PYC-SMAD7 treatment.
Figure 3. IL-22RA2 is a putative target responsible for PYC-SMAD7-derived IL-22 signaling suppression.
(a) Overlapping *DEGs across transcriptomes of IMQ, AD, and TS skin by Tat-PYC-SMAD7 treatment. (b) Enriched KEGG pathways for overlapping DEGs. (c) Volcano plots for transcriptomes of inflamed skin samples, with Il22ra2 as a common DEG upregulated by Tat-PYC-SMAD7 treatment. (d) mRNA fold change by qPCR and IL22RA2 levels by ELISA using lysates of lesional skin samples. (e) Enrichment plot and (f) heatmap for IL-22 signaling-upregulated genes. (g) IL-22 levels detected by ELISA and (h) western blot for IL-22 signaling markers using protein lysis of lesional skin samples. Samples were qualified using two-tailed unpaired t- or Mann-Whitney U test. Data are representative of three independent experiments with five samples per group. Data represent mean ± SEM. AD, atopic dermatitis; DEG, differentially expressed gene; FC, fold change; IMQ, imiquimod; KEGG, Kyoto Encyclopedia of Genes and Genomes; pSTAT3, phosphorylated signal transducer and activator of transcription 3; STAT3, signal transducer and activator of transcription 3; TS, tape stripping.
Because IL-22RA2 expression directly affects IL-22 signaling and because IL-22 signaling contributes to the development of IMQ-induced psoriasis lesions (Van Belle et al., 2012), we investigated whether Tat-PYC-SMAD7 had a direct effect on IL-22 signaling. Gene set enrichment analyses against Il22 gene sets indicated decreased levels of IL-22-upregulated genes in Tat-PYC-SMAD7-treated samples compared with those in vehicle treatment in IMQ, AD, and TS skin (Figure 3e and f). However, IL-22 levels in Tat-PYC-SMAD7-treated skin were not significantly changed compared with those in vehicle-treated skin (Figure 3g), indicating that IL-22 signaling inhibition was not a result of IL-22 reduction. Consistent with IL-22 signaling suppression, we observed reduced phosphorylated STAT3 in Tat-PYC-SMAD7-treated skin, whereas expression of IL-22 and IL-22 receptor IL-22RA1 were not significantly changed, and that of IL-22RA2 was increased (Figure 3h).
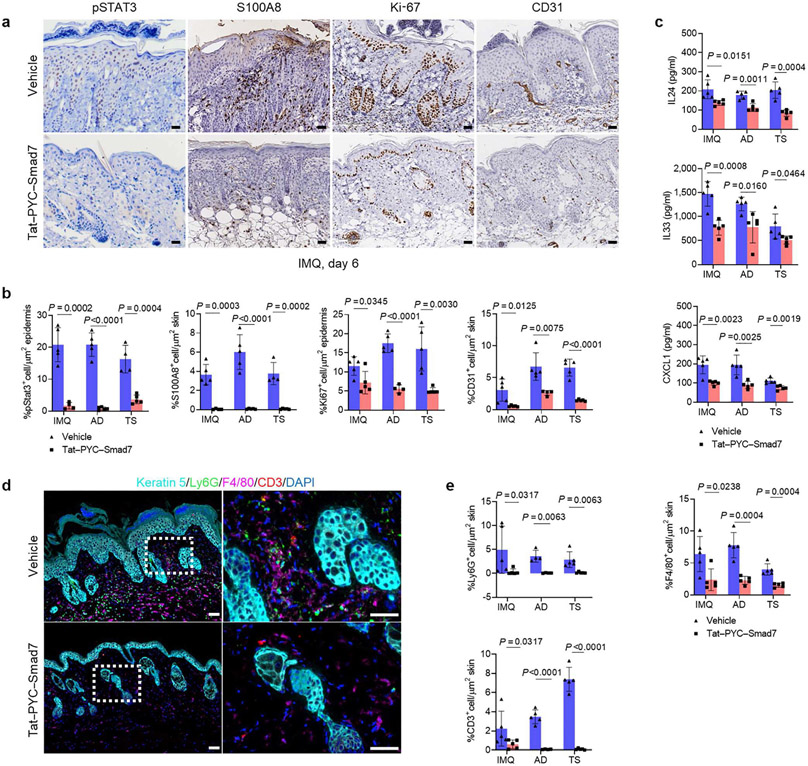
Corresponding to diminished IL-22 signaling, Gene Ontology analysis indicated downregulation of biological processes, including positive regulation of epithelial cell proliferation, neutrophil chemotaxis, and positive regulation of angiogenesis in Tat-PYC-SMAD7-treated samples (Supplementary Figure S4e). Consistently, immunohistochemistry staining showed a significant decrease in the staining of IL-22 signaling markers in Tat-PYC-SMAD7-treated lesions, including nuclear phosphorylated STAT3, S100A8, Ki-67+ proliferative keratinocytes, and CD31+ endothelial cells (Figure 4a and b). In addition, tissue lysate from vehicle- and Tat-PYC-SMAD7-treated lesions showed attenuated levels of IL-22-regulated cytokines, including IL-24, IL-33, and CXCL1 that are associated with cell proliferation, keratinocyte activation, and neutrophil chemotaxis (Martin et al., 2017) (Figure 4c). Finally, multiplex fluorescent immunohistochemistry for spatial immune analysis showed a drastically reduced number of Ly6G+ neutrophils, CD3+T cells, and F4/80+ macrophages infiltrating local lesions of Tat-PYC-SMAD7-treated skin compared with that in vehicle-treated skin (Figure 4d and e). These data together showed a profound impact of Tat-PYC-SMAD7 in dampening IL-22 signaling and its downstream pathogenic events.
