Abstract
Genes (ebrAB) responsible for ethidium resistance were cloned from chromosomal DNA of Bacillus subtilis ATCC 9372. The recombinant plasmid produced elevated resistance against ethidium bromide, acriflavine, pyronine Y, and safranin O not only in Escherichia coli but also in B. subtilis. It also caused an elevated energy-dependent efflux of ethidium in E. coli. EbrA and EbrB showed high sequence similarity with members of the small multidrug resistance (SMR) family of multidrug efflux pumps. Neither ebrA nor ebrB was sufficient for resistance, but introduction of the two genes carried on different plasmids conferred drug resistance. Thus, both EbrA and EbrB appear to be necessary for activity of the multidrug efflux pump. In known members of the SMR family, only one gene produces drug efflux. Thus, EbrAB is a novel SMR family multidrug efflux pump with two components.
Drug efflux from cells is one of the major mechanisms of drug resistance. Multidrug efflux pumps are widely distributed in membranes ranging from bacterial cells to animal cells and remove toxic substances from the cytoplasm or membranes in an energy-dependent manner. The multidrug efflux pumps are responsible for multidrug resistance in bacterial cells and in cancer cells. Thus, the presence of multidrug efflux pumps is a serious problem in the treatment of infectious diseases and cancer. Several major groups of multidrug extrusion systems are known in microorganisms (2, 11, 17). One such group is the small multidrug resistance (SMR) family, and members of this family have been found in many microorganisms (19). Transporters of the SMR family are rather small and usually possess four transmembrane domains in one polypeptide. The SMR family includes more than 40 proteins in eubacteria, and a few of them have been studied in detail (19, 24). These include Smr (Staphylococcus aureus) (6) and EmrE (Escherichia coli) (25). It is very likely that the SMR family drug transporters are drug/ H+ antiporters. During the course of our studies of multidrug transporters in bacterial cells, we found a unique system in Bacillus subtilis ATCC 9372: a two-component drug transporter that belongs to the SMR family.
MATERIALS AND METHODS
Bacteria and growth.
B. subtilis ATCC 9372 was used as a donor of chromosomal DNA. E. coli KAM3 (15), a derivative of K-12 that lacks a restriction system and AcrAB (13), was used as the cloning host and for drug susceptibility testing. B. subtilis ISW1214 (hsrM leuA8 metB5; Tets) was purchased from TaKaRa Co. and used for the drug susceptibility test. B. subtilis and E. coli cells were grown in Luria-Bertani (LB) broth (10) under aerobic conditions at 37°C. Where indicated, drugs were added to the medium.
Drug susceptibility test.
The MICs of drugs were determined in Mueller-Hinton broth (Difco) containing various drugs at various concentrations as indicated. Cells in the test medium (105 cells/ml) were incubated at 37°C for 24 h, and thereafter the growth was judged.
Gene manipulation.
