Abstract
Epilepsy is a neural network disorder caused by uncontrolled neuronal hyperexcitability induced by an imbalance between excitatory and inhibitory networks. Abnormal synaptogenesis plays a vital role in the formation of overexcited networks. Recent evidence has confirmed that thrombospondin-1 (TSP-1), mainly secreted by astrocytes, is a critical cytokine that regulates synaptogenesis during epileptogenesis. Furthermore, numerous studies have reported that TSP-1 is also involved in other processes, such as angiogenesis, neuroinflammation, and regulation of Ca2+ homeostasis, which are closely associated with the occurrence and development of epilepsy. In this review, we summarize the potential contributions of TSP-1 to epilepsy development.
Keywords: Thrombospondin-1, Epileptogenesis, Synaptogenesis, Hyperexcitability
Introduction
Epilepsy is one of the most common central nervous system (CNS) diseases, affecting over 50 million individuals globally [1]. Currently, anti-epileptic drugs are recognized as the major therapeutic strategy; however, these drugs have unsatisfactory effects on the therapy for epilepsy, and drug resistance represents a non-negligible problem [2]. Clinical investigation has confirmed that only 66% efficiency of anti-seizure drugs is achieved, even in high-income countries [3], and half of patients with epilepsy still present seizures after treatment with anti-epileptic drugs [4]. Therefore, it is essential to investigate additional prospective targets and strategies for epilepsy therapy through extensive research on the mechanism.
Generally, epilepsy is considered to be a process of synchronization and amplification mediated by an imbalance between excitatory and inhibitory synaptic transmission [5], and the abnormal connectivity of synapses in the brain contributes greatly to epileptic network formation and increases sensitivity to seizures [5, 6]. Astrocytes, the most abundant cells in the CNS, can not only support neurons but also regulate the stabilization and plasticity of synapses [7]. Christopherson et al. reported that co-culture with mature astrocytes is essential for synaptogenesis of retinal ganglion cells (RGCs). The results suggest that astrocytes secrete vital extracellular signaling molecules for synapse formation. Further analysis confirmed that the macromolecular protein secreted by astrocytes is thrombospondin-1 (TSP-1) [8].
A member of the thrombospondin family, TSP-1 is a multidomain extracellular matrix protein [9, 10] that is expressed highly in the developing brain, exhibits low expression in the adult brain [11], and regulates neuronal migration, as well as synapse formation and growth [12, 13]. Previous studies have confirmed that increased secretion of TSP-1 from activated astrocytes contributes to synaptogenesis and angiogenesis, eventually promoting epilepsy development [14, 15]. A critical protein secreted by astrocytes, TSP-1 supports dendritic spine development, which plays a vital role in modulating synaptic and circuit alterations [16]. Previous studies have reported that the expression of astrocyte-secreted TSP-1 reaches a peak that is consistent with the synaptogenic period and the initiation of excitatory synaptogenesis in vivo [17]. The above work suggests that astrocytic TSP-1 has a vital effect on synaptogenesis. Interestingly, as an endogenous angiogenesis inhibitor, TSP-1 reportedly contributes to angiogenesis [18]. Furthermore, it has been confirmed that TSP-1/transforming growth factor (TGF-β1)/phosphorylated small mothers against decapentaplegic (pSmad2/3)/vascular endothelial growth factor (VEGF) pathway, which is regulated by P2 receptors (especially P2Y4), promotes angiogenesis in rats with kainic acid-induced status epilepticus [18]. The results above demonstrate that TSP-1 plays an important role in the formation of epileptic networks through the regulation of synaptogenesis and angiogenesis.
Besides the contributions to synaptogenesis and angiogenesis [18, 19], studies have suggested that TSP-1 is also involved in multiple complex processes, which are thought to be closely associated with epilepsy formation and development, including apoptosis [20], neuronal injury [21], neuroinflammation [22], oxidative stress [23], and receptor or ion channel dysfunction [24]. In this review, we summarize the demonstrated and potential contributions of TSP-1 to epilepsy development and provide a systematic and theoretical basis for future studies on the mechanisms underlying epilepsy development and the potential strategies through TSP-1 targeting.
Structure of TSP-1
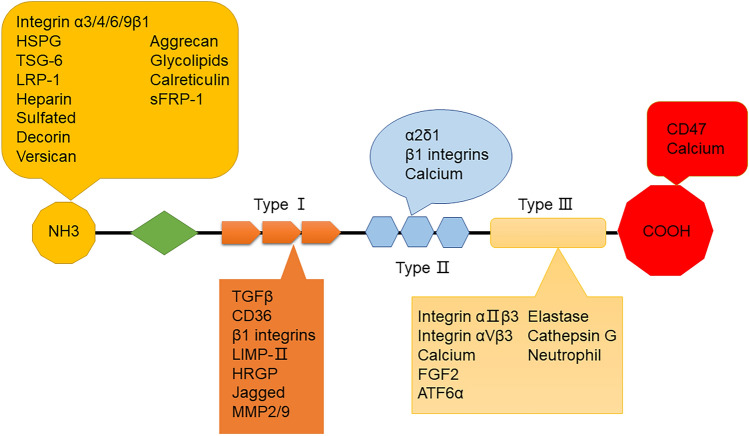
TSP-1 was the first member of the TSP family to be reported. It is also the most extensively studied extracellular matrix protein [25]. The TSP family contains five proteins: TSP 1–5 [26]. TSP-1 was discovered by Baenziger et al. [27] in 1971. Lawder et al. [28] have revealed that intact TSP-1 protein is a homotrimer weighing 420 kDa and mature polypeptide chains of TSP-1 are 180 kDa [25]. Since its discovery, TSP-1 has been defined as a glycoprotein associated with the α-granules during platelet activation [29]. TSP-1 has a complex multidomain structure, which is considered the basis of interactions with various molecules. TSP-1 comprises trimer subunits with disulfide bonds and each subunit includes 1152 amino-acid residues [30], which are linked by disulfide bonds between cysteines 252 and 256 [25]. Each subunit of the TSP-1 trimer comprises β-sandwich domains made up of the amino-terminal (N-terminal) and carboxyl-terminal (C-terminal) connected with a flexible and long arm [30, 31]. The arm region includes two cysteine residues (participating in the formation of the trimer), a procollagen homologized region, and three types of repeated domains—types I, II, and III [32]. The different domains of the TSP-1 protein exhibit different characteristics and functions under various conditions. For example, the N-terminal might interact with heparin, heparan sulfate proteoglycan, and low-density lipoprotein receptor-related protein 1 (LRP 1) [33]. The type III repeats of TSP-1 contain numerous aspartic acid residues that form one or two sequences of each repeat, similar to Ca2+-binding motifs, forming a structural foundation for binding with Ca2+. This feature is vital for the functional properties of TSP-1 [34]. It is clear that the multiple functions of TSP-1 may mainly depend on the interactions of its various structural domains with cellular molecules.
TSP-1-Interacting Molecules
The structural characteristics of TSP-1 provide reliable action sites for multiple types of molecules. Although the complete specific networks of the TSP-1 interaction remain unclear, various TSP-1-interacting molecules have been successively identified. Interestingly, studies have shown that the multifarious functions of TSP-1 depend on the molecules that interact with it [35, 36], including cytokines, growth factors, and structural components of cell receptors [37] (Fig. 1). Moreover, bioinformatics analyses have shown that TSP-1-mediated signaling affects numerous processes, such as neural development, angiogenesis, cell proliferation, adhesion, and motility [38]. Therefore, it is necessary to understand the contributions of TSP-1 in the epileptic network by appreciating of multiple functions of molecules interacting with TSP-1. Some major molecules interacting with TSP-1 are listed as follows.
Fig. 1.
The simple structure and interacting molecules of TSP-1.
CD47
CD47 is a highly hydrophobic glycoprotein, which is composed of an IgV-like extracellular domain, a five-span transmembrane region, and a short intracytoplasmic tail lacking a signaling motif [39]. Lehrman et al. [40] found that CD47 colocalizes with the pre- and postsynaptic markers, which indicated that CD47 might be localized in synapses. Previous work has reported that the binding sequences of CD47 are identified by the C-terminal of TSP-1 [41]; however, how TSP-1/CD47 signaling regulates synaptogenesis and epileptogenesis in the brain remains unclear. Nonetheless, the interactions of TSP-1 and CD47 regulate multiple functions like cellular adhesion, apoptosis, migration, and neuroimmunity, which are believed to be related closely to neurodegenerative disorders [42]. In addition, the TSP-1/CD47 interaction reportedly plays a key role in the modulation of cell invasion and angiogenesis in glioblastoma [43]. Such findings suggest that TSP-1/CD47 might be associated with the related pathological progress of epileptic networks.
CD36
CD36 is considered to possess a CLESH-1 homology motif that interacts with the type I repeats of TSP-1 [44]. Similar to CD47, CD36 is identified as a receptor for TSP-1 and is vital for inflammation and angiogenesis. A previous study has demonstrated that TSP-1/CD36 exerts anti-tumor activities by inhibiting angiogenesis in the tumor microenvironment [45]. Conversely, the evidence demonstrated that TSP-1 can increase VEGF receptor 2 levels by binding CD36 [46]. The Ortiz-Masià research group has reported that hypoxia induces the phagocytosis of macrophages significantly, increasing the expression of TSP-1 and CD36, and TSP-1/CD36 may contribute to this inflammatory process [47]. Besides, CD36/TSP-1 is reported to participate in the induction of oxidative stress [48].
TGF-β1
TGF-β1 is a regulatory protein that is activated by TSP-1 [49] and participates in angiogenesis, scar deposition, inflammation, astrocytic phenotype, and mobility regulation [50]. A recent study reported that TSP-1 plays a major role in the molecular mechanism of synaptogenesis, and the progression has been demonstrated to be connected with the interaction between TSP-1 and neuroligin 1/TGF-β1 [51]. Besides, TSP-1/TGF-β1/Smad signaling pathways can influence angiogenesis via diversely regulating the expression of VEGF [52]. These processes are closely related to epileptic network formation.
