Abstract
With the discovery of the therapeutic activity of peptides, they have emerged as a promising class of anti-cancer agents due to their specific targeting, low toxicity, and potential for high selectivity. In particular, as peptide-drug conjugates enter clinical, the coupling of targeted peptides with traditional chemotherapy drugs or cytotoxic agents will become a new direction in cancer treatment. To facilitate the drug development of cancer therapy peptides, we have constructed DCTPep, a novel, open, and comprehensive database for cancer therapy peptides. In addition to traditional anticancer peptides (ACPs), the peptide library also includes peptides related to cancer therapy. These data were collected manually from published research articles, patents, and other protein or peptide databases. Data on drug library include clinically investigated and/or approved peptide drugs related to cancer therapy, which mainly come from the portal websites of drug regulatory authorities and organisations in different countries and regions. DCTPep has a total of 6214 entries, we believe that DCTPep will contribute to the design and screening of future cancer therapy peptides.
Subject terms: Databases, Drug discovery
Background & Summary
Cancer is a leading cause of death and a significant barrier to increasing life expectancy worldwide1. Cancer treatment has improved in the past few decades, but chemotherapy remains the mainstay of cancer treatment. Multidrug resistance is a major problem associated with anticancer chemotherapy2,3. Data show that 90% of cancer deaths can be attributed to multidrug resistance4. Due to structural differences with small-molecule compounds, bioactive peptides have received much attention and are believed to be alternative candidates for multidrug-resistant cancer therapy5–7.
Anticancer peptides (ACPs) are biologically active peptides with antitumor activities that exist widely in a variety of organisms, including mammals, amphibians, insects, plants, and microorganisms. ACPs have many advantages in the treatment of tumours, such as low molecular weight (compared to protein-based therapy), simple structure, high anticancer activity, high selectivity, fewer side effects, easy modifications, and less possibility to cause resistance8,9. Although ACPs have been extensively studied, its mechanism of action is not fully understood. At present, the known mechanisms of ACPs mainly include inhibition of tumour cell proliferation or migration10,11, inhibition of tumour blood vessel formation12, causing cancer cell lysis13, and induction of cancer cell apoptosis14. In addition, peptides can also serve as a targeted therapeutic agent that can target and directly bind specific cancer cells or cancer related biomarkers, and can also serve as a peptide carrier linked to traditional anticancer drugs15,16.
Although the enormous potential of peptides in cancer therapeutics, there is a relative scarcity of dedicated databases specifically storing cancer therapy peptides information. Most of the ACPs information is dispersed in bioactive peptide databases, such as DRAMP17, APD18, DBAASP19, HORDB20, CPPsite21, and SATPdb22, which mainly focus on antimicrobial peptides or hormones. The CancerPPD23 database is a known database for annotating ACPs and anticancer proteins; however, its data have not been updated since 2015. Many antimicrobial peptide databases also store information about the anticancer activity of some antimicrobial peptides, but it does not contain detail annotation of ACPs. For example, they did not fully provide information on cancer cells or molecular targets of ACPs, nor do they include peptide drugs. Therefore, we constructed an open, comprehensive database of cancer therapy peptides, DCTPep, that not only includes traditional ACPs, but also peptides with targeted effects on cancer therapeutics. DCTPep can be freely accessed and downloaded from http://dctpep.cpu-bioinfor.org/.
Developing targeted therapies that selectively act on cancer cells has always been an ideal approach for cancer treatment. A promising targeted therapy is drug conjugates, which involve linking targeting carriers with chemotherapy drugs or cytotoxic agents through a linker, such as antibody-drug conjugates (ADCs) and peptide-drug conjugates (PDCs)24. Currently, the most common drug conjugates used in cancer treatment in clinical practice are ADCs. However, with the increasing presence of peptides in clinical, PDCs has also emerged. PDCs have the potential to overcome the limitations of ADCs, such as smaller molecular weight and ease of synthesis25. Nowadays, only two PDCs, 177 Lu-dotatate (DCTPepD0013) and Melflufen (DCTPepD0108), have been approved for clinical cancer treatment, of which Melflufen being withdrawn from the market by the FDA. However, there are still many PDCs in cancer clinical development or about to enter clinical trials. The potential of PDCs cannot be ignored. Peptides play a crucial role as carriers in PDCs. Therefore, DCTPep not only focuses on collecting ACPs but also emphasizes the collection of cancer targeted peptides. The carrier peptides in PDCs include cell-penetrating peptides (CPP) and cell-targeting peptides (CTP)26. The classification field in the database also follows a similar category, including cell-penetrating peptides, cancer-targeting peptides, and targeted peptide conjugates.
