Abstract
Prostatic stromal tumors, encompassing prostatic sarcoma and stromal tumors of uncertain malignant potential (STUMP), represent an exceedingly rare category of prostatic diseases, with a prevalence of less than 1%. We present a rare case involving a man in his early 40s diagnosed with STUMP. Despite presenting with normal prostate-specific antigen (PSA) concentrations, the patient experienced persistent dysuria and gross hematuria for >7 months, leading to an initial misdiagnosis of benign prostatic hyperplasia. Persistent symptoms prompted further investigation, with magnetic resonance imaging (MRI) revealing a suspicious lesion on the left side of the prostate, initially thought to be malignant. Transrectal prostatic biopsy subsequently confirmed the presence of mucinous liposarcoma, with no medical history of diabetes, coronary heart disease, or hypertension. The treatment approach comprised robot-assisted laparoscopic radical prostatectomy, culminating in a postoperative pathological definitive diagnosis of STUMP. This case underscores the indispensable role of early MRI in the diagnostic process, highlighting the necessity of detailed pathological examination for a conclusive diagnosis. Our report aims to illuminate the diagnostic challenges and potential treatment pathways for STUMP, emphasizing its consideration in the differential diagnosis of prostatic tumors to advance clinical outcomes in this rare but important condition.
Keywords: Stromal tumor of uncertain malignant potential, magnetic resonance imaging, prostatectomy, robot-assisted surgery, histopathology, dysuria, hematuria, definitive diagnosis
Introduction
Prostatic stromal tumors are divided into prostatic sarcoma and stromal tumor of uncertain malignant potential (STUMP), which account for <1% of prostate cancers. 1 Prostatic stromal tumors are characterized mainly by atypical and distinctive mesenchymal cellular hyperplasia in the prostate gland and have been categorized as a specific mesenchymal tumor because of the unique development in prostatic tissue. 2 As a unique entity, these tumors include multiple subtypes. Early studies described STUMP as a prostate phyllodes tumor, atypical stromal hyperplasia, cystic liposarcoma, and cystic epithelial stromal tumor. 3 The age of patients with STUMP ranges widely from 23 to 81 years,4–6 and occurs mainly in those aged 50 to 70 years (Table 1). Typical clinical manifestations comprise urinary tract obstruction, hematuria, elevated serum prostate-specific antigen (PSA) concentration, and abnormal digital rectal examination findings, and may even affect sexual function. 7 In rare cases, STUMP may present as a huge bladder mass. 8 Five STUMP patterns have been described, including degenerative atypia matrix, a high density of spindle cells, and mucus-like spindle cells. The remaining patterns are a phyllodes-like pattern4,9,10 and a newly discovered round cell subtype, 11 of which degenerative atypia is the most common. However, the clinical presentation of STUMP varies widely, ranging from incidental findings to distant metastases and even death, and the great heterogeneity of its clinical behavior complicates the diagnosis. 12
Table 1.
Summary of previously reported cases of prostatic stromal tumors.