Figure 4. Tat-PYC-SMAD7 dampens IL-22 signaling and relevant pathological events.
(a) Representative images and (b) quantification of IHC staining for IL-22 signaling markers pSTAT3, S100A8, Ki-67, and CD31 in skin sections of IMQ, AD, and TS samples. (c) Levels of IL-24, IL-33, and CXCL1 by ELISA from IMQ, AD, and TS skin lysis. (d) Representative images and (e) quantification of MFI staining on sections of IMQ, AD, and TS skin lesions. Dotted lines: epidermal-dermal boundary. White dot frames indicate areas in higher magnification to the right of each image. Bars = 25 μm for all slides. Samples from each group were qualified using two-tailed unpaired t or Mann-Whitney U test for statistics. Data are representative of three independent experiments with 4–5 samples per group. Data represent mean ± SEM. AD, atopic dermatitis; IHC, immunohistochemistry; MFI, multiplex fluorescent immunohistochemistry; IMQ, imiquimod; pSTAT3, phosphorylated signal transducer and activator of transcription 3; TS, tape stripping.
IL-22RA2 inhibition attenuated the anti-inflammatory effect of SMAD7
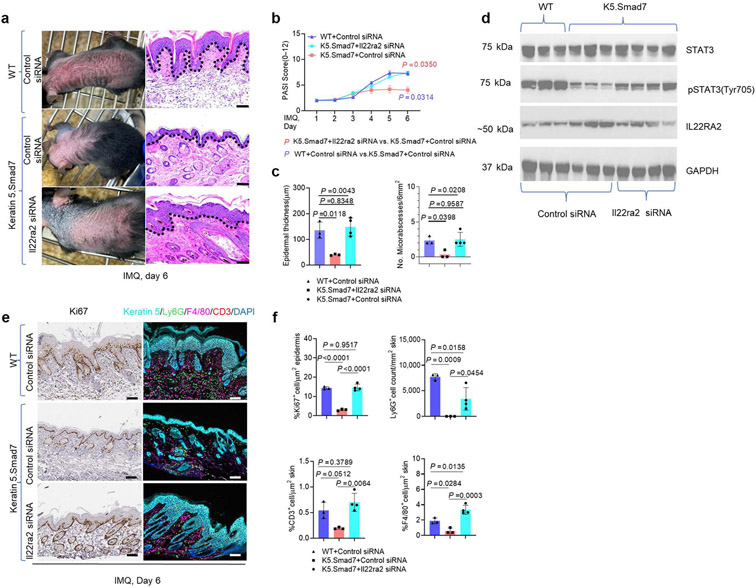
To determine whether transcriptional upregulation of Il22ra2 by SMAD7 contributed to the amelioration of IMQ-induced inflammation, we utilized in vivo delivery of Il22ra2 small interfering RNA (siRNA) for blockade of endogenous IL-22RA2 in IMQ-treated skin in K5.Smad7 mice and WT littermates. After the IMQ application, K5.Smad7 skin injected with control siRNA showed resistance to psoriatic inflammation (Figure 5a-c). In contrast, siRNA knockdown of Il22ra2, IMQ-induced erythema, thickening, and patchy scaling were observed in K5.Smad7 skin, similar to those observed in IMQ-applied WT skin with control siRNA delivery (Figure 5a-c). Tissue lysates showed that IL-22RA2 upregulation in K5.Smad7 IMQ-treated skin was blocked by Il22ra2 siRNA delivery, accompanied by increased phosphorylated STAT3 (Figure 5d). Correspondingly, immunohistochemistry and multiplex fluorescent immunohistochemistry staining showed a more pronounced epidermal proliferation and inflammatory cell infiltration in Il22ra2 siRNA-treated K5.Smad7 IMQ-treated skin (Figure 5e and f), suggesting IL-22RA2 as a critical mediator contributing to the anti-inflammatory functions of SMAD7.
