Abstract
Background
The use of non-specific immunosuppressants (NSIS) to treat multiple sclerosis (MS) remains prevalent in certain geographies despite safety concerns, likely due to resource limitations.
Objective
To use MSBase registry data to compare real-world outcomes in adults with relapsing-remitting MS (RRMS) treated with dimethyl fumarate (DMF) or NSIS (azathioprine, cyclosporine, cyclophosphamide, methotrexate, mitoxantrone or mycophenolate mofetil) between January 1, 2014 and April 1, 2022.
Methods
Treatment outcomes were compared using inverse probability of treatment weighting (IPTW) Cox regression. Outcomes were annualized relapse rates (ARRs), time to discontinuation, time to first relapse (TTFR) and time to 24-week confirmed disability progression (CDP) or 24-week confirmed disability improvement (CDI; in patients with baseline Expanded Disability Status Scale [EDSS] score ≥2).
Results
After IPTW, ARR was similar for DMF (0.13) and NSIS (0.16; p = 0.29). There was no difference in TTFR between cohorts (hazard ratio [HR]: 0.98; p = 0.84). The DMF cohort experienced longer times to discontinuation (HR: 0.75; p = 0.001) and CDP (HR: 0.53; p = 0.001), and shorter time to CDI (HR: 1.99; p < 0.008), versus the NSIS cohort.
Conclusion
This analysis supports the use of DMF to treat patients with relapsing forms of MS, and may have implications for MS practices in countries where NSIS are commonly used to treat RRMS.
Keywords: Dimethyl fumarate, non-specific immunosuppressants, relapsing-remitting multiple sclerosis, real-world, effectiveness
Introduction
Non-specific immunosuppressants (NSIS), such as azathioprine, cyclophosphamide, methotrexate and mitoxantrone, have been used in the treatment of multiple sclerosis (MS) for up to 60 years. 1 The only NSIS to be indicated for MS in the United States and across Europe is mitoxantrone, with its indication in Europe being for highly active relapsing MS in patients for whom no alternative treatments are available.2,3 Despite a lack of data on their safety and efficacy from controlled clinical trials, other NSIS are frequently used off-label to treat MS.
NSIS reduce the proliferation of all rapidly dividing cells, including immune cells, through interfering with the cell's life cycle either via a blocking, cytostatic action or by stopping DNA replication and inducing cell death.1,4,5 As a consequence, NSIS not only reduce autoimmune activity, but also immune activity including protection from infectious agents and cancer prevention. 5 Safety concerns such as increased risk of infection or neoplasia need to be considered alongside the potential benefits of using NSIS. 4 Long-term azathioprine, for example, is associated with an increased rate of malignancy,6,7 and has only modest effectiveness for treatment of MS.8,9 NSIS are generally considered to have an unfavourable risk-to-benefit ratio for MS. 5
Such concerns around NSIS have contributed to the increased use of disease-modifying therapies (DMTs) including interferons, glatiramer acetate, teriflunomide, alemtuzumab, sphingosine-1-phosphate receptor modulators (S1PRMs), fumarates, natalizumab and anti-CD20 monoclonal antibodies (mAbs). 10 Dimethyl fumarate (DMF) is an oral fumarate approved in the US for the treatment of relapsing forms of MS and in the EU for the treatment of relapsing-remitting MS (RRMS).11,12 DMF is one of the most commonly used DMTs worldwide and has shown clinical efficacy through phase 3 trials and over numerous years of patient exposure.13–15 As of 31 December 2023, approximately 607,448 patients had been treated with DMF, representing over 1.4 million patient-years of exposure. 16 Use of NSIS for the treatment of MS continues in some countries around the world, despite the availability of DMTs such as DMF. There is relatively little comparative evidence on the clinical outcomes of NSIS and DMTs, and no prior studies have assessed the effectiveness of DMF versus NSIS for the treatment of MS. As DMF is now approved in countries such as China, where NSIS are still used as standard of care, 17 this study utilized patient data from the MSBase registry database to compare real-world clinical effectiveness outcomes of DMF and NSIS.
Methods
Study design
MSBase is an international, online registry for the collection and evaluation of real-world outcomes data in MS. 18 MSBase was initiated in 2004 and comprises over 86,000 patient records from 45 participating countries. 19 This retrospective analysis utilized data from across 29 countries extracted from the MSBase registry to compare outcomes for DMF and NSIS in the primary analysis sample. The MSBase registry is registered with World Health Organization International Clinical Trials Registry Platform (ACTRN12605000455662). 20 MSBase was approved by Melbourne Health Human Research Ethics Committee and by each site's institutional review board. All patients had provided written or verbal consent.
Patients
Patients with RRMS aged ≥18 years (based on age at index date) who initiated DMF or an NSIS as monotherapy from January 1, 2014, to April 1, 2022 were identified in the MSBase registry database. For the purpose of this study, the NSIS group was considered to include azathioprine, cyclosporine, cyclophosphamide, methotrexate, mitoxantrone and mycophenolate mofetil; ‘non-specific’ was used to distinguish these agents from other immunosuppressants including anti-CD20 mAbs, such as ocrelizumab, and anti-CD52 mAbs, such as alemtuzumab, both of which are approved in the US for the treatment of relapsing MS.21,22
Eligible patients were required to be on therapy for at least 6 months, due to the potential use of NSIS as induction therapy23–25 and subsequent discontinuation in the first months. We were not able to analyze safety profiles of DMF and NSIS because of the low data density of this outcome.
Outcomes
Data for baseline demographics, disease status and history and treatment history were extracted. Outcomes evaluated in the DMF and NSIS cohorts were time to discontinuation, annualized relapse rate (ARR), time to first relapse (TTFR) and time to 24-week confirmed disability progression (CDP); time to 24-week confirmed disability improvement (CDI) was assessed in patients with baseline Expanded Disability Status Scale (EDSS) score ≥2. Patients were followed from treatment initiation until censored at time of event, end of on-treatment follow-up or last recorded registry visit.
Statistical methods
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Inverse probability of treatment weighting (IPTW) weighted Cox regression was used to model outcomes. To adjust for potential confounding, propensity scores were generated using logistic regression to estimate the predicted probability of receiving DMF relative to NSIS as a function of age, sex, duration of MS disease, baseline EDSS score, country, pre-baseline relapse activity and sequence of prior DMTs at the index date. Weighted standardized differences were derived to assess post-weighting confounder balance.