Lipoprotein Receptor-Related Protein-1 (LRP1)
TSP-1 reportedly interacts with LRP-1 [53]. The interaction could promote VEGF clearance, inhibiting angiogenesis. Notably, it has been reported that activated astrocytes secrete TSP-1, which, upon binding to LRP1 in the synapse, promotes synaptic recovery [54]. In addition, TSP-1 has been shown to be associated with LRP-1 in T cells. T cell motility is triggered through the combination of TSP-1 with LRP1 and the co-related receptor calreticulin [55]. Such evidence indicates that the interactions between LRP-1 and TSP-1 regulate the function of synapses, angiogenesis, and immunity, and therefore might be an underlying contributor to epileptogenesis.
α2δ-1
As a voltage-gated Ca2+ channel receptor, α2δ-1 is considered to be a key target of the commonly prescribed anti-epileptic drug Gabapentin [56]. Gabapentin is thought to block the binding of TSP-1 and α2δ-1, and in turn, inhibit the formation of synapses [57]. A previous study found that excitatory neurons (including RGCs) express high levels of α2δ-1, which localizes in synapses [17]. Overexpression of α2δ-1 increases excitatory synaptogenesis, whereas knockout leads to defects in synapse formation [17]. Hence, TSP-1-α2δ-1 is crucial for synapse formation, which participates in epileptogenesis.
Consequently, the interaction of TSP-1 with diverse proteins contributes to a wide spectrum of cellular functions. Therefore, it is beneficial to understand the mechanisms of epileptic network formation by focusing on these potential molecular interactions with TSP-1 in the brain.
Increased Expression of TSP-1 Promotes Synaptogenesis in Epileptic Network Formation
Astrocyte-Secreted TSP-1 Promotes Synaptogenesis
Studies have revealed that astrocytes, the most abundant glial cells in the brain, play an important role in synaptic connectivity, regulation of synaptic function, and provision of growth factors [24, 58]. Because astrocytes are necessary for synaptogenesis in co-cultured RGCs, Christopherson et al. [11] further analyzed the soluble macromolecular proteins secreted by astrocytes. Based on the relative molecular weight characteristics and a heparin-binding region, the active substance promoting synapse formation was demonstrated to be TSP-1. Further studies have also confirmed that TSP-1 purified from human platelets significantly increases the number of synapses in cultured RGCs even in the absence of astrocytes [8]. Similarly, animal experiments have confirmed that astrocyte-secreted TSP-1 contributes to synapse formation and is a vital factor in synaptogenesis [11]. A study on the distribution of TSP-1 in newborn mouse brains showed that on day 8 after birth, TSP-1 is distributed widely in the cortex, superior thalamus, and retina, whereas it almost disappears by day 21 [59]. Tagnaouti et al. [60] and Danjo et al. [61] revealed that the transcripts of TSP-1 increase significantly during mouse embryonic development, whereas TSP-1 expression is reduced significantly during the late period of development and early adulthood. These results suggest that elevated TSP-1 levels are highly consistent with the period when synaptic formation is highly active [59].
Furthermore, TSP-1 plays an important role in the regulation of nervous system function by promoting synapse formation [62]. TSP-1 knockout results in a significantly reduced number of synapses in the cortex of rats [10]. Moreover, in Alzheimer's disease and brain trauma, studies have found that synaptic formation and plasticity in TSP-1 knock-out mice are significantly inhibited, leading to abnormalities in learning, memory, and behavioral function [63, 64]. In human astrocytoma infected by Zika virus, studies have shown that the expression of TSP-1 is closely consistent with synapse formation and plasticity [65]. The investigation of neural development-related intellectual disability confirmed that TSP-1 expression in astrocytes of fragile X syndrome is reduced remarkably [66]. Similarly, decreased TSP-1 expression has been reported in Down’s syndrome astrocytes, accompanied by defects in synapse formation and neural function [66, 67].
However, how does TSP-1 promote synaptogenesis? Further experimental data have shown that the critical transcriptional factor, TGF-β1, is upregulated and activated, accompanied by increased TSP-1 levels, to promote the expression of synaptophysin in RGCs [68]. Although the synaptic number of mouse neurons, which were co-cultured with human astrocytes, increased by 260%, administration of a TGF-β1 pathway inhibitor completely prevented the increase of synaptogenesis. Moreover, compared with neuronal culture alone, TGF-β1 administration in the culture medium resulted in a significantly increased number of synapses, and the synaptic ultrastructure was similar to that of normal [69]. Such evidence suggests that TSP-1 plays a vital role in synaptogenesis via regulation of the downstream molecule, TGF-β1.
Besides, TSP-1 is involved in the repair and regeneration by inducing synaptogenesis [70]. Studies have reported that TSP-1 secreted by astrocytes promotes the synaptic regeneration of neurons and the growth of axons [10, 71]. In addition, TSP-1 accelerates synaptogenesis in young hippocampal neurons in the human brain [72]. Khakh et al. [73] reported that, in a hyperactivity model, medium spiny neurons (MSNs) of the striatum activate astrocyte-related signaling pathways. During this pathological progression, the level of TSP-1 secreted by astrocytes increases and is accompanied by enhanced excitatory synaptic connectivity and transmission. All these changes are reversed by inhibiting TSP-1. Such studies reveal the key role of astrocyte-secreted TSP-1 in synapse formation.
Therefore, the in-depth study of the function and mechanism of TSP-1 in synapse formation is of great importance for understanding the functional regulation of the CNS and the contribution of TSP-1 to epileptic network formation.
Regulation of TSP-1 Secretion
A vital protein that is mainly secreted by astrocytes, TSP-1 secretion is considered to be regulated by various factors, including cytokines, extracellular matrix components, and intracellular signaling pathways [74]. For instance, researchers have found that some extracellular matrix components like adenosine triphosphate (ATP) or collagen promote TSP-1 secretion [75, 76]. Meanwhile, Hisaoka-Nakashima et al. [77] reported that lysophosphatidic acid activation stimulates TSP-1 secretion via the extracellular signal-regulated kinase (ERK), MAPK, and JNK signaling networks in astrocytes. Conversely, it has been reported that activation of the phosphatidylinositol 3 kinase (PI3K)/Akt/mTOR singling pathway downregulates the TSP-1 secretion in human tumor-derived endothelial cells [78]. In addition, some transcription factors, such as NF-κB and AP-1, have been demonstrated to influence TSP-1 secretion via the regulation of genetic transcription under different conditions [79].
Previous studies have revealed multiple mechanisms that regulate TSP-1 secretion from astrocytes. In general, this regulation is divided broadly into two types: intracellular and extracellular [80, 81]. Some intracellular signaling pathways in astrocytes, such as Wnt and K-ras, have been reported to repress the secretion of TSP-1 in colonic tumorigenesis [82]. Therefore, stimulation of the expression of TSP-1 is considered a potential therapeutic strategy. Moreover, cytokines (such as TNF-α) have been reported to induce the synthesis and secretion of TSP-1 via the Akt and P38 MAPK signaling pathways [83]. Conversely, astrocytes can regulate the secretion of TSP-1 through extracellular signals [14]. Specifically, when astrocytes are stimulated by external factors, extracellular receptors are activated, which in turn prompt astrocytes to secrete TSP-1 [84]. This secretion process can be regulated through various signaling pathways, including ERK [85] and PI3K [78].
In addition, the extracellular receptor P2Y4 is a specific receptor that is located on the membrane of astrocytes [86]. Recently, it has been confirmed that activation of the P2Y4 receptor increases intracellular Ca2+ [87]; furthermore, increased astrocytic Ca2+ signaling can promote TSP-1 release [88]. Therefore, it is reasonable to speculate that P2Y4 receptor and astrocytic Ca2+ signaling are involved in TSP-1 secretion. In addition, the activation of the P2Y4 receptor can affect TSP-1 synthesis and secretion by regulating the expression of transcriptional factors and cytokines within cells [14, 89]. For example, Hu et al. [90] reported that TSP-1 is upregulated significantly in activated astrocytes in the striatum of mice, accompanied by increased synaptic inputs from upstream neurons and the growth of MSNs, causing behavioral hyperactivity. Furthermore, the increased TSP-1 secretion is regulated by the purinergic P2-type receptor [91], especially P2Y4, which are vital regulator of TSP-1 secretion in activated astrocytes [14, 69]. In addition, Koizumi et al. [60] reported that, in a neurological pain model, the expression of metabotropic glutamate receptor 5 (mGluR5) in somatosensory cortex astrocytes promotes TSP-1 secretion by increasing Ca2+ activity, eventually leading to excessive synapse formation and incorrect connectivity of the pain network. Xiao et al. [92] also revealed that hyper-glucose conditions lead to astrocyte dysfunction and reduce TSP-1 secretion, which might be associated with the activation of toll-like receptor 9 signaling.
The Possible Mechanism of TSP-1 in Synaptogenesis
TSP-1 reportedly triggers a series of signal transduction pathways by binding related molecules on the surface of neurons, thus promoting the formation and development of synapses. Among them, TSP-1 can bind with CD47 on the surface of neurons and activate CD47-related signaling pathways (such as CD47-SIRRP α), which promotes synapse formation [93]. In addition, TSP-1 can bind to other molecules on the surfaces of neurons, such as αvβ3 integrins and LRP1, which have been confirmed to influence synapse formation by regulating extracellular matrix assembly and intracellular signaling [94, 95]. Studies have also shown that astrocyte-derived TSP-1 can regulate the assembly and breakdown of extracellular matrix around neurons, thereby affecting synapse formation and development [96]. Specifically, TSP-1 can increase adhesion and interaction between presynaptic and postsynaptic neurons, besides promoting the assembly of extracellular matrix, thus promoting excitatory synapse formation and development [65]. In addition, TSP-1 can affect synapse formation and development by regulating extracellular matrix molecular activity: TSP-1 can reportedly inhibit the activity of Glypican-4, thereby promoting the formation and development of excitatory synapses [60].