The gene responsible for ethidium resistance was cloned from B. subtilis ATCC 9372 cells as follows. Chromosomal DNA was prepared from B. subtilis ATCC 9372 by the method of Lovett and Keggins (12). The DNA was partially digested with Sau3AI, and fragments with 4 to 10 kbp were separated by sucrose density gradient centrifugation. The DNA fragments were ligated into pUC19, which had been digested with BamHI, and dephosphorylated with bacterial alkaline phosphatase. Competent cells of E. coli KAM3 prepared by the method of Hanahan (8) were transformed with the ligated recombinant plasmids and were spread on LB agar plates containing 9 μg of ethidium bromide per ml and 60 μg of ampicillin per ml. Plasmids contained in the transformants were isolated, reintroduced into KAM3 cells, and spread on the same type of plates again. One of the resulting hybrid plasmids were designated pBET5. The DNA insert in the pBET5 was about 3.5 kbp. Two HincII sites were present in pBET5, one in the multicloning site derived from pUC19 and another one in the insert. The pBET5 was digested with HincII and self ligated. The resulting recombinant plasmid pBET52 carries ebrAB. Two HindIII sites were present in pBET5, one site in the ebrB gene and another in the multicloning site derived from pUC19. The pBET5 was digested with HindIII and self ligated. The resulting pBET53 carries ebrA but not ebrB. pBET52 was cleaved with ClaI, digested with mung bean nuclease, which resulted in blunt ends, and self ligated, to produce pBET51. Thus, the ebrA gene was inactivated by removing two nucleotides from the ClaI site while leaving the ebrB gene intact. The DNA insert in the pBET53 was subcloned into pSTV29, a derivative of pACYC184, and pTS93 was obtained. pHAB was constructed as follows. The DNA insert in the pBET52 was cut out and ligated to a B. subtilis shuttle vector, pHY300PLK (Ampr Tetr) (purchased from TaKaRa Co.). The plasmids used in this study, their vectors, and B. subtilis genes carried are listed in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Vector | B. subtilis genes carried |
|---|---|---|
| pBET5 | pUC19 | ebrA ebrB |
| pBET51 | pUC19 | ebrB |
| pBET52 | pUC19 | ebrA ebrB |
| pBET53 | pUC19 | ebrA |
| pTS93 | pSTV29 (a derivative of pACYC184) | ebrA |
| pHAB | pHY300PLK (a shuttle vector) | ebrA ebrB |
The nucleotide sequence was determined by the dideoxy chain termination method (20) using a DNA sequencer (ALF express; Pharmacia Biotech). Sequence data were analyzed with GENETYX sequence analysis software (Software Development Co.). The SwissProt and GenBank databases were screened for sequence similarities.
Ethidium efflux assay in cells.
E. coli KAM3 cells harboring each hybrid plasmid were grown in LB broth (10) under aerobic conditions at 37°C. The cells were harvested at the late exponential phase of growth, washed twice with a minimal medium (23), and suspended in the same medium to an optical density of about 0.2 at 650 nm. Carbonylcyanide m-chlorophenylhydrazone (CCCP) and ethidium bromide were added to the cell suspension to 40 μM and 2.5 μM, respectively. The cell suspension was shaken for 1 h at 37°C to deplete the energy of cells and to load the cells with ethidium. Thereafter, cells were harvested and washed twice with the minimal medium supplemented with ethidium bromide (2.5 μM, final concentration) and resuspended in the same medium to an optical density of about 0.1 at 650 nm. The cell suspension was preincubated at 37°C for 5 min, and the assay was initiated. The fluorescence of the assay mixture was measured with excitation and emission wavelengths of 500 nm and 580 nm (1), respectively, using the Hitachi fluorescence spectrophotometer F-2000.
Other.
Chemicals and enzymes used in this study were from commercial sources.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases with the accession no. AB029306.
RESULTS
Cloning of ethidium resistance genes.
E. coli mutant KAM3 lacks both the restriction system (hsd mutant) and the principal multidrug efflux pump AcrAB and is suitable for cloning of multidrug resistance gene(s) from other organisms (15). In fact, we have cloned multidrug efflux genes from Vibrio parahaemolyticus (15), Pseudomonas aeruginosa (14), and others (unpublished results) with KAM3 as host. We also tried to clone a multidrug resistance gene(s) from the chromosome of gram-positive bacterium B. subtilis with KAM3 as host. Ethidium resistance is a useful marker for cloning a drug efflux gene(s), because cells have to extrude ethidium to escape its toxicity. We obtained two recombinant plasmids by using ethidium as drug for the selection. Restriction analysis revealed that these two plasmids carried the same DNA region. We further analyzed one of the plasmids, pBET5. Table 2 shows MICs of several drugs for KAM3/pUC19 (control) and KAM3/pBET5. When compared to the control, KAM3/pBET5 cells required a MIC that was eight times higher with ethidium bromide and four times higher with acriflavine, pyronine Y, safranin O, and tetraphenylphosphonium (TPP) chloride. No significant change in the MIC was observed with other drugs tested (Table 2).