We conducted several robustness and sensitivity analyses. First, a 1:1 propensity score matching analysis was performed using a caliper of 0.05, and conducted without replacement. Second, the population was trimmed to the 95th percentile of the propensity score to assess the impact of outliers. Third, analyses compared outcomes between the DMF cohort and the NSIS subset who received azathioprine only. Fourth, DMF and NSIS cohorts only included patients who were treatment-naive prior to index treatment. Lastly, outcomes were assessed in patients with more closely matched baseline EDSS scores (score <6.0; standardized difference <15%). IPTW was recalculated for each analysis. All analyses were conducted in Stata version 17 (StataCorp, College Station, Texas).
Data availability
The data that support the findings of this study are not available for sharing since the author group are not permitted to share patient-level data with anyone not specified in the original permission report. MSBase data are more broadly available to researchers upon request subject to satisfying the MSBase internal approval procedures.
Results
Patients
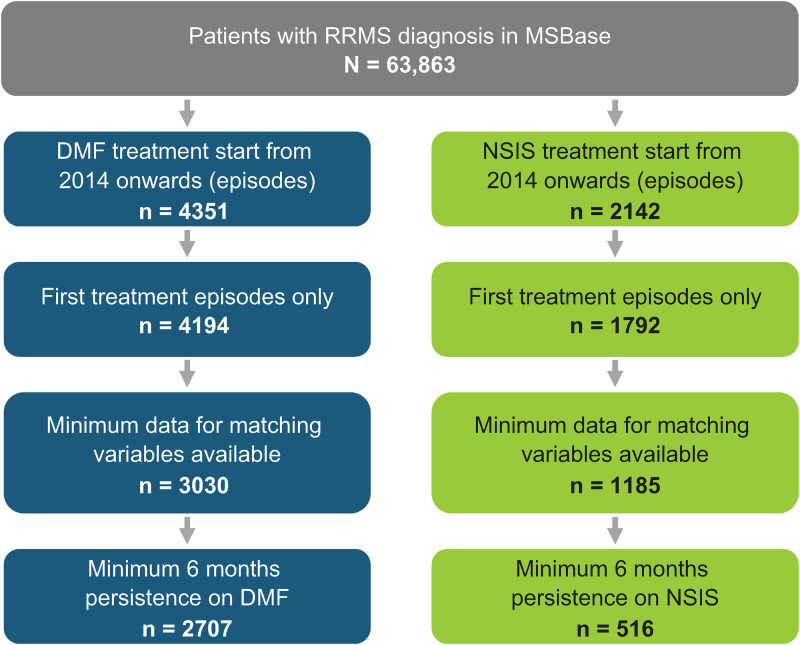
At data extraction (January 2, 2022), there were 78,409 patient records in the database, of whom 63,863 patients had a diagnosis of RRMS. After applying the inclusion criteria, 2707 patients in the DMF cohort and 516 patients in the NSIS cohort were identified who had initiated therapy from 2014 onward and had a minimum of 6 months’ exposure (Figure 1).
Figure 1.
Patient attrition for DMF and NSIS cohorts.
DMF: dimethyl fumarate; NSIS: non-specific immunosuppressant; RRMS: relapsing-remitting multiple sclerosis.
Patients were distributed across 29 countries, with the highest proportions of patients in the DMF cohort coming from Australia (15.6%), Italy (13.7%), Spain (13.7%) and Turkey (12.4%), and in the NSIS cohort coming from Turkey (30.6%), Italy (14.3%), Iran (12.6%) and Egypt (11.6%). In the NSIS cohort, patients received azathioprine (n = 331), methotrexate (n = 87), cyclophosphamide (n = 55), mitoxantrone (n = 21), mycophenolate mofetil (n = 21) or cyclosporine (n = 1). Patient demographic and disease characteristics at baseline are shown in Table 1.
Table 1.
Patient demographics and baseline characteristics for unadjusted and weighted analyses.
| Unadjusted | Weighteda | |||||
|---|---|---|---|---|---|---|
| Characteristic | DMF (n = 2707) |
NSIS (n = 516) |
Standardized difference | DMF (n = 2707) |
NSIS (n = 516) |
Weighted standardized differenceb |
| Age, mean (SD), years | 39.0 (11.4) | 42.4 (12.9) | −0.278 | 44.8 (17.8) | 42.4 (12.9) | −0.155 |
| Sex, n (%) | ||||||
| Female | 1885 (69.6) | 382 (74.0) | −0.098 | 1903 (70.3) | 382 (74.0) | −0.101 |
| Male | 822 (30.4) | 134 (26.0) | 804 (29.7) | 134 (26.0) | ||
| Disease duration, mean (SD), years | 8.3 (7.9) | 9.9 (9.4) | −0.191 | 9.5 (9.3) | 9.9 (9.4) | −0.015 |
| EDSS, median (IQR) | 2 (1–3) | 4 (2–6) | 0.919 | 2.1 (1.1, 3.3) | 4 (2, 6) | 0.183 |
| Relapses in prior year, mean (SD) | 0.65 (0.76) | 0.64 (0.84) | 0.010 | 0.75 (0.90) | 0.64 (0.84) | –0.145 |
| Pre-baseline MS therapies,c n (%) | ||||||
| 0 | 383 (14.2) | 123 (23.8) | 0.202 | 387 (14.3) | 123 (23.8) | 0.131 |
| 1 | 626 (23.1) | 107 (20.7) | 614 (22.7) | 107 (20.7) | ||
| 2 | 472 (17.4) | 86 (16.7) | 465 (17.2) | 86 (16.7) | ||
| ≥3 | 1226 (45.3) | 200 (38.8) | 1241 (45.8) | 200 (38.8) | ||
DMF: dimethyl fumarate; EDSS: Expanded Disability Status Scale; IQR: interquartile range; MS: multiple sclerosis; NSIS: non-specific immunosuppressants; SD: standard deviation.
aNs for the weighted pseudopopulation (N ≈ 3200) were rounded to nearest integer.
bPropensity score derived based on age, sex, disease duration, baseline EDSS, country, pre-baseline relapse activity and treatment order.
cMS therapy defined as either disease-modifying therapy or immunosuppressant.