Figure 5. IL-22RA2 inhibition blocks the anti-inflammatory effects of SMAD7.
(a) Representative gross images and corresponding H&E skin sections of IMQ-induced lesions with in vivo delivery of either control or Il22ra2-directed siRNA. (b) PASI scores of IMQ-induced skin phenotypes of WT and K5.Smad7 mice during IMQ application. (c) Average epidermal thickness and number of microabscesses in H&E. (d) Western blot using protein lysis of lesional skin samples. Representative images (e) and (f) quantification of IHC and MFI staining on sections of skin lesions. Dotted lines: epidermal-dermal boundary. Bars = 100 μm for all slides. A total of 3–4 skin samples from each group were qualified using two-way ANOVA or one-way ANOVA for statistics. Data are representative of at least two independent experiments with 3–4 samples per group. Data represent mean ± SEM. IHC, immunohistochemistry; IMQ, imiquimod; pSTAT3, phosphorylated signal transducer and activator of transcription 3; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3; WT, wild type.
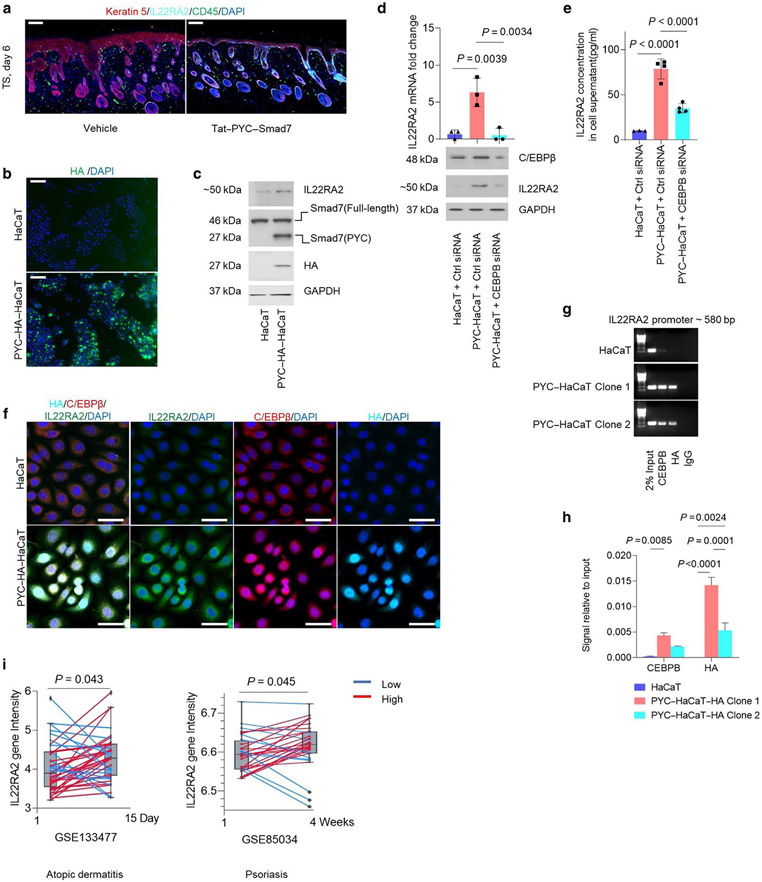
PYC-SMAD7 promoted C/EBPβ DNA binding to IL-22RA2 promoter that conferred IL22RA2 transcription
To determine the cellular source of IL-22RA2, we performed immunofluorescent costaining and found the expression of IL-22RA2 in Tat-PYC-SMAD7-treated IMQ epidermis (Figure 6a). To verify whether PYC-SMAD7 directly regulates IL-22RA2 expression, we stably transfected human HaCaT keratinocytes with a PYC-SMAD7-HA expression construct (PYC-HaCaT). HA staining indicated predominantly nuclear staining of HA-PYC-SMAD7 (Figure 6b), consistent with the observation in Tat-PYC-SMAD7-treated skin (Figure 2b). mRNA level of C-terminal Smad7 was 60-fold higher (Supplementary Figure S6a), and the protein level of PYC-SMAD7 was about the same as that of endogenous full-length SMAD7 in PYC-HaCaT compared with that in HaCaT cells (Figure 6c). IL-22RA2 was significantly increased in PYC-HaCaT cells at RNA and protein levels compared with that in HaCaT cells (Supplementary Figure S6a and Figure 6c), suggesting PYC-SMAD7-dependent IL-22RA2 upregulation. We searched for transcriptional enhancer-binding sites in IL22RA2 gene regulatory sequence and found that the C/EBPβ-binding site is a predicted regulator of IL-22RA2 expression (Gene-Hancer Identifier: GH06J137240). Transcriptional functions of C/EBPβ have been reported to be repressed by SMAD3/SMAD4 through physical interaction (Choy and Derynck, 2003). C/EBPβ also plays a key role in the coordination of TGFβ cytostatic gene responses (Gomis et al., 2006). Knockdown of C/EBPβ in PYC-HaCaT cells effectively reduced Il22ra2 mRNA and protein levels (Figure 6d and e). Moreover, costaining of HA, C/EBPβ, and IL-22RA2 in HaCaT and PYC-HaCaT showed C/EBPβ in the cytoplasm of HaCaT cells but mostly in the nucleus of PYC-HaCaT cells (Figure 6f). PYC-HaCaT cells exhibited a stronger expression of IL-22RA2 (Figure 6f). These results suggest that PYC-SMAD7 facilitates C/EBPβ nuclear translocation and