| No. | Age (years) | Clinical features | Diagnostic specimen | Immunohistochemistry | Treatment | Follow-up time (months) | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | Urinary retention, lower urinary tract symptoms, hematuria | TURP | Vimentin (+), CD34 (+), SMA (+) | TURP | 78 | Dysuria | Shen et al.6 (2021) |
| 2 | 31 | Lower urinary tract symptoms | Biopsy | SMA (±), CD34 (+), ER (±), PR (±) | None | 74 | No recurrence or metastasis | Shen et al.6 (2021) |
| 3 | 73 | Urinary retention, lower urinary tract symptoms | TURP | TURP | / | / | Shen et al.6 (2021) | |
| 4 | 67 | Urinary retention, lower urinary tract symptoms | Biopsy | Vimentin (+), SMA (+), CD34 (±), PR (±) | TURP | 68 | No recurrence or metastasis | Shen et al.6 (2021) |
| 5 | 66 | Lower urinary tract symptoms, hematuria | RP | Vimentin (+), CD34 (+), PR (+) | RP | / | / | Shen et al.6 (2021) |
| 6 | 77 | Lower urinary tract symptoms, hematuria | Biopsy | TURP | / | / | Shen et al.6 (2021) | |
| 7 | 66 | Lower urinary tract symptoms, hematuria | RP | Vimentin (+), SMA (+), CD34 (±) | RP | 57 | No recurrence or metastasis | Shen et al.6 (2021) |
| 8 | 73 | Urinary retention, lower urinary tract symptoms | TURP | TURP | 57 | No recurrence or metastasis | Shen et al.6 (2021) | |
| 9 | 81 | Urinary retention, lower urinary tract symptoms | TURP | TURP | 56 | No recurrence or metastasis | Shen et al.6 (2021) | |
| 10 | 71 | Lower urinary tract symptoms | TURP | SMA (+), CD34 (+), ER (±), PR (+) | TURP | 56 | No recurrence or metastasis | Shen et al.6 (2021) |
| 11 | 71 | Urinary retention, lower urinary tract symptoms | Biopsy | SMA (+) | None | / | / | Shen et al.6 (2021) |
| 12 | 51 | No obvious symptoms | RP | Vimentin (+), SMA (+), ER (±), PR (+) | None | 27 | No recurrence or metastasis | Shen et al.6 (2021) |
| 13 | 39 | Lower urinary tract symptoms, urinary retention | Biopsy | Vimentin (+), ER (+), PR (+) | None | 10 | No recurrence or metastasis | Shen et al.6 (2021) |
| 14 | 78 | Lower urinary tract symptoms | RP | SMA (+), CD34 (+), PR (+) | RP | / | / | Shen et al.6 (2021) |
| 15 | 58 | Lower urinary tract symptoms, urinary retention | Biopsy | None | / | / | Shen et al.6 (2021) | |
| 16 | 53 | No obvious symptoms | RP | CD34 (±), ER (±), PR (+) | RP | 86 | No recurrence or metastasis | Shen et al.6 (2021) |
| 17 | 75 | Lower urinary tract symptoms | TURP | Vimentin (+), SMA (±), CD34 (+), ER (+), PR (+) | TURP | 85 | No recurrence or metastasis | Shen et al.6 (2021) |
| 18 | 33 | Lower urinary tract symptoms, urinary retention | Biopsy | TURP | 7 | Dysuria | Shen et al.6 (2021) | |
| 19 | 37 | Hematuria, lower urinary tract symptoms | RP | CD34 (±) | RP | 16 | Recurrence and death | Shen et al.6 (2021) |
| 20 | 25 | Lower urinary tract symptoms, urinary retention | Biopsy | Vimentin (+), SMA (±), CD34 (+) | Prostatectomy | / | / | Shen et al.6 (2021) |
| 21 | 61 | Hematuria | RP | Vimentin (+), SMA (+), CD34 (+) | RP and total pelvic exenteration | 6 | Recurrence | Shen et al.6 (2021) |
| 22 | 40 | Lower urinary tract symptoms, urinary retention | TURP | Vimentin (+), SMA (±), CD34 (±) | TURP and radical cystectomy | 24 | Recurrence | Shen et al.6 (2021) |
| 23 | 23 | Lower urinary tract symptoms, urinary retention | TURP | Vimentin (+), SMA (+), CD34 (±) | TURP and radical cystectomy | / | / | Shen et al.6 (2021) |
| 24 | 29 | Urinary emptying disorder and hematuria | Biopsy | Not available | TURP+RP | 2 | No recurrence or metastasis | Syarif et al.7 (2023) |
| 25 | 53 | Left back pain and lower urinary tract symptoms | Biopsy | CD117 (+), CD34 (+), PR (+), Desmin (+), SMA (−) | RP | 12 | No recurrence or metastasis | Wang et al.8 (2014) |
| 26 | 62 | Abnormal DRE | Biopsy | Not available | None | 12 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 27 | 64 | Abnormal DRE | Biopsy | Not available | None | 34 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 28 | 67 | Abnormal DRE | Biopsy | Not available | TURP | 86 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 29 | 63 | Urinary retention | TURP | Not available | TURP | 11 | Death of other cause | Gaudin et al.9 (1998) |