Characteristics of the DMF cohort were changed following IPTW, while those of the NSIS cohort remained unchanged (Table 1). Cohorts were generally well balanced across baseline parameters with small weighted standardized differences after weighting, although a residual imbalance in EDSS was observed. EDSS scores were higher in the NSIS cohort in both the unweighted sample (median [IQR], 4 [2–6]) compared with the DMF cohort (median [IQR], 2 [1–3]) as well as the IPTW sample (median [IQR], 4 [2–6]) compared with the DMF cohort (median [IQR], 2.1 [1.1–3.3]). Mean (SD) age was 39.0 (11.4) versus 42.4 (12.9) years before weighting and 44.8 (17.8) versus 42.4 (12.9) years after IPTW in DMF and NSIS groups, respectively. Mean (SD) years of disease duration was 8.3 (7.9) versus 9.9 (9.4) years before weighting and 9.5 (9.3) versus 9.9 (9.4) years after IPTW in DMF and NSIS groups, respectively. Patient demographics and baseline characteristics for all robustness and subgroup analyses are presented in Supplemental Tables 1–5.
Mean (SD) on-treatment follow-up times were 2.46 (1.68) years on DMF and 2.09 (1.43) years on NSIS, pre-weighting, and 2.45 (1.52) years and 2.05 (1.37) years, respectively, post-IPTW. Mean (SD) number of EDSS assessments over follow-up on treatment was 4.81 (3.34) in the DMF cohort and 4.96 (3.44) in the NSIS cohort.
Outcomes after IPTW
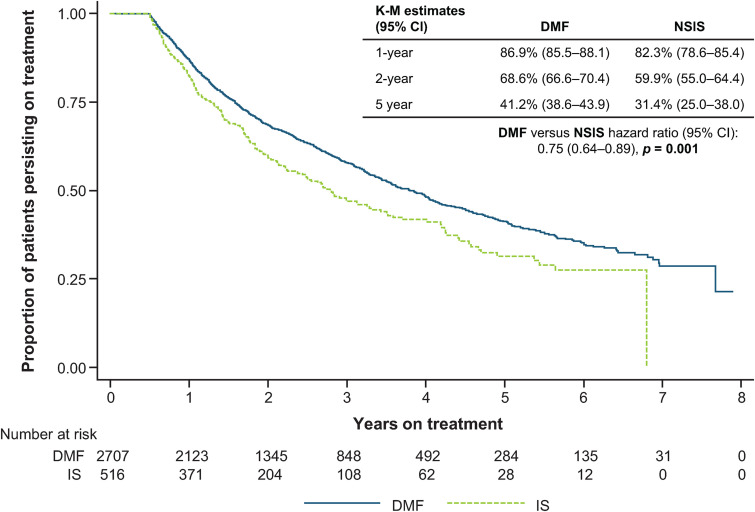
Kaplan-Meier estimates for proportions of patients who had not discontinued treatment (i.e. those persisting on treatment) in the DMF and NSIS cohorts were, respectively, 86.9% (95% CI: 85.5–88.1) and 82.3% (95% CI: 78.6–85.4) at 1 year, and 68.6% (95% CI: 66.6–70.4) and 59.9% (95% CI: 55.0–64.4) at 2 years (Figure 2). Time to discontinuation was significantly longer for DMF versus NSIS, with a hazard ratio (HR) of 0.75 (95% CI: 0.64–0.89; p = 0.001).
Figure 2.
Time to discontinuation for DMF versus NSIS after IPTW Cox regression analysis.
CI: confidence interval; DMF: dimethyl fumarate; IPTW: inverse probability of treatment weighting; K-M: Kaplan-Meier; NSIS: non-specific immunosuppressant.
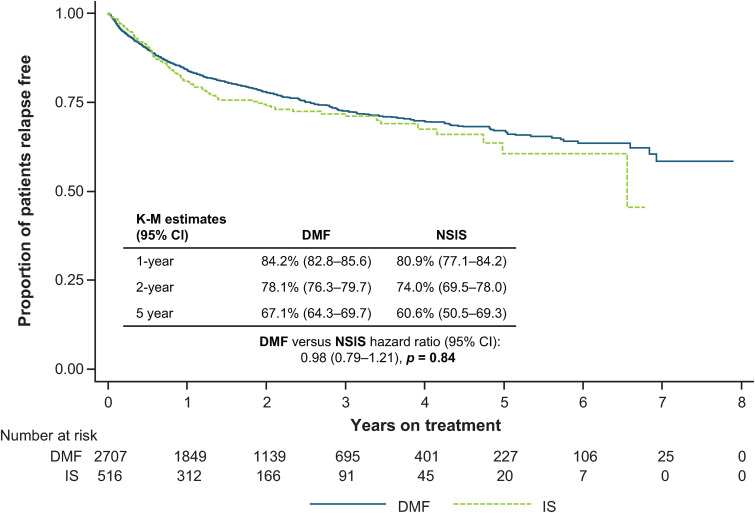
ARR was similar for DMF (0.13 [95% CI: 0.12–0.14]) and NSIS (0.16 [95% CI: 0.14–0.19]; p = 0.29). There was no difference in TTFR between the cohorts (HR: 0.98 [95% CI: 0.79–1.21]; p = 0.84) (Figure 3).
Figure 3.
TTFR for DMF versus NSIS after IPTW.
CI: confidence interval; DMF: dimethyl fumarate; IPTW: inverse probability of treatment weighting; IS: immunosuppressant; K-M: Kaplan-Meier; NSIS: non-specific immunosuppressant; TTFR: time to first relapse.
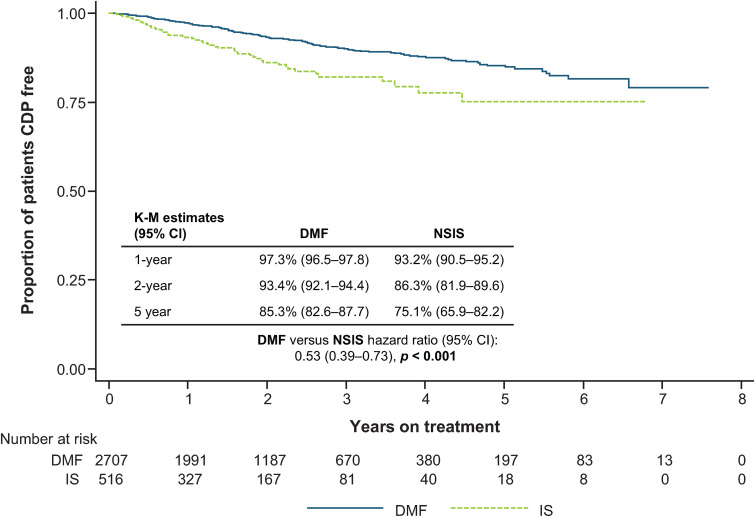
A higher proportion of patients on DMF remained free of 24-week CDP at 1, 2 and 5 years versus patients on NSIS (HR: 0.53 [95% CI: 0.39–0.73]; p < 0.001) (Figure 4).