TABLE 2.
Changes in drug susceptibility in E. coli transformant
| Drug | MIC (μg/ml) for:
|
|
|---|---|---|
| KAM3/pUC19 | KAM3/pBET5 | |
| Ethidium bromide | 1 | 8 |
| Acriflavine | 4 | 16 |
| Pyronine Y | 1 | 4 |
| Safranin O | 4 | 16 |
| TPP Cl | 8 | 32 |
| Rhodamine 6G | 2 | 2 |
| Erythromycin | 1 | 1 |
| Chloramphenicol | 1 | 1 |
| Tetracycline | 0.5 | 0.5 |
| Kanamycin | 1 | 1 |
| CCCP | 8 | 8 |
Sequence and characteristics of the gene products.
Partial sequencing of the DNA insert in the pBET5 plasmid revealed that it carried the putative ebrAB genes, which were suggested from the genome sequence of B. subtilis 168 (9). Since we cloned the genes from B. subtilis ATCC 9372, we determined the sequence of the whole ebrAB region. We found that there were some differences in the sequence of the ebrAB region between strain ATCC 9372 and strain 168 (91% identity). The putative ebrAB genes were preceded by a possible promoter-like sequence and followed by a possible terminator-like sequence. The ebrAB region was located just downstream from the lac promoter of pUC19 but in the opposite orientation in the plasmid pBET5. Cells harboring pBET5 showed elevated drug resistance, as described above. Therefore, we conclude that the original promoter of the ebrAB genes derived from B. subtilis is functional in the E. coli cells. There were no terminator-like sequence or promoter-like sequence between the two putative genes, ebrA and ebrB. Thus, it is likely that ebrA and ebrB are members of one operon. Both ebrA and ebrB were preceded by Shine-Dalgarno sequences (22) at a proper distance.
The deduced EbrA and EbrB proteins consist of 105 and 118 amino acid residues, respectively, similar to the size of EbrA and EbrB in strain 168 (9). The identity in the amino acid sequence of the EbrA (and EbrB) between strain ATCC 9372 and strain 168 was 94% and similarity was 99%. Strikingly, the deduced amino acid sequence of the EbrA was highly similar (80%) to that of EbrB. A homology search in the SwissProt database revealed that the EbrA and EbrB have sequence similarity (80 to 85%) with EmrE and Ebr of E. coli and Smr of S. aureus. Thus, EbrA and EbrB are both members of the SMR family of drug transporters (19). In addition, significant sequence similarities were detected between EbrAB and SugEs of E. coli (5), Proteus vulgaris (3), and Citrobacter freundii (19). Hydropathy plots for EbrA and EbrB as calculated by the method of Eisenberg et al. (4) showed four hydrophobic, presumably transmembrane, regions as expected for the SMR family (6, 18, 24). EbrB possesses a hydrophilic region at the carboxyl terminus.
Both EbrA and EbrB are needed for drug resistance.
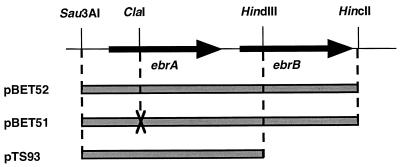
The known drug efflux pumps of the SMR family consist of one component (19). We tested whether both EbrA and EbrB are necessary for drug resistance. We constructed a plasmid carrying the normal ebrAB genes (pBET52), a plasmid carrying the normal ebrA gene alone (pTS93), and a plasmid carrying a defective ebrA gene and a normal ebrB gene (pBET51) (Fig. 1). E. coli KAM3 cells harboring pBET52 had an elevated ethidium bromide MIC (16 μg/ml) compared with cells harboring pUC19 (2 μg/ml). Cells harboring either pBET51 or pTS93 showed no change in resistance compared with cells harboring pUC19. Therefore, it seems that neither ebrA nor ebrB is sufficient for ethidium bromide resistance. However, cells harboring both pTS93 and pBET51 showed the same level of resistance (MIC of ethidium bromide, 16 μg/ml) as compared to cells harboring pBET52. Based on these results, we conclude that ebrB gene in pBET51 is functional, and therefore there is no polar effect in pBET51 in which an upstream ebrA gene is defective. Similar resistance patterns were obtained with cells harboring both pTS93 and pBET51 when acriflavine, pyronine Y, safranin O, or TPP Cl was used instead of ethidium bromide (data not shown). Thus, we conclude that both ebrA and ebrB are necessary for drug resistance. We also conclude that the EbrAB system is a new type of SMR family member, a drug efflux pump consisting of two small components.