Figure 1 and Table 1 presents the comparative results of DCTPep datasets with ACP datasets in other peptide databases. Compared to DBAASP, CancerPPD and SATPdb, DCTPep possesses over 3000 unique entries. DCTPep provides a vast amount of cancer therapy peptide data, including clinically relevant peptide drugs curated in the drug library, filling the gaps in existing data and offering assistance in the design and screening of novel cancer therapeutic peptides. Particularly, the targeted peptide data will offer more options for PDC design. In order to better understand the mechanism of action of cancer therapy peptides, we have added target annotations and collected over 60 targets for these peptides that are not included in other ACPs databases. The dataset is freely available to all via the web without the need to login or registration and is not password protected. We believe that DCTPep will become a valuable resource for the development of novel bioactive peptides, particularly in the field of cancer therapeutics.
Fig. 1.
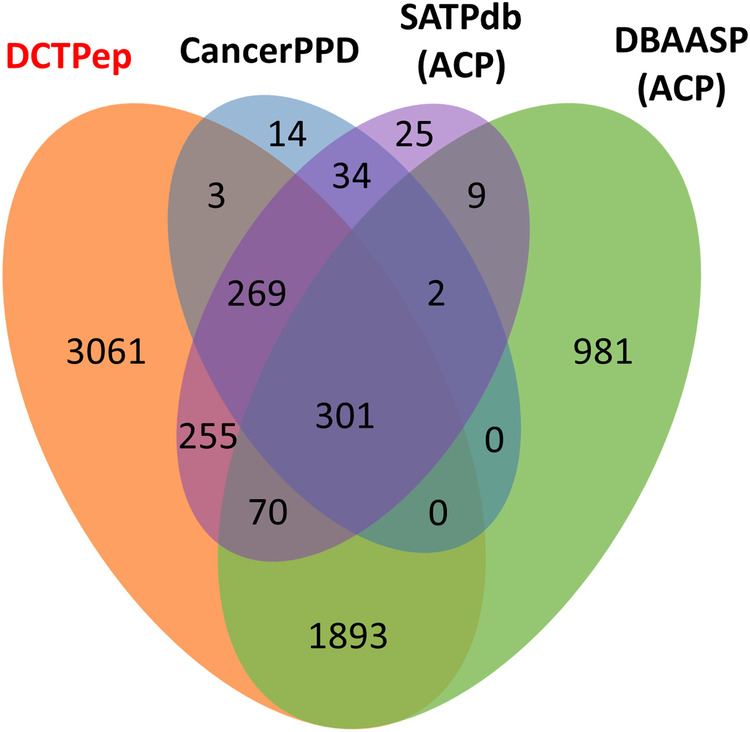
Venn diagram illustrating the numbers of overlapping and non-overlapping peptide sequences related to cancer therapy from the DCTPep, CancerPPD, SATPdb and DBAASP.
Table 1.
Comparison of peptides related to cancer therapy in DCTPep with other peptide databases (data as of 2023.12.20).
| Features | DCTPep | CancerPPD | DRAMP 3.0 | APD3 | DBAASP v3 | SATPdb |
|---|---|---|---|---|---|---|
| Entries related to cancer therapy peptide | 6106 | 624 | 455 | 276 | 3,409 | 1,099 |
| ACP drugs | 108 | NA | NA | NA | NA | NA |
| Cancer cell line | >400 | 249 | NA | NA | NA | NA |
| Targets | >60 | NA | NA | NA | NA | NA |
| predicted structures of ACPs | 1646 (Based on AlphaFold) | 617 (Based on molecular dynamics) | NA | NA | 1372 (Based on molecular dynamics) | NA |
| Download dataset | Yes | Yes | Yes | Yes | Yes | Yes |
Methods
Data collection and compilation
In order to develop DCTPep, extensive searches were conducted on published articles, patents, and public databases. The data of DCTPep was stored in two sub libraries: peptide library and drug library. The inclusion criteria for the peptide library in the DCTPep were as follows: 1. The sequence of amino acids is reported; 2. Mature peptide sequences without precursor and signal regions; 3. The length of the sequence does not exceed 100 amino acids; 4. Peptides that exhibit anticancer/antitumor activity or target specific molecules/biomarkers overexpressed in cancer cells; 5. Cell-penetrating peptides that can enhance the delivery of drugs into cancer cells. The inclusion criteria for drug library were similar to those for peptide library: 1. Peptides and their derivatives or amino acid derivatives related to cancer treatment; 2. Entered clinical research or approved by FDA, EMA or HC.