Figure 4.
Time to 24-week CDP for DMF versus NSIS after IPTW.
CDP: confirmed disability progression; CI: confidence interval; DMF: dimethyl fumarate; IPTW, inverse probability of treatment weighting; IS: immunosuppressant; K-M: Kaplan-Meier; NSIS: non-specific immunosuppressant.
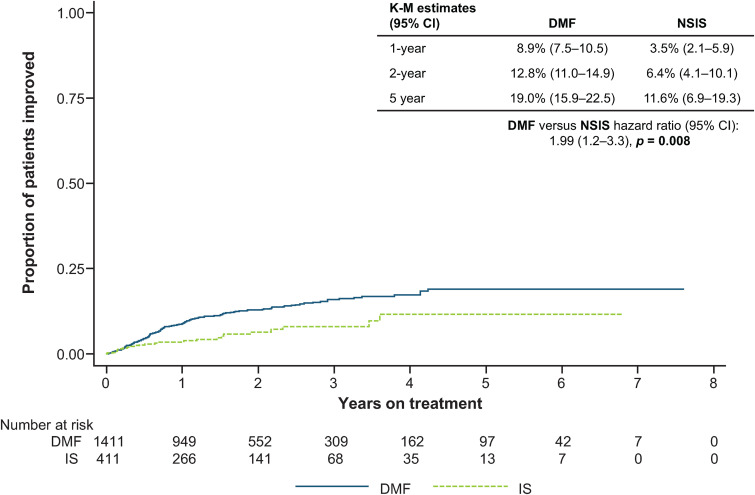
Of patients with baseline EDSS score ≥2 (DMF, n = 1411; NSIS, n = 411), estimated proportions achieving 24-week CDI were higher in the DMF cohort versus the NSIS cohort (HR: 1.99 [95% CI: 1.2–3.3]; p = 0.008) (Figure 5).
Figure 5.
Time to 24-week CDI for DMF versus NSIS after PS-IPTW in patients with baseline EDSS score ≥2.
CDI: confirmed disability improvement; CI: confidence interval; DMF: dimethyl fumarate; EDSS: Expanded Disability Status Scale; IPTW: inverse probability of treatment weighting; IS: immunosuppressant; K-M: Kaplan-Meier; PS: propensity score; NSIS: non-specific immunosuppressant.
Outcomes of robustness and subgroup analyses
Outcomes after propensity score matching
Following 1:1 propensity score matching, there were 346 patients in the DMF cohort and 346 patients in the NSIS cohort, representing 12.8% and 67.1% of the primary DMF and NSIS cohorts, respectively. ARR was 0.17 (95% CI: 0.15–0.21) for DMF and 0.19 (95% CI: 0.16–0.22) for NSIS (p = 0.50). No significant differences were observed between the cohorts across any of the other outcomes assessed (Table 2).
Table 2.
Outcomes for primary analysis, robustness analyses and subgroup analyses.
| Analysis | Number of patients in DMF cohort | Number of patients in NSIS cohort | ARR (95% CI) DMF vs NSIS | IPTW Cox proportional hazard ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Time to treatment discontinuation | Time to first relapse | Time to 24-week CDP | Time to 24-week CDI in patients with baseline EDSS score ≥2 | ||||
| Primary analysis | 2707 | 516 | 0.13 (0.12–0.14) vs. 0.16 (0.14–0.19) p = 0.29 |
0.75 (0.64–0.89) p = 0.001 |
0.98 (0.79–1.21) p = 0.84 |
0.53 (0.39–0.73) p < 0.001 |
1.99 (1.2–3.3) p = 0.008 (DMF, n = 1411) (NSIS, n = 411) |
| 1:1 propensity score matched analysis | 346 | 346 | 0.17 (0.15–0.21) vs. 0.19 (0.16–0.22) p = 0.50 |
0.90 (0.72–1.12) p = 0.35 |
0.89 (0.67–1.19) p = 0.45 |
0.85 (0.51–1.39) p = 0.51 |
1.59 (0.89–2.84) p = 0.12 |
| Top and bottom 5% of patient population trimmed based on propensity score | 2470 | 296 | 0.13 (0.12–0.14) vs. 0.20 (0.16–0.24) p = 0.0001 |
0.76 (0.61–0.96) p = 0.023 |
0.68 (0.51–0.90) p = 0.007 |
0.66 (0.40–1.09) p = 0.10 |
2.06 (1.26–3.37) p = 0.004 (DMF, n = 1324) (NSIS, n = 203) |
| Only patients who received azathioprine included in the NSIS cohort | 2707 | 331 | 0.13 (0.12–0.14) vs. 0.19 (0.16–0.22) p = 0.001 |
0.88 (0.74–1.05) p = 0.15 |
0.79 (0.64–0.98) p = 0.044 |
0.63 (0.45–0.90) p = 0.012 |
1.61 (0.99–2.62)
p = 0.05 (DMF, n = 1411) (NSIS, n = 244) |
| Treatment-naive patients in DMF and NSIS group only | 383 | 123 | 0.10 (0.08–0.12) vs. 0.19 (0.14–0.25) p = 0.0006 |
0.70 (0.48–1.02) p = 0.06 |
0.58 (0.37–0.89) p = 0.014 |
0.19 (0.09–0.41) p < 0.001 |
2.68 (1.06–6.75) p = 0.037 (DMF, n = 161) (NSIS, n = 101) |
| Patient subset matched based on EDSS scores | 2707 | 304 | 0.13 (0.12–0.14) vs. 0.18 (0.15–0.22) p = 0.0008 |
0.77 (0.64–0.93) p = 0.006 |
0.68 (0.54–0.86) p = 0.001 |
0.55 (0.36–0.83) p = 0.005 |
1.09 (0.64–1.85) p = 0.75 (DMF, n = 2707) (NSIS, n = 304) |
ARR: annualized relapse rate; CDI: confirmed disability improvement; CDP: confirmed disability progression; CI: confidence interval; DMF: dimethyl fumarate; EDSS: Expanded Disability Status Scale; IPTW: inverse probability of treatment weighting; NSIS: non-specific immunosuppressant.