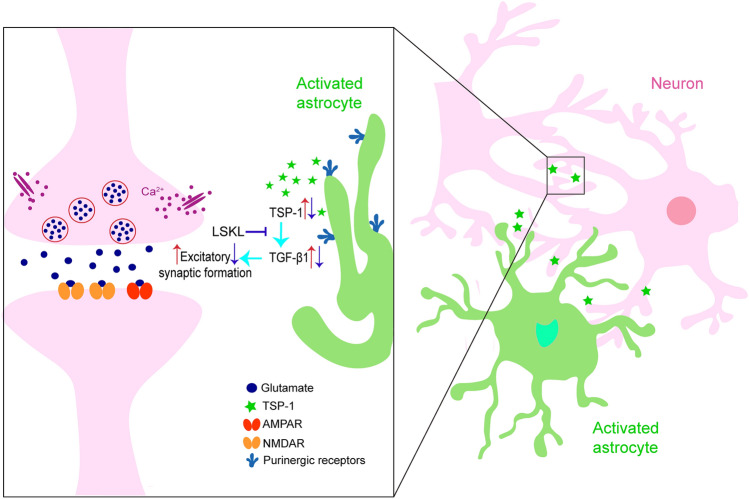
TSP-1 binding to the Leu-Ser-Lys-Leu (LSKL) peptide sequence in the TGF-β1 complex leads to TGF-β1 activation, and preventing TSP-1 binding by administration of exogenous LSKL impedes TGF-β1 activation [70]. Our previous results also confirm that an increase in the number of synapses in the brains of amygdala-kindled seizure rats accompanies elevated levels of TSP-1 and TGF-β1. Similar to the roles of intervention in TSP-1 expression, restraining the TGF-β1 activation by administering exogenous LSKL significantly attenuated synaptogenesis and epileptic development [14]. Such results suggest that the TSP-1/TGF-β1 pathway is a critical promoter of synaptogenesis and epileptogenesis [14] (Fig. 2). Further investigations are required to uncover the precise mechanisms underlying the role of TSP-1 in synapse formation.
Fig. 2.
Regulation of TSP-1 secreted by activated astrocytes and potential mechanism of TSP-1 in excitatory synaptic formation.
Synaptogenesis is the Basis of Epileptic Network Formation
The occurrence and development of epilepsy involve the progression of abnormal neural network formation based on synaptogenesis [97]. Numerous clinical and animal studies have confirmed that some complex and intractable epilepsies originate from abnormal neuronal protrusions in the temporal lobe region [98]. Evidence shows that neurons are highly connected through excitatory synapses to overcome the feedback inhibition of interneurons and eventually form epileptic networks [8]. As a key participant in neuronal activity, abnormal excitatory synaptogenesis is critical in building hyperexcitatory neural circuits that contribute to epilepsy [99, 100]. Recent studies have indicated that the transmission regions of epileptic signals in the brain are strongly correlated with the number of synaptic changes [14]. In addition, there is evidence that net excitation or inhibition in the new neural circuits depends on the number of synapses formed on principal cells or interneurons [101]. Although the two types of synapses are found in normal and epileptic rats, the newly formed synapses sprouting in the inner molecular layer of the dentate gyrus (DG) are mainly on the principal cells and contribute to the recurrent excitatory circuitry [101, 102]. It has been reported that mossy fiber sprouting plays a critical role in the reorganization of circuits during seizures [103]. During epileptic seizures, the surviving mossy cells participate in the formation of an aberrant synaptic network, establishing excessive positive feedback in the granule cells of the DG and CA3 in the hippocampus, which are considered pivotal for promoting the connectivity of excitatory synapses [104]. In addition, Freiman et al. [105] found that sprouted mossy fibers not only lead to excitatory synaptic connectivity but also produce extra neural innervation on preserved inhibitory interneurons, eventually causing an imbalance between excitation and inhibition in the brain.
Therefore, synaptogenesis, especially excitatory synaptogenesis, plays a key role in epileptic network formation and is a critical factor in inducing epileptogenesis [106]. In addition, TSP-1 has been verified as a vital factor in excitatory synapse formation [107]. Consequently, targeting TSP-1 is beneficial for inhibiting epileptic network formation through the intervention in synaptogenesis [14].
The Upregulation of TSP-1 can Contribute to Epileptogenesis by Promoting Angiogenesis
Contribution of TSP-1 to Angiogenesis Regulation
Physiologically, the angiogenic progress is maintained by the balance of the angiogenic promotors and inhibitors [108]. TSP-1 is considered an endogenous angiogenesis inhibitor for repair when the tissue is injured under physiological conditions [109]. The anti-angiogenic function of TSP-1 can be mediated by its structural three type I repeats [110]. However, when the pro- or anti-angiogenic factors are dysregulated under pathological conditions, it is believed to favor angiogenesis [98]. TSP-1 is thought to be a vital factor regulating angiogenic progress under a pathological internal environment. Research has demonstrated that TSP-1 promotes angiogenesis through binding with CD47 (integrin-associated protein), in turn enhancing the repair of post-ischemic damage [111]. The extracellular matrix is a key part of the angiogenesis process, supporting cell migration and vascularization [112–114]. In patients with gliomas, the increased TSP-1 levels, which are mainly distributed in intracellular spaces or the cytoplasm, are closely related to the process of angiogenesis in the gliomas [115]. In the human prostate cancer cell line, dynamic changes in TSP-1 expression level have been confirmed during angiogenesis, and its expression level is positively correlated with the degree of angiogenesis [115, 116]. A previous study confirmed that TSP-1 promotes vascular proliferation by activating α9β1 integrin in a quail chorioallantoic membrane system experiment. Inhibition of α9β1 integrin reduces the TSP-1-mediated proangiogenic activity in vivo [117], which indicates that TSP-1/α9β1 integrin signaling is important for angiogenesis. Yang et al. [118] found that thrombin participates in angiogenesis in rats with cerebral hemorrhage by increasing TSP-1/2 expression. After administering hirudin, a thrombin inhibitor, the levels of TSP-1/2 are reduced, and angiogenesis is inhibited. In addition, in an ischemic stroke model, upregulated miR-487b promotes neovascularization by directly targeting TSP-1. Further analysis has suggested that increased TSP-1 expression is due to miR-487b binding to TSP-1 mRNA. The results indicate that miR-487b may mediate angiogenesis in ischemic stroke by increasing TSP-1 levels [119].
Previous studies have shown that the activation of Smad 2/3 mediated by TGF-β1 can induce angiogenesis by accelerating the expression of angiogenic factors, such as VEGF [120]. Furthermore, it has been confirmed that TGF-β1 is activated by TSP-1 [121] and that the N-terminal domain of TSP-1 is responsible for angiogenesis [122]. Moreover, TSP-1 secretion is mainly regulated by the P2Rs receptor (mainly the P2Y4 receptor) in astrocytes [15]. In rats with KA-induced status epilepticus, synchronously elevated levels of TSP-1, TGF-β1, and phosphorylated Samd 2/3 are accompanied by angiogenesis. Angiogenesis is significantly attenuated by blocking P2Y4, reducing TSP-1 expression, or TGF-β1 activation. This strongly indicates that the P2Y4/TSP-1/TGF-β1/pSmad2/3 pathway is a pivotal component of angiogenesis in epilepsy [18].
In addition, TSP-1 affects angiogenesis by regulating the expression and activity of the pro-angiogenic factors, which are believed to be essential for angiogenesis [116]. Some cytokines have been reported to be involved in promoting angiogenesis, like VEGF and basic fibroblast growth factor [116]. For instance, NF-κB is considered an important transcription factor that induces the expression of the VEGF gene [123], and TSP-1 increases VEGF gene transcription by activating the transcription factor NF-κB [124]. Ghorbanzadeh et al. [125] reported that the TSP-1/NF-κB/VEGF signaling pathway participates in promoting angiogenesis in the heart of aged rats.
In summary, TSP-1 plays an important role in angiogenesis regulation. It is involved in the regulation of various links of angiogenesis by regulating the proliferation and migration of endothelial cells, inducing their apoptosis, regulating their differentiation and function, and regulating the expression and activity of angiogenesis-related factors [115, 125, 126]. The in-depth studies of the role of TSP-1 in the regulation of angiogenesis help to reveal the molecular mechanisms of angiogenesis in related diseases.
Angiogenesis is a Crucial Participant in Epileptogenesis
Angiogenesis, an important event in many diseases and first described by Spielmeyer in 1925, is a key factor involved in the development of epilepsy [127]. Accumulating evidence since 2007 suggests that intractable temporal lobe epilepsy (TLE) is associated closely with angiogenesis [128, 129]. Numerous genes are responsible for the VEGF signaling pathway in patients with TLE, which is associated with GABA or glutamate synaptic transmission [130]. Clinical studies have also reported that vascularization and increased permeability of the blood-brain barrier (BBB), leads to large leakage of serum proteins and inflammation in the hippocampus of patients with TLE [128, 131]. The BBB depends on intercellular tight junctions and adherence [132]. The study demonstrated that, in epilepsy, angiogenesis may lead to BBB permeability; furthermore, BBB leakage is associated with structural aberrations during epileptic seizure and activates the glutamate signaling pathway via A2 cytosolic phospholipase [133]. In addition, BBB leakage causes several serum proteins, such as albumin, to be released into the parenchyma, which can lead to epileptic focus production [134, 135]. In KA-induced status epilepticus, reduced TSP-1 expression results in attenuated angiogenesis, vascular leakage, and inhibition of epileptogenesis [18].
Angiogenesis is a key pathological process that contributes to epileptogenesis, and TSP-1 has been confirmed to be an active participant in angiogenesis. Moreover, previous studies have confirmed that TSP-1 participates in epileptogenesis via angiogenesis.
TSP-1 Participates in Epilepsy by Regulating the Level of Oxidative Stress
TSP-1 Exerts a Crucial Role in Oxidative Stress
Astrocytes can respond to changes in oxidative stress levels in the brain and play diverse roles in regulating oxidative stress [136]. Besides their roles in the decomposition and clearance of free radicals, astrocytes can also be the main source of detrimental reactive oxygen species (ROS), leading to neuronal injury [137]. TSP-1 also promotes ROS generation [138]. The expression of TSP-1 is upregulated in many oxidative stress-related diseases, such as ischemia-reperfusion injury and atherosclerosis [139]. For example, synchronously increased TSP-1 levels and oxidative stress have been detected within an hour of ischemia [137]. Recently, Thom et al. [140] described a pre-feedback inflammatory/oxidative stress cycle mediated by TSP-1. They reported that in brain CO poisoning, astrocytes induce TSP-1 expression; subsequently, TSP-1 is released into the blood and promotes CD36 (a ligand of TSP-1) expression and oxidative stress. Such studies indicate that increasing TSP-1 levels could be an important contributor to oxidative stress.