To collect peptide data, keywords were used to search in academic search engines such as Google Scholar, Web of Science, PubMed, and Google Patents. The keywords included “ACP”, “antiangiogenic peptides”, “cancer therapy peptide”, “cancer targeted peptide”, and “peptide conjugates”. After collecting research papers, patents, and clinical research literature, data were manually extracted. In addition to manually extracting information of cancer therapy peptide from literature, also included other information related to peptides (such as three-dimensional structures) in UniProt27, PDB28, and other databases. The physicochemical information of peptides is calculated using Expasy Protparam server (https://web.expasy.org/protparam/, accessed on March 2024) and SciDBMaker29.
The data of drug library mainly originated from the portal websites of drug regulatory authorities and organisations in several countries and regions. In addition, it was supplemented by the drug databases DrugBank30, PubChem31, NCI Thesaurus32 and Global Substance Registration System (GSRS)33. By entering keywords such as “peptides and their derivatives”, “amino acids and their derivatives”, and “anticancer” into the aforementioned website or database, relevant information can be found.
Structural prediction and evaluation
Due to the difficulties in experimental determination of peptide and protein structures, most of the peptides lack experimental determined structures. AlphaFold34 was used to predict the potential 3D structures of DCTPep peptides. Default structure parameters for AlphaFold prediction were used: peptide was modeled as a monomer; Multiple sequence alignment (MSA) information databases: full_dbs (all gene databases)34. Each peptide generates 5 structures, and the structure with the highest score is selected based on predicted local distance difference test (pLDDT)34. To evaluate the reliability of AlphaFold predicted peptide structures, 30 peptides with experimental determined structures were selected and their structures were predicted by AlphaFold. The differences between predicted structure and experimentally determined structure were calculated by Root-Mean-Square Deviation (RMSD)35. Given two conformations, α and β of N residues, let rα and rβ be the respective coordinates of their residues at position i, for 1, …, N. RMSD between α and β as Eq. (1):
| 1 |
Where Q is the unitary rotation matrix that optimally aligns the vectors. Disulfide bonds are also considered to see if AlphaFold can correctly predict the disulfide bonds. Whatcheck36 and Procheck37 are used to assess the quality of the predicted structures. Whatcheck36 evaluates multiple parameters such as bond lengths, bond angles, and torsion angles of the input structure. Procheck37 assesses the stereochemical quality of the input structure and provides various graphical outputs. Ramachandran plot38 is used to evaluate the rationality of the structure, where peptide bond dihedral angles Ψ(psi) and Φ(phi) combinations are expected to located in most favored regions and allowed regions (core regions) in the plot. Ideally, a protein structure should have over 90% dihedral angles Φ-Ψ of residues in these core regions37.
Data Records
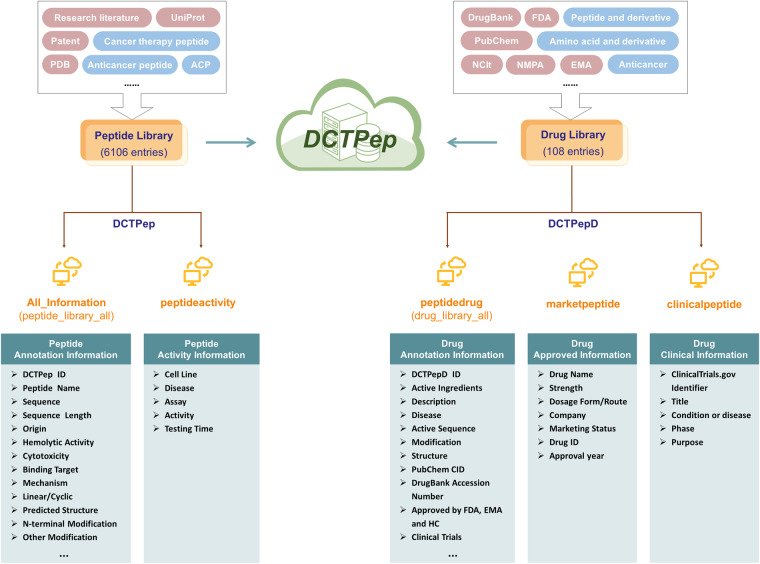
The datasets of DCTPep are available at Figshare39 and contains the following files: All_information (annotation information table for storing peptide library entries), peptideactivity (activity information annotation of peptide library entries), peptidedrug (annotation information table for storing active Ingredients of drug library entries), marketpeptide (approved drug preparations information annotation of drug library entries), clinicalpeptide (clinical peptide information annotation of drug library entries), peptide_library_all (peptide library data stored in Fasta format) and prediction pdb (compressed packets for storing predicted structures). The architecture of the DCTPep is shown in Fig. 2. DCTPep contains a total of 6214 peptide entries, of which 6106 are stored in the peptide library and 108 are stored in the drug library (DCTPepD), involving over 60 targets and over 380 cancer cell lines.