IPTW Cox regression was performed. IPTW-adjusted propensity score derived based on age, sex, disease duration, baseline EDSS, country, pre-baseline relapse activity and treatment order.
Trimming of top and bottom 5% based on propensity score
After the top and bottom 5% of the cohorts were trimmed based on propensity score, there were 2470 patients in the DMF cohort and 296 patients in the NSIS cohort. The results were generally similar to the overall population. ARR was 0.13 (95% CI: 0.12–0.14) for DMF and 0.20 (95% CI: 0.16–0.24) for NSIS (p = 0.0001). Other outcomes favourable for DMF versus NSIS were time to treatment discontinuation, TTFR and time to 24-week CDI in patients with baseline EDSS score ≥2 (Table 2). No significant difference was observed in time to 24-week CDP between DMF and NSIS.
Comparison of DMF and azathioprine cohorts
When outcomes were compared between patients on DMF (n = 2707) and those on the most commonly used NSIS, azathioprine (n = 331), all outcomes apart from time to treatment discontinuation were significantly different in favour of DMF (Table 2).
Outcomes in treatment-naive patients
When assessed in patients who were treatment-naive prior to index treatment (DMF, n = 383; NSIS, n = 123), outcomes that were significantly in favour of DMF versus NSIS were ARR, TTFR, time to 24-week CDP and time to 24-week CDI in patients with baseline EDSS score ≥2 (Table 2). Time to treatment discontinuation in these patients was not significantly different between the cohorts (HR: 0.70 [95% CI: 0.48–1.02]; p = 0.06).
Matching of EDSS scores
Outcomes were assessed in the subset of patients weighted so baseline EDSS scores were more closely matched (score <6.0; standardized difference <15%). ARR was significantly lower for DMF (0.13 [95% CI: 0.12–0.14]) versus NSIS (0.18 [95% CI: 0.15–0.22]; p = 0.0008) (Table 2). Time to treatment discontinuation, TTFR and time to 24-week CDP outcomes favoured DMF compared with NSIS. No significant difference was observed in time to 24-week CDI in patients with baseline EDSS score ≥2 between the DMF and NSIS cohorts (HR: 1.09 [95% CI: 0.64–1.95]; p = 0.75).
Discussion
This analysis of real-world data from the international MSBase registry indicates that patients with RRMS treated with DMF had significantly longer times to discontinuation and 24-week CDP than patients on NSIS, and significantly shorter time to 24-week CDI. ARR and TTFR were comparable between the treatment cohorts. These outcomes may be of value since there are limited data comparing clinical efficacy or real-world outcomes of NSIS with either DMF or with other DMTs more widely. Use of NSIS as treatment for MS persists in many countries. In China, there were no oral DMTs approved prior to 2018, and therefore, NSIS remain among the most commonly used drugs to treat MS due to limited availability of DMTs. 17 Ensuring access to oral DMTs in these countries may become an important issue for patients with MS.
For DMF, Kaplan-Meier estimates of proportions discontinuing treatment at 12 and 24 months (13.1% and 31.4%, respectively) were lower than other reports of real-world use of DMF in 531 patients at single centres in the US (∼28% and ∼45%, respectively) and in 2697 patients in a French claims database analysis (32.0% and 46.5%, respectively), possibly due to differences in study design or patient population.26,27 However, the 24-month estimate was similar to the overall discontinuation rates reported for the randomized phase 3 DEFINE and CONFIRM studies (31% and 30%, respectively) over the 96-week study durations.13,14 These comparisons of discontinuation rates are challenging since discontinuations due to tolerability issues typically occur within the first 6 months on DMF,28,29 and patients with <6 months of treatment were excluded from the analyses. Therefore, the differences in discontinuation rates reported here and in other real-world studies of DMF are likely to be due to the exclusion of patients with <6 months’ exposure from the current analysis.
Further to the relative paucity of comparative efficacy data for NSIS, randomized controlled studies of NSIS in patients with MS are limited. Clinical trials have shown favourable outcomes for mitoxantrone and azathioprine in terms of reducing relapse rates, however, efficacy against disability progression has not been established.1,9,30–32 This is consistent with the findings of this analysis, as while there was no difference in ARR or TTFR between the cohorts, DMF had a significantly longer time to 24-week CDP than NSIS. As there were no data concerning adverse events (AEs) or safety, time to discontinuation served as a surrogate marker for tolerability in patients who received DMF or NSIS and were documented in the MSBase registry. Time to discontinuation was longer for patients on DMF than those on NSIS and could potentially indicate a better safety and/or tolerability profile; however, as described above, patients with <6 months’ treatment were excluded from the analyses, therefore eliminating the time period during which discontinuations of DMF due to tolerability issues occur most frequently.
The propensity score-IPTW process weighted the DMF cohort to reflect specific baseline characteristics of the NSIS cohort more closely, therefore rendering the cohorts more comparable for the assessment of outcomes. This resulted in an increase in mean age from 39.0 years in the unadjusted DMF cohort to 44.8 years in the weighted DMF cohort, and an increase in mean disease duration from 8.3 years to 9.5 years, respectively. The outcomes reported for the weighted DMF cohort could be considered as hypothetical outcomes for the NSIS cohort were these patients to have received DMF instead, with the caveat that some unmeasured confounding factors remain such as potential differences in MRI.
Robustness analyses were also employed. An analysis of trimming down the top and bottom 5% of the population based on propensity score to remove outliers indicated that the DMF cohort had a significantly longer time to treatment discontinuation than the NSIS cohort, consistent with the primary analysis. In contrast to the primary analysis, removing outliers resulted in a longer TTFR among DMF-treated patients versus NSIS-treated patients. ARR outcomes significantly favoured DMF compared with NSIS after trimming. After weighting to match cohorts on baseline EDSS scores more closely, all outcomes favoured DMF apart from time to 24-week CDI in patients with baseline EDSS score ≥2.
