Abstract
Background
Inter-hospital transfer (IHT, the transfer of patients between hospitals) occurs regularly and exposes patients to risks of discontinuity of care, though outcomes of transferred patients remains largely understudied.
Objective
To evaluate the association between IHT and healthcare utilisation and clinical outcomes.
Design
Retrospective cohort.
Setting
CMS 2013 100 % Master Beneficiary Summary and Inpatient claims files merged with 2013 American Hospital Association data.
Participants
Beneficiaries≥age 65 enrolled in Medicare A and B, with an acute care hospitalisation claim in 2013 and 1 of 15 top disease categories.
Main outcome measures
Cost of hospitalisation, length of stay (LOS) (of entire hospitalisation), discharge home, 3 -day and 30- day mortality, in transferred vs non-transferred patients.
Results
The final cohort consisted of 53 420 transferred patients and 53 420 propensity-score matched non-transferred patients. Across all 15 disease categories, IHT was associated with significantly higher costs, longer LOS and lower odds of discharge home. Additionally, IHT was associated with lower propensity-matched odds of 3-day and/or 30- day mortality for some disease categories (acute myocardial infarction, stroke, sepsis, respiratory disease) and higher propensity-matched odds of mortality for other disease categories (oesophageal/gastrointestinal disease, renal failure, congestive heart failure, pneumonia, renal failure, chronic obstructivepulmonary disease, hip fracture/dislocation, urinary tract infection and metabolic disease).
Conclusions
In this nationally representative study of Medicare beneficiaries, IHT was associated with higher costs, longer LOS and lower odds of discharge home, but was differentially associated with odds of early death and 30 -day mortality depending on patients’ disease category. These findings demonstrate heterogeneity among transferred patients depending on the diagnosis, presenting a nuanced assessment of this complex care transition.
INTRODUCTION
The transfer of patients between acute care facilities (inter-hospital transfer or IHT) occurs regularly, with 1.5% of all Medicare patients undergoing IHT,1 and greater frequency among select patient populations, including patients who are critically ill2 or suffering from an acute myocardial infarction (AMI).3–5
Similar to other care transitions like patient discharge,6 or intra-hospital patient handoffs,7–9 IHT exposes patients to known risks of discontinuity of care, such as errors in communication and gaps in information transfer. Moreover, patients undergoing IHT may be even more vulnerable to these risks than patients undergoing other care transitions, given the severity of illness in this patient population,1 10 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature highlights the risk associated with IHT, demonstrating that transferred patients use more resources11 12 and experience worse outcomes compared with non-transferred patients,10 although these data involved limited patient populations, are older studies and variably adjusted for illness severity and other confounding factors.13–15
In the context of unclear outcomes of transferred patients and variability in transfer practices,1 determining which patients should be transferred is often ambiguous and subjective,16 with poor concordance between cited reasons for transfer among patients, transferring physicians and receiving physicians.17 Though cited reasons for transfer include providing patients access to unique specialty services,18 actual receipt of such specialty care after transfer is varied.5 19 20 The Emergency Medical Treatment and Active Labour Act (EMTALA) laws dictate that hospitals transfer patients requiring a more specialised service unavailable at the transferring institution, or when ‘medical benefits…outweigh the increased risks to the individual…’, although in practice, this provides little guidance to practitioners.18
In this study, we aimed to gain a better understanding of which patients have better (or worse) outcomes after transfer by evaluating the association between IHT and clinically important patient outcomes among a variety of patient diagnoses, using nationally representative data and rigorous methodology to account for differences between transferred and non-transferred patients. Such information will be essential in order to gain a more nuanced understanding of the transfer process and help practitioners carefully consider the risks and benefits seen among transferred patients.
METHODS
Data
We performed a retrospective cohort analysis using two nationally representative datasets: (1) Centre for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all Fee-for-Service programme Medicare enrolees’ demographic information, date of death and hospitalisation claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,21 which contains hospital-level characteristics for all acute care hospitals in the USA. The lead author affirms that the results presented are an honest, accurate and transparent account of the study findings.
Study cohort
Beneficiaries were eligible for inclusion if they were age ≥65 years, continuously enrolled in Medicare A and B and with an acute care hospitalisation claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalised at federal or non-acute care hospitals or critical access hospitals given their mission to stabilise and then transfer patients to referral hospitals.22 We further refined our cohort to include only beneficiaries with previously identified most frequent 15 disease categories at transfer using ICD-9 codes1 to improve the validity of our comparative analyses of transferred vs non-transferred patients within the same disease category (see online supplementary appendix 1 for ICD-9 codes included with each diagnosis).