However, previous studies have demonstrated that TSP-1 may have a neuroprotective effect on Aβ-treated mouse hippocampal neuroblastoma cells. Further results have shown that TSP-1 inhibits the transmission of Drp-1 into mitochondria and mediates mitochondrial fission by maintaining phosphorylated-Drp1 levels and the stability of mitochondrial function [141]. Mitochondrial dysfunction is one of the major processes involved in oxidative stress [142]. The above studies provide novel insights into the relationship between TSP-1 and oxidative stress. There are two contrasting roles of TSP-1 in oxidative stress regulation, and its specific roles during epileptogenesis remain to be explored.
Oxidative Stress Level is Critical in Epilepsy Formation and Development
The brain is sensitive to oxidative stress due to hyperoxic metabolism. Overproduction of free radicals has been confirmed to lead to neuronal hyperexcitability and injury, which participate in epilepsy initiation and progression [143]. Mitochondria are the main sites of ROS production during various biochemical processes, including the tricarboxylic acid cycle [144]. Overproduction of ROS can damage mitochondrial function, which, in turn, affects the synthesis of many enzyme complexes in the electron chain [145] and leads to mitochondrial disorders [146]. This pathological progression is conducive to epilepsy formation and development [146]. Multiple animal models of epilepsy, such as pilocarpine and kainic acid, have confirmed mitochondrial dysfunction and elevated levels of oxidative stress [147]. Moreover, excessive ROS-induced mitochondrial injury leads to energy metabolism disorders, which play a vital role in the occurrence and development of epilepsy [148]. Oxidative stress induced by excessive ROS production may eventually lead to the onset of epilepsy [149]. Conversely, decreasing ROS levels reduce the release of excitatory amino-acids and attenuate epileptic seizures [148, 150]
In summary, TSP-1 is secreted mainly by activated astrocytes and plays a vital role in oxidative stress in the brain. Based on the close relationship between oxidative stress and epilepsy, TSP-1 may play a pivotal role in epilepsy development by oxidative stress.
TSP-1 Might Participate in the Progress of Epilepsy by Mediating Neuroinflammation
A previous study detected transiently increased levels of TSP-1 in inflamed tissues [151]. CD47, a natural ligand of TSP-1, is a homologous trimeric extracellular matrix protein that mediates neuroinflammatory pathways [152, 153]. Studies have reported that the TSP-1/CD47 pathway plays a major role in numerous neurological disorders, such as decompression sickness [154], glioblastoma [43], spinal cord injury [155], stroke [42], Alzheimer’s disease [156, 157], and neonatal meningitis [151]. For example, in the Escherichia coli K1-induced-neonatal meningitis model, upregulation of TSP-1 and CD47 is accompanied by inhibition of dendritic cell maturation. Subsequently, blocking TSP-1 or CD47 protects the brains of newborn mice against neuroinflammation and damage caused by E. coli K1 [151]. In addition, CD36 is a key molecule that interacts with TSP-1 in neuroinflammation [158]. A previous study demonstrated that the activation of phosphatidylserine/CD36/TGF-β signaling significantly counters anti-inflammatory effects on the neuroinflammation-induced injury of rats [159]. Although the specific molecular mechanism is still unclear, it is hypothesized that TSP-1 may regulate neuroinflammation by BBB breakdown [160]. In addition, previous research has revealed that TSP-1 can result in the leakage of the BBB, causing the neuroinflammatory response and upregulation of the levels of some chemokines like macrophage inflammatory protein 2. This progress is regulated by Lysophosphatidic acid receptor 1 (LPA1) via the ERK, MAPK, and JNK signaling network [161].
Epileptic seizures are closely associated with elevated and persistent inflammatory reactions in the brain [162]. Inflammatory reactions are induced by the release of inflammatory agents like IL-1β, which can activate NMDA receptors [163]. Neuroinflammation-induced BBB damage is believed to be a crucial factor in epilepsy [164]. Conversely, systemic or focal inflammation would lead to aberrant neural connectivity and over-excitatory network formation, eventually causing epileptogenesis [165, 166]. Therefore, it is reasonable to assume that TSP-1-mediated neuroinflammation may be involved in epilepsy development. The effects of TSP-1 on neuroinflammation during epileptogenesis require further confirmation.
TSP-1 Might Play a Pivotal Role in Epilepsy by Regulating Neuronal Injury
Previous studies have demonstrated that TSP-1 expression is generally low in mature brains under physiological conditions [167]. However, significantly increased TSP-1 levels have been detected during neuronal injury, indicating that TSP-1 might be involved in neural damage [168]. As a vital member of the matricellular family, TSP-1 regulates apoptosis under numerous physiological and pathological conditions [169]. Previous studies have reported that TSP-1 is expressed mainly in activated or stressed astrocytes and endothelial cells to induce apoptosis and is believed to be one of the most critical factors causing injury [170–172]. When neurons begin to die, TSP-1 secretion is increased synchronously due to ATP release [173], and the TSP-1-CD47 pathway builds a bridge between cell phagocytosis and death [174]. This leads to a peak in microglial proliferation and phagocytosis due to induction by damaged neurons [166]. Lo et al. [175] reported that TSP-1 binding to CD47 mediates cell death through caspase-3-dependent or -independent pathways. Secondly, the binding of TSP-1 and CD47 can inhibit cellular regeneration and migration [176]. Furthermore, TSP-1 can affect the growth and connection of neurons by regulating the composition and structure of the extracellular matrix [177]. Recently, Norenberg et al. [178] found that astrocytes are dysfunctional and lose their neurotrophic function during neuronal injury, which may be associated with the TSP-1-TDP-43 signaling pathway.
Neuronal injury is a critical feature of epileptogenesis. Increased levels of excitatory neurotransmitters, including glutamate and acetylcholine, are critical factors in the pathophysiology of neural injury [179]. Neuronal injury promotes epileptogenesis [21]. In addition, astrocytes are thought to partly maintain the balance between excitation and inhibition by absorbing excessive glutamate during seizures. However, neural injury hampers this progress due to elevated synaptic release and disordered glutamate uptake [180]. Therefore, TSP-1 may participate in epilepsy formation and development by regulating neuronal injury.
TSP-1 Probably Contributes to Epileptogenesis by Interacting with Ca2+ and the Ca2+ Voltage-Gated Channel Receptor α2δ-1
A previous study has reported that increased dendritic release of Ca2+ plays a key role in synchronous neuronal firing induced by crosstalk between neurons and astrocytes [181]. Especially astrocytic Ca2+ is involved in many supportive roles in regulating synaptic transmission. Moreover, substantial evidence has demonstrated that Ca2+ is an active participant in the release of glutamate from active astrocytes [130, 131], and is a promotive contributor to epilepsy.
TSP-1 is an important regulator of Ca2+ homeostasis as the C-terminal signature domains form a C-terminal β-sandwich, which carries many Ca2+ binding sites, forming coordination bonds with it, thus changing the conformation and function [34, 182]. In addition, TSP-1 can maximally combine with 30 Ca2+ ions at a time [34, 117], and experimental data have revealed that TSP-1 participates in regulating the influx and concentration of Ca2+ in cells [183, 184]. For example, Martin-Manso et al. [183] have found an elevated level of intracellular Ca2+ after the addition of TSP-1. Besides, research has also indicated that TSP-1 treatment may lead to an influx of Ca2+ in human red blood cells [184].
In addition, TSP-1 regulates synapse formation and plasticity by interacting with Ca2+ channel receptor α2δ-1 [185]. The α2δ-1 subunit, which is mainly located at neurons [186], is considered pivotal for the formation and maturation of excitatory synapses in the cortex and plays an important role in the trafficking and kinetics of the Ca2+ voltage-gated channel [187, 188]. For instance, Kim et al. [189] have reported that elevated levels of Ca2+ promote the release of TSP-1, which induces excitatory synaptogenesis via binding with the α2δ-1 receptor. Besides, excitatory synapse formation is controlled by interactions with TSP-1 and the α2δ-1 receptor via Rac1, which suggests the potential mechanisms of both the physiology and pathology of synaptic development [190, 191]. These mechanisms help maintain the steady-state Ca2+ and Ca2+ voltage-gated channel within neurons to prevent excessive Ca2+ accumulation from causing damage to neurons [189, 190]. TSP-1 regulates excitatory synaptic formation by binding with α2δ-1 of the Ca2+ channel in the intracerebral hemorrhage [188] and kindling models [5].
Previous studies have demonstrated that the interaction of TSP-1 and Ca2+ or the Ca2+ voltage-gated channel plays numerous roles in the nervous system; it is involved in promoting neurodevelopment and synaptic formation, neuroprotection and repair, and regulation of neuroinflammation and immune responses, among other processes. Such molecular progresses are believed to be critical factors for the formation of epileptic networks. Although the specific mechanisms of the interactions of TSP-1 and Ca2+ in regulating epilepsy formation are still unclear, TSP-1 is causally linked to Ca2+ [59, 187], and targeting TSP-1 is a prospective epileptic therapy.
Conclusions
In summary, although epilepsy is one of the most complex medical challenges today, the specific mechanisms of the neuronal disease remain unclear. Therefore, exploring the pathogenesis of epilepsy is vital for the development of therapeutic targets and strategies.
TSP-1 is a prospective protein discovered in recent years that contributes significantly to epileptic network formation. Studies have suggested that TSP-1 may contribute to epilepsy formation and development in multiple ways, such as by regulating synaptogenesis, angiogenesis, neuroinflammation, and Ca2+ homeostasis (Fig. 3). Based on the potentially vital role of TSP-1 in epileptogenesis, targeting TSP-1 may be a prospective strategy for treating epileptogenesis and epileptic seizures.
Fig. 3.