subsequently promotes IL-22RA2 expression. From the JASPAR database, putative DNA binding sites of C/EBPβ within human IL22RA2 promoter were identified. We performed chromatin immunoprecipitation using PYC-HaCaT cell clones that have similar levels of C/EBPβ and endogenous full-length SMAD7 expression (Supplementary Figure S6b and c). mRNA levels of IL22RA2 reached ~40-fold and 8-fold in PYC-SMAD7 clone 1 and clone 2, respectively, compared with that in HaCaT cells (Supplementary Figure S6c). Furthermore, chromatin immunoprecipitation assay confirmed that C/EBPβ and PYC-SMAD7 (detected by HA-Tag antibody) both bind to IL22RA2 promoter ~580 base pair site in PYC-HaCaT cells, whereas no binding of C/EBPβ was detected at IL22RA2 promoter in HaCaT cells (Figure 6g and h). Therefore, PYC-SMAD7 promotes C/EBPβ binding to the IL22RA2 promoter.
Figure 6. PYC-SMAD7 promotes C/EBPβ DNA binding conferring IL22RA2 transcription expression.
(a) IF visualizing IL-22RA2-expressing cells in Tat-PYC-SMAD7-treated skin. (b) IF visualizing HA-tag in PYC-SMAD7-HA transfected HaCaT cells and WT HaCaT cells. (c) Western blot using cell lysate from HaCaT and PYC-SMAD7-HA transfected HaCaT cells. (d) mRNA change by qPCR and protein expression by western blot and (e) IL-22RA2 levels by ELISA from HaCaT and PYC-SMAD7-HA transfected HaCaT cells transfected with CEBPB or Ctrl siRNA for 48 h. (f) IF showing the nucleus/cytoplasmic presences of IL-22RA2, C/EBPβ, and HA. (g) ChIP assay and (h) ChIP-qPCR for binding to ~580 base pair site of IL-22RA2 promoter. (i) IL22RA2 gene intensity of microarray data from GEO database. Data were qualified using two-tailed unpaired or paired t-test or two-way or one-way ANOVA for statistics. Data are representative of two or three independent experiments. Data represent mean ± SEM. PYC-HA-HaCaT or PYC-HaCaT: PYC-SMAD7-HA transfected HaCaT cells. ChIP, chromatin immunoprecipitation; Ctrl, control; GEO, Gene Omnibus Expression; h, hour; HA, hemagglutinin; IF, immunofluorescent; siRNA, small interfering RNA; TS, tape stripping.
Elevated IL-22RA2 could be a therapeutic target for human skin inflammation
To assess whether patients with inflammation could benefit from local modulation of IL-22RA2, we explored open-access data from the Gene Expression Omnibus database. We chose the crisaborole clinical trials that target skin barrier dysfunction (NCT03233529) (Bissonnette et al., 2019) in patients with AD and adalimumab that targets TNF signaling (NCT00932113) (Goldminz et al., 2015) for patients with psoriasis. We found significantly higher levels of IL22RA2 gene expression in lesions from patients with AD receiving topical application of crisaborole and patients with psoriasis given treatments of adalimumab than the initial levels (Figure 6i). These data suggested that IL-22RA2 could be a predictive marker for therapeutic response to drugs treating skin inflammation. With its effect of elevating IL-22RA2, SMAD7-based Tat protein could be a promising therapeutic agent for skin inflammatory diseases.