Transferred patients were defined as beneficiaries with corresponding ‘transfer in’ and ‘transfer out’ claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.1 19 Beneficiaries transferred to the same hospital, those with greater than one transfer within the same hospitalisation, or those cared for at hospitals with ‘outlier’ transfer in rates equal to 100% or transfer out rates greater than 35% were excluded from analysis given the suggestion of non-standard claims practices.1
Outcomes
Our primary outcome was 30-day mortality from index admission (ie, date of admission to the first hospital, regardless of transfer status). We additionally examined secondary outcomes suggested by prior literature to be affected by IHT and/or other inpatient care transitions (hypothesising they would also be affected by IHT), including cost (as measured by allowed charges and charges per day), length of stay (LOS), discharge home and ‘early death’ (3-day mortality).7–10
Allowed charges is a variable within the CMS dataset which encompasses each patient’s acute care hospitalisation base charge and DRG-weight. CMS standardisation methodology23 was used to remove variation based on geographic location and obtain standardised inpatient cost for the entire hospitalisation (including transferring and accepting hospital costs for transferred patients). Charges per day was also evaluated by dividing total allowed charges by LOS. Monetary figures are reported in 2013 US dollars.
LOS (for the entire acute care hospitalisation, that is, including time spent at both transferring and accepting hospitals for transferred patients) and 30-day mortality from date of index admission were measured using dates of hospitalisation and dates of death available within the CMS dataset. Similarly, discharge home (vs other locations, such as a skilled nursing facility) is a distinct variable within the CMS dataset.
We defined 3-day mortality as death within 3 days of hospital transfer. As each transferred patient was matched to a non-transferred patient via propensity matching (as described below), the same 3-day mortality timeframe was used for each matched pair. For example, if the transferred patient was transferred on day 2, then both the transferred patient and their matched control would be counted for 3-day mortality if either died from day 2 to day 5 after admission to the index hospital.
Matching and control variables
All analyses controlled for both patient and hospital-level characteristics known to be associated with the primary exposure (IHT) and/or influence any of the examined outcomes.1 10 24–26
Patient characteristics were obtained from the CMS data files and included: Demographics (age, sex, race); DRG-weight; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score27; presence of Medicaid coinsurance; number of hospitalisations in the past 12 months and median income per household by census tract.
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorised into small, medium and large (<100, 100–399, ≥400 beds); geographic location; ownership (eg, for-profit); teaching status; setting (urban vs rural); case mix index for all patients cared for at the hospital and presence of 55 selected specialty services compiled into a composite score, as was used in our prior research.1
Statistical analysis
Because transferred patients are inherently different than non-transferred patients,1 10 we used propensity score analyses as our primary method to control for these differences. This methodology allows for adjustment for observed variables that may influence the exposure (decision to transfer) and outcome using a single composite variable, thereby addressing confounding by indication.28 29 To conduct the propensity score analyses, we performed a logistic regression model for each disease category to calculate the propensity (probability) of undergoing IHT. As research suggests that non-parsimonious models yield less biased effect estimates,30 31 all available patient and hospital characteristics above were included in each propensity model. The fitted probability from each model was used as the propensity score, which was assigned to each patient, reflecting their probability of having received the ‘exposure, that is, undergone IHT. The c statistic for the propensity models ranged from 0.80 to 0.89, depending on the disease category, indicating good discrimination.
Within each diagnosis, patients were then matched on their propensity score, using a greedy matching technique,32 33 in which every transferred patient was matched to a non-transferred patient with the closest propensity score. We used common support matching such that we only included patients with propensity scores between the larger minimum and smaller maximum of the transferred and non-transferred patients, thereby cutting off the tails and excluding patients with an extreme propensity score in either direction (ie, a transferred patient was excluded if they had a propensity score higher than anyone in the non-transferred group and vice versa). Once matching was complete, baseline characteristics of transferred and non-transferred patients within each disease category were compared with evaluate the effectiveness of the matching.
To analyse the effects of IHT on each outcome, we performed a series of regression models stratified by disease category, incorporating the propensity score and using generalised estimating equations to account for the clustering of patients within hospitals.34 Linear regression models were used for continuous outcomes (allowed charges, charges per day, LOS) and logistic regression models for dichotomous outcomes (discharge home, 3-day and 30-day mortality) to obtain adjusted OR.
All tests were considered statistically significant at a two-sided α=0.05. Estimating baseline 30-day mortality rates between 4% and 11% depending on disease category,35 36 an estimated sample size of 3274–7985 would provide 80% power to detect a 1% absolute change in adjusted 30-day mortality rates in transferred versus non-transferred patients. All analyses were carried out using V.9.4 of SAS statistical software (SAS, Cary, North Carolina, USA).
RESULTS
There were 6.6 million beneficiaries who had an acute care hospitalisation to non-federal, non-critical access hospitals in 2013. After excluding beneficiaries without one of the top 15 disease categories on transfer (n=3 187 237), those transferred to the same facility (n=3790), experiencing more than one transfer during their hospitalisation (n=2329), or being cared for at one of the 11 hospitals identified with ‘outlier’ transfer in/out rates (n=416), our final cohort consisted of 54 995 transferred and 3 258 325 non-transferred beneficiaries. There were significant differences between transferred and non-transferred patients within each disease category before propensity matching (table 1).
Table 1.