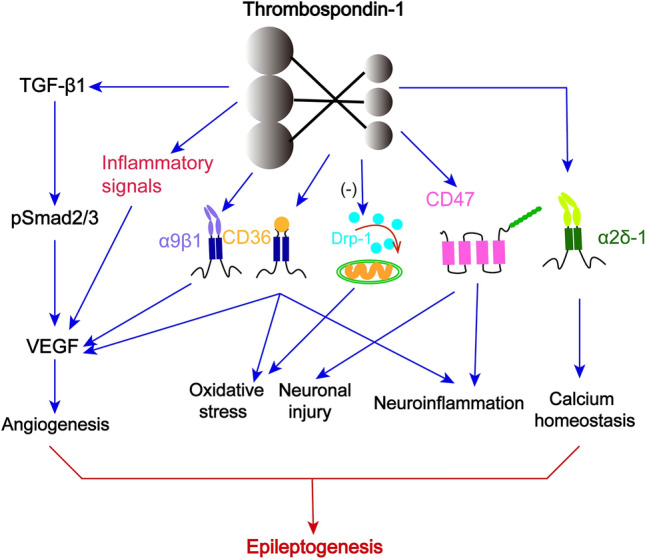
Other factors underlying the regulation of TSP-1 in epileptogenesis, including angiogenesis, neuronal injury, oxidative stress, neuroinflammation, and Ca2+ homeostasis.
Acknowledgements
This review was supported by the Natural Science Foundation of Shandong Province (ZR2021MH034 and ZR2022MH059) and the National Natural Science Foundation of China (81573412). We’d like to thank Editage for English language editing.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Yao Cheng and Yujie Zhai contributed equally to this review.
Contributor Information
Shucui Li, Email: lishucui929@163.com.
Hongliu Sun, Email: sunhongliu@bzmc.edu.cn.
References
- 1.Aronica E, Mühlebner A. Neuropathology of epilepsy. Handb Clin Neurol. 2017;145:193–216. doi: 10.1016/B978-0-12-802395-2.00015-8. [DOI] [PubMed] [Google Scholar]
- 2.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 3.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 4.Tian N, Boring M, Kobau R, Zack MM, Croft JB. Active epilepsy and seizure control in adults - United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67:437–442. doi: 10.15585/mmwr.mm6715a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendus D, Rankin-Gee EK, Mustapha M, Porter BE. Increased sensitivity to kindling in mice lacking TSP1. Neuroscience. 2015;305:302–308. doi: 10.1016/j.neuroscience.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg EM, Coulter DA. Mechanisms of epileptogenesis: A convergence on neural circuit dysfunction. Nat Rev Neurosci. 2013;14:337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codazzi F, Pelizzoni I, Zacchetti D, Grohovaz F. Iron entry in neurons and astrocytes: A link with synaptic activity. Front Mol Neurosci. 2015;8:18. doi: 10.3389/fnmol.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 9.Foulsham W, Dohlman TH, Mittal SK, Taketani Y, Singh RB, Masli S, et al. Thrombospondin-1 in ocular surface health and disease. Ocul Surf. 2019;17:374–383. doi: 10.1016/j.jtos.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng C, Lau SKM, Doering LC. Astrocyte-secreted thrombospondin-1 modulates synapse and spine defects in the fragile X mouse model. Mol Brain. 2016;9:74. doi: 10.1186/s13041-016-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin KT, Eroglu C. Molecular mechanisms of astrocyte-induced synaptogenesis. Curr Opin Neurobiol. 2017;45:113–120. doi: 10.1016/j.conb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennekinne L, Colasse S, Triller A, Renner M. Differential control of thrombospondin over synaptic glycine and AMPA receptors in spinal cord neurons. J Neurosci. 2013;33:11432–11439. doi: 10.1523/JNEUROSCI.5247-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Ma L, Zhang Y, Pan X, Wang C, Zhang J, et al. A purinergic P2 receptor family-mediated increase in thrombospondin-1 bolsters synaptic density and epileptic seizure activity in the amygdala-kindling rat model. Front Cell Neurosci. 2018;12:302. doi: 10.3389/fncel.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang M, Zhu W, Pan X, Wang Q, Gao X, et al. Role of elevated thrombospondin-1 in kainic acid-induced status epilepticus. Neurosci Bull. 2020;36:263–276. doi: 10.1007/s12264-019-00437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres MD, Garcia O, Tang C, Busciglio J. Dendritic spine pathology and thrombospondin-1 deficits in Down syndrome. Free Radic Biol Med. 2018;114:10–14. doi: 10.1016/j.freeradbiomed.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31:170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhu W, Yu H, Yu J, Zhang M, Pan X, et al. P2Y4/TSP-1/TGF-β1/pSmad2/3 pathway contributes to acute generalized seizures induced by kainic acid. Brain Res Bull. 2019;149:106–119. doi: 10.1016/j.brainresbull.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Nishitsuji K, Ikezaki M, Manabe S, Uchimura K, Ito Y, Ihara Y. Thrombospondin type 1 repeat-derived C-mannosylated peptide attenuates synaptogenesis of cortical neurons induced by primary astrocytes via TGF-Β. Glycoconj J. 2022;39:701–710. doi: 10.1007/s10719-021-10030-y. [DOI] [PubMed] [Google Scholar]
- 20.Henshall DC, Simon RP. Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab. 2005;25:1557–1572. doi: 10.1038/sj.jcbfm.9600149. [DOI] [PubMed] [Google Scholar]
- 21.Pototskiy E, Dellinger JR, Bumgarner S, Patel J, Sherrerd-Smith W, Musto AE. Brain injuries can set up an epileptogenic neuronal network. Neurosci Biobehav Rev. 2021;129:351–366. doi: 10.1016/j.neubiorev.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15:144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geronzi U, Lotti F, Grosso S. Oxidative stress in epilepsy. Expert Rev Neurother. 2018;18:427–434. doi: 10.1080/14737175.2018.1465410. [DOI] [PubMed] [Google Scholar]
- 24.Badawy RAB, Freestone DR, Lai A, Cook MJ. Epilepsy: Ever-changing states of cortical excitability. Neuroscience. 2012;222:89–99. doi: 10.1016/j.neuroscience.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Huang T, Sun L, Yuan X, Qiu H. Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget. 2017;8:84546–84558. doi: 10.18632/oncotarget.19165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schöllhorn L, Bock F, Cursiefen C. Thrombospondin-1 as a regulator of corneal inflammation and lymphangiogenesis: Effects on dry eye disease and corneal graft immunology. J Ocul Pharmacol Ther. 2015;31:376–385. doi: 10.1089/jop.2015.0020. [DOI] [PubMed] [Google Scholar]
- 27.Baenziger NL, Brodie GN, Majerus PW. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971;68:240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayser H, Mann K, Machaidze G, Nimtz M, Ringler P, Müller SA, et al. Isolation, characterisation and molecular imaging of a high-molecular-weight insect biliprotein, a member of the hexameric arylphorin protein family. J Mol Biol. 2009;389:74–89. doi: 10.1016/j.jmb.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 29.Liu YS, Yang M. The effect of 5-hydroxtryptamine on the regulation of megakaryocytopoiesis. Hematology. 2006;11:53–56. doi: 10.1080/10245330500322370. [DOI] [PubMed] [Google Scholar]
- 30.Adams JC. Thrombospondin-1. Int J Biochem Cell Biol. 1997;29:861–865. doi: 10.1016/S1357-2725(96)00171-9. [DOI] [PubMed] [Google Scholar]
- 31.Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, Li M, Yin L, Fu G, Liu Z. Role of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases (Review) Int J Mol Med. 2020;45:1275–1293. doi: 10.3892/ijmm.2020.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duquette M, Nadler M, Okuhara D, Thompson J, Shuttleworth T, Lawler J. Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity. Matrix Biol. 2014;37:15–24. doi: 10.1016/j.matbio.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims JN, Lawler J. Thrombospondin-1-based antiangiogenic therapy. J Ocul Pharmacol Ther. 2015;31:366–370. doi: 10.1089/jop.2015.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur S, Roberts DD. Why do humans need thrombospondin-1? J Cell Commun Signal. 2023;17:485–493. doi: 10.1007/s12079-023-00722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Lawler J. Thrombospondin-based antiangiogenic therapy. Microvasc Res. 2007;74:90–99. doi: 10.1016/j.mvr.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: An immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100:120–134.e6. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald JF, Dimitry JM, Frazier WA. An amyloid-like C-terminal domain of thrombospondin-1 displays CD47 agonist activity requiring both VVM motifs. Biochemistry. 2003;42:10001–10011. doi: 10.1021/bi0341408. [DOI] [PubMed] [Google Scholar]
- 42.Gheibihayat SM, Cabezas R, Nikiforov NG, Jamialahmadi T, Johnston TP, Sahebkar A. CD47 in the brain and neurodegeneration: An update on the role in neuroinflammatory pathways. Molecules. 2021;26:3943. doi: 10.3390/molecules26133943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanase C, Enciu AM, Codrici E, Popescu ID, Dudau M, Dobri AM, et al. Fatty acids, CD36, thrombospondin-1, and CD47 in glioblastoma: Together and/or separately? Int J Mol Sci. 2022;23:604. doi: 10.3390/ijms23020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simantov R, Febbraio M, Crombie R, Asch AS, Nachman RL, Silverstein RL. Histidine-rich glycoprotein inhibits the antiangiogenic effect of thrombospondin-1. J Clin Invest. 2001;107:45–52. doi: 10.1172/JCI9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur S, Bronson SM, Pal-Nath D, Miller TW, Soto-Pantoja DR, Roberts DD. Functions of thrombospondin-1 in the tumor microenvironment. Int J Mol Sci. 2021;22:4570. doi: 10.3390/ijms22094570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bongrazio M, Da Silva-Azevedo L, Bergmann EC, Baum O, Hinz B, Pries AR, et al. Shear stress modulates the expression of thrombospondin-1 and CD36 in endothelial cells in vitro and during shear stress-induced angiogenesis in vivo. Int J Immunopathol Pharmacol. 2006;19:35–48. doi: 10.1177/205873920601900104. [DOI] [PubMed] [Google Scholar]
- 47.Ortiz-Masià D, Díez I, Calatayud S, Hernández C, Cosín-Roger J, Hinojosa J, et al. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PLoS One. 2012;7:e48535. doi: 10.1371/journal.pone.0048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zong P, Feng J, Li CX, Jellison ER, Yue Z, Miller B, et al. Activation of endothelial TRPM2 exacerbates blood-brain barrier degradation in ischemic stroke. Cardiovasc Res. 2023;2023:cvad126. doi: 10.1093/cvr/cvad126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Y, Halade GV, Lindsey ML. Extracellular matrix and fibroblast communication following myocardial infarction. J Cardiovasc Transl Res. 2012;5:848–857. doi: 10.1007/s12265-012-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Chen W, Liu W, Wu J, Shao Y, Zhang X. The role of thrombospondin-1 and transforming growth factor-beta after spinal cord injury in the rat. J Clin Neurosci. 2009;16:818–821. doi: 10.1016/j.jocn.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- 52.Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210:807–818. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz A, Yepes M. Urokinase-type plasminogen activator promotes synaptic repair in the ischemic brain. Neural Regen Res. 2018;13:232–233. doi: 10.4103/1673-5374.226384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sizova O, John LS, Ma Q, Molldrem JJ. Multi-faceted role of LRP1 in the immune system. Front Immunol. 2023;14:1166189. doi: 10.3389/fimmu.2023.1166189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oyasu M, Kuroda S, Nakashita M, Fujimiya M, Kikkawa U, Saito N. Immunocytochemical localization of a neuron-specific thrombospondin-1-like protein, NELL2: Light and electron microscopic studies in the rat brain. Brain Res Mol Brain Res. 2000;76:151–160. doi: 10.1016/S0169-328X(99)00342-3. [DOI] [PubMed] [Google Scholar]