| 30 | 51 | Urinary retention | SP | Vimentin (+), CD34 (+), Desmin (±), PR (+) | SP | 60 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 31 | 47 | Constipation, nocturia, palpable prostate mass | TUB | Not available | Exploratory laparotomy, partial prostatectomy and excision of mass | / | Not available | Gaudin et al.9 (1998) |
| 32 | 48 | Gross hematuria | TUB | Not available | TUB | 6.5 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 33 | 65 | Abnormal DRE | Biopsy | Not available | None | 2 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 34 | 27 | Rectal fullness, prostate mass | Biopsy | Not available | Hormones, RCP | 35 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 35 | 43 | Urinary retention, prostate mass | TURP | Vimentin (+), CD34 (±), SMA (+) | TURP | 18 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 36 | 52 | Palpable rectal mass | TURP | Vimentin (+), CD34 (+), Desmin (+), PR (±) | TURP and modified pelvic exenteration | 76 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 37 | 53 | No obvious symptoms | RP for PCa | Not available | RP | 10 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 38 | 51 | Urinary retention, hematuria | Biopsy | Vimentin (+), CD34 (+), SMA (+), Desmin (+), PR (+) | TURP | 136 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 39 | 39 | Urinary retention | TURP | Vimentin (+), CD34 (±), ER (+), PR (+) | TURP, finasteride, and RP | 71 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 40 | 65 | Urinary retention | TURP | Not available | TURP | 6 | Death of other cause | Gaudin et al.9 (1998) |
| 41 | 71 | Urinary retention, hematuria | Biopsy | Not available | RP | 0.5 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 42 | 55 | Urinary retention | TURP | Vimentin (+), CD34 (±), PR (+) | TURP+RP | 21 | No recurrence or metastasis | Gaudin et al.9 (1998) |
| 43 | 60 | Urinary retention | Biopsy | Desmin (+), CD34 (−), PR (−) | RARP | 103 | No recurrence or metastasis | Dokubo et al.12 (2023) |
| 44 | 65 | Abnormal DRE | Biopsy | Desmin (+), CD34 (−) | RARP | 57 | No recurrence or metastasis | Dokubo et al.12 (2023) |
| 45 | 64 | Mild obstructive voiding symptoms | Biopsy | Vimentin (+), Desmin (+), PR (+) | RARP | 6 | No recurrence or metastasis | Chan et al.13 (2022) |
| 46 | 71 | Mild obstructive voiding symptoms | Biopsy | Not available | RP | 17 | No recurrence or metastasis | Ladner et al.14 (2021) |
| 47 | 52 | Progressive urinary retention | Biopsy | Not available | RP | 18 | Death | Muglia et al.16 (2011) |
| 48 | 63 | No obvious symptoms | Biopsy | CD34 (+), PR (−), Desmin (+) | Not available | Not available | Not available | Johnson and Carter17(2015) |
| 49 | 67 | Urinary retention | TURP | Not available | TURP | 60 | No recurrence or metastasis | De Berardinis et al.18 (2012) |
| 50 | 53 | Febrile prostatitis, urinary retention | Biopsy | Not available | TURP | 13 | STUMP still detectable, but no signs of sarcoma | Laturnus et al.23 (2010) |
| 51 | 77 | Weak urinary stream | Biopsy | CD34 (+), Desmin (+), SMA (−) | RP | 19 | No recurrence or metastasis | Inagaki et al.24 (2015) |
| 52 | 56 | Urinary retention | Not available | Not available | TURP | Not available | Not available | Wee et al.26 (2005) |
| 53 | 56 | Urinary frequency | Biopsy | Not available | RP | 12 | No recurrence | Okada et al.28 (2013) |
| 54 | 60 | No obvious symptoms | Biopsy | CD34 (+), PR (+), SMA (+) | RARP | 6 | No recurrence or metastasis | Suzuki et al.32 (2020) |
CD117, cluster of differentiation 117; CD34, cluster of differentiation 34; DRE, digital rectal examination; ER, estrogen receptor; PCa, prostate cancer; PR, progesterone receptor; PSA, prostate-specific antigen; RARP, robot-assisted radical prostatectomy; RCP, radical cyst prostatectomy; RP, radical prostatectomy; SMA, smooth muscle actin; SP, suprapubic prostatectomy; STUMP, stromal tumors of unknown malignant potential; TUB, transurethral biopsy; TURP, transurethral resection of the prostate.