DISCUSSION
Transcriptional regulation of IL-22RA2 by PYC-SMAD7 plays a critical role against inflammation in epithelial defenses and reflects a context-dependent regulation of IL-22RA2
Our study shows that IL-22RA2 is a mediator responsible for Tat-PYC-SMAD7-derived IL-22 signaling inhibition. This finding aligns with previous studies showing an anti-inflammatory role of IL-22RA2 in the skin (Voglis et al., 2018). IL-22RA2 could be significant for skin homeostasis because its expression was low in lesional skin from patients with psoriasis and AD and higher when lesions improved with treatment (Figure 6i). Similarly, in the intestine, IL-22-IL-2RA2 balance is known to regulate tissue repair and tumorigenesis in the colon (Huber et al., 2012). Despite similar functions of IL-22RA2 in the skin and intestine, local sources of IL-22RA2 could be different. In the intestine, local dendritic cells and circulating eosinophils are considered the primary source (Huber et al., 2012; Kempski et al., 2020; Martin et al., 2016), whereas in the skin, keratinocytes appear to be the main source of IL-22RA2. Because local delivery of Tat-PYC-SMAD7 barely exerts any systemic effects (Boss et al., 2022) and because systemic levels of IL-22 were not significantly decreased by Tat-PYC-SMAD7 (Figure 3g), IL-22RA2 should be primarily functional in situ. Consistently, RNA-sequencing data showed IL22RA2 transcription in the interfollicular epidermis in mouse skin (Joost et al., 2016), where immunofluorescent staining also indicated IL-22RA2 expression in TS skin (Figure 6a). Given that interfollicular epidermis exhibits typical features of psoriasis (Wagner et al., 2010), keratinocyte-derived IL-22RA2 could play a critical role in epithelial defenses at the inflammatory frontier.
In the intestine, IL-22RA2 is regulated by IL-18 inflammasomes or through noncanonical NF-κB pathway in dendritic cells (Huber et al., 2012; Kempski et al., 2020). However, IL-18 treatment failed to trigger the expression of IL-22RA2 in HaCaT cells (data not shown). These different mechanisms of activation may reflect a context-dependent regulation of IL-22RA2 and correspond with a context-dependent effect of SMAD7 in the intestine and skin (Alfei et al., 2019). Meanwhile, our data provide evidence to support PYC-SMAD7 as a transcriptional coregulator. This is consistent with previous reports about the DNA-binding property of C-SMAD7 (Shi et al., 2008). Moreover, SMAD7 antagonizes TGFβ signaling in the nucleus by interrupting functional SMAD-DNA complex formation (Zhang et al., 2007). Given that C-SMAD7 exhibited a DNA-binding function with higher binding affinity to DNA elements than full-length SMAD7 (Shi et al., 2008; Zhang et al., 2007), this property of C-SMAD7 may contribute to a stronger anti-inflammatory effect of Tat-PYC-SMAD7 than that of Tat-SMAD7.
Anti-IL-17/IL-22 and anti-TGFβ/NF-κB function of Tat-PYC-SMAD7 collectively contribute to therapeutic effects on Th1/17- and Th2-type inflammation
Our results revealed consistent repression of IL-17 signaling after Tat-PYC-SMAD7 treatment across all three models (Supplementary Figure S5). We also observed a consistent reduction in type II cytokine Il33 (Imai, 2019) after Tat-PYC-SMAD7 treatment (Figure 4c). Because IL-17 signaling was also involved in the pathogenesis of AD (Eyerich et al., 2009; Koga et al., 2008; Noda et al., 2015), IL-17 and IL-22 pathways converge in treating psoriasis and AD through Tat-PYC-SMAD7. We did not find a direct suppression of IL-23 signaling by SMAD7 overexpression or Tat-PYC-SMAD7 treatment, suggesting that either SMAD7 is insufficient to inhibit TGFβ-mediated IL-23 induction, or IL-23 is also regulated by other pathways (Lowes et al., 2013; Mellett et al., 2018).