FIG. 1.
Construction of plasmids carrying ebrA and/or ebrB. The plasmid pBET52 carries normal ebrA and ebrB. Plasmid pBET51 carries intact ebrB and defective ebrA in which two nucleotides were removed from the ClaI site. Plasmid pTS93 carries intact ebrA. Both pBET52 and pBET51 are derivatives of pUC19, and pTS93 is a derivative of pACYC184.
Ethidium efflux activity.
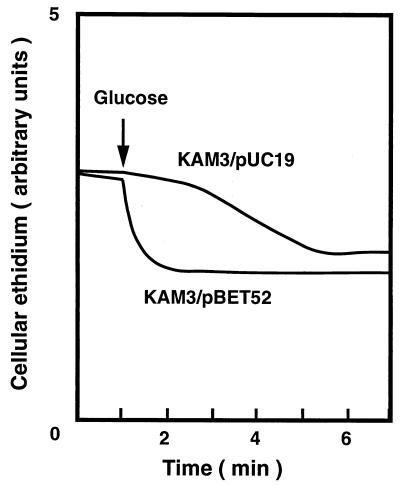
As mentioned above, sequence similarity with other SMR family multidrug efflux proteins suggested that the putative EbrAB is a multidrug efflux pump. We tested this possibility by measuring ethidium efflux. Energy-starved cells were first loaded with ethidium. Thereafter, glucose was added to energize the cells. As shown in Fig. 2, rapid ethidium efflux was observed with KAM3/pBET52 cells just after the addition of glucose. On the other hand, only a slow efflux of ethidium was observed with cells of KAM3/pUC19. Addition of an H+ conductor, CCCP, greatly reduced the ethidium efflux elicited by glucose (data not shown). Thus, the driving force for the ethidium efflux is likely an electrochemical potential of H+. Addition of Na+ or Li+ did not produce any significant effect on ethidium efflux (data not shown).
FIG. 2.
Accumulation of ethidium in cells. Energy-starved cells of E. coli KAM3/pUC19 and KAM3/pBET52 were loaded with ethidium bromide. Cellular ethidium was monitored continuously by measuring the fluorescence of ethidium at the excitation and emission wavelengths of 500 nm and 580 nm, respectively. After 1 min (arrow), glucose was added to the cell suspension at a final concentration of 20 mM to energize the cells.
We observed similar glucose-induced ethidium efflux with cells of KAM3/pBET51 and KAM3/pTS93, but not with E. coli KAM3/pBET51, KAM3/pTS93, and KAM3/pUC19 (data not shown). Thus, ethidium resistance and ethidium efflux activity in cells corresponded well. These results also support the idea that products of both ebrA and ebrB genes are involved in drug efflux.
EbrAB is functional in B. subtilis.
