Abstract
The implementation of Artificial Intelligence (AI) in healthcare is enhancing diagnostic accuracy in clinical setups. The use of AI in healthcare is steadily increasing with advancing technology, extending beyond disease diagnosis to encompass roles in feto-maternal health. AI harnesses Machine Learning (ML), Natural Language Processing (NLP), Artificial Neural Networks (ANN), and computer vision to analyze data and draw conclusions. Considering maternal health, ML analyzes vast datasets to predict maternal and fetal health outcomes, while NLP interprets medical texts and patient records to assist in diagnosis and treatment decisions. ANN models identify patterns in complex feto-maternal medical data, aiding in risk assessment and intervention planning whereas, computer vision enables the analysis of medical images for early detection of feto-maternal complications. AI facilitates early pregnancy detection, genetic screening, and continuous monitoring of maternal health parameters, providing real-time alerts for deviations, while also playing a crucial role in the early detection of fetal abnormalities through enhanced ultrasound imaging, contributing to informed decision-making. This review investigates into the application of AI, particularly through predictive models, in addressing the monitoring of feto-maternal health. Additionally, it examines potential future directions and challenges associated with these applications.
Keywords: Artificial intelligence, feto-maternal health, fetal monitoring, machine learning, Artificial neural networks
Introduction
Maternal and fetal health are of paramount importance during pregnancy. Ensuring the well-being of both the mother and the developing fetus is a complex task that involves continuous monitoring and timely intervention.1 Feto-maternal health covers the care and assessment of the health status of both the expectant mother and the growing fetus from the moment of conception to the delivery of the baby. Feto-maternal health involves a series of intricate processes that are vital for a successful pregnancy and a healthy birth outcome. Maternal health during pregnancy is not only about the physical health of the expectant mother but also includes her emotional and psychological well-being.2 Factors such as proper nutrition, adequate prenatal care, and management of chronic conditions are critical to ensure the health of the mother and her ability to support the developing fetus.3–6 Simultaneously, monitoring fetal health is equally crucial. This involves assessing the growth and development of the fetus, ensuring the presence of a healthy placenta, and monitoring fetal heart rate (FHR) and movement.7,8 Early detection of any abnormalities or complications is essential for timely intervention, which can significantly influence the course of pregnancy and the health of both mother and fetus.
Early identification of pregnancy-related issues and immediate medical attention is important in feto-maternal context. Complications during pregnancy, if not identified or managed on time, can lead to adverse outcomes such as preterm birth, stillbirth, maternal mortality, or long-term health issues for the newborn.9,10 Moreover, early detection and monitoring helps in the prevention and management of conditions like preeclampsia, gestational diabetes, and fetal growth restriction.11,12 These conditions could be fatal if not detected or treated during the early stages of pregnancy. The significance of early detection and monitoring is not limited to high-risk pregnancies alone, even in seemingly low-risk pregnancies, unexpected issues can arise. Therefore, a robust system for continuous monitoring and early detection is essential to ensure the health and safety of both the mother and the fetus.
Existing methodologies for detecting fetomaternal abnormalities, such as blood screening, do exist, but they have limitations and may require upgrading with advanced methods, particularly to address challenges in early detection. Resource constraints, such as limited access to specialized healthcare professionals and diagnostic equipment, further hinder timely and comprehensive assessments. Additionally, conventional approaches often struggle with interpreting complex medical data and predicting potential risks accurately. These limitations underscore the necessity for Artificial Intelligence (AI) -assisted approaches, which can leverage advanced algorithms, Machine Learning (ML) techniques, and predictive models to enhance decision-making, optimize healthcare delivery, and ultimately improve patient outcomes in feto-maternal health.
AI has emerged as a transformative force in healthcare, offering a new dimension to the way we approach disease diagnosis, its treatment, and monitoring.13–15 AI comprises a spectrum of fundamental components, incorporating ML, Natural Language Processing (NLP), Artificial Neural Networks (ANNs), and computer vision, among others.16 These elements empower computers to emulate human intelligence, facilitating advanced problem-solving and decision-making. In the context of healthcare, AI has been increasingly applied to various medical domains, including radiology, cardiology, surgery, oncology and obstetrics.17–19 AI algorithms can process and analyze large volumes of data, making it possible to identify subtle patterns and deviations that might go unnoticed through traditional medical tests.17 In recent years, AI has found a significant role in the field of feto-maternal health.20 It offers innovative solutions for early detection, continuous monitoring, and personalized care. These algorithms can work in real-time, providing healthcare professionals with actionable insights, thereby potentially improving maternal and fetal health outcomes.21
This review article explores the myriad applications of AI in the context of feto-maternal health, covering the pregnancy journey from conception to delivery. It examines the current state of AI in this field, highlights its achievements, challenges, and prospects, and aims to provide a comprehensive understanding of how AI is reshaping the landscape of feto-maternal health care.
Fundamentals of Feto-Maternal Health
During pregnancy, maternal health becomes an essential focus, necessitating a holistic approach to monitor and support the well-being of the mother. This involves monitoring her physical health, managing any preexisting health conditions, and addressing complications that may arise during pregnancy.22 Timely medical intervention is crucial to safeguard the mother’s health. Factors like adequate nutrition and weight management are vital during pregnancy, as maternal nutrition significantly affects fetal development.23 Additionally, the psychological well-being of the expectant mother must be considered, as issues like prenatal depression and anxiety need to be addressed to ensure complete maternal health.24
Fetal development occurs in distinct stages, each marked by significant milestones in the growth and maturation of the fetus. Monitoring these stages is essential for assessing the wellness of the fetus. The initial weeks of pregnancy involve embryonic development, during which key organs and structures begin to form.24,25 This phase is particularly susceptible to teratogenic effects,26 making early monitoring crucial. As pregnancy progresses, the fetus undergoes rapid growth and further development of organs and systems. Understanding the developmental stage is vital for assessing the overall health of the fetus. Conventionally fetal health is often assessed through key parameters, such as FHR, tracking fetal movement and amniotic fluid levels, which are critical for evaluating the health of the fetus.27 Furthermore, monitoring maternal vital signs, such as blood pressure and heart rate, is crucial for identifying any abnormal conditions that may arise in expectant mothers during pregnancy. Due to advances in computer programming techniques, feto-maternal parameters can now be analyzed, monitored, and inferred using AI-enabled algorithms.13 These fundamental aspects of feto-maternal health serve as the foundation for comprehending how AI can enhance monitoring and early detection techniques, ultimately improving feto-maternal health outcomes.
AI in Feto-Maternal Health
The foundational components of AI like ML, NLP, ANNs along with computer vision empower systems to identify patterns in data and subsequently make predictions or assessments based on these patterns.28,29 NLP is critical for extracting useful insights from massive amounts of textual data. Deep learning is built on ANNs, which are inspired by the neural architecture of the human brain. These interwoven layers of algorithms perform exceptionally well in jobs requiring pattern detection and nonlinear interactions within data.29 Another important aspect of AI is computer vision, which gives machines the ability to perceive and grasp visual information from the outside world, assisting in picture analysis for extracting valuable insights, such as in medical imaging or experimental results. These fundamentals of AI conduct a cross-woven analysis of the data, providing conclusive and accurate outputs that can be used to solve problems across various fields.