IL-22 signaling has been identified as a common target of psoriasis and AD (Dudakov et al., 2015). IL-22 signaling could be mainly responsible for keratinocyte proliferation and T-cell infiltration because the numbers of proliferative Ki-67+ and CD3+ T cells in IL-22RA2 siRNA-transfected K5.Smad7 IMQ-treated skin was almost equal to that of WT IMQ-treated skin (Figure 5e and f). This is in line with previous reports showing that IL-22 strongly induces hyperplasia of the reconstituted human epidermis and promotes T-cell chemokine expression in keratinocytes (Boniface et al., 2005). In addition to IL-17/IL-22 signaling, TGFβ and NF-κB signaling pathways are also known targets of Tat-PYC-SMAD7 because TGFβ itself and NF-κB-induced chemokines often contribute to monocyte chemotaxis or motility (Li et al., 2019). This could explain why IL-22RA2 knockdown did not completely block K5.Smad7 effect on reducing F4/80+ macrophage and Ly6G+ neutrophil infiltration (Figure 5e and f).
Our analysis showed TGFβ signaling as a common target of Tat-PYC-SMAD7 for three skin inflammatory conditions. Tat-PYC-SMAD7 is expected to attenuate Th1/17-type inflammation through C-SMAD7-dependent TGFβ signaling inhibition (Yan et al., 2016). However, loss of TGFβ signaling resulting in defective immune tolerance has been linked to the risk of type-II allergic diseases, such as AD and asthma (Hoffjan et al., 2004; Li et al., 2007; Papaioannou et al., 2019). Therefore, TGFβ1 may not be an initiator but plays a pathogenic role in established or sustained Th2-type inflammations. To support this, a dramatic increase of nuclear phosphorylated SMAD2 expression in bronchial epithelial, alveolar, and infiltrating inflammatory cells was found in allergen-challenged lungs (Rosendahl et al., 2001). Moreover, mast cell-mediated allergic airway inflammation and fibrosis were ameliorated by inhibiting the TGFβ signaling pathway (Kim et al., 2020). Therefore, our study supports that anti-IL-22 and anti-TGFβ function of Tat-PYC-SMAD7 synergistically contribute to therapeutic effects on Th1/17- and Th2-type inflammation.
Tat-PYC-SMAD7 has the potential as a targeted topical therapy for skin inflammation
Our data showed that Tat-PYC-SMAD7 efficiently penetrates the epidermis with a compromised skin barrier, indicating the feasibility of topical delivery of SMAD7-based protein. Because Tat-PYC-SMAD7 targets local specific proinflammatory pathways, it may reduce off-target side effects compared with TCS and thus could be better tolerated during repeated dosing. For example, TCS-induced fibrotic striae would likely be prevented by Tat-PYC-SMAD7 owing to the anti-fibrotic property of SMAD7 (Hengge et al., 2006). Furthermore, Tat-PYC-SMAD7 diminishes major signaling cascades, including TGFβ, NFκB signaling, and IL-22/Jak/STAT3 involved in skin inflammation. This broad effect could make Tat-PYC-SMAD7 one of the most potent anti-inflammatory proteins in the skin. From a translational perspective, Jak inhibitors have shown proof of efficacy in clinical trials for patients with psoriasis (NCT02969018) and AD (NCT03568318). Clinical trial performed on efficacy of intravenous fezakinumab (NCT0941537) indicated a neutralizing anti-IL-22 antibody achieving clinical improvements over placebo, particularly in patients with severe AD, suggesting that the IL-22 pathway could be an efficacious target for skin inflammation. Given the broad physiological functions of IL-22, topical regulation of IL-22RA2 by Tat-PYC-SMAD7 could minimize off-target side effects and thereby has advantages over systemic IL-22/STAT3 inhibition. In addition, because proinflammatory pathways are often intertwined during skin conditions, the pleiotropic effects of Tat-PYC-SMAD7 could offer advantages over drugs targeting specific pathways.
In summary, topical application of Tat-PYC-SMAD7 and its translocation to cell nucleus conferred C\EBPβ-dependent IL-22RA2 expression, which by blocking IL-22 signaling led to decreased IL-22 signaling-mediated inflammation. Tat-PYC-SMAD7, containing functional domains of SMAD7, dampens multiple proinflammatory signaling pathways, revealing the potential of SMAD7-based Tat protein as a local therapeutic agent for skin inflammatory diseases. Short-term DNFB model and TS models have limitations in inducing strong type II immune responses and may not fully represent the complexity of human AD. Future studies using more comprehensive animal models to test the anti-inflammatory effects of Tat-PYC-SMAD7 in a more clinically relevant setting are warranted. Given overlapping clinical and histological features of a chemical-induced mouse model with certain autoimmune conditions (Hawkes et al., 2018), Tat-PYC-SMAD7 treatment could have the potential to control inflammation in some autoimmune disease entities, such as lichen planus and lupus erythematosus. Thus, topical application of Tat-PYC-SMAD7 could become a treatment option for a wide spectrum of intractable skin inflammatory conditions.