Fig. 2.
Architecture of the datasets in DCTPep.
Table 2 displays detailed annotation information of the data in the peptide library. Each entry in the peptide library consists of the following sections: general information, activity information, structural information, physicochemical information, literature information, and links. The peptides in the peptide library included cancer therapeutic peptides such as traditional ACP and cancer targeted peptides. Low cytotoxicity and hemolytic activity are also important criteria for developing peptide-based drugs. Therefore, in addition to anticancer activity and targets, activity information also includes cytotoxicity and hemolytic activity. All annotation information is manually extracted from the literature, and corresponding paper or patent source information is provided. The physicochemical information is calculated by Protparam and SciDBMaker29. For the same peptide, the emphasis of the information recorded in different databases may vary. Therefore, DCTPep provides corresponding peptide entry codes in other peptide databases.
Table 2.
Peptide library data annotation field list.
| Fields | Description | |
|---|---|---|
| General information | DCTPep ID | The identifier of peptides in DCTPep. |
| Peptide Name | Name of each peptide in DCTPep. | |
| Sequence | Single letter sequence of peptides. | |
| Sequence Length | Number of residues in the peptide sequence. | |
| Uniprot ID | Corresponding Uniprot link ID. | |
| Source | Organism source. | |
| Type | Native peptide or synthetic peptide. | |
| Classification | Various possible classifications. Including ACP, Cancer targeted peptides, Membrane-targeted mechanism… | |
| Activity information | Anticancer activity | Anticancer activity verified by experiment (Data from literature). |
| Hemolytic Activity | Hemolytic activity information against red blood cells (RBCs). | |
| Cytotoxicity | Cytotoxicity information against normal (non-cancerous) cell line. | |
| Target | The action site of peptides against cancer cell. | |
| Affinity | Binding affinity between peptides and targets. | |
| Mechanism | Mechanisms by peptides exert anticancer agents. | |
| Structure information | PDB ID | Corresponding PDB link ID. |
| Predicted Structure | Structure predicted by Alphafold. | |
| Helicity | Percentage of α-helix. | |
| Linear/Cyclic | Linear or cyclic of peptides. | |
| Disulfide/Other Bond | Disulfide bond (DSB) or other bond, such as N-C termini peptide bond (NCB). | |
| N/C-terminal Modification | The modifications of N/C-terminal according to the references. | |
| Other Modification | Special amino acids (out of 20 common amino acids). | |
| Chiral | The L/D amino acid composition of peptides. | |
| Physicochemical Information | Formula, mass, pI, Net charge and other information, calculated by Protparam and SciDBMaker. | |
| Literature Information | The information of peptides come from all kinds of papers or patents, and the section provides the way to find the full text. | |
| Link | Corresponding link to other peptide databases. | |
The data in the drug library includes peptide drugs that have been approved or are in clinical research stage. Table 3 shows detailed annotation information for drug library data. Each entry consists of four sections: general information, structural information, external codes, and drug approval. The external codes provide identification codes for drug entries in other public databases, allowing users to obtain more comprehensive information on related entries from other sources. Approved drug formulations and clinical information can be found in the drug approval section. A total of 28 approved anticancer peptide drugs and 80 peptides in various clinical trial stages are included in the drug library.
Table 3.
Drug library data annotation field list.
| Fields | Description | |
|---|---|---|
| General information | DCTPepD ID | Identification code for DCTPepD drug library, the field provides the unique accessing number linking to the corresponding DCTPepD entry. |
| Active Ingredients | Active pharmaceutical ingredient. Substance in which the drug actually works. | |
| Description | Drug description. Derived from descriptions in NCI or literature sources. | |
| Synonyms | Other names of drug. | |
| Disease | Applicable diseases. | |
| Classification | Drug Categories. | |
| Structure information | Molecular Formula, Molecular Weight, Active Sequence, Sequence Length, Modification, and other structure information. | |
| External Codes | External identification code, also provides the accessing link to PubChem, DrugBank, NCI Thesaurus and GSRS. | |
| Drug approval | Drug indication | Stemmed from DrugBank or clinical trials. |
| Approved information | Approved drug formulation information, sourced from Drugs@FDA, European Medicines Agency (EMA), and Health Canada. | |
| Clinical information | Information sourced from ClinicalTrials.gov. | |
Technical Validation
Alphafold demonstrated unprecedented accuracy in 14th Critical Assessment of protein Structure Prediction (CASP14)34. The study conducted by McDonald et al.40 also indicated that AlphaFold can accurately predict peptides with α-helices, β-sheets, and rich in disulfide bonds. To evaluate the accuracy of AlphaFold, 30 ACPs with experimentally determined structures were predicted by AlphaFold.