Case report
We present the case of a man in his early 40s who experienced frequent urination, urgency, thin urine stream, weak urination, and other symptoms of dysuria without apparent triggers. Occasional hematuria but no low back pain was described, and the patient’s blood PSA concentration was normal. He had smoked for more than 20 years and denied drinking alcohol.
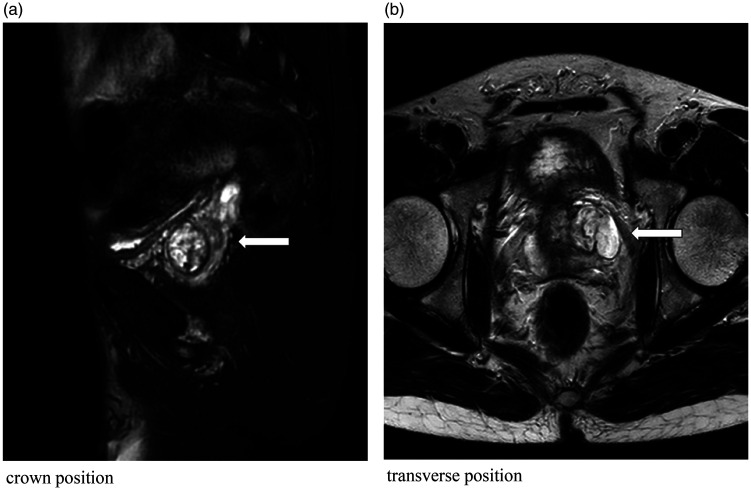
He was initially treated at the Social Welfare Hospital and underwent ultrasonography, which indicated prostatic hyperplasia. Although there was slight alleviation of the symptoms with medication, he continued to experience recurrent episodes of dysuria and eventually developed urinary retention, requiring an indwelling catheter. He was subsequently hospitalized, and upon rectal palpation, a firm nodule was detected in the left lobe of the prostate, with no obvious tenderness. Magnetic resonance imaging (MRI) revealed a left lateral prostatic mass (Figure 1) measuring approximately 24 × 22 mm, which was considered a malignant lesion. To clarify the diagnosis, we performed transurethral prostatic biopsy, and the pathology of the aspirate indicated prostatic mucinous liposarcoma, which was considered stage T2bN0M0. To obtain a precise diagnosis, we performed robot-assisted laparoscopic radical prostatectomy under general anesthesia.
Figure 1.
Magnetic resonance images; ((a) coronal view and (b) transverse view): The prostatic volume is increased above normal, and the images show mixed signals dominated by slightly high-intensity signals surrounded by a low-signal-intensity capsule. No obviously abnormal signals are evident in the remaining prostatic tissue, and the prostatic capsule is intact (arrows).