AI plays a crucial role in disease diagnosis, treatment management and decision-making process among health care professionals. It relies on algorithms that are trained to recognize patterns within data, allowing for the prediction and decision-making based on this data (Figure 1).13,30–32 This capability is particularly pertinent in healthcare, where large volumes of medical data need to be analyzed to assist healthcare professionals. Learning models in AI can continuously improve their accuracy in diagnosing and monitoring medical conditions, including those related to feto-maternal health. In recent times, the integration of AI has proven instrumental in distinguishing between high-risk and low-risk pregnancies. Studies have assessed the accuracy of AI in predicting preterm births.33,34 Furthermore, in the evaluation of hypertensive-related disorders such as preeclampsia or gestational diabetes, AI has demonstrated an ability to accurately predict the onset of these conditions using predictive models like obstetric ultrasounds or knowledge base methods like metabolic panels or maternal blood profiles.35 These evaluations aim to mitigate neonatal loss and enhance overall birth outcomes. Despite the limited understanding of AI’s application in pregnant women, a recent survey conducted by Armero et al revealed that 69% of women agreed with the use of AI in maternal healthcare settings.36 The existing body of research collectively underscores AI’s multifaceted applications in feto-maternal health, as demonstrated by several key studies. For instance, improvements in fetal examination accuracy and efficiency have been demonstrated in cardiotocography, ultrasonography, and magnetic resonance imaging, as well as in the early detection of maternal-fetal disorders such as preterm birth and aberrant fetal growth.37 Furthermore, AI can contribute to continuous monitoring by carefully observing maternal health parameters, such as blood pressure and glucose levels, and providing real-time alerts for any deviations.38,39 These applications highlight the vast potential of AI in feto-maternal health, where it promises to enhance prenatal care and address the complex challenges associated with maternal and fetal health.
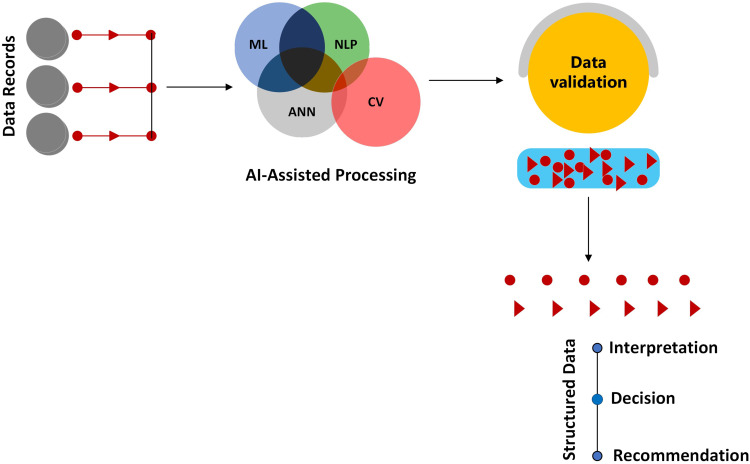
Figure 1.
This figure illustrates the systematic processing of the data records through various AI models. The journey begins with raw data inputs like patient records or medical imagery directed towards specialized AI models such as Artificial Neural Networks (ANN), Machine Learning (ML), Natural Language Processing (NLP), and Computer Vision (CV). The extracted information is then structured and organized, allowing for a coherent interpretation.
By now, we can ascertain that AI subsets, particularly ML, serve as pivotal models in fetal medicine alongside knowledge-based methods. ML methodologies, widely adopted, include ANNs and classical techniques like support vector machines and genetic algorithms, extending to recent innovations such as random forest and random booster trees.29–32 ANNs, especially those in deep learning, excel in image processing, segmentation, and classification. On the other hand knowledge-based methods, like bioinformatic processes aid towards analyzing omics datasets that contribute to formulating hypotheses regarding pathophysiology associated with conditions like preeclampsia, preterm birth, and the monitoring of maternal parameters.40 ML methods predominantly handle ultrasound imaging, numerical, and clinical datasets, aiding in risk assessment for conditions like preterm delivery, neonatal outcomes, aneuploidy, and other feto-maternal parameters including maternal blood sugar, blood pressure, and fetal heart rate, among others.41 Additionally, routine statistical techniques like regression analysis and data visualization, exemplified by K-means clustering, form integral parts of the ML approach. These techniques aid in the assessment of both maternal and neonatal outcomes. As AI technology progresses, its role in feto-maternal health is poised to expand, promising more opportunities for early detection and intervention, thereby enhancing treatment outcome.
AI-Based Prenatal Screening
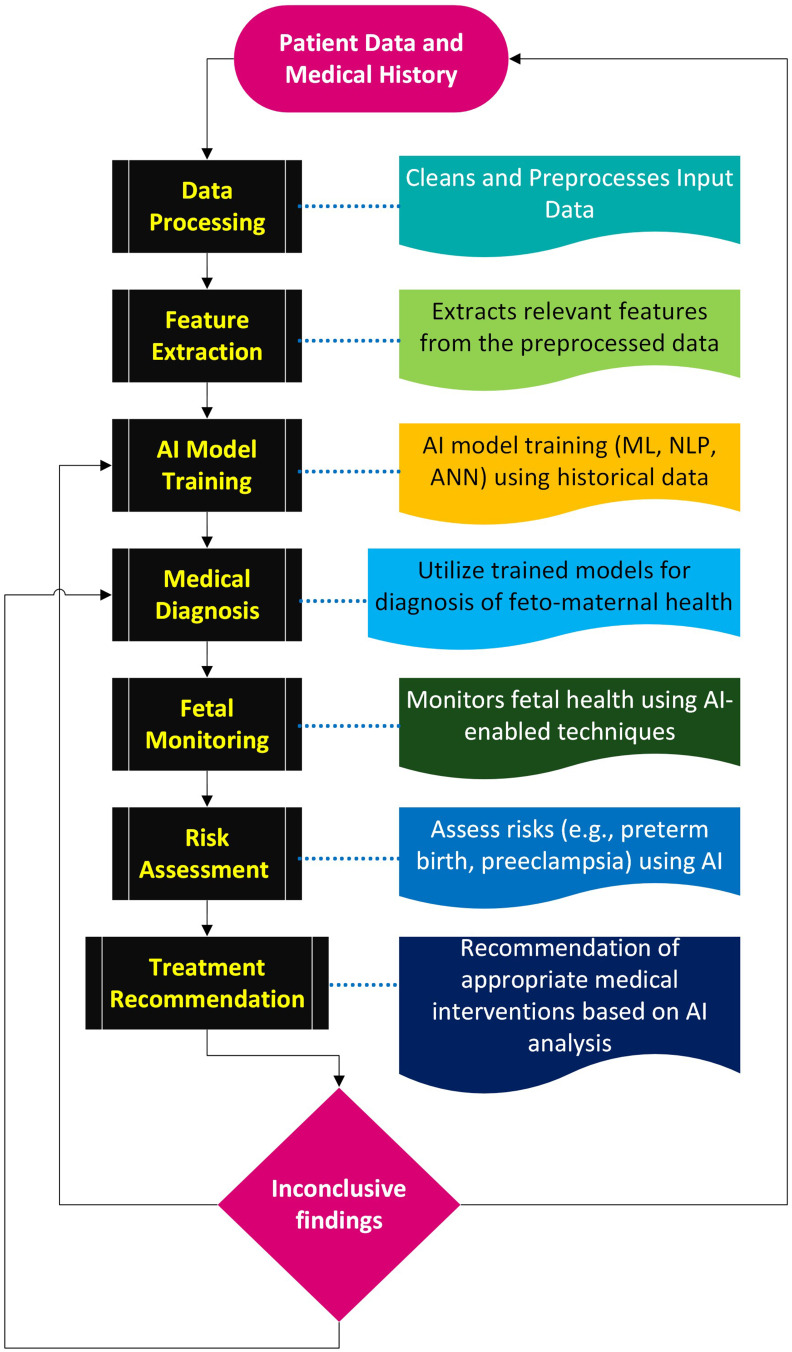
Prenatal screening is a critical aspect of feto-maternal health that includes various components, including the genetic screening and risk assessment, and the monitoring of maternal health parameters. AI-assisted screening is a systematic processing of the data records through various AI models (Figure 2). This section explores these essential elements, emphasizing the pivotal role AI plays in improving the accuracy and efficiency of prenatal screening.