- 60.Danjo Y, Shigetomi E, Hirayama YJ, Kobayashi K, Ishikawa T, Fukazawa Y, et al. Transient astrocytic mGluR5 expression drives synaptic plasticity and subsequent chronic pain in mice. J Exp Med. 2022;219:e20210989. doi: 10.1084/jem.20210989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tagnaouti N, Loebrich S, Heisler F, Pechmann Y, Fehr S, De Arcangelis A, et al. Neuronal expression of muskelin in the rodent central nervous system. BMC Neurosci. 2007;8:28. doi: 10.1186/1471-2202-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott-Drew S, ffrench-Constant C. Expression and function of thrombospondin-1 in myelinating glial cells of the central nervous system. J Neurosci Res. 1997;50:202–214. doi: 10.1002/(SICI)1097-4547(19971015)50:2<202::AID-JNR9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 63.Cheng C, Yu Z, Zhao S, Liao Z, Xing C, Jiang Y, et al. Thrombospondin-1 gene deficiency worsens the neurological outcomes of traumatic brain injury in mice. Int J Med Sci. 2017;14:927–936. doi: 10.7150/ijms.18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin C, Wang K, Zhang L, Bai L. Stem cell therapy for Alzheimer’s disease: An overview of experimental models and reality. Animal Model Exp Med. 2022;5:15–26. doi: 10.1002/ame2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sher AA, Glover KKM, Coombs KM. Zika virus infection disrupts astrocytic proteins involved in synapse control and axon guidance. Front Microbiol. 2019;10:596. doi: 10.3389/fmicb.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia O, Torres M, Helguera P, Coskun P, Busciglio J. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down’s syndrome. PLoS One. 2010;5:e14200. doi: 10.1371/journal.pone.0014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang B, Zou L, Li M, Zhou L. Astrocyte: A foe or a friend in intellectual disability-related diseases. Front Synaptic Neurosci. 2022;14:877928. doi: 10.3389/fnsyn.2022.877928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu K, Ge J, Summers JB, Li F, Liu X, Ma P, et al. TSP-1 secreted by bone marrow stromal cells contributes to retinal ganglion cell neurite outgrowth and survival. PLoS One. 2008;3:e2470. doi: 10.1371/journal.pone.0002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diniz LP, Almeida JC, Tortelli V, Vargas Lopes C, Setti-Perdigão P, Stipursky J, et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem. 2012;287:41432–41445. doi: 10.1074/jbc.M112.380824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tran MD, Furones-Alonso O, Sanchez-Molano J, Bramlett HM. Trauma-induced expression of astrocytic thrombospondin-1 is regulated by P2 receptors coupled to protein kinase cascades. Neuroreport. 2012;23:721–726. doi: 10.1097/WNR.0b013e32835688fe. [DOI] [PubMed] [Google Scholar]
- 71.Lin Z, Gu Y, Zhou R, Wang M, Guo Y, Chen Y, et al. Serum exosomal proteins F9 and TSP-1 as potential diagnostic biomarkers for newly diagnosed epilepsy. Front Neurosci. 2020;14:737. doi: 10.3389/fnins.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang B, Guo W, Huang Y. Thrombospondins and synaptogenesis. Neural Regen Res. 2012;7:1737–1743. doi: 10.3969/j.issn.1673-5374.2012.22.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jun N, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, et al. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell. 2019;177:1280–1292.e20. doi: 10.1016/j.cell.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Min-DeBartolo J, Schlerman F, Akare S, Wang J, McMahon J, Zhan Y, et al. Thrombospondin-I is a critical modulator in non-alcoholic steatohepatitis (NASH) PLoS One. 2019;14:e0226854. doi: 10.1371/journal.pone.0226854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ungefroren H, Gieseler F, Kaufmann R, Settmacher U, Lehnert H, Rauch BH. Signaling crosstalk of TGF-β/ALK5 and PAR2/PAR1: A complex regulatory network controlling fibrosis and cancer. Int J Mol Sci. 2018;19:1568. doi: 10.3390/ijms19061568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kisseleva EP, Krylov AV, Stepanova OI, Lioudyno VI. Transplantable subcutaneous hepatoma 22a affects functional activity of resident tissue macrophages in periphery. Int J Cell Biol. 2011;2011:793034. doi: 10.1155/2011/793034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hisaoka-Nakashima K, Yokoe T, Watanabe S, Nakamura Y, Kajitani N, Okada-Tsuchioka M, et al. Lysophosphatidic acid induces thrombospondin-1 production in primary cultured rat cortical astrocytes. J Neurochem. 2021;158:849–864. doi: 10.1111/jnc.15227. [DOI] [PubMed] [Google Scholar]
- 78.Bussolati B, Assenzio B, Deregibus MC, Camussi G. The proangiogenic phenotype of human tumor-derived endothelial cells depends on thrombospondin-1 downregulation via phosphatidylinositol 3-kinase/Akt pathway. J Mol Med. 2006;84:852–863. doi: 10.1007/s00109-006-0075-z. [DOI] [PubMed] [Google Scholar]
- 79.Pacurari M, Mitra A, Turner T. Idiopathic pulmonary comorbidities and mechanisms. Int J Inflam. 2021;2021:3963659. doi: 10.1155/2021/3963659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Planas-Fontánez TM, Sainato DM, Sharma I, Dreyfus CF. Roles of astrocytes in response to aging, Alzheimer’s disease and multiple sclerosis. Brain Res. 2021;1764:147464. doi: 10.1016/j.brainres.2021.147464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rama Rao KV, Kielian T. Neuron-astrocyte interactions in neurodegenerative diseases: Role of neuroinflammation. Clin Exp Neuroimmunol. 2015;6:245–263. doi: 10.1111/cen3.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jo WS, Mizukami Y, Duerr EM, Zukerberg LR, Chung DC. Wnt signaling can repress thrombospondin-1 expression in colonic tumorigenesis. Cancer Biol Ther. 2005;4:1361–1366. doi: 10.4161/cbt.4.12.2201. [DOI] [PubMed] [Google Scholar]
- 83.Fairaq A, Goc A, Artham S, Sabbineni H, Somanath PR. TNFα induces inflammatory stress response in microvascular endothelial cells via Akt- and P38 MAP kinase-mediated thrombospondin-1 expression. Mol Cell Biochem. 2015;406:227–236. doi: 10.1007/s11010-015-2440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orozco-Morales M, Avilés-Salas A, Hernández-Pedro N, Catalán R, Cruz-Rico G, Colín-González AL, et al. Clinicopathological and prognostic significance of CD47 expression in lung neuroendocrine tumors. J Immunol Res. 2021;2021:6632249. doi: 10.1155/2021/6632249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giehl K, Graness A, Goppelt-Struebe M. The small GTPase Rac-1 is a regulator of mesangial cell morphology and thrombospondin-1 expression. Am J Physiol Renal Physiol. 2008;294:F407–F413. doi: 10.1152/ajprenal.00093.2007. [DOI] [PubMed] [Google Scholar]
- 86.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yurdagul A, Jr, Finney AC, Woolard MD, Orr AW. The arterial microenvironment: The where and why of atherosclerosis. Biochem J. 2016;473:1281–1295. doi: 10.1042/BJ20150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho J, Huh Y. Astrocytic calcium dynamics along the pain pathway. Front Cell Neurosci. 2020;14:594216. doi: 10.3389/fncel.2020.594216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogáti R, Katona É, Shemirani AH, Balogh E, Bárdos H, Jeney V, et al. The effect of activated FXIII, a transglutaminase, on vascular smooth muscle cells. Int J Mol Sci. 2022;23:5845. doi: 10.3390/ijms23105845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Z, Hu H. Star-like cells spark behavioural hyperactivity in mice. Nature. 2019;571:43–44. doi: 10.1038/d41586-019-01949-2. [DOI] [PubMed] [Google Scholar]
- 91.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 92.Zhao Y, Pu D, Sun Y, Chen J, Luo C, Wang M, et al. High glucose-induced defective thrombospondin-1 release from astrocytes via TLR9 activation contributes to the synaptic protein loss. Exp Cell Res. 2018;363:171–178. doi: 10.1016/j.yexcr.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 93.Takeuchi K, Ariyoshi Y, Shimizu A, Okumura Y, Cara-Fuentes G, Garcia GE, et al. Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation. Xenotransplantation. 2021;28:e12708. doi: 10.1111/xen.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy-Ullrich JE. Thrombospondin-1 signaling through the calreticulin/LDL receptor related protein 1 axis: Functions and possible roles in glaucoma. Front Cell Dev Biol. 2022;10:898772. doi: 10.3389/fcell.2022.898772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maeda N, Maenaka K. The roles of matricellular proteins in oncogenic virus-induced cancers and their potential utilities as therapeutic targets. Int J Mol Sci. 2017;18:2198. doi: 10.3390/ijms18102198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeong JK, Kim JG, Kim HR, Lee TH, Park JW, Lee BJ. A role of central NELL2 in the regulation of feeding behavior in rats. Mol Cells. 2017;40:186–194. doi: 10.14348/molcells.2017.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heller JP, Rusakov DA. Morphological plasticity of astroglia: Understanding synaptic microenvironment. Glia. 2015;63:2133–2151. doi: 10.1002/glia.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pascual MRQ. Temporal lobe epilepsy: Clinical semiology and neurophysiological studies. Semin Ultrasound CT MR. 2007;28:416–423. doi: 10.1053/j.sult.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Samanta D. Epilepsy in Angelman syndrome: A scoping review. Brain Dev. 2021;43:32–44. doi: 10.1016/j.braindev.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang YH, Huang TL, Chen X, Yu SX, Li W, Chen T, et al. Glioma-derived TSP2 promotes excitatory synapse formation and results in hyperexcitability in the peritumoral cortex of glioma. J Neuropathol Exp Neurol. 2021;80:137–149. doi: 10.1093/jnen/nlaa149. [DOI] [PubMed] [Google Scholar]