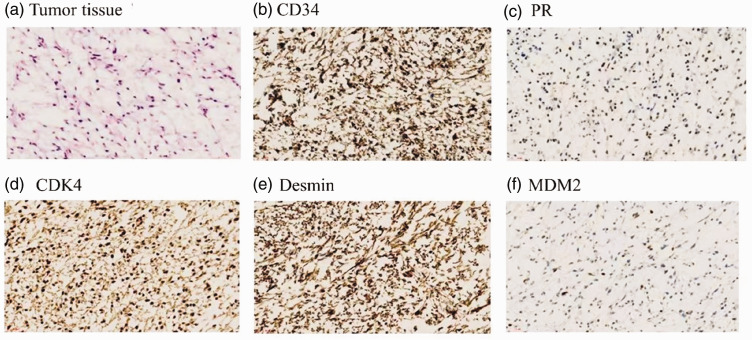
The pathological examination of the postoperative specimens revealed an enlarged left lobe of the prostate, with a greyish-white mass evident in the section. The primary mass was localized within the left lobe of the prostate, with a portion displaying a jelly-like and soft texture. The mass measured approximately 3.5 cm × 2.7 cm × 2.0 cm. Microscopic examination revealed the presence of heterogeneous round and spindle-shaped cells in a mucus background. Notably, a subset of these heterogeneous cells exhibited markedly increased nuclear-staining depth, consistent with the morphological characteristics of STUMP. Furthermore, immunohistochemical analysis revealed negative staining for smooth muscle actin (SMA), MyoD1, myogenin, S-100, and pan-cytokeratin , and the MDM2 gene was amplified. Conversely, there was some positive staining for progesterone receptors (PR), and staining was diffusely positive for cyclin D-dependent kinase 4, desmin, and cluster of differentiation 34 (CD34) (Figure 2). These findings further supported the diagnosis of STUMP.
Figure 2.
Pathological and immunohistochemical findings; (a–f) Biopsy-obtained pathological sections (×400) showing heterogeneous round and spindle-shaped cells in a mucus background. Positive staining for (b) CD34, (c) PR, (d) CDK4, (e) desmin, and (f) MDM2 is widespread among the tumor cells. Staining in panel (a): hematoxylin and eosin. CD34, cluster of differentiation 34; PR, progesterone receptor; CDK4, cyclin D-dependent kinase 4.
Discussion
Initially, this patient’s prostatic puncture biopsy was diagnosed as mucinous liposarcoma of the prostate. This condition is often confused with STUMP, likely owing to the limited volume of a puncture biopsy specimen. Subsequently, after a postoperative pathological examination, the patient’s diagnosis was revised to STUMP. Most reported STUMP cases are solitary, with only a tiny fraction co-occurring with prostatic adenocarcinomas.13,14 Early clinical symptoms in patients with STUMP are unremarkable. When the tumor is large, it may exert pressure on the bladder, and patients may have symptoms such as frequent urination, urgency, and dysuria. Compression of the lower ureter can cause hydronephrosis, and compression of the rectum leads to difficult bowel movements or an anal bulge, and scrotal and inguinal radiating pain. Rectal palpation of the prostate mainly reveals apparent prostatic enlargement, disappearance of the central sulcus, uneven surface, medium texture, and tenderness.
The primary imaging modality for evaluating STUMP is MRI, which typically reveals a solid appearance of the tumor with occasional findings of small focal cystic changes or minor focal hemorrhages. 15 These lesions show a consistent pattern, characterized by uniform low signal intensity on T1-weighted images, heterogeneous signal intensity on T2-weighted images, 16 slight hyperintensity on diffusion-weighted imaging, slight hyperintensity on apparent diffusion coefficient mapping, and specific features of continuous or gradual enhancement. 15 It is important to note that PSA is produced by prostatic epithelial cells, whereas stromal tumors originate from mesenchymal tissue, which may not result in a marked elevation of PSA concentrations. However, in a previous case report of STUMP, the PSA concentration was >14.7 nmol/L, 17 which required differentiation from prostate cancer. Immunohistochemical analysis of STUMP typically reveals positive expression of markers such as CD34 and vimentin, 18 with varying degrees of positive staining for myogenic markers, such as SMA, desmin, and muscle-specific actin. 15 Given their origin in the hormone-dependent specialized mesenchyme, these tumors usually express PRs, while exhibiting lower expression of estrogen receptors. 19 Additionally, S100 protein and c-kit (CD117) are generally not detected in STUMP. 9