Figure 2.
AI-enhanced workflow for feto-maternal health: The process involves rigorous data preprocessing, feature extraction, and AI model training for precise medical diagnoses. Iterative feedback loops, including real-time fetal monitoring and risk assessment with tailored treatment recommendations. In cases of inconclusive results, a dynamic feedback mechanism directs the data back to the AI model training or medical diagnosis steps, or even to reconsider the patient data, ensuring continuous refinement until conclusive outcomes are achieved.
Abbreviations: ML, Machine Learning; NLP, Natural Language Processing; ANN, Artificial Neural Networks.
Early Detection of Pregnancy
Early detection of pregnancy is a foundational step in maternal healthcare. AI tools have been instrumental in enhancing the accuracy and efficiency of pregnancy detection. These AI-driven systems like omics system, leverage data from various sources, such as hormone levels, physical symptoms, body temperature and fertility tracking, to identify the earliest signs of pregnancy.42 The benefit of early pregnancy detection lies in initiating prenatal care promptly, allowing healthcare providers to monitor and support the expectant mother from the very beginning of her pregnancy journey. In a recent study involving in vitro fertilized embryos, static images of the inner cell mass and trophectoderm of developing embryos were utilized to detect clinical pregnancy. The study showcased that the predictive performance of AI was enhanced when employing improved images of the inner cell mass and trophectoderm. Similarly, another study utilized computer vision and deep learning to develop the non-invasive Life Whisperer AI model for predicting embryo viability, utilizing single static images of day 5 blastocysts.43,44 These early detections can significantly influence maternal and fetal health outcomes.
AI offers innovative solutions to enhance the efficiency and accuracy of pregnancy detection, but it also faces several limitations and challenges that must be addressed to fully realize its potential. One of the primary limitations is the variability in pregnancy symptoms presents a significant challenge for AI systems. Early signs of pregnancy, such as missed periods, hormonal profile, and length of menstruation, can vary widely among individuals. AI algorithms must account for this variability to accurately identify signs of pregnancy.
Genetic Screening and Risk Assessment
AI in genetic screening and risk assessment is a substantial advancement in prenatal care. It provides an ample understanding of genetic factors that may affect the developing fetus.39,45 AI analyze genetic data to identify potential risks and inform expectant parents about genetic conditions that might be present. For instance, a study tested a feedforward neural network dependent on genetic algorithms on 381 pregnant women. The findings demonstrated that employing genetic algorithms to optimize the structure of the neural network technique could enhance accuracy in diagnosing Down syndrome using information from first-trimester screening tests.46 This knowledge enables informed decision-making regarding prenatal testing, potential interventions, and long-term planning. Genetic screening and risk assessment empower healthcare professionals to provide personalized care and counselling, ensuring the best possible outcomes. Despite AI’s promising potential in genetic screening, it encounters challenges that warrant careful consideration. For instance, data quality and accessibility remain key barriers, as genetic datasets may contain inconsistencies, errors, or biases that can affect the accuracy and reliability of AI algorithms. Inadequate representation of diverse populations in genetic databases may lead to disparities in risk assessment and healthcare outcomes, highlighting the need for inclusive and representative datasets
Monitoring Maternal Health Parameters
Maternal health parameters are continuously monitored during pregnancy to ensure the well-being of the mother. AI-based systems can continuously track maternal health parameters such as blood pressure, glucose levels, and weight. AI algorithms analyze the data and provide real-time alerts to healthcare providers when deviations from the normal ranges are detected. For instance, Gulzar et al provide a comprehensive review of wearable AI-backed sensors used in monitoring blood sugar, blood pressure, maternal heart rate and fetal movement.47 These sensors captured data via wireless channels and classify recorded data using k-nearest neighbors algorithm, support vector machine, K-mean, and random forest algorithm. Internet-of-things based stress monitoring system that uses an adaptive algorithm, have achieved a high accuracy in estimating stress levels in pregnant women.48 Allen et al, investigated the efficacy of MyBP, an automated SMS-facilitated home blood pressure monitoring program, in facilitating healthy behavior changes among expectant mothers with hypertension.49 This technology transmits vital parameters from a patient to a hospital in real time, enabling the pre-planning of labor and emergency care. These studies collectively demonstrate the potential of technology driven solutions in enhancing maternal health.
Data quality and interoperability remain significant barriers, as healthcare systems often rely on disparate sources of data that may be incomplete, inaccurate, or inconsistent. This poses a significant challenge when monitoring maternal health parameters with AI algorithms. Integrating data from different sources and formats into AI algorithms requires robust data governance frameworks and interoperable health information systems to ensure data integrity and reliability. Furthermore, the complex and multifactorial nature of pregnancy-related complications poses challenges in accurately predicting individual outcomes based solely on historical data and risk factors. In instances where AI interprets data unrelated to pregnancy, its algorithms may generate false positives or false negatives, potentially resulting in unnecessary interventions.
AI for Fetal Monitoring
With rising trend of AI’s application in fields such as surgery, radiology and precision medicine, AI is playing an increasingly important role in Obstetrics and Gynecology as evidenced by the growing body of research on its application in pregnancy (Figure 3). One means of application is to monitor the fetus at various stages of pregnancy. Fetal monitoring is a method through which healthcare professionals monitor the health of the fetus, including parameters such as FHR, either during pregnancy or labor. Prenatal ultrasonography stands out as the primary and highly effective method for monitoring the fetus during pregnancy. AI has proven effective in automating the detection of standard planes in fetal ultrasound, measuring biometric parameters, diagnosing diseases, and providing valuable support to traditional imaging methods. Additionally, it shortens the learning curves for beginners, enhances the quality control of clinical workflows, and ensures a more rational distribution of medical resources.