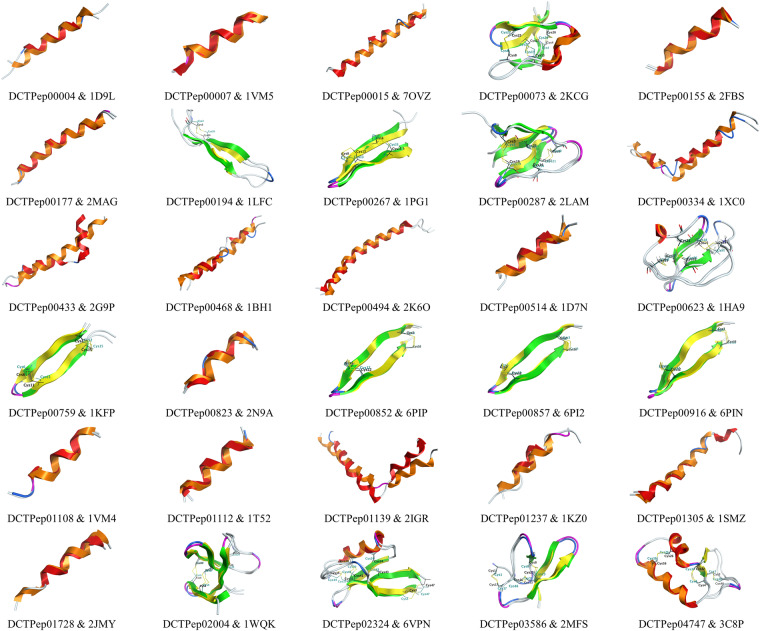
Table 4 and Fig. 3 displays the comparison results between predicted structures and experimental structures, including RMSD and disulfide bond positions. The results indicate that the predicted structures have high accuracy. The deviations between the predicted and experimental structures are small, with an average of Cα (α-carbon atom) RMSD value is 1.621 Å. For structures containing disulfide bonds, AlphaFold can accurately predict the positions of the disulfide bonds. Some of the predicted structures of peptides can be directly obtained from the AlphaFold Protein Structure Database41, for example, AF-P82393-F1 (DCTPep00006) and AF-P80400-F1 (DCTPep00097).
Table 4.
Comparison between predicted structures and experimental structures.
| DCTPep_ID | PDB_ID | pLDDT | Cα RMSD(Å) | Disulfide bond position* |
|---|---|---|---|---|
| DCTPep00004 | 1D9L | 81.43 | 2.314 | / |
| DCTPep00007 | 1VM5 | 92.59 | 0.629 | / |
| DCTPep00015 | 7OVZ | 85.33 | 2.510 | / |
| DCTPep00073 | 2KCG | 89.84 | 0.758 | TRUE |
| DCTPep00155 | 2FBS | 87.92 | 0.419 | / |
| DCTPep00177 | 2MAG | 84.56 | 0.632 | / |
| DCTPep00194 | 1LFC | 76.00 | 2.060 | TRUE |
| DCTPep00267 | 1PG1 | 84.8 | 2.145 | TRUE |
| DCTPep00287 | 2LAM | 84.35 | 2.345 | TRUE |
| DCTPep00334 | 1XC0 | 75.86 | 3.583 | / |
| DCTPep00433 | 2G9P | 83.30 | 3.740 | / |
| DCTPep00468 | 1BH1 | 79.11 | 3.435 | / |
| DCTPep00494 | 2K6O | 83.08 | 2.148 | / |
| DCTPep00514 | 1D7N | 82.51 | 1.927 | / |
| DCTPep00623 | 1HA9 | 84.38 | 1.029 | TRUE |
| DCTPep00759 | 1KFP | 88.10 | 1.632 | TRUE |
| DCTPep00823 | 2N9A | 92.31 | 0.972 | / |
| DCTPep00852 | 6PIP | 91.06 | 0.618 | TRUE |
| DCTPep00857 | 6PI2 | 91.14 | 0.688 | TRUE |
| DCTPep00916 | 6PIN | 90.74 | 0.880 | TRUE |
| DCTPep01108 | 1VM4 | 91.98 | 0.598 | / |
| DCTPep01112 | 1T52 | 82.43 | 0.916 | / |
| DCTPep01139 | 2IGR | 71.96 | 3.413 | / |
| DCTPep01237 | 1KZ0 | 79.60 | 2.538 | / |
| DCTPep01305 | 1SMZ | 82.25 | 1.276 | / |
| DCTPep01728 | 2JMY | 83.34 | 1.985 | / |
| DCTPep02004 | 1WQK | 86.35 | 1.281 | TURE |
| DCTPep02324 | 6VPN | 97.15 | 0.878 | TURE |
| DCTPep03586 | 2MFS | 88.20 | 0.689 | TURE |
| DCTPep04747 | 3C8P | 95.60 | 0.579 | TURE |
| Average | — | — | 1.621 | — |
*“TURE” indicates consistent disulfide bond formation positions; “FALSE” indicates inconsistent disulfide bond formation positions; “/” indicates no disulfide bond formation.