MATERIALS AND METHODS
Generation and identification of K5.N-Smad7 mice
The Institutional Animal Care and Use Committee at the University of Colorado Denver Anschutz Medical Campus (Aurora, CO) approved all animal experiments. Human N-SMAD7 (1-258 amino acid) with a 5′ flag epitope was inserted into the K5 vector (Ramírez et al., 1994). The K5.N-Smad7 or K5.PYC-Smad7 transgenic vector was microinjected into mouse embryos obtained from mating between FVB/NJ females and males.
A full description of all materials and methods is available in Supplementary Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS
XJW and CDY were supported by National Institutes of Health grants DE024659, DE028718, and AR078669. YK was supported by a Milstein Medical Asian American Partnership Foundation Award in Dermatology and Sequencing Plot Award from Genomics and Microarray Core University of Colorado Denver. We thank the Human Immune Monitoring Shared Resource within the University of Colorado Human Immunology and Immunotherapy Initiative for their expert assistance in the analysis of Multiplex Spectral Imaging, Gates Center for Regenerative Medicine at the University of Colorado’s Anschutz Medical Campus for histological support, the RNA Biosciences Initiative at the University of Colorado for assistance with RNA sequencing, and Biostatistics and Bioinformatics Shared Resource at University of Colorado Cancer Center for RNA-sequencing analysis.
Abbreviations:
- AD
atopic dermatitis
- HA
hemagglutinin
- IMQ
imiquimod
- siRNA
small interfering RNA
- STAT
signal transducer and activator of transcription
- TCS
topical corticosteroid
- Th
T helper
- TS
tape stripping
- WT
wild type
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material is linked to the online version of the paper at www.jidonline.org and at https://doi.org/10.1016/j.jid.2023.04.029.
CONFLICT OF INTEREST
XJW and CDY are inventors of the patent filed by the University of Colorado for using SMAD7-based biologics as therapeutic agents. XJW is the founder of Allander Biotechnologies, which developed Tat-PYC-SMAD7. Allander Biotechnologies has an exclusive license from the University of Colorado in developing SMAD7-based therapy. The remaining authors state no conflict of interest.
Data availability statement
Datasets related to this article can be accessed under the accession number GSE231815 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE231815
REFERENCES
- Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 2019;571:265–9. [DOI] [PubMed] [Google Scholar]
- Aranez V, Ambrus J Jr. Immunologic adverse effects of biologics for the treatment of atopy. Clin Rev Allergy Immunol 2020;59:220–30. [DOI] [PubMed] [Google Scholar]
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Pavel AB, Diaz A, Werth JL, Zang C, Vranic I, et al. Crisaborole and atopic dermatitis skin biomarkers: an intrapatient randomized trial. J Allergy Clin Immunol 2019;144:1274–89. [DOI] [PubMed] [Google Scholar]
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174:3695–702. [DOI] [PubMed] [Google Scholar]
- Boss MK, Ke Y, Bian L, Harrison LG, Lee Bl, Prebble A, et al. Therapeutic intervention using a Smad7-based Tat protein to treat radiation-induced oral mucositis. Int J Radiat Oncol Biol Phys 2022;112:759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé Y, Rola-Pleszczynski M. Controversy surrounding the increased expression of TGF beta 1 in asthma. Respir Res 2007;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel) 2019;6:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy L, Derynck R. Transforming growth factor-β inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 2003;278:9609–19. [DOI] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015;33:747–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71:116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldminz AM, Suárez-Fariñas M, Wang AC, Dumont N, Krueger JG, Gottlieb AB. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol 2015;151:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis RR, Alarcón C, Nadal C, Van Poznak C, Massagué J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell 2006;10:203–14. [DOI] [PubMed] [Google Scholar]
- Han G, Li F, Singh TP, Wolf P, Wang XJ. The pro-inflammatory role of TGFβ1: a paradox? Int J Biol Sci 2012;8:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Adalsteinsson JA, Gudjonsson JE, Ward NL. Research techniques made simple: murine models of human psoriasis. J Invest Dermatol 2018;138:e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Li AG, Wang D, Han S, Zheng B, Goumans MJ, et al. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J 2002;21:2580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006;54:1–15. quiz 6–8. [DOI] [PubMed] [Google Scholar]
- Hoffjan S, Ostrovnaja I, Nicolae D, Newman DL, Nicolae R, Gangnon R, et al. Genetic variation in immunoregulatory pathways and atopic phenotypes in infancy. J Allergy Clin Immunol 2004;113:511–8. [DOI] [PubMed] [Google Scholar]