- 101.Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: An emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- 102.Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–558. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 103.Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: A network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol. 2005;93:437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W, Thamattoor AK, LeRoy C, Buckmaster PS. Surviving mossy cells enlarge and receive more excitatory synaptic input in a mouse model of temporal lobe epilepsy. Hippocampus. 2015;25:594–604. doi: 10.1002/hipo.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Puhahn-Schmeiser B, Leicht K, Gessler F, Freiman TM. Aberrant hippocampal mossy fibers in temporal lobe epilepsy target excitatory and inhibitory neurons. Epilepsia. 2021;62:2539–2550. doi: 10.1111/epi.17035. [DOI] [PubMed] [Google Scholar]
- 106.Ishibashi M, Egawa K, Fukuda A. Diverse actions of astrocytes in GABAergic signaling. Int J Mol Sci. 2019;20:2964. doi: 10.3390/ijms20122964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fujimura M, Usuki F, Nakamura A. Methylmercury induces hyperalgesia/allodynia through spinal cord dorsal horn neuronal activation and subsequent somatosensory cortical circuit formation in rats. Arch Toxicol. 2021;95:2151–2162. doi: 10.1007/s00204-021-03047-7. [DOI] [PubMed] [Google Scholar]
- 108.Wu Q, Finley SD. Mathematical model predicts effective strategies to inhibit VEGF-eNOS signaling. J Clin Med. 2020;9:1255. doi: 10.3390/jcm9051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Eekelen M, Sasportas LS, Kasmieh R, Yip S, Figueiredo JL, Louis DN, et al. Human stem cells expressing novel TSP-1 variant have anti-angiogenic effect on brain tumors. Oncogene. 2010;29:3185–3195. doi: 10.1038/onc.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jeanne A, Sick E, Devy J, Floquet N, Belloy N, Theret L, et al. Identification of TAX2 peptide as a new unpredicted anti-cancer agent. Oncotarget. 2015;6:17981–18000. doi: 10.18632/oncotarget.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernando KHN, Yang HW, Jiang Y, Jeon YJ, Ryu B. Ishige okamurae extract and its constituent ishophloroglucin A attenuated in vitro and in vivo high glucose-induced angiogenesis. Int J Mol Sci. 2019;20:5542. doi: 10.3390/ijms20225542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walewska E, Wołodko K, Skarzynski D, Ferreira-Dias G, Galvão A. The interaction between nodal, hypoxia-inducible factor 1 alpha, and thrombospondin 1 promotes luteolysis in equine corpus luteum. Front Endocrinol. 2019;10:667. doi: 10.3389/fendo.2019.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyata Y, Sakai H. Thrombospondin-1 in urological cancer: Pathological role, clinical significance, and therapeutic prospects. Int J Mol Sci. 2013;14:12249–12272. doi: 10.3390/ijms140612249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J, Yang W, Zhao D, Han Y, Liu B, Zhao H, et al. Correlation between TSP-1, TGF-β and PPAR-γ expression levels and glioma microvascular density. Oncol Lett. 2014;7:95–100. doi: 10.3892/ol.2013.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gustavsson H, Tesan T, Jennbacken K, Kuno K, Damber JE, Welén K. ADAMTS1 alters blood vessel morphology and TSP1 levels in LNCaP and LNCaP-19 prostate tumors. BMC Cancer. 2010;10:288. doi: 10.1186/1471-2407-10-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, et al. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 118.Yang AL, Zhou HJ, Lin Y, Luo JK, Cui HJ, Tang T, et al. Thrombin promotes the expression of thrombospondin-1 and-2 in a rat model of intracerebral hemorrhage. J Neurol Sci. 2012;323:141–146. doi: 10.1016/j.jns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Feng N, Wang Z, Zhang Z, He X, Wang C, Zhang L. MiR-487b promotes human umbilical vein endothelial cell proliferation, migration, invasion and tube formation through regulating THBS1. Neurosci Lett. 2015;591:1–7. doi: 10.1016/j.neulet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 120.Soltani A, Walters EH, Reid DW, Shukla SD, Nowrin K, Ward C, et al. Inhaled corticosteroid normalizes some but not all airway vascular remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2359–2367. doi: 10.2147/COPD.S113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uchida H, Kuroki M, Shitama T, Hayashi H, Kuroki M. Activation of TGF-beta1 through up-regulation of TSP-1 by retinoic acid in retinal pigment epithelial cells. Curr Eye Res. 2008;33:199–203. doi: 10.1080/02713680701852090. [DOI] [PubMed] [Google Scholar]
- 122.Morandi V. The N-terminal domain of thrombospondin-1: A key for the dual effect of TSP-1 in angiogenesis and cancer progression? Sci World J. 2009;9:133–136. doi: 10.1100/tsw.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prangsaengtong O, Park JY, Inujima A, Igarashi Y, Shibahara N, Koizumi K. Enhancement of lymphangiogenesis in vitro via the regulations of HIF-1α expression and nuclear translocation by deoxyshikonin. Evid Based Complement Alternat Med. 2013;2013:148297. doi: 10.1155/2013/148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghorbanzadeh V, Pourheydar B, Dariushnejad H, Ghalibafsabbaghi A, Chodari L. Curcumin improves angiogenesis in the heart of aged rats: Involvement of TSP1/NF-κB/VEGF-A signaling. Microvasc Res. 2022;139:104258. doi: 10.1016/j.mvr.2021.104258. [DOI] [PubMed] [Google Scholar]
- 125.Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: A sticky affair with cancers. J Oncol. 2012;2012:351089. doi: 10.1155/2012/351089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li S, Dong J, Ta G, Liu Y, Cui J, Li X, et al. Xuan Bi Tong yu Fang promotes angiogenesis via VEGF-Notch1/Dll4 pathway in myocardial ischemic rats. Evid Based Complement Alternat Med. 2020;2020:5041629. doi: 10.1155/2020/5041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baulac M. MTLE with hippocampal sclerosis in adult as a syndrome. Rev Neurol. 2015;171:259–266. doi: 10.1016/j.neurol.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 128.Morin-Brureau M, Rigau V, Lerner-Natoli M. Why and how to target angiogenesis in focal epilepsies. Epilepsia. 2012;53:64–68. doi: 10.1111/j.1528-1167.2012.03705.x. [DOI] [PubMed] [Google Scholar]
- 129.Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, et al. Angiogenesis is associated with blood–brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 130.Castro-Torres RD, Ureña-Guerrero ME, Morales-Chacón LM, Lorigados-Pedre L, Estupiñan-Díaz B, Rocha L, et al. New aspects of VEGF, GABA, and glutamate signaling in the neocortex of human temporal lobe pharmacoresistant epilepsy revealed by RT-qPCR arrays. J Mol Neurosci. 2020;70:916–929. doi: 10.1007/s12031-020-01519-6. [DOI] [PubMed] [Google Scholar]
- 131.Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Castañeda-Cabral JL, Colunga-Durán A, Ureña-Guerrero ME, Beas-Zárate C, Nuñez-Lumbreras MLA, Orozco-Suárez S, et al. Expression of VEGF- and tight junction-related proteins in the neocortical microvasculature of patients with drug-resistant temporal lobe epilepsy. Microvasc Res. 2020;132:104059. doi: 10.1016/j.mvr.2020.104059. [DOI] [PubMed] [Google Scholar]
- 133.Rempe RG, Hartz AMS, Soldner ELB, Sokola BS, Alluri SR, Abner EL, et al. Matrix metalloproteinase-mediated blood-brain barrier dysfunction in epilepsy. J Neurosci. 2018;38:4301–4315. doi: 10.1523/JNEUROSCI.2751-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Löscher W, Friedman A. Structural, molecular, and functional alterations of the blood-brain barrier during epileptogenesis and epilepsy: A cause, consequence, or both? Int J Mol Sci. 2020;21:591. doi: 10.3390/ijms21020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han H, Mann A, Ekstein D, Eyal S. Breaking bad: The structure and function of the blood-brain barrier in epilepsy. AAPS J. 2017;19:973–988. doi: 10.1208/s12248-017-0096-2. [DOI] [PubMed] [Google Scholar]
- 136.Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care. 2010;13:87–92. doi: 10.1097/MCO.0b013e328333b829. [DOI] [PubMed] [Google Scholar]