When outcomes were compared between DMF and azathioprine, the largest contributor to the NSIS cohort, DMF had significantly lower ARR, significantly longer TTFR and 24-week CDP and significantly shorter time to 24-week CDI. There was no difference between DMF and azathioprine regarding time to treatment discontinuation. This is consistent with azathioprine having a more favourable short-term safety profile than other NSIS such as mitoxantrone and cyclophosphamide, but having moderate efficacy. 31 Subgroup analysis of treatment-naive patients showed DMF to have better outcomes on effectiveness measures compared with NSIS.
DMF has well-established efficacy, tolerability and safety profiles. Data from the phase 3 clinical studies have shown DMF to reduce the frequency of relapse compared with placebo, although the effect on disability progression was not conclusive.13,14 However, in an integrated analysis of the phase 3 DEFINE and CONFIRM studies, DMF showed a statistically significant effect on 12-week and 24-week CDP compared with placebo, possibly due to the increased sample size allowed by this analysis. 33 The most common AEs associated with DMF treatment are flushing and gastrointestinal disorders, including nausea, diarrhoea and abdominal pain; gastrointestinal events are the most common AEs leading to discontinuation, usually within the first month of treatment. 12 , 14 ,34–37 Safety data for up to 13 years from the ENDORSE study support that DMF has a favourable long-term safety profile, with most AEs being mild to moderate. 15 While NSIS are powerful suppressants of the immune system, their use comes with considerable safety concerns. There is a risk of potentially fatal congestive heart failure with mitoxantrone, which may occur during therapy or even months or years after discontinuation. Cardiotoxicity risk increases with cumulative dose and may occur whether cardiac risk factors are present or not. Mitoxantrone also has an associated risk of secondary acute promyelocytic leukemia.2,3,31 With azathioprine, the major safety concern is the increased risk of skin cancers, non-Hodgkin lymphoma or other malignancies with long-term use.6,7,31 A cohort study of patients with inflammatory bowel disease indicated that treatment with azathioprine was associated with an increased incidence of cancer, with adjusted rate ratio (95% CI) of 1.41 (1.15–1.74) compared with patients who had not been treated with azathioprine. 38 Safety concerns with cyclophosphamide are severe immunosuppression, leading to serious and potentially fatal infections and potentially fatal cardiotoxicity. Frequent, non-fatal side effects include alopecia, infertility, amenorrhoea, nausea, vomiting, haemorrhagic cystitis, leukopenia and pulmonary interstitial fibrosis. In addition, cyclophosphamide is a teratogen.4,31,39 Given the safety concerns around NSIS, a significant limitation of this study was the lack of safety data that would have enabled comparison of the safety profiles of DMF and NSIS. However, the variant safety profiles across different NSIS would complicate the comparison of safety data between DMF and NSIS.
This study also had methodological limitations. Using IPTW, this analysis controlled for several confounders, including age, sex, MS disease duration, baseline EDSS score, country, pre-baseline relapse activity and treatment order, to ensure outcomes could be compared between the treatment cohorts. Despite this comprehensive list, there may be other potential confounders that have not been controlled for that could bias the results. Although baseline characteristics were generally well balanced based on a ± 15% threshold of standardized differences, some imbalances between the patient cohorts emerged, especially in terms of EDSS scores, for which there was a standardized difference of 0.183 post-weighting. Therefore, several sensitivity analyses, including closer matching of EDSS scores, were performed to test the robustness of the results. In the analysis of patient subsets matched based on EDSS scores, outcomes including ARR and TTFR were favourable for DMF. Additionally, a 1:1 propensity score matching analysis showed no significant differences between DMF and NSIS for ARR, time to treatment discontinuation, TTFR and time to 24-week CDP. Although there was a tendency for better outcomes in the DMF group, these did not reach statistical significance, possibly due to a lower sample size in this analysis.
Another limitation is that patients who had been on treatment for <6 months were excluded from this analysis due to potential use of NSIS as induction therapy with subsequent discontinuation within the first months. By doing so, a bias in favour of DMF could have been introduced whereby only patients who tolerated the drug in the first 6 months and persisted on treatment were included, as it is known that tolerability issues occur mostly in the first months of DMF use.28,29 Potential use of NSIS as induction therapy for longer than 6 months with subsequent discontinuation could have further biased the results.
Conclusion
This analysis indicated favourable outcomes for DMF compared with NSIS in terms of time to discontinuation, time to 24-week CDP and time to 24-week CDI. These outcomes may have implications for MS practices in countries where NSIS are commonly used to treat relapsing forms of MS. However, generalization to other geographies should be conservative as medical practices differ across countries. The outcomes reported here support the use of DMF in the treatment of patients with relapsing forms of MS.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173241247182 for Comparative effectiveness of dimethyl fumarate versus non-specific immunosuppressants: Real-world evidence from MSBase by Tim Spelman, Sara Eichau, Raed Alroughani, Serkan Ozakbas, Samia J Khoury, Francesco Patti, Eva Kubala Havrdova, Cavit Boz, Murat Terzi, Jens Kuhle, Pierre Grammond, Jeanette Lechner-Scott, Orla Gray, Maria Pia Amato, Guy Laureys, Vahid Shaygannejad, Robert Hyde, Haijue Wang, Ivan Bozin, Nicholas Belviso, Chao Quan, Feng Zeng, Anneke van der Walt, Helmut Butzkueven and in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
The authors thank the MSBase registry participants, investigators and staff. Biogen provided funding for medical writing support in the development of this manuscript; David Pertab, PhD, of Excel Scientific Solutions, wrote the first draft of the manuscript based on input from the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Footnotes