Fig. 3.
Alignment and superimposition plot of predicted structures and experimental structures. Predicted structures: helix-orange, strand-green, turn-magenta, Cys-dark cyan; Experimental structures: helix-red, strand-yellow, turn-blue, Cys-black.
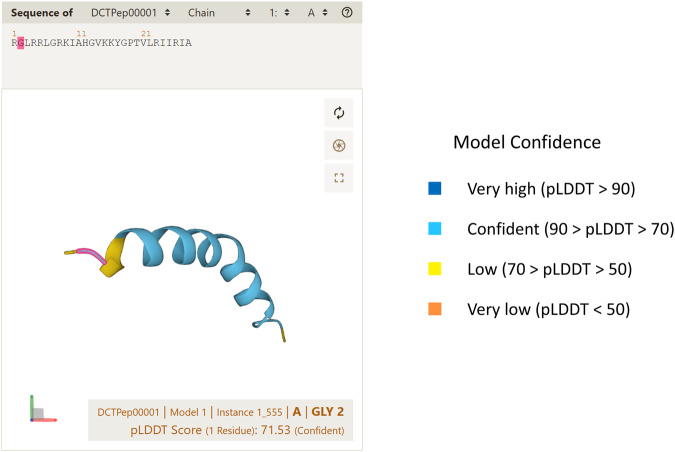
pLDDT is an important parameter for assessing the confidence of predictions34. While using pLDDT alone to define the accuracy of predicted peptide structures may not be entirely accurate, it can still reflect their accuracy to some extent. DCTPep integrates the Mol* Viewer42 to display the predicted structures, where the pLDDT of each residue can be visualized in the structure43 (Fig. 4).
Fig. 4.
Example of the predicted structure of DCTPep00001 showing by Mol* Viewer.
The quality assessment of the predicted structures was performed using Whatcheck36 and Procheck37 (Table 5), and the results indicate that the predicted structures are reliable. The average error rate of Whatcheck is 11.52%, which is at a relatively low level. In the Ramachandran plot generated by Procheck, the average core regions occupancy rate is 95.11%, only the DCTPep00623 has a low occupancy rate of core regions. The average disallowed regions occupancy rate is 0.26%, only DCTPep00267 has one residue present in the disallowed regions. These errors are within an acceptable range.
Table 5.
The results of predicted structures in Whatcheck and Procheck.
| DCTPep_ID | Whatcheck | Procheck | ||||
|---|---|---|---|---|---|---|
| Total metrics | Error | Error rate | Core regions | Additional/generous allowed regions | Disallowed regions | |
| DCTPep00004 | 40 | 4 | 10.00% | 100.00% | 0.00% | 0.00% |
| DCTPep00007 | 40 | 4 | 10.00% | 100.00% | 0.00% | 0.00% |
| DCTPep00015 | 42 | 4 | 9.52% | 100.00% | 0.00% | 0.00% |
| DCTPep00073 | 42 | 5 | 11.90% | 87.50% | 12.50% | 0.00% |
| DCTPep00155 | 42 | 4 | 9.52% | 100.00% | 0.00% | 0.00% |
| DCTPep00177 | 42 | 4 | 9.52% | 100.00% | 0.00% | 0.00% |
| DCTPep00194 | 43 | 6 | 13.95% | 100.00% | 0.00% | 0.00% |
| DCTPep00267 | 40 | 5 | 12.50% | 92.30% | 0.00% | 7.70% |
| DCTPep00287 | 44 | 5 | 11.36% | 81.80% | 18.20% | 0.00% |
| DCTPep00334 | 43 | 4 | 9.30% | 96.20% | 3.80% | 0.00% |
| DCTPep00433 | 43 | 4 | 9.30% | 100.00% | 0.00% | 0.00% |
| DCTPep00468 | 42 | 4 | 9.52% | 100.00% | 0.00% | 0.00% |
| DCTPep00494 | 45 | 5 | 11.11% | 90.60% | 9.40% | 0.00% |
| DCTPep00514 | 38 | 4 | 10.53% | 100.00% | 0.00% | 0.00% |
| DCTPep00623 | 42 | 6 | 14.29% | 69.20% | 30.80% | 0.00% |
| DCTPep00759 | 41 | 5 | 12.20% | 86.70% | 13.30% | 0.00% |
| DCTPep00823 | 39 | 4 | 10.26% | 100.00% | 0.00% | 0.00% |
| DCTPep00852 | 40 | 6 | 15.00% | 92.90% | 7.10% | 0.00% |
| DCTPep00857 | 40 | 5 | 12.50% | 92.90% | 7.10% | 0.00% |
| DCTPep00916 | 40 | 4 | 10.00% | 92.90% | 7.10% | 0.00% |
| DCTPep01108 | 40 | 4 | 10.00% | 100.00% | 0.00% | 0.00% |
| DCTPep01112 | 38 | 4 | 10.53% | 100.00% | 0.00% | 0.00% |
| DCTPep01139 | 40 | 6 | 15.00% | 100.00% | 0.00% | 0.00% |
| DCTPep01237 | 39 | 4 | 10.26% | 100.00% | 0.00% | 0.00% |
| DCTPep01305 | 39 | 4 | 10.26% | 100.00% | 0.00% | 0.00% |
| DCTPep01728 | 39 | 5 | 12.82% | 100.00% | 0.00% | 0.00% |
| DCTPep02004 | 45 | 6 | 13.33% | 90.30% | 9.70% | 0.00% |
| DCTPep02324 | 45 | 6 | 13.33% | 95.10% | 4.90% | 0.00% |
| DCTPep03586 | 43 | 7 | 16.28% | 87.50% | 8.30% | 0.00% |
| DCTPep04747 | 43 | 5 | 11.63% | 97.40% | 2.60% | 0.00% |
| Average | — | — | 11.52% | 95.11% | 4.49% | 0.26% |
Acknowledgements
This work was funded by the National Natural Science Foundation of China (grant number 82273834) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD2014-65). The authors would like to thank for the computational support from the High-Performance Computing Centre at China Pharmaceutical University.
Author contributions
H.Z. conceived the project; X.S. and N.Z. designed and developed the database; X.S., Y.L. and T.M. extracted and organized data; X.S. and Y.L. tested the database; X.S. and T.M. proofread the database; X.S. written the manuscript; H.Z. reviewed the manuscript; X.L. provided constructive suggestions for the project; H.Z. guide and supervise the project.