- Holzmann S, Tripp CH, Schmuth M, Janke K, Koch F, Saeland S, et al. A model system using tape stripping for characterization of Langerhans cell-precursors in vivo. J Invest Dermatol 2004;122:1165–74. [DOI] [PubMed] [Google Scholar]
- Hong S, Lim S, Li AG, Lee C, Lee YS, Lee EK, et al. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol 2007;8:504–13. [DOI] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012;491:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci 2019;96:2–7. [DOI] [PubMed] [Google Scholar]
- Joost S, Zeisel A, Jacob T, Sun X, La Manno G, Lönnerberg P, et al. Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst 2016;3:221–37.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempski J, Giannou AD, Riecken K, Zhao L, Steglich B, Lücke J, et al. IL22BP mediates the antitumor effects of lymphotoxin against colorectal tumors in mice and humans. Gastroenterology 2020;159:1417–30.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Hur G, Lee SW, Lee SJ, Lee S, Kim SH, et al. AGK2 ameliorates mast cell-mediated allergic airway inflammation and fibrosis by inhibiting FcεRI/TGF-β signaling pathway. Pharmacol Res 2020;159:105027. [DOI] [PubMed] [Google Scholar]
- Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol 2008;128:2625–30. [DOI] [PubMed] [Google Scholar]
- Li F, Bian L, Iriyama S, Jian Z, Fan B, Luo J, et al. Smad7 ameliorates TGF-beta-mediated skin inflammation and associated wound healing defects but not susceptibility to experimental skin carcinogenesis. J Invest Dermatol 2019;139:940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Romieu I, Wu H, Sienra-Monge JJ, Ramírez-Aguilar M, del Río-Navarro BE, et al. Genetic polymorphisms in transforming growth factor beta-1 (TGFB1) and childhood asthma and atopy. Hum Genet 2007;121:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol 2013;34:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JC, Bériou G, Heslan M, Bossard C, Jarry A, Abidi A, et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol 2016;9:539–49. [DOI] [PubMed] [Google Scholar]
- Martin JC, Wolk K, Bériou G, Abidi A, Witte-Händel E, Louvet C, et al. Limited presence of IL-22 binding protein, a natural IL-22 inhibitor, strengthens psoriatic skin inflammation. J Immunol 2017;198:3671–8. [DOI] [PubMed] [Google Scholar]
- Mellett M, Meier B, Mohanan D, Schairer R, Cheng P, Satoh TK, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol 2018;138:2010–23. [DOI] [PubMed] [Google Scholar]
- Munera-Campos M, Carrascosa JM. Innovation in atopic dermatitis: from pathogenesis to treatment. Actas Dermo Sifiliogr 2020;111:205–21. [DOI] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997;389:631–5. [DOI] [PubMed] [Google Scholar]
- Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol 2015;136:1254–64. [DOI] [PubMed] [Google Scholar]
- Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 2010;126:976–84.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou E, Yánez DC, Ross S, Lau Cl, Solanki A, Chawda MM, et al. Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation. J Clin Invest 2019;129:3153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez A, Bravo A, Jorcano JL, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation 1994;58:53–64. [DOI] [PubMed] [Google Scholar]
- Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-beta/activin-Smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol 2001;25:60–8. [DOI] [PubMed] [Google Scholar]
- Shi X, Chen F, Yu J, Xu Y, Zhang S, Chen YG, et al. Study of interaction between Smad7 and DNA by single-molecule force spectroscopy. Biochem Biophys Res Commun 2008;377:1284–7. [DOI] [PubMed] [Google Scholar]
- Shimura S, Takai T, Iida H, Maruyama N, Ochi H, Kamijo S, et al. Epicutaneous allergic sensitization by cooperation between allergen protease activity and mechanical skin barrier damage in mice. J Invest Dermatol 2016;136:1408–17. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Michaels KA, Sutter AJ, Diaconu D, Fritz Y, Xing X, et al. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med 2017;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol 2017a;76:377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol 2017b;76:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol 2012;188:462–9. [DOI] [PubMed] [Google Scholar]
- Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol 2009;123:59–66.e4. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009;182:5836–45. [DOI] [PubMed] [Google Scholar]
- Voglis S, Moos S, Kloos L, Wanke F, Zayoud M, Pelczar P, et al. Regulation of IL-22BP in psoriasis. Sci Rep 2018;8:5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol 2010;6:704–14. [DOI] [PubMed] [Google Scholar]
- Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, et al. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/Smad signaling. J Biol Chem 2016;291:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther 2020;5:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 2007;27:4488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets related to this article can be accessed under the accession number GSE231815 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE231815