Authors’ Note: Some of these data have been previously presented at the 14th Congress of the Pan-Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS), Singapore, November 24–26, 2022 and the Chinese Society of Neurology – 25th National Conference of Neurology, China, April 13–16, 2023.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TS: received compensation for serving on scientific advisory boards, honoraria for consultancy and funding for travel from Biogen. SE: received speaker honoraria and consultant fees from Bayer, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. RA: received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche and Sanofi-Genzyme. SO: no potential conflicts of interest to disclose. SJK: received compensation for scientific advisory board activity from Merck and Roche. FP: served on advisory boards for Alexion, Biogen, Merck, Novartis, Roche and Sanofi; received research grants from FISM, Italian Health Minister, Italian University Minister and Reload Association; and received honoraria as a speaker from Almirall, Bristol Myers Squibb and Janssen. EKH: received honoraria/research support from Biogen, Merck Serono, Novartis, Roche and Teva; has been member of advisory boards for Actelion, Biogen, Celgene, EMD Serono, Novartis, and Sanofi Genzyme; and has been supported by the Czech Ministry of Education research project Cooperatio LF1 and National Institute for Neurological Research (Programme EXCELES, ID project No LX22NPO5107) funded by the European Union-Next Generation EU. CB: received conference travel support from Bayer-Schering, Biogen, Merck, Novartis and Teva; has participated in clinical trials by Novartis, Roche and Sanofi. MT: received travel grants from Bayer-Schering, Merck, Novartis and Teva; has participated in clinical trials by Novartis, Roche and Sanofi. JK: received speaker fees, research support, travel support and/or served on advisory boards for Alnylam, Bayer, Biogen, Bristol Myers Squibb, Celgene, Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Progressive MS Alliance, Quanterix, Roche, Sanofi, Stata DX, Swiss MS Society, Swiss National Research Foundation (320030_189140/1) and University of Basel. PG: has served on advisory boards for Biogen, EMD Serono, Novartis, Pendopharm, Roche and Sanofi-Genzyme; has received grant support from Genzyme and Roche; and has received research grants for his institution from Biogen, EMD Serono and Sanofi-Genzyme. JL-S: travel compensation from Biogen, Merck, Novartis and Roche. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Biogen, Merck, Novartis, Roche and Teva. OG: received honoraria as a consultant on scientific advisory boards for Biogen, Genzyme, Merck, Novartis and Roche; has received honoraria as a steering committee member for Novartis and Roche; received travel grants from Biogen, Merck, Novartis, Roche and Sanofi; participated in clinical trials by Biogen; her organization has received research funding from Biogen. MPA: received honoraria as consultant on scientific advisory boards by Biogen, Bayer-Schering, Merck, Sanofi and Teva; has received research grants by Biogen, Bayer-Schering, Merck, Novartis and Teva. GL: received financial compensation for congress attendance, consultancy, research and education from Almirall, Biogen, Bristol Myers Squibb, Celgene, Novartis, Roche, Sanofi and Teva. VS: no potential conflicts of interest to disclose. RH: was an employee of Biogen and holds stock in Biogen. HW, IB, NB and FZ: employees of and hold stock/stock options in Biogen. CQ: served on advisory boards for Biogen, Novartis, Roche and Sanofi. AvdW: no potential conflicts of interest to disclose. HB: received institutional (Monash University) funding from Alexion, Biogen, CSL, F. Hoffmann-La Roche Ltd, Merck and Novartis; has carried out contracted research for Biogen, F. Hoffmann-La Roche Ltd, Merck, and Novartis; has taken part in speaker bureaus for Biogen, F. Hoffmann-La Roche Ltd, Genzyme, Merck, Novartis, and UCB; and has received personal compensation from Oxford Health Policy Forum for the Brain Health Steering Committee.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Biogen.
ORCID iDs: T Spelman https://orcid.org/0000-0001-9204-3216
S Eichau https://orcid.org/0000-0001-9159-3128
F Patti https://orcid.org/0000-0002-6923-0846
O Gray https://orcid.org/0000-0002-7799-0116
MP Amato https://orcid.org/0000-0003-3325-3760
C Quan https://orcid.org/0000-0003-1040-3930
A van der Walt https://orcid.org/0000-0002-4278-7003
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tim Spelman, MSBase Foundation, Melbourne, Australia; Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden.
Sara Eichau, Hospital Universitario Virgen Macarena, Sevilla, Spain.
Raed Alroughani, Amiri Hospital, Sharq, Kuwait.
Serkan Ozakbas, Dokuz Eylul University, Konak/Izmir, Turkey.
Samia J Khoury, American University of Beirut Medical Center, Beirut, Lebanon.
Francesco Patti, Department of Medical and Surgical Sciences and Advanced Technologies, GF Ingrassia, Catania, Italy.
Eva Kubala Havrdova, Department of Neurology, First Medical Faculty, Charles University in Prague and General University Hospital, Prague, Czech Republic.
Cavit Boz, KTU Medical Faculty Farabi Hospital, Trabzon, Turkey.
Murat Terzi, 19 Mayis University, Samsun, Turkey.
Jens Kuhle, Department of Neurology, University Hospital and University of Basel, Basel, Switzerland; Multiple Sclerosis Centre and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB), Departments of Biomedicine and Clinical Research, University Hospital and University of Basel, Basel, Switzerland.
Pierre Grammond, CISSS Chaudière-Appalache, Lévis, QC, Canada.
Jeanette Lechner-Scott, University of Newcastle, Newcastle, NSW, Australia.
Orla Gray, South Eastern HSC Trust, Belfast, UK.
Maria Pia Amato, University of Florence, Florence, Italy.
Guy Laureys, University Hospital Ghent, Ghent, Belgium.
Vahid Shaygannejad, Isfahan University of Medical Sciences, Isfahan, Iran.
Robert Hyde, Biogen, Baar, Switzerland.
Haijue Wang, Biogen, Cambridge, MA, USA.
Ivan Bozin, Biogen, Baar, Switzerland.
Nicholas Belviso, Biogen, Cambridge, MA, USA.
Chao Quan, Department of Neurology, Huashan Hospital, National Center for Neurological Disorders, Fudan University, Shanghai, China.
Feng Zeng, Biogen, Cambridge, MA, USA.
Anneke van der Walt, Monash University, Melbourne, Australia.
Helmut Butzkueven, The Alfred Hospital, Melbourne, Australia.
References
- 1.Confavreux C, Vukusic S. Non-specific immunosuppressants in the treatment of multiple sclerosis. Clin Neurol Neurosurg 2004; 106: 263–269. [DOI] [PubMed] [Google Scholar]
- 2.Pfizer. Novantrone® (Mitoxantrone) prescribing information. https://www.pfizermedicalinformation.com/en-us/mitoxantrone/dosage-admin (accessed July 23, 2023).
- 3.Pfizer. Novantrone® (Mitoxantrone) summary of product characteristics. https://www.ema.europa.eu/en/documents/referral/novantrone-article-30-referral-annex-iii_en.pdf (2016, accessed July 21, 2023).