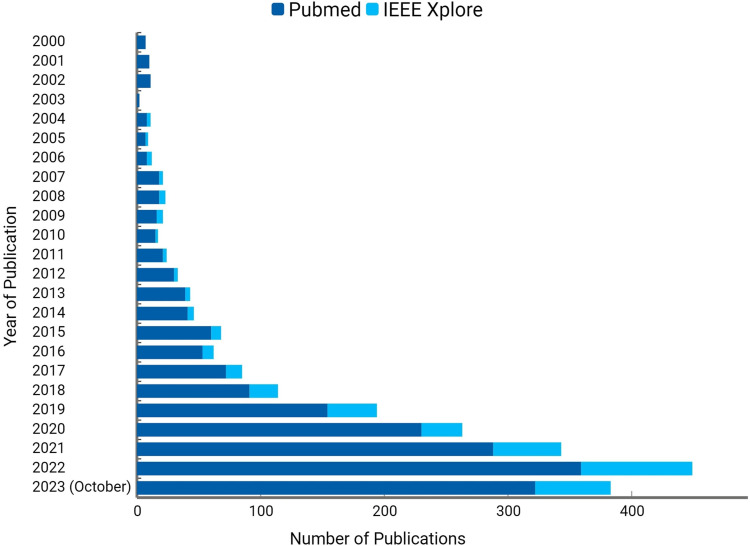
Figure 3.
The figure depicts the number of studies identified on PubMed and IEEE Xplore using the keyword “Artificial Intelligence in Pregnancy”: The presentation of data aims to illustrate an overall trend in these studies. It’s crucial to note that the publication numbers serve as an indicative list and may not fully represent the entire volume of publications. This count specifically includes studies conducted on human subjects (women) and has been identified with a single keyword. The count on IEEE Xplore includes both conference papers and journal articles, while the count on PubMed is limited to journal articles alone.
FHR monitoring has long been a cornerstone of prenatal care. It offers critical insights into the fetal condition, helping healthcare professionals assess the safety of the fetus. AI-driven algorithms can analyze complex FHR patterns, detecting subtle anomalies that may not be readily apparent with traditional monitoring methods.50 Some supervised deep learning models like HeartNet, SonoNet could predict the viewing plane, orientation and transverse scanning planes of fetal heart respectively.47 In a separate study, algorithms like logistic regression, random forest classifier, extreme gradient boosting approaches have autonomously determined fetal health status and have demonstrated an accuracy of 96.7% and an F1-Score of 0.963 in the pathologic class.51 This study employed five ML algorithms to construct computation-friendly multiclass classification models for predicting fetal health. These models were trained using secondary cardiotocography datasets comprising normal, suspected, and pathological cases obtained from the University of California Irvine ML Repository. By processing continuous electronic fetal monitoring data, these algorithms provide a detailed assessment of the FHR, its variability, and any accelerations or decelerations. This high level of precision allows for a more comprehensive evaluation of fetal health, empowering healthcare providers to make well-informed decisions and take early action when necessary.
Predictive modeling for fetal well-being is another vital application of AI in the realm of fetal monitoring. ML algorithms are deployed to analyze a broad spectrum of data, encompassing FHR patterns, maternal vital signs, and other clinical parameters.52 Through these analyses, predictive models are developed, offering insights into the likelihood of fetal distress, preterm birth, or other complications. AI excels in identifying hidden patterns and correlations within extensive datasets, making it particularly adept at identifying early warning signs of potential issues.48 AI algorithms continually scrutinize fetal and maternal data, searching for deviations from established norms and patterns. When irregularities are detected, the system generates real-time alerts, which serve as critical signals for healthcare providers to initiate immediate actions. These alerts are a valuable resource, enabling prompt interventions like changing the mother’s position, administering oxygen, or preparing for an emergency cesarean section when necessary. By offering real-time decision support, AI plays a pivotal role in facilitating rapid responses to critical situations, thus minimizing risks and enhancing the chances of positive outcomes for both the mother and the fetus.
While AI algorithms can analyze fetal monitoring and provide insights into fetal health, there may be instances where the interpretation accuracy is not optimal. Factors such as variations in fetal movement, maternal physiology, or equipment calibration could affect the reliability of AI-generated assessments. Furthermore, AI algorithms trained on specific datasets may lack generalizability when applied to diverse patient populations or clinical settings. Variations in demographic characteristics, medical histories, or healthcare practices among subjects could pose challenges in algorithm generalization, leading to suboptimal performance in real-world scenarios. Ensuring the robustness and adaptability of AI algorithms, may decrease aforementioned limitations.
Preterm Birth Risk Assessment
Annually, approximately 15 million births globally are projected to be preterm, making it the primary contributor to neonatal and childhood mortality worldwide.53 This phenomenon is accountable for one million deaths among children under the age of five.54 The impact of preterm birth on newborns and children represents a significant burden of disease globally. AI-enabled algorithms can analyze a multitude of factors, including maternal health data, fetal growth patterns, and prior pregnancy history to create predictive models for preterm birth. Previous feasibility studies have explored extensive sample sizes to assess the effectiveness of ML processes in stratifying the risks of preterm birth. For instance, a ML model employing six algorithms—namely eXtreme Gradient Boosting, Elastic Net, Quantile Ordinal Regression, Linear Regression, Ridge Regression, and Decision Tree, utilizing data from 3,876,666 mothers in Brazil, successfully predicted the week of delivery with an accuracy margin of within two weeks.55 While the eXtreme Gradient Boosting approach was noted for its lower precision, the study reported five distinct variables: the mother’s age, cesarean-section history, number of previous deliveries, availability of ultrasound examinations, and the percentage of primary care teams in the municipality documenting oral care consultations, that were able to estimate weeks of delivery. Likewise, other studies integrated predictive models, such as recurrent neural networks and a big data-based algorithm known as Map Reduce-based ML algorithms (MR-PB-PFS), into their cohort studies, to estimate the risk of preterm birth using extensive national datasets.56,57 In one of these studies, the models correctly identified chronic blood pressure as a significant feature for predicting preterm birth. In another study, it was concluded that temporal deep learning could anticipate extreme preterm birth up to 8 weeks before its actual occurrence.
Despite challenges like reduced detection rate, data variability, model generalization, and algorithm complexity, learning approaches like logistic regression, gradient boosting decision trees, and ANN with activation functions like rectified and scaled exponential linear units, demonstrate efficacy in predicting early stillbirth, late stillbirth, and preterm birth. These models deliver healthcare providers with insights into the likelihood of preterm delivery, allowing for early intervention and personalized care plans.