We tested whether the putative EbrAB pump is functional in B. subtilis. A plasmid, pHAB, carrying the ebrAB genes was introduced into B. subtilis ISW1214, and changes in drug resistance were tested. As shown in Table 3, a several-fold increase in MICs was observed with ethidium bromide, acriflavine, pyronine Y, and safranin O but not with other antimicrobial drugs tested in ISW1214/pHAB compared with ISW1214. Thus, the EbrAB system is functional in B. subtilis cells. A slightly smaller increase in MIC was observed with B. subtilis ISW1214/pHAB cells (Table 3) than with E. coli KAM3/pBET5 cells (Table 2). This may be partly due to the fact that B. subtilis ISW1214 has a wild-type phenotype regarding drug sensitivity; perhaps it possesses intrinsic drug efflux pumps, such as Bmr (16) and EbrAB, while E. coli KAM3 is deleted in the major drug efflux pump AcrAB. Another factor(s) which may affect the MIC would be the copy number of the plasmids in cells and/or efficiency of the gene expression.
TABLE 3.
Changes in drug susceptibility in B. subtilis transformant
| Drug | MIC (μg/ml) for:
|
|
|---|---|---|
| ISW1214 | ISW1214/pHAB | |
| Ethidium bromide | 4 | 16 |
| Acriflavine | 2 | 4 |
| Pyronine Y | 4 | 8 |
| Safranin O | 8 | 16 |
| Rhodamine 6G | 1 | 1 |
| Erythromycin | 0.25 | 0.25 |
| Chloramphenicol | 0.5 | 0.5 |
| Ofloxacin | 0.06 | 0.06 |
DISCUSSION
The presence of putative ebrAB genes in the chromosomal DNA of B. subtilis has been revealed by whole-genome sequencing (9). Judging from the similarity of the deduced amino acid sequences between EbrA or EbrB and members of SMR family of multidrug efflux proteins, it seemed that the EbrAB is responsible for ethidium bromide resistance. We cloned the ebrAB genes from B. subtilis ATCC 9372 and expressed them in E. coli cells. In fact, our data suggest that the EbrAB is a multidrug efflux pump and is involved in multidrug resistance against cationic lipophilic dyes such as ethidium bromide, acriflavine, pyronine Y, and safranin O. Three signature sequences that are specific to the SMR family members (19) were all present in deduced amino acid sequences of EbrA and EbrB (data not shown). Thus, we believe that the EbrAB is a member of the SMR family.
Each member of the SMR family so far reported is encoded by a single gene. Smr of S. aureus (6) and EmrE of E. coli (25) have been purified to homogeneity and reconstituted into liposomes. The reconstituted liposomes then showed drug efflux activity, indicating that a single polypeptide is sufficient for function although it may work as homo-oligomer (26). Interestingly, however, we found two consecutive genes in the ebr region of B. subtilis. It seemed possible that either of the products from the two genes (ebrA and ebrB) could function as a drug efflux pump. However, introduction of either gene alone into cells did not confer resistance. On the other hand, concomitant introduction of the two genes made cells resistant. Thus, both EbrA and EbrB are necessary for drug efflux activity. Sasatsu and coworkers reported that there were two consecutive ebr genes in plasmid pTZ22, a transferable plasmid of S. aureus (21). Deletion of one of the genes only lowered the resistance level to ethidium (21). One copy of the ebr gene was thus enough for the drug efflux in their case. Thus, our case is the first example of a drug efflux pump of the SMR family that seems to be composed of two very similar, but different, components.
The EbrAB system was functional in both E. coli and B. subtilis. It is very likely that the promoter of the ebrAB operon and products of the operon from a gram-positive bacterium, B. subtilis, are functional in a gram-negative bacterium, E. coli.
Two more sets of putative genes similar to the ebrAB genes are present in the whole genome of B. subtilis 168, yvdR and yvdS and ykkD and ykkC (9). The deduced products of these putative genes showed roughly 50% sequence similarity with EbrA and EbrB. It would be interesting to test whether they encode drug efflux pumps.
Grinius and Goldberg reported that Glu-13 of the Smr, a unique acidic residue located in the hydrophobic domain, is directly involved in the drug/H+ antiporter (7). They also reported that Glu-24 of the Smr is involved in determining the specificity of drug resistance. These two Glu residues are conserved in both EbrA and EbrB. Paulsen and coworkers reported that Tyr-59 and Trp-62 play an essential role in drug resistance in QacC (Smr) (18). These two residues are also conserved in EbrA and EbrB.