- 4.Neuhaus O, Kieseier BC, Hartung HP. Immunosuppressive agents in multiple sclerosis. Neurotherapeutics 2007; 4: 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierhansl L, Hartung HP, Aktas O, et al. Thinking outside the box: non-canonical targets in multiple sclerosis. Nat Rev Drug Discov 2022; 21: 578–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs 2018; 32: 939–949. [DOI] [PubMed] [Google Scholar]
- 7.Confavreux C, Saddier P, Grimaud J, et al. Risk of cancer from azathioprine therapy in multiple sclerosis: a case-control study. Neurology 1996; 46: 1607–1612. [DOI] [PubMed] [Google Scholar]
- 8.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 9.Filippini G, Del Giovane C, Vacchi L, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2013; 6: CD008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA 2021; 325: 765–779. [DOI] [PubMed] [Google Scholar]
- 11.Biogen. TECFIDERA® (dimethyl fumarate) delayed-release capsules, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/204063s029lbl.pdf (2023, accessed July 4, 2023).
- 12.Biogen Netherlands BV. TECFIDERA® summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/tecfidera-epar-product-information_en.pdf (2022, accessed July 4, 2023).
- 13.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 14.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 15.Gold R, Arnold DL, Bar-Or A, et al. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: final ENDORSE study results. Mult Scler 2022; 28: 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biogen. Data on file. 2024.
- 17.Jia D, Zhang Y, Yang C. The incidence and prevalence, diagnosis, and treatment of multiple sclerosis in China: a narrative review. Neurol Sci 2022; 43: 4695–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spelman T, Ozakbas S, Alroughani R, et al. Comparative effectiveness of cladribine tablets versus other oral disease-modifying treatments for multiple sclerosis: results from MSBase registry. Mult Scler 2023; 29: 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MSBase Neuro-Immunology Registry. https://www.msbase.org/ (accessed February 2023).
- 20.Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler 2006; 12: 769–774. [DOI] [PubMed] [Google Scholar]
- 21.Genentech. OCREVUS® (ocrelizumab) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761053s031lbl.pdf (2023, accessed November 24, 2023).
- 22.Genzyme. LEMTRADA® (alemtuzumab) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/103948s5187lbl.pdf (2023, accessed November 24, 2023).
- 23.Prosperini L, Mancinelli CR, Solaro CM, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics 2020; 17: 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Page E, Leray E, Taurin G, et al. Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry 2008; 79: 52–56. [DOI] [PubMed] [Google Scholar]
- 25.Fenu G, Lorefice L, Frau F, et al. Induction and escalation therapies in multiple sclerosis. Antiinflamm Antiallergy Agents Med Chem 2015; 14: 26–34. [DOI] [PubMed] [Google Scholar]
- 26.Vollmer B, Nair KV, Sillau SH, et al. Comparison of fingolimod and dimethyl fumarate in the treatment of multiple sclerosis: two-year experience. Mult Scler J Exp Transl Clin 2017; 3: 2055217317725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosco-Levy P, Debouverie M, Brochet B, et al. Comparative effectiveness of dimethyl fumarate in multiple sclerosis. Br J Clin Pharmacol 2022; 88: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 28.Fox EJ, Vasquez A, Grainger W, et al. Gastrointestinal tolerability of delayed-release dimethyl fumarate in a multicenter, open-label study of patients with relapsing forms of multiple sclerosis (MANAGE). Int J MS Care 2016; 18: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min J, Cohan S, Alvarez E, et al. Real-world characterization of dimethyl fumarate-related gastrointestinal events in multiple sclerosis: management strategies to improve persistence on treatment and patient outcomes. Neurol Ther 2019; 8: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tramacere I, Del Giovane C, Salanti G, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2015; 9: CD011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kieseier BC, Jeffery DR. Chemotherapeutics in the treatment of multiple sclerosis. Ther Adv Neurol Disord 2010; 3: 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massacesi L, Tramacere I, Amoroso S, et al. Azathioprine versus beta interferons for relapsing-remitting multiple sclerosis: a multicentre randomized non-inferiority trial. PLoS One 2014; 9: e113371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viglietta V, Miller D, Bar-Or A, et al. Efficacy of delayed-release dimethyl fumarate in relapsing-remitting multiple sclerosis: integrated analysis of the phase 3 trials. Ann Clin Transl Neurol 2015; 2: 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gold R, Giovannoni G, Phillips JT, et al. Sustained effect of delayed-release dimethyl fumarate in newly diagnosed patients with relapsing-remitting multiple sclerosis: 6-year interim results from an extension of the DEFINE and CONFIRM studies. Neurol Ther 2016; 5: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinea A, Amezcua L, Vargas W, et al. Real-world safety and effectiveness of dimethyl fumarate in Hispanic or Latino patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther 2020; 9: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams MJ, Amezcua L, Okai A, et al. Real-world safety and effectiveness of dimethyl fumarate in Black or African American patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther 2020; 9: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alroughani R, Ahmed SF, Behbehani R, et al. Effectiveness and safety of dimethyl fumarate treatment in relapsing multiple sclerosis patients: real-world evidence. Neurol Ther 2017; 6: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasternak B, Svanstrom H, Schmiegelow K, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol 2013; 177: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 39.Ingenus Pharmaceuticals. Cyclophosphamide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf (2020, accessed July 21, 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173241247182 for Comparative effectiveness of dimethyl fumarate versus non-specific immunosuppressants: Real-world evidence from MSBase by Tim Spelman, Sara Eichau, Raed Alroughani, Serkan Ozakbas, Samia J Khoury, Francesco Patti, Eva Kubala Havrdova, Cavit Boz, Murat Terzi, Jens Kuhle, Pierre Grammond, Jeanette Lechner-Scott, Orla Gray, Maria Pia Amato, Guy Laureys, Vahid Shaygannejad, Robert Hyde, Haijue Wang, Ivan Bozin, Nicholas Belviso, Chao Quan, Feng Zeng, Anneke van der Walt, Helmut Butzkueven and in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Data Availability Statement
The data that support the findings of this study are not available for sharing since the author group are not permitted to share patient-level data with anyone not specified in the original permission report. MSBase data are more broadly available to researchers upon request subject to satisfying the MSBase internal approval procedures.