Preeclampsia and Gestational Diabetes Prediction
Preeclampsia and gestational diabetes have significant consequences for maternal and fetal health. Preeclampsia is a pregnancy complication characterized by high blood pressure and signs of damage to other organs, typically occurring after 20 weeks of pregnancy.58,59 Gestational diabetes is a condition characterized by elevated blood glucose levels during pregnancy. Different algorithms can analyze patient data like blood pressure measurements, blood sugar levels, and other relevant health parameters to create predictive models. Approaches like cost-sensitive deep neural networks have been employed to analyze a comprehensive and existing dataset containing a diverse cohort for predicting preeclampsia.60 The authors identified clinical and demographic variables correlated with preeclampsia across an entire cohort, encompassing diverse populations. This study stands out due to its utilization of a large dataset and its inclusion of diverse demographics. An interpretable learning method like Explainable Boosting Machine (EBM) has demonstrated effectiveness in predicting preterm preeclampsia, as well as maternal morbidity, antepartum stillbirth, and shoulder dystocia. The model notably identified body mass index (BMI) as the most significant risk factor for preterm preeclampsia, alongside hypertension, previous stillbirths, maternal age, nulliparity, and various others. EBMs offer advantages in that they rank and score the risk factors for each outcome they validate.61 Integrated electronic health record data coupled with genetic information and synergized with polygenic risk scores for systolic blood pressure, prove valuable for the predictive modeling of preeclampsia. ML techniques such as XGBoost and linear regression contribute to the effectiveness of these predictive models.62 Marić et al developed a model utilizing gradient boosting and elastic net algorithms for early preeclampsia prognosis.63 The model yielded positive outcomes, achieving a receiver operating characteristic curve of 0.79 (95% CI 0.75–0.83), with a false positive rate of 8.1% and a sensitivity of 45.2%. Notably, the developed model autonomously identified significant risk factors for preeclampsia from routine pregnancy data. Similarly, variant of recurrent neural networks such as long short-term memory were applied to the dataset derived from hospital records.64 Validation methods employed included leave-one-out cross-validation and tenfold cross-validation. The study achieved optimal accuracy rates for both training data (96.62%) and testing data (90.22%) by utilizing a combination of the leave-one-out cross-validation approach, 30 hidden neurons, a maximum of 100 epochs, and a single parameter series pattern.64
The multivariate logistic regression model, a well-established algorithm is widely recognized for predicting diabetes and its associated complications. Additionally, other methodologies like random forest, extreme gradient boosting, and light gradient boosting machine, have been employed in addressing diabetes-related challenges. An increasing array of research utilizes these approaches to pinpoint risk factors for gestational diabetes and develop early prediction models for the disease.65 We examine a few of these approaches and their methodologies which have been used elsewhere. In a study by Wu et al, an algorithm was employed to predict gestational diabetes as early as the first trimester, a departure from the conventional second-trimester prediction.66 The study utilized four distinct ML methods: deep neural network, logistic regression, k-nearest neighbor and support vector machine. A 7-variable LR model was developed, which accurately predicted risk factors such as lower BMI, free T3, and T4 as indicators of gestational diabetes. The variables were chosen based on their absolute Spearman and Pearson correlation coefficients in relation to both the gestational diabetes and control groups. While the models demonstrated high accuracy in early pregnancy gestational diabetes prediction, the predictive scores varied among the methods. In a separate investigation involving 1139 pregnant Chinese women, including 186 with gestational diabetes, researchers devised a straightforward algorithm for predicting gestational diabetes in the first trimester. The algorithm incorporated pre-pregnancy body mass index, abdomen circumference in the first trimester, age, polycystic ovary syndrome, gravidity, irregular menstruation, and family history of diabetes as key factors.67 These models effectively projected pregnant women with an increased likelihood of developing preeclampsia and gestational diabetes. Early identification and effective management of these disorders are essential to prevent complications such as premature birth and maternal health issues.
Fetal Abnormality Detection
Ultrasound imaging stands out as a secure and effective diagnostic method for evaluating fetal growth. Assessing fetal growth during pregnancy is challenging but ultrasound imaging has significantly enhanced this aspect of Obstetrics and Gynecology. The integration of AI holds great promise in ultrasound imaging leading to improved diagnostic speed and accuracy. AI, employing neural networks extracts and measures biometric parameters through segmentation techniques using ultrasound image planes.68 These values are then fed into a neural network for detecting fetal anomalies. Various learning algorithms, particularly Feedforward neural networks based on backpropagation, have been thoroughly analyzed and compared. Additionally, ANN method has been proposed for identifying fetal abnormalities in 2-D ultrasound images from 14 to 40 weeks.69 This model successfully detects intrauterine growth retardation and abnormal fetuses using head and abdominal circumference. Moreover, a real-time AI system has been suggested for automatically identifying standard reference planes of the fetal brain (transthalamic, transventricular, and transcerebellar) during routine ultrasound scans.68 It offers advanced imaging and diagnostic capabilities that can detect anomalies in fetal development enabling healthcare providers to assess and address issues at an early stage. AI-enhanced ultrasound and medical imaging analysis are powerful tools in this context, capable of detecting structural abnormalities. For example, a novel model named DGACNN, constructed based on DANomaly and GACNN (Wgan-GP and CNN), was developed to detect structural abnormalities in fetal ultrasound and echocardiography images associated with cardiac health.70 The study elucidated how the architecture of DGACNN can be leveraged to scan unlabeled and unannotated video slices for the recognition of fetal heart disease. According to the authors, the proposed model achieved a test accuracy of 84%, surpassing the performance of expert cardiologists in identifying fetal heart complications. Meshaka et al in his scoping review identified the capacity of AI in fetal Magnetic Resonance Imaging specifically for spotting congenital and acquired anomalies using algorithms like U-net, CASE-Net, V-net, and SVRnet among others.71 Together, these studies underscore the capability of AI-enhanced tools to improve the identification of fetal structural abnormalities, exhibiting promising outcomes in accuracy and performance.
While AI algorithms can segment ultrasound images to extract biometric parameters for fetal growth assessment, achieving precise segmentation accuracy remains a challenge. Variations in image quality, fetal positioning, and anatomical structures can introduce errors in segmentation, impacting the reliability of measurements and diagnostic outcomes. Furthermore, the performance of AI algorithms in ultrasound imaging heavily relies on the quality and standardization of imaging protocols and equipment. Variations in ultrasound machine settings, probe positioning, and operator technique can introduce inconsistencies in image quality, affecting the accuracy and reliability of AI-assisted fetal anomaly detection.
Challenges and Limitations
Implementing AI in feto-maternal health encounters multifaceted challenges across various dimensions. Firstly, the quality and availability of data stand as a foundational concern. AI models heavily rely on robust and diverse datasets for effective training and in some healthcare settings data quality may be suboptimal potentially leading to the development of biased or less accurate models.52 Moreover, ethical and privacy concerns arise due to the sensitive nature of medical data during pregnancy necessitating a delicate balance between reaping the benefits of AI and safeguarding patient confidentiality and autonomy.72 The interoperability and integration of AI systems into existing healthcare infrastructure present another layer of complexity. Compatibility with diverse healthcare systems and seamless integration with electronic health records are essential for successful implementation as disruptions in workflow can hinder acceptance among healthcare providers. Regulatory and legal challenges add to the complexity requiring adherence to evolving frameworks while addressing liability concerns associated with potential AI-related errors.