- 137.Ning M, Sarracino DA, Kho AT, Guo S, Lee SR, Krastins B, et al. Proteomic temporal profile of human brain endothelium after oxidative stress. Stroke. 2011;42:37–43. doi: 10.1161/STROKEAHA.110.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao JB, Tang WD, Wang HX, Xu Y. Predictive value of thrombospondin-1 for outcomes in patients with acute ischemic stroke. Clin Chim Acta. 2015;450:176–180. doi: 10.1016/j.cca.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 139.Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998;5:401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- 140.Ruhela D, Bhopale VM, Kalakonda S, Thom SR. Astrocyte-derived microparticles initiate a neuroinflammatory cycle due to carbon monoxide poisoning. Brain Behav Immun Health. 2021;18:100398. doi: 10.1016/j.bbih.2021.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kang S, Byun J, Son SM, Mook-Jung I. Thrombospondin-1 protects against Aβ-induced mitochondrial fragmentation and dysfunction in hippocampal cells. Cell Death Discov. 2018;4:31. doi: 10.1038/s41420-017-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88:23–45. doi: 10.1016/j.eplepsyres.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frantseva MV, Velazquez JL, Hwang PA, Carlen PL. Free radical production correlates with cell death in an in vitro model of epilepsy. Eur J Neurosci. 2000;12:1431–1439. doi: 10.1046/j.1460-9568.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 144.Yang N, Guan QW, Chen FH, Xia QX, Yin XX, Zhou HH, et al. Antioxidants targeting mitochondrial oxidative stress: Promising neuroprotectants for epilepsy. Oxid Med Cell Longev. 2020;2020:6687185. doi: 10.1155/2020/6687185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Puttachary S, Sharma S, Stark S, Thippeswamy T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res Int. 2015;2015:745613. doi: 10.1155/2015/745613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chowdhury AR, Zielonka J, Kalyanaraman B, Hartley RC, Murphy MP, Avadhani NG. Mitochondria-targeted paraquat and metformin mediate ROS production to induce multiple pathways of retrograde signaling: A dose-dependent phenomenon. Redox Biol. 2020;36:101606. doi: 10.1016/j.redox.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Souza MA, Mota BC, Gerbatin RR, Rodrigues FS, Castro M, Fighera MR, et al. Antioxidant activity elicited by low dose of caffeine attenuates pentylenetetrazol-induced seizures and oxidative damage in rats. Neurochem Int. 2013;62:821–830. doi: 10.1016/j.neuint.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 148.Rowley S, Liang LP, Fulton R, Shimizu T, Day B, Patel M. Mitochondrial respiration deficits driven by reactive oxygen species in experimental temporal lobe epilepsy. Neurobiol Dis. 2015;75:151–158. doi: 10.1016/j.nbd.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shekh-Ahmad T, Lieb A, Kovac S, Gola L, Christian Wigley W, Abramov AY, et al. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol. 2019;26:101278. doi: 10.1016/j.redox.2019.101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cenini G, Lloret A, Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid Med Cell Longev. 2019;2019:2105607. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mittal R, Gonzalez-Gomez I, Prasadarao NV. Escherichia coli K1 promotes the ligation of CD47 with thrombospondin-1 to prevent the maturation of dendritic cells in the pathogenesis of neonatal meningitis. J Immunol. 2010;185:2998–3006. doi: 10.4049/jimmunol.1001296. [DOI] [PubMed] [Google Scholar]
- 152.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 153.Bornstein P. Thrombospondins: Structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 154.Thom SR, Bhopale VM, Bhat AR, Arya AK, Ruhela D, Qiao G, et al. Neuroinflammation with increased glymphatic flow in a murine model of decompression sickness. J Neurophysiol. 2023;129:662–671. doi: 10.1152/jn.00005.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106:658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- 156.Li P, Marshall L, Oh G, Jakubowski JL, Groot D, He Y, et al. Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat Commun. 2019;10:2246. doi: 10.1038/s41467-019-10101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 158.Gong L, Gu Y, Han X, Luan C, Liu C, Wang X, et al. Spatiotemporal dynamics of the molecular expression pattern and intercellular interactions in the glial scar response to spinal cord injury. Neurosci Bull. 2023;39:213–244. doi: 10.1007/s12264-022-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang L, Tang H, Sherchan P, Lenahan C, Boling W, Tang J, et al. The activation of phosphatidylserine/CD36/TGF-β1 pathway prior to surgical brain injury attenuates neuroinflammation in rats. Oxid Med Cell Longev. 2020;2020:4921562. doi: 10.1155/2020/4921562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang Y, Chen S, Luo Y, Han Z. Crosstalk between inflammation and the BBB in stroke. Curr Neuropharmacol. 2020;18:1227–1236. doi: 10.2174/1570159X18666200620230321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao L, Peng L, Sherchan P, Tang H, Liu Y, Xiao J, et al. Inhibition of lysophosphatidic acid receptor 1 relieves PMN recruitment in CNS via LPA1/TSP1/CXCR2 pathway and alleviates disruption on blood-brain barrier following intracerebral haemorrhage in mice. Fluids Barriers CNS. 2023;20:33. doi: 10.1186/s12987-023-00434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alyu F, Dikmen M. Inflammatory aspects of epileptogenesis: Contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr. 2017;29:1–16. doi: 10.1017/neu.2016.47. [DOI] [PubMed] [Google Scholar]
- 163.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Musto AE, Gjorstrup P, Bazan NG. The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia. 2011;52:1601–1608. doi: 10.1111/j.1528-1167.2011.03081.x. [DOI] [PubMed] [Google Scholar]
- 166.Musto AE, Rosencrans RF, Walker CP, Bhattacharjee S, Raulji CM, Belayev L, et al. Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci Rep. 2016;6:30298. doi: 10.1038/srep30298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hong Z, Chen H, Hong H, Lin L, Wang Z. TSP-1 expression changes in diabetic rats with spinal cord injury. Neurol Res. 2009;31:878–882. doi: 10.1179/174313209X403887. [DOI] [PubMed] [Google Scholar]
- 168.Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.STR.0000047100.84604.BA. [DOI] [PubMed] [Google Scholar]
- 169.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 170.Maikos JT, Shreiber DI. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma. 2007;24:492–507. doi: 10.1089/neu.2006.0149. [DOI] [PubMed] [Google Scholar]
- 171.Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin Neurosci Res. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang L, Li L, Wang B, Qian DM, Song XM, Hu M. Human cytomegalovirus infection modulates thrombospondins 1 and 2 in primary fetal astrocytes. Neuroreport. 2013;24:526–535. doi: 10.1097/WNR.0b013e32836206d1. [DOI] [PubMed] [Google Scholar]
- 173.Jaber SM, Polster BM. An in vitro model yields ‘importin’ new insights into chronic traumatic encephalopathy: Damaged astrocytes stop ‘thrombospondin’ to the injury: An Editorial Highlight for ‘Defective synthesis and release of astrocytic thrombospondin-1 mediates the neuronal TDP-43 proteinopathy, resulting in defects in neuronal integrity associated with chronic traumatic encephalopathy: in vitro studies’. J Neurochem. 2017;140:531–535. doi: 10.1111/jnc.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 175.Xie Y, Zhang J, Jin W, Tian R, Wang R. Role of Thrombospondin-1 in sepsis-induced myocardial injury. Mol Med Rep. 2021;24:869. doi: 10.3892/mmr.2021.12509. [DOI] [PubMed] [Google Scholar]
- 176.Masli S, Sheibani N, Cursiefen C, Zieske J. Matricellular protein thrombospondins: Influence on ocular angiogenesis, wound healing and immuneregulation. Curr Eye Res. 2014;39:759–774. doi: 10.3109/02713683.2013.877936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jayakumar AR, Tong XY, Shamaladevi N, Barcelona S, Gaidosh G, Agarwal A, et al. Defective synthesis and release of astrocytic thrombospondin-1 mediates the neuronal TDP-43 proteinopathy, resulting in defects in neuronal integrity associated with chronic traumatic encephalopathy: in vitro studies. J Neurochem. 2017;140:645–661. doi: 10.1111/jnc.13867. [DOI] [PubMed] [Google Scholar]
- 179.Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: Seizures do not inevitably cause neuronal loss. Brain. 2005;128:1344–1357. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- 180.Xu S, Sun Q, Fan J, Jiang Y, Yang W, Cui Y, et al. Role of astrocytes in post-traumatic epilepsy. Front Neurol. 2019;10:1149. doi: 10.3389/fneur.2019.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 182.Misenheimer TM, Mosher DF. Calcium ion binding to thrombospondin 1. J Biol Chem. 1995;270:1729–1733. doi: 10.1074/jbc.270.4.1729. [DOI] [PubMed] [Google Scholar]
- 183.Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res. 2008;68:7090–7099. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bissinger R, Petkova-Kirova P, Mykhailova O, Oldenborg PA, Novikova E, Donkor DA, et al. Thrombospondin-1/CD47 signaling modulates transmembrane cation conductance, survival, and deformability of human red blood cells. Cell Commun Signal. 2020;18:155. doi: 10.1186/s12964-020-00651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bazzazi H, Isenberg JS, Popel AS. Inhibition of VEGFR2 activation and its downstream signaling to ERK1/2 and calcium by thrombospondin-1 (TSP1): In silico investigation. Front Physiol. 2017;8:48. doi: 10.3389/fphys.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang Y, Yang F, Yang F, Li CL, Wang Y, Li Z, et al. Gabapentinoid insensitivity after repeated administration is associated with down-regulation of the α(2)δ-1 subunit in rats with central post-stroke pain hypersensitivity. Neurosci Bull. 2016;32:41–50. doi: 10.1007/s12264-015-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Calzada MJ, Kuznetsova SA, Sipes JM, Rodrigues RG, Cashel JA, Annis DS, et al. Calcium indirectly regulates immunochemical reactivity and functional activities of the N-domain of thrombospondin-1. Matrix Biol. 2008;27:339–351. doi: 10.1016/j.matbio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang B, Li X, Yu N, Yang L, Nan C, Guo L, et al. Intracerebral hemorrhage alters α2δ1 and thrombospondin expression in rats. Exp Ther Med. 2022;23:327. doi: 10.3892/etm.2022.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 2016;126:1983–1997. doi: 10.1172/JCI82859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Risher WC, Kim N, Koh S, Choi JE, Mitev P, Spence EF, et al. Thrombospondin receptor α2δ-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J Cell Biol. 2018;217:3747–3765. doi: 10.1083/jcb.201802057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kadurin I, Ferron L, Rothwell SW, Meyer JO, Douglas LR, Bauer CS, et al. Proteolytic maturation of α2δ represents a checkpoint for activation and neuronal trafficking of latent calcium channels. Elife. 2016;5:e21143. doi: 10.7554/eLife.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]