Code availability
DCTPep can be freely accessed at http://dctpep.cpu-bioinfor.org/. The data are stored in the Figshare repository available at 10.6084/m9.figshare.25796353.v1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xingzhen Lao, Email: lao@cpu.edu.cn.
Heng Zheng, Email: zhengh18@hotmail.com.
References
- 1.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 2.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017;7:339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfarouk KO, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukowski K, Kciuk M, Kontek R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arumugam V, Venkatesan M, Ramachandran S, Sundaresan U. Bioactive Peptides from Marine Ascidians and Future Drug Development–A Review. Int. J. Pept. Res. Ther. 2018;24:13–18. doi: 10.1007/s10989-017-9662-9. [DOI] [Google Scholar]
- 6.O’Brien-Simpson NM, Hoffmann R, Chia CSB, Wade JD. Editorial: Antimicrobial and Anticancer Peptides. Front. Chem. 2018;6:13. doi: 10.3389/fchem.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kardani K, Bolhassani A. Antimicrobial/anticancer peptides: bioactive molecules and therapeutic agents. Immunotherapy. 2021;13:669–684. doi: 10.2217/imt-2020-0312. [DOI] [PubMed] [Google Scholar]
- 8.Xie M, Liu D, Yang Y. Anti-cancer peptides: classification, mechanism of action, reconstruction and modification. Open Biol. 2020;10:200004. doi: 10.1098/rsob.200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiangjong W, Chutipongtanate S, Hongeng S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review) Int J Oncol. 2020;57:678–696. doi: 10.3892/ijo.2020.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan A, Hettiarachchy NS, Marshall M, Raghavan S, Kristinsson H. Shrimp shell peptide hydrolysates inhibit human cancer cell proliferation: Shrimp shell peptide hydrolysates inhibit cancer cell proliferation. J. Sci. Food Agric. 2011;91:1920–1924. doi: 10.1002/jsfa.4464. [DOI] [PubMed] [Google Scholar]
- 11.Khamessi O, Ben Mabrouk H, ElFessi-Magouri R, Kharrat R. RK1, the first very short peptide from Buthus occitanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem. Biophys. Res. Commun. 2018;499:1–7. doi: 10.1016/j.bbrc.2018.01.133. [DOI] [PubMed] [Google Scholar]
- 12.Gong F, et al. A Novel Peptide from Abalone (Haliotis discus hannai) to Suppress Metastasis and Vasculogenic Mimicry of Tumor Cells and Enhance Anti-Tumor Effect In Vitro. Mar. Drugs. 2019;17:244. doi: 10.3390/md17040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajski G, Garaj-Vrhovac V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Ren SX, et al. FK-16 Derived from the Anticancer Peptide LL-37 Induces Caspase-Independent Apoptosis and Autophagic Cell Death in Colon Cancer Cells. PLoS One. 2013;8:e63641. doi: 10.1371/journal.pone.0063641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peyressatre M, Prevel C, Pellerano M, Morris MC. Targeting cyclin-dependent kinases in human cancers: from small molecules to Peptide inhibitors. Cancers (Basel). 2015;7:179–237. doi: 10.3390/cancers7010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang C, Zhang W, Li X. Bioactive peptides for anticancer therapies. Biomater Transl. 2023;4:5–17. doi: 10.12336/biomatertransl.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi G, et al. DRAMP 3.0: an enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2022;50:D488–D496. doi: 10.1093/nar/gkab651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirtskhalava M, et al. DBAASP v3: database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021;49:D288–D297. doi: 10.1093/nar/gkaa991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N, Dong F, Shi G, Lao X, Zheng H. HORDB a comprehensive database of peptide hormones. Sci. Data. 2022;9:187. doi: 10.1038/s41597-022-01287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal P, et al. CPPsite 2.0: a repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016;44:D1098–1103. doi: 10.1093/nar/gkv1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, et al. SATPdb: a database of structurally annotated therapeutic peptides. Nucleic Acids Res. 2016;44:D1119–1126. doi: 10.1093/nar/gkv1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagi A, et al. CancerPPD: a database of anticancer peptides and proteins. Nucleic Acids Research. 