Algorithmic bias and fairness pose significant challenges as AI models can inherit biases from their training data potentially leading to disparities in healthcare outcomes.73 Generalizability across different healthcare systems and populations demands careful consideration ensuring that AI solutions remain effective in diverse settings. Financial constraints in smaller healthcare facilities and limited understanding of AI among healthcare professionals further complicate the adoption process. The technology’s potential to complement human expertise rather than replace it needs to be emphasized and effective education and training are necessary to bridge the knowledge gap among healthcare practitioners. Finally, the validation and clinical adoption of AI systems requires meticulous efforts to overcome regulatory, technical and clinical hurdles along the path from research and development to practical implementation.74
Future Directions
In the future setting of AI in feto-maternal health two key directions stand out. Firstly, there is a promising shift towards personalized and adaptive healthcare solutions driven by AI. The technology’s ability to analyze extensive data sets and identify intricate patterns positions it as a powerful tool for tailoring medical care to individual patient needs. This includes considerations of genetic factors, unique medical history, lifestyle and environmental influences. As AI system evolve, it holds the potential to adapt to a patient’s changing health profile, offering precise individualized approaches to care. For expectant mothers this could mean prenatal care plans and interventions precisely aligned with their specific health requirements ultimately improving maternal and fetal health outcomes.
The second critical direction involves extending the reach of AI into remote and underserved areas. In regions lacking uniform access to quality prenatal care, telehealth and telemedicine technologies empowered by AI offer a solution. These technologies enable remote monitoring and consultation providing specialized care and guidance to expectant mothers in underserved areas. AI algorithms can analyze remotely collected data, offering insights into maternal and fetal health. By addressing healthcare disparities through AI in remote regions, the outcomes include improved maternal and fetal health, early detection of complications and enhanced pregnancy experiences for women in these areas. Furthermore, the integration of AI with wearable devices and home monitoring solutions emerges as a promising frontier. Wearables with AI-driven sensors can continuously monitor maternal and fetal health parameters, providing timely alerts to expectant mothers. This not only facilitates prompt medical attention but also reduces the burden on healthcare facilities allowing for more efficient resource allocation. The utilization of AI in fetal anomaly detection is also expected to advance significantly enhancing the precision and scope of anomaly identification and providing greater confidence in the health assessment of the developing fetus. However, the expanding role of AI in feto-maternal health comes with its share of challenges including concerns about data quality, ethical considerations, regulatory complexities, potential algorithm bias, generalizability across populations and the necessity of building trust and understanding among healthcare professionals and expectant mothers. Validating AI systems for clinical use and seamless integration into healthcare workflows requires dedicated efforts.
Conclusion
AI’s influence on feto-maternal health is transformative and far-reaching. The integration of AI applications holds substantial promise for revolutionizing clinical practices and improving patient outcomes in feto-maternal health through enhanced diagnostic accuracy, predictive modeling, and personalized care interventions. While challenges and limitations persist, the future directions of AI in this field promise to further enhance prenatal care, minimize risks, and improve the welfare of both expectant mothers and their developing fetus. As AI continues to advance, it is essential to address challenges responsibly and ethically ensuring that the promise of AI in feto-maternal health is realized while safeguarding the well-being and privacy of patients. This analysis recognizes that while it touches upon AI applications in monitoring fetal-maternal health, the coverage is not comprehensive. Given the expansive nature of the subject providing an in-depth discussion of every application surpasses the limits set by this review. In recognizing the limitations of a comprehensive overview, the review seeks to inspire further research, discussions and advancements in AI-driven solutions tailored to monitoring and enhancing the health of both mothers and their unborn children.
Acknowledgments
We are thankful to Dr Vivek Bhatia for his critical feedback on this review.
Funding Statement
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1445).
Abbreviations
AI, Artificial Intelligence; ML, Machine Learning; NLP, Natural Language Processing; ANN, Artificial Neural Networks; FHR, Fetal Heart Rate; EBM, Explainable Boosting Machine; BMI, Body Mass Index.
Data Sharing Statement
All data discussed are included in the review.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Richardson L, Kim S, Menon R, Han A. Organ-on-chip technology: the future of feto-maternal interface research. Front Physiol. 2020;11:715. doi: 10.3389/fphys.2020.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirzakhani K, Ebadi A, Faridhosseini F, Khadivzadeh T. Well-being in high-risk pregnancy: an integrative review. BMC Pregnancy Childbirth. 2020;20(1):526. doi: 10.1186/s12884-020-03190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderdice F, McNeill J, Lynn F. A systematic review of systematic reviews of interventions to improve maternal mental health and well-being. Midwifery. 2013;29(4):389–399. doi: 10.1016/j.midw.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 4.Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):4–26. doi: 10.1111/j.1365-3016.2012.01291.x [DOI] [PubMed] [Google Scholar]
- 5.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96(4):1509–1565. doi: 10.1152/physrev.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassi ZS, Dean SV, Mallick D, Bhutta ZA. Preconception care: delivery strategies and packages for care. Reprod Health. 2014;11(Suppl 3):S7. doi: 10.1186/1742-4755-11-S3-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David AL, Spencer RN. Clinical assessment of fetal well-being and fetal safety indicators. J Clin Pharmacol. 2022;62(Suppl 1):S67–S78. doi: 10.1002/jcph.2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baschat AA, Galan HL, Lee W, et al. The role of the fetal biophysical profile in the management of fetal growth restriction. Am J Obstet Gynecol. 2022;226(4):475–486. doi: 10.1016/j.ajog.2022.01.020 [DOI] [PubMed] [Google Scholar]
- 9.Swamy GK, Skjšrven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. 2008;299(12):1429–1436. doi: 10.1001/jama.299.12.1429 [DOI] [PubMed] [Google Scholar]