Both STUMP and prostatic stromal sarcoma (PSS) are derived from the prostatic mesenchyme, making the distinction between them complicated. These tumors share clinical similarities and both express PRs, while both lack estrogen receptor expression. 19 However, PSS typically exhibits a higher diffusion-weighted imaging signal and lower apparent diffusion coefficient signal in MRI compared with STUMP. 15 Notably, this difference is insufficient to clearly distinguish the two. Therefore, the differential diagnosis of STUMP and PSS relies mainly on histopathological characteristics and immunohistochemical markers. 20 For PSS patients, positive staining for myogenic antibodies such as desmin, SMA, and MyoD14,21 is common, whereas STUMP generally involves positive CD34 and vimentin expression. 21 Although the genetic characteristics of STUMP have not been fully elucidated, the literature reports chromosomal alterations across all histological subtypes of STUMP. The most common alteration is the loss of chromosome 10, followed by loss of chromosomes 9 and 7. 22
Currently, a unified and clear STUMP treatment plan has not been established, as the clinical manifestations of STUMP vary from individual to individual, and the patient’s age, tumor size, tumor growth pattern, and degree of invasion affect the treatment choice. Therefore, treatment must be individualized, Additionally the tumor can remain stable for many years without treatment;23,24 however, some STUMP patients may have local recurrence, 25 and a very small number of tumors may evolve into PSS. It is worth noting that compared with radical resection, patients who undergo transurethral resection of the prostate appear to be more prone to recurrence,5,26 and radical resection of the prostate provides hope for patients with early STUMP.27,28
We opted for Da Vinci robot-assisted total prostatectomy (RARP) as our surgical approach, driven by its multifaceted benefits. 12 RARP affords superior surgical visualization owing to its high-definition three-dimensional optics, enhancing intraoperative anatomical discernment and thereby minimizing surgery-associated adverse events. The ergonomic design of the robotic system allows for intuitive surgeon hand movements, mitigating surgeon fatigue and enhancing surgical precision and consistency. 29 Additionally, the RARP technique harnesses the principles of fascial anatomy, facilitating meticulous dissection of the prostate from adjacent structures; thus, diminishing the likelihood of leaving behind malignant tissue. 30 This precise dissection preserves vital structures, notably, the urethral sphincter and neurovascular bundles pertinent to erectile function; thereby, attenuating the risks of postoperative complications, such as urinary incontinence and sexual dysfunction. 31 Fluorodeoxyglucose-positron emission tomography may help assess the malignant potential of STUMP, as the accumulation of fluorodeoxyglucose in these tumors is usually low, which may help in the development of surgical plans. 32 Therefore, in the treatment and follow-up of STUMP, these factors must be considered comprehensively while considering individual patient conditions to achieve the best therapeutic effect.
Conclusion
The clinical presentations of STUMP vary widely, often leading to potential misdiagnosis as prostatic hyperplasia if the diagnosis is based solely on clinical symptoms. Although MRI is beneficial in the early diagnosis of prostatic stromal tumors, a definitive diagnosis remains reliant on pathological examination. Regarding the differential diagnosis, particular emphasis should be placed on distinguishing STUMP from prostatic mesenchymal sarcoma. Treatment strategies should be meticulously customized to each patient’s unique profile, necessitating long-term follow-up to optimize the overall prognosis for individuals affected by STUMP.
The reporting of this study conforms to the CARE guidelines. 33
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605241253756 for Prostatic stromal tumor of uncertain malignant potential: a case report and literature review by Tao Zhu, Cong Yin, Cen Liufu, Jiahao Jiang, Junhua Luo and Yan Wang in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605241253756 for Prostatic stromal tumor of uncertain malignant potential: a case report and literature review by Tao Zhu, Cong Yin, Cen Liufu, Jiahao Jiang, Junhua Luo and Yan Wang in Journal of International Medical Research
Acknowledgement
The authors thank Professor Liangkuan Bi for his dedicated advice and assistance in the diagnosis of this case.
Author contributions: Tao Zhu conceived and designed the study. Cen Liufu and Jiahao Jiang provided administrative support. Junhua Luo provided study materials or patient data. Yan Wang collected evidence and supervised the study. Tao Zhu and Cong Yin analyzed and interpreted the data. All authors wrote the manuscript and provided final approval of the manuscript to be submitted.