2015;43:D837–D843. doi: 10.1093/nar/gku892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alas M, Saghaeidehkordi A, Kaur K. Peptide-Drug Conjugates with Different Linkers for Cancer Therapy. J Med Chem. 2021;64:216–232. doi: 10.1021/acs.jmedchem.0c01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindberg, J., Nilvebrant, J., Nygren, P. A. & Lehmann, F. Progress and Future Directions with Peptide-Drug Conjugates for Targeted Cancer Therapy. Molecules. 26 (2021). [DOI] [PMC free article] [PubMed]
- 26.Hoppenz P, Els-Heindl S, Beck-Sickinger AG. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front Chem. 2020;8:571. doi: 10.3389/fchem.2020.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Consortium U. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bittrich S, et al. RCSB Protein Data Bank: improved annotation, search and visualization of membrane protein structures archived in the PDB. Bioinformatics. 2022;38:1452–1454. doi: 10.1093/bioinformatics/btab813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammami R, Zouhir A, Naghmouchi K, Ben Hamida J, Fliss I. SciDBMaker: new software for computer-aided design of specialized biological databases. BMC Bioinf. 2008;9:121. doi: 10.1186/1471-2105-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wishart DS, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388–D1395. doi: 10.1093/nar/gkaa971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sioutos N, et al. NCI Thesaurus: a semantic model integrating cancer-related clinical and molecular information. J Biomed Inform. 2007;40:30–43. doi: 10.1016/j.jbi.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Peryea T, et al. Global Substance Registration System: consistent scientific descriptions for substances related to health. Nucleic Acids Res. 2021;49:D1179–D1185. doi: 10.1093/nar/gkaa962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betancourt MR, Skolnick J. Universal similarity measure for comparing protein structures. Biopolymers. 2001;59:305–309. doi: 10.1002/1097-0282(20011015)59:5<305::AID-BIP1027>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Hooft RW, Vriend G, Sander C, Abola EE. Errors in protein structures. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]
- 37.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- 38.Ramachandran GN, Ramakrishnan C, Sasisekharan V. Stereochemistry of polypeptide chain configurations. Journal of molecular biology. 1963;7:95–99. doi: 10.1016/S0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- 39.Sun X. 2024. DCTPep, the data of cancer therapy peptides. Figshare. [DOI] [PMC free article] [PubMed]
- 40.McDonald EF, Jones T, Plate L, Meiler J, Gulsevin A. Benchmarking AlphaFold2 on peptide structure prediction. Structure. 2023;31:111–119.e112. doi: 10.1016/j.str.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varadi M, et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sehnal D, et al. Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021;49:W431–W437. doi: 10.1093/nar/gkab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruff KM, Pappu RV. AlphaFold and Implications for Intrinsically Disordered Proteins. J Mol Biol. 2021;433:167208. doi: 10.1016/j.jmb.2021.167208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sun X. 2024. DCTPep, the data of cancer therapy peptides. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
DCTPep can be freely accessed at http://dctpep.cpu-bioinfor.org/. The data are stored in the Figshare repository available at 10.6084/m9.figshare.25796353.v1.