- 10.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C; GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(S1):1–22. doi: 10.1186/1471-2393-10-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerby P, Bujold E. Early detection and prevention of intrauterine growth restriction and its consequences. JAMA Pediatr. 2020;174(8):749–750. doi: 10.1001/jamapediatrics.2020.1106 [DOI] [PubMed] [Google Scholar]
- 12.Villar J, Carroli G, Wojdyla D, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194(4):921–931. doi: 10.1016/j.ajog.2005.10.813 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed Z, Mohamed K, Zeeshan S, Dong X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database. 2020;2020:baaa010. doi: 10.1093/database/baaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohoon TJ, Bhavnani SP. Toward precision health: applying artificial intelligence analytics to digital health biometric datasets. Per Med. 2020;17(4):307–316. doi: 10.2217/pme-2019-0113 [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi YK, Hughes L, Ismagilova E, et al. Artificial Intelligence (AI): multidisciplinary perspectives on emerging challenges, opportunities, and agenda for research, practice and policy. Int J Info Manag. 2021;57:101994. [Google Scholar]
- 16.Lauriola I, Lavelli A, Aiolli F. An introduction to deep learning in natural language processing: models, techniques, and tools. Neurocomputing. 2022;470:443–456. doi: 10.1016/j.neucom.2021.05.103 [DOI] [Google Scholar]
- 17.Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668–2679. doi: 10.1016/j.jacc.2018.03.521 [DOI] [PubMed] [Google Scholar]
- 18.Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi: 10.1186/s41747-018-0061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delanerolle G, Yang X, Shetty S, et al. Artificial intelligence: a rapid case for advancement in the personalization of Gynaecology/Obstetric and Mental Health care. Womens Health. 2021;17:17455065211018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pammi M, Aghaeepour N, Neu J. Multiomics, artificial intelligence, and precision medicine in perinatology. Pediatr Res. 2023;93(2):308–315. doi: 10.1038/s41390-022-02181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajirasouliha I, Elemento O. Precision medicine and artificial intelligence: overview and relevance to reproductive medicine. Fertil Steril. 2020;114(5):908–913. doi: 10.1016/j.fertnstert.2020.09.156 [DOI] [PubMed] [Google Scholar]
- 22.Hill B, Skouteris H, Boyle JA, et al. Health in preconception, pregnancy and postpartum global alliance: international network pregnancy priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J Clin Med. 2020;9(3):822. doi: 10.3390/jcm9030822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassi ZS, Mansoor T, Salam RA, Das JK, Bhutta ZA. Essential pre-pregnancy and pregnancy interventions for improved maternal, newborn and child health. Reprod Health. 2014;11(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahbazi MN. Mechanisms of human embryo development: from cell fate to tissue shape and back. Development. 2020;147(14):dev190629. doi: 10.1242/dev.190629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boss AL, Chamley LW, James JL. Placental formation in early pregnancy: how is the centre of the placenta made? Hum Reprod Update. 2018;24(6):750–760. doi: 10.1093/humupd/dmy030 [DOI] [PubMed] [Google Scholar]
- 26.Valladares DA, Rasmussen SA. An update on teratogens for pediatric healthcare providers. Curr Opin Pediatr. 2022;34(6):565–571. doi: 10.1097/MOP.0000000000001177 [DOI] [PubMed] [Google Scholar]
- 27.Narain S, McEwan A. Antenatal assessment of fetal well-being. Obstet Gynaecol Reprod Med. 2023;33(8):217–224. doi: 10.1016/j.ogrm.2023.05.002 [DOI] [Google Scholar]
- 28.Haug CJ, Drazen JM, Drazen JM, Kohane IS, Leong T-Y. Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med. 2023;388(13):1201–1208. doi: 10.1056/NEJMra2302038 [DOI] [PubMed] [Google Scholar]
- 29.Taye MM. Understanding of machine learning with deep learning: architectures, workflow, applications and future directions. Computers. 2023;12(5):91. doi: 10.3390/computers12050091 [DOI] [Google Scholar]
- 30.Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. 2020;9(2):14. doi: 10.1167/tvst.9.2.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greener JG, Kandathil SM, Moffat L, Jones DT. A guide to machine learning for biologists. Nat Rev Mol Cell Biol. 2022;23(1):40–55. doi: 10.1038/s41580-021-00407-0 [DOI] [PubMed] [Google Scholar]
- 32.Janiesch C, Zschech P, Heinrich K. Machine learning and deep learning. Electron Mark. 2021;31(3):685–695. doi: 10.1007/s12525-021-00475-2 [DOI] [Google Scholar]
- 33.Ramakrishnan R, Rao S, He JR. Perinatal health predictors using artificial intelligence: a review. Womens Health. 2021;17:17455065211046132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akazawa M, Hashimoto K. Prediction of preterm birth using artificial intelligence: a systematic review. J Obstet Gynaecol. 2022;42(6):1662–1668. doi: 10.1080/01443615.2022.2056828 [DOI] [PubMed] [Google Scholar]
- 35.Dhombres F, Bonnard J, Bailly K, Maurice P, Papageorghiou AT, Jouannic JM. Contributions of artificial intelligence reported in obstetrics and gynecology journals: systematic review. J Med Internet Res. 2022;24(4):e35465. doi: 10.2196/35465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armero W, Gray KJ, Fields KG, Cole NM, Bates DW, Kovacheva VP. A survey of pregnant patients’ perspectives on the implementation of artificial intelligence in clinical care. J Am Med Inform Assoc. 2022;30(1):46–53. doi: 10.1093/jamia/ocac200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HY, Cho GJ, Kwon HS. Applications of artificial intelligence in obstetrics. Ultrasonography. 2023;42(1):2–9. doi: 10.14366/usg.22063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn KH, Lee KS. Artificial intelligence in obstetrics. Obstet Gynecol Sci. 2022;65(2):113–124. doi: 10.5468/ogs.21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emin EI, Emin E, Papalois A, Willmott F, Clarke S, Sideris M. Artificial intelligence in obstetrics and gynaecology: is this the way forward? In Vivo. 2019;33(5):1547–1551. doi: 10.21873/invivo.11635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson L, Boland MR. Towards deep phenotyping pregnancy: a systematic review on artificial intelligence and machine learning methods to improve pregnancy outcomes. Brief Bioinform. 2021;22(5):bbaa369. doi: 10.1093/bib/bbaa369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mennickent D, Rodríguez A, Opazo MC, et al. Machine learning applied in maternal and fetal health: a narrative review focused on pregnancy diseases and complications. Front Endocrinol. 2023;14:1130139. doi: 10.3389/fendo.2023.1130139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazantsev A, Ponomareva J, Kazantsev P, Digilov R, Huang P. Development of e-health network for in-home pregnancy surveillance based on artificial intelligence. In: Proceedings of 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics. IEEE; January 5; 2012:82–84. [Google Scholar]