The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Science Technology and Innovation Commission of Shenzhen Municipality [grant numbers JCYJ20220531094217040, JCYJ20220530150812027, and JCYJ20220531094207017], the Scientific Research Foundation of Peking University Shenzhen Hospital [grant number KYQD2023308], and the Shenzhen High-level Hospital Construction Fund and ‘San-ming' Project of Medicine in Shenzhen [grant number SZSM202111007]. All figures were created using Adobe Illustrator.
ORCID iDs: Cong Yin https://orcid.org/0000-0001-6334-4677
Junhua Luo https://orcid.org/0000-0002-1885-4595
Data availability statement
The data that support the findings of this study are available from the corresponding author (Junhua Luo) upon reasonable request.
Ethics statement
We obtained written informed consent from the patient described in this report. Institutional review board approval was not required because of the retrospective nature of the study.
References
- 1.Bostwick DG, Egevad L. Prostatic stromal proliferations: a review. Pathology 2021; 53: 12–25. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016; 70: 106–119. [DOI] [PubMed] [Google Scholar]
- 3.Murer LM, Talmon GA. Stromal tumor of uncertain malignant potential of the prostate. Arch Pathol Lab Med 2014; 138: 1542–1545. [DOI] [PubMed] [Google Scholar]
- 4.Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol 2006; 30: 694–704. [DOI] [PubMed] [Google Scholar]
- 5.Bostwick DG, Hossain D, Qian J, et al. Phyllodes tumor of the prostate: long-term followup study of 23 cases. J Urol 2004; 172: 894–899. [DOI] [PubMed] [Google Scholar]
- 6.Shen Q, Zhou Z, Liu Z, et al. Clinical and pathological features of prostatic stromal tumor of uncertain malignant potential: a retrospective study of 23 Chinese cases. Urol Int 2021; 105: 206–214. [DOI] [PubMed] [Google Scholar]
- 7.Syarif S, Azis A, Natsir AS, et al. A rare prostatic stromal tumor – stromal tumor of uncertain malignant potential (STUMP) and prostatic stromal sarcomas (PSS) as cultural sexual health problem in a young adult: a case report. Urol Case Rep 2023; 48: 102381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang MW, Li C, Zhang Q, et al. Prostatic stromal tumor of uncertain malignant potential presenting as a huge bladder mass: an unusual case. Asian J Androl 2014; 16: 794–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol 1998; 22: 148–162. [DOI] [PubMed] [Google Scholar]
- 10.Hansel DE, Herawi M, Montgomery E, et al. Spindle cell lesions of the adult prostate. Mod Pathol 2007; 20: 148–158. [DOI] [PubMed] [Google Scholar]
- 11.Sadimin ET, Epstein JI. Round cell pattern of prostatic stromal tumor of uncertain malignant potential: a subtle newly recognized variant. Hum Pathol 2016; 52: 68–73. [DOI] [PubMed] [Google Scholar]
- 12.Dokubo II, Tay LJ, Rutigliani L, et al. Prostatic stromal tumour of uncertain malignant potential treated with robotic-assisted radical prostatectomy: medium-term oncological and functional outcome of two cases. Ann R Coll Surg Engl 2023; 105: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan K, Piedad J, Mudiyanselage A, et al. A rare case of prostatic stromal tumour of uncertain malignant potential surrounding ejaculatory ducts in a patient with concurrent prostate adenocarcinoma. Cureus 2022; 14: e31690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladner T, Schultz T, Moore J, et al. A case of incidental STUMP discovery in a patient with concurrent prostatic adenocarcinoma. J Surg Case Rep 2021; 2021: rjab576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Wu J, Shen Q, et al. Magnetic resonance imaging features of prostatic stromal tumour of uncertain malignant potential. J Med Imaging Radiat Oncol 2022; 66: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 16.Muglia VF, Saber G, Maggioni G, Jr, et al. MRI findings of prostate stromal tumour of uncertain malignant potential: a case report. Br J Radiol 2011; 84: e194–e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MH, Carter HB. Stromal tumor of uncertain malignant potential (STUMP) with PSA >500 ng/ml: a case report. Urol Case Rep 2015; 3: 175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Berardinis E, Busetto GM, Antonini G, et al. Incidental prostatic stromal tumor of uncertain malignant potential (STUMP): histopathological and immunohistochemical findings. Urologia 2012; 79: 65–68. [DOI] [PubMed] [Google Scholar]