ACKNOWLEDGMENTS
We thank Dr. Manuel Varela of Eastern New Mexico University for critically reading the manuscript.
This work was supported in part by grants from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Bolhuis H, Molenaar D, Poelarends G, van Veen H W, Poolman B, Driessen A J M, Konings W N. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J Bacteriol. 1994;176:6957–6964. doi: 10.1128/jb.176.22.6957-6964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolhuis H, van Veen H W, Poolman B, Driessen A J, Konings W N. Mechanisms of multidrug transporters. FEMS Microbiol Rev. 1997;21:55–84. doi: 10.1111/j.1574-6976.1997.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 3.Cole S T. Nucleotide sequence and comparative analysis of the frd operon encoding the fumarate reductase of Proteus vulgaris. Extensive sequence divergence of the membrane anchors and absence of an frd-linked ampC cephalosporinase gene. Eur J Biochem. 1987;167:481–488. doi: 10.1111/j.1432-1033.1987.tb13362.x. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 5.Greener T, Govezensky D, Zamir A. A novel multicopy suppressor of a groEL mutation includes two nested open reading frames transcribed from different promoters. EMBO J. 1993;12:889–896. doi: 10.1002/j.1460-2075.1993.tb05729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinius L, Dreguniene G, Goldberg E B, Liao C H, Projan S J. A staphylococcal multidrug resistance gene product is a member of a new protein family. Plasmid. 1992;27:119–129. doi: 10.1016/0147-619x(92)90012-y. [DOI] [PubMed] [Google Scholar]
- 7.Grinius L L, Goldberg E B. Bacterial multidrug resistance is due to a single membrane protein which functions as a drug pump. J Biol Chem. 1994;269:29998–30004. [PubMed] [Google Scholar]
- 8.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 9.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 10.Lennox E S. Transduction of linked genetic characters of host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 11.Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 12.Lovett P S, Keggins K M. Bacillus subtilis as a host for molecular cloning. Methods Enzymol. 1979;68:342–357. doi: 10.1016/0076-6879(79)68025-4. [DOI] [PubMed] [Google Scholar]
- 13.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 14.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neyfakh A A, Bidnenko V E, Chen L B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 18.Paulsen I T, Brown M H, Dunstan S J, Skurray R A. Molecular characterization of the Staphylococcal multidrug resistance export protein QacC. J Bacteriol. 1995;177:2827–2833. doi: 10.1128/jb.177.10.2827-2833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulsen I T, Skurray R A, Tam R, Saier M J, Turner R J, Weiner J H, Goldberg E B, Grinius L L. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasatsu M, Shibata Y, Noguchi N, Kono M. High-level resistance to ethidium bromide and antiseptics in Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:109–113. doi: 10.1016/0378-1097(92)90514-o. [DOI] [PubMed] [Google Scholar]
- 22.Shine J, Dalgarno L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S, Lerner S A, Lin E C C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967;93:642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yelin R, Rotem D, Schuldiner S. EmrE, a small Escherichia coli multidrug transporter, protects Saccharomyces cerevisiae from toxins by sequestration in the vacuole. J Bacteriol. 1999;181:949–956. doi: 10.1128/jb.181.3.949-956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yerushalmi H, Lebendiker M, Schuldiner S. EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J Biol Chem. 1995;270:6856–6863. doi: 10.1074/jbc.270.12.6856. [DOI] [PubMed] [Google Scholar]
- 26.Yerushalmi H, Lebendiker M, Schuldiner S. Negative dominance studies demonstrate the oligomeric structure of EmrE, a multidrug antiporter from Escherichia coli. J Biol Chem. 1996;271:31044–31048. doi: 10.1074/jbc.271.49.31044. [DOI] [PubMed] [Google Scholar]