- 43.Kim HM, Ko T, Kang H, et al. Improved prediction of clinical pregnancy using artificial intelligence with enhanced inner cell mass and trophectoderm images. Sci Rep. 2024;14(1):3240. doi: 10.1038/s41598-024-52241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.VerMilyea M, Hall JMM, Diakiw SM, et al. Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF. Hum Reprod. 2020;35(4):770–784. doi: 10.1093/humrep/deaa013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feduniw S, Golik D, Kajdy A, et al. Application of artificial intelligence in screening for adverse perinatal outcomes-a systematic review. Healthcare. 2022;10(11):2164. doi: 10.3390/healthcare10112164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamshidnezhad A, Hosseini SM, Mohammadi-Asl J, Mahmudi M. An intelligent prenatal screening system for the prediction of Trisomy-21. Inf Med Unlocked. 2021;24:100625. doi: 10.1016/j.imu.2021.100625 [DOI] [Google Scholar]
- 47.Gulzar Ahmad S, Iqbal T, Javaid A, et al. Sensing and artificial intelligent maternal-infant health care systems: a review. Sensors. 2022;22(12):4362. doi: 10.3390/s22124362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oti O, Azimi I, Anzanpour A, Rahmani AM, Axelin A, Liljeberg P. IoT-based healthcare system for real-time maternal stress monitoring. In: Proceedings of the 2018 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies. September 26; 2018:57–62. [Google Scholar]
- 49.Allen ME, Irizarry T, Einhorn J, et al. SMS-facilitated home blood pressure monitoring: a qualitative analysis of resultant health behavior change. Patient Educ Couns. 2019;102(12):2246–2253. doi: 10.1016/j.pec.2019.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komatsu M, Sakai A, Komatsu R, et al. Detection of cardiac structural abnormalities in fetal ultrasound videos using deep learning. Appl Sci. 2021;11(1):371. doi: 10.3390/app11010371 [DOI] [Google Scholar]
- 51.Rayhana T, Arefina AS, Chowdhury SA. Automatic detection of fetal health status from cardiotocography data using machine learning algorithms. J Bangladesh Acad Sci. 2021;45(2):155–167. doi: 10.3329/jbas.v45i2.57206 [DOI] [Google Scholar]
- 52.Xiao S, Zhang J, Zhu Y, et al. Application and progress of artificial intelligence in fetal ultrasound. J Clin Med. 2023;12(9):3298. doi: 10.3390/jcm12093298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21(2):74–79. doi: 10.1016/j.siny.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 54.Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150(1):31–33. doi: 10.1002/ijgo.13195 [DOI] [PubMed] [Google Scholar]
- 55.Rocha TAH, de Thomaz EBAF, de Almeida DG, et al. Data-driven risk stratification for preterm birth in Brazil: a population-based study to develop of a machine learning risk assessment approach. Lancet Reg Health Am. 2021;3:100053. doi: 10.1016/j.lana.2021.100053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao C, Osmundson S, Velez Edwards DR, Jackson GP, Malin BA, Chen Y. Deep learning predicts extreme preterm birth from electronic health records. J Biomed Inform. 2019;100:103334. doi: 10.1016/j.jbi.2019.103334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khatibi T, Kheyrikoochaksarayee N, Sepehri MM. Analysis of big data for prediction of provider-initiated preterm birth and spontaneous premature deliveries and ranking the predictive features. Arch Gynecol Obstet. 2019;300(6):1565–1582. doi: 10.1007/s00404-019-05325-3 [DOI] [PubMed] [Google Scholar]
- 58.Pelícia SMC, Fekete SMW, Corrente JE, Rugolo LMSS. Impact of early-onset preeclampsia on feeding tolerance and growth of very low birth weight infants during hospitalization. Rev Paul Pediatr. 2022;41:e2021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sufriyana H, Wu YW, Su EC. Prediction of preeclampsia and intrauterine growth restriction: development of machine learning models on a prospective cohort. JMIR Med Inform. 2020;8(5):e15411. doi: 10.2196/15411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett R, Mulla ZD, Parikh P, Hauspurg A, Razzaghi T, Mohammadzadeh A. An imbalance-aware deep neural network for early prediction of preeclampsia. PLoS One. 2022;17(4):e0266042. doi: 10.1371/journal.pone.0266042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosschieter TM, Xu Z, Lan H, et al. Interpretable predictive models to understand risk factors for maternal and fetal outcomes. J Healthc Inform Res. 2023;8(1):65–87. doi: 10.1007/s41666-023-00151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kovacheva VP, Eberhard BW, Cohen RY, Maher M, Saxena R, Gray KJ. Preeclampsia prediction using machine learning and polygenic risk scores from clinical and genetic risk factors in early and late pregnancies. Hypertension. 2024;81(2):264–272. doi: 10.1161/HYPERTENSIONAHA.123.21053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marić I, Tsur A, Aghaeepour N, et al. Early prediction of preeclampsia via machine learning. Am J Obstet Gynecol MFM. 2020;2(2):100100. doi: 10.1016/j.ajogmf.2020.100100 [DOI] [PubMed] [Google Scholar]
- 64.Sakinah N, Tahir M, Badriyah T, Syarif I. LSTM with Adam optimization-powered high accuracy preeclampsia classification. In: 2019 International Electronics Symposium (IES). Surabaya, Indonesia; September 27–28; 2019:314–319. [Google Scholar]
- 65.Artzi NS, Shilo S, Hadar E, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26(1):71–76. doi: 10.1038/s41591-019-0724-8 [DOI] [PubMed] [Google Scholar]
- 66.Wu YT, Zhang CJ, Mol BW, et al. Early prediction of gestational diabetes mellitus in the Chinese Population via Advanced Machine Learning. J Clin Endocrinol Metab. 2021;106(3):e1191–e1205. doi: 10.1210/clinem/dgaa899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Lv B, Chen X, et al. An early model to predict the risk of gestational diabetes mellitus in the absence of blood examination indexes: application in primary health care centres. BMC Pregnancy Childbirth. 2021;21(1):814. doi: 10.1186/s12884-021-04295-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drukker L. Real-time identification of fetal anomalies on ultrasound using artificial intelligence: what’s next? Ultrasound Obstet Gynecol. 2022;59(3):285–287. doi: 10.1002/uog.24869 [DOI] [PubMed] [Google Scholar]
- 69.Rawat V, Jain A, Shrimali V, Raghuvanshi S. Performance analysis of different learning algorithms of feed forward neural network regarding fetal abnormality detection. In: Thanh Nguyen N, Kowalczyk R, editors. Transactions on Computational Collective Intelligence XXX. Springer; 2018:118–132. Lecture Notes in Computer Science, vol 11120. Springer, Cham. [Google Scholar]
- 70.Gong Y, Zhang Y, Zhu H, et al. Fetal congenital heart disease echocardiogram screening based on DGACNN: adversarial one-class classification combined with video transfer learning. IEEE Trans Med Imaging. 2020;39(4):1206–1222. doi: 10.1109/TMI.2019.2946059 [DOI] [PubMed] [Google Scholar]
- 71.Meshaka R, Gaunt T, Shelmerdine SC. Artificial intelligence applied to fetal MRI: a scoping review of current research. Br J Radiol. 2023;96(1147):20211205. doi: 10.1259/bjr.20211205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meskó B, Topol EJ. The imperative for regulatory oversight of large language models (or generative AI) in healthcare. NPJ Digit Med. 2023;6(1):120. doi: 10.1038/s41746-023-00873-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J, Zhang ZM. Ethics and governance of trustworthy medical artificial intelligence. BMC Med Inform Decis Mak. 2023;23(1):7. doi: 10.1186/s12911-023-02103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mithany RH, Aslam S, Abdallah S, et al. Advancements and challenges in the application of artificial intelligence in surgical arena: a literature review. Cureus. 2023;15(10):e47924. [DOI] [PMC free article] [PubMed] [Google Scholar]