- 19.Torlakovic E, Lilleby W, Berner A, et al. Differential expression of steroid receptors in prostate tissues before and after radiation therapy for prostatic adenocarcinoma. Int J Cancer 2005; 117: 381–386. [DOI] [PubMed] [Google Scholar]
- 20.McKenney JK. Mesenchymal tumors of the prostate. Mod Pathol 2018; 31: S133–S142. [DOI] [PubMed] [Google Scholar]
- 21.Medina Perez M, Valero Puerta JA, Martin DP. [Atypical stromal hyperplasia of the prostate (stromal proliferation of uncertain malignant potential)]. Arch Esp Urol 2000; 53: 722–723. [PubMed] [Google Scholar]
- 22.Pan CC, Epstein JI. Common chromosomal aberrations detected by array comparative genomic hybridization in specialized stromal tumors of the prostate. Mod Pathol 2013; 26: 1536–1543. [DOI] [PubMed] [Google Scholar]
- 23.Laturnus JM, Gebhard M, Sommerauer M, et al. [Stromal tumour of uncertain malignant potential of the prostate (STUMP) - a case report.] Aktuelle Urol 2010; 41: 197–199. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki Y, Fukuda S, Fukuda S, et al. [Stromal tumors of uncertain malignant potential (STUMP) of the prostate: a case report.] Hinyokika Kiyo 2015; 61: 245–248. [PubMed] [Google Scholar]
- 25.Liu Y, Liu ZJ, Shen Q, et al. [A clinical analysis of 14 cases of prostatic stromal tumor of uncertain malignant potential.] Beijing Da Xue Bao Yi Xue Ban 2020; 52: 621–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wee HM, Ho SH, Tan PH. Recurrent prostatic stromal tumour of uncertain malignant potential (STUMP) presenting with urinary retention 6 years after transurethral resection of prostate (TURP). Ann Acad Med Singap 2005; 34: 441–442. [PubMed] [Google Scholar]
- 27.Boshchenko VS, Maspanov DA, Lozovsky MS, et al. [Organ-sparing treatment of prostate stromal tumor of uncertain malignancy potential]. Urologiia 2023; 3: 102–106. [PubMed] [Google Scholar]
- 28.Okada M, Tanaka T, Fukuta F, et al. [A case of stromal tumors of uncertain malignant potential treated by radical prostatectomy.] Hinyokika Kiyo 2013; 59: 137–140. [PubMed] [Google Scholar]
- 29.Menon M, Tewari A, Peabody J, VIP Team. Vattikuti Institute prostatectomy: technique. J Urol 2003; 169: 2289–2292. [DOI] [PubMed] [Google Scholar]
- 30.Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 2014; 65: 303–313. [DOI] [PubMed] [Google Scholar]
- 31.Haga N, Miyazaki T, Tsubouchi K, et al. Comprehensive approach for preserving cavernous nerves and erectile function after radical prostatectomy in the era of robotic surgery. Int J Urol 2021; 28: 360–368. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki I, Kijima T, Owada A, et al. Case of prostate stromal tumour of uncertain malignant potential where positron emission tomography with 18F-fluorodeoxyglucose was useful for surgical planning. BMJ Case Rep 2020; 13: e235738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagnier JJ, Kienle G, Altman DG, et al. CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605241253756 for Prostatic stromal tumor of uncertain malignant potential: a case report and literature review by Tao Zhu, Cong Yin, Cen Liufu, Jiahao Jiang, Junhua Luo and Yan Wang in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605241253756 for Prostatic stromal tumor of uncertain malignant potential: a case report and literature review by Tao Zhu, Cong Yin, Cen Liufu, Jiahao Jiang, Junhua Luo and Yan Wang in Journal of International Medical Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Junhua Luo) upon reasonable request.