Baseline patient characteristics before and after propensity matching
| Characteristic | Before matching |
After matching |
||
|---|---|---|---|---|
| Transferred (n=54 995) | Non-transferred (n=3 258 325) | Transferred (n=53 420) | Non-transferred (n=53 420) | |
| Patient characteristics | ||||
| Age, mean (SD) | 77.4 (0.04) | 80.3 (0.03) | 77.4 (0.04) | 77.9 (0.05) |
| Male, n (%) | 27 511 (50.0) | 1,340,241 (41.1) | 26 703 (50.0) | 27 551 (51.6) |
| Race, n (%) | ||||
| White | 48 145 (87.5) | 2 792 697 (85.7) | 46 835 (87.7) | 46 961 (87.9) |
| Black | 4540 (8.3) | 306 613 (9.4) | 4364 (8.2) | 4274 (8.0) |
| Hispanic | 684 (1.2) | 59 105 (1.8) | 663 (1.2) | 641 (1.2) |
| Other | 1626 (3.0) | 99 910 (3.1) | 1558 (2.9) | 1544 (2.9) |
| DRG-weight, mean (SD) | 1.6 (0.004) | 1.6 (0.002) | 1.6 (0.005) | 1.6 (0.005) |
| HCC Risk Score, mean (SD)* | 3.5 (0.01) | 2.8 (0.006) | 3.5 (0.01) | 3.6 (0.02) |
| Medicaid coinsurance, n (%) | 1.3 (0.01) | 1.4 (0.006) | 1.3 (0.01) | 1.4 (0.01) |
| Hospitalisations in past 12 months, n (%) | 1.57 (0.004) | 1.65 (0.002) | 1.57 (0.005) | 1.57 (0.005) |
| Median income per household by census tract, mean (SD) | 52 215 (579) | 52 952 (619) | 52 218 (581) | 52 271 (527) |
| Hospital characteristics † | ||||
| Size, n(%): | ||||
| Small (<99 beds) | 16 196 (29.4) | 313 325 (9.6) | 15 883 (29.7) | 15 188 (28.4) |
| Medium (100–399 beds) | 33 684 (61.2) | 1 903 872 (58.4) | 32 810 (61.4) | 33 734 (63.1) |
| Large (≥400 beds) | 5115 (9.3) | 1 041 128 (32.0) | 4727 (8.8) | 4498 (8.4) |
| Geographic location, n(%): | ||||
| Northeast | 13 210 (24.0) | 642 216 (19.7) | 12 825 (24.0) | 13 382 (25.1) |
| Midwest | 12 349 (22.5) | 764 911 (23.5) | 12 084 (22.6) | 11 896 (22.3) |
| South | 23 612 (42.9) | 1 379 349 (42.3) | 22 914 (42.9) | 22 603 (42.3) |
| West | 5824 (10.6) | 471 849 (14.5) | 5597 (10.5) | 5539 (10.4) |
| Ownership, n(%): | ||||
| For-Profit | 2407 (4.4) | 4 97 823 (15.3) | 2092 (3.9) | 1893 (3.5) |
| Not for Profit | 12 895 (23.4) | 1 110 144 (34.1) | 12 474 (23.2) | 12 871 (24.1) |
| Public | 39 693 (72.2) | 1 650 358 (50.7) | 38 854 (72.7) | 38 656 (72.4) |
| Teaching status, n(%): | ||||
| Major | 9246 (16.8) | 504 802 (15.5) | 9028 (16.9) | 9100 (17.0) |
| Minor | 38 177 (69.4) | 2 410 980 (74.0) | 37 047 (69.4) | 37 122 (69.5) |
| Non-teaching | 7572 (13.8) | 3 42 543 (10.5) | 7345 (13.7) | 7198 (13.5) |
| Urban location, n(%) | 49 260 (89.6) | 3 167 897 (97.2) | 47 808 (89.5) | 48 346 (90.5) |
| Case mix index, mean (SD) | 1.4 (0.004) | 1.6 (0.005) | 1.4 (0.004) | 1.4 (0.005) |
| Composite score of other hospital specialty services, mean (SD)‡ | 22.2 (0.29) | 28.8 (0.34) | 22.0 (0.29) | 22.0 (0.28) |
CMS-Hierarchical Condition Category (HCC) risk score.27
Presented hospital characteristics for the transferred beneficiaries are characteristics of index hospital.
Score ranged from 0 to 55. DRG, diagnosis-related group.
Propensity score matching was successful for a total of 53 420 transferred patients (97.1% of all included transferred patients) to 53 420 non-transferred patients, of which the most common primary disease categories were AMI (23.4%), congestive heart failure (CHF, 13.8%), arrhythmia (12.4%), sepsis (12.4%), pneumonia (6.5%), stroke (6.3%), gastrointestinal (GI) bleed (5.4%), renal failure (3.9%), oesophageal/GI disease (3.5%), chronic obstructive pulmonary disease (COPD, 3.3%), hip fracture/dislocation (3.0%), urinary tract infection (UTI, 1.7%), chest pain (1.6%), metabolic disease (1.5%) and respiratory disease (1.4%). After matching by propensity score, baseline characteristics were much more similar between transferred and non-transferred groups (table 1).
In both unadjusted and propensity-matched analysis (matched results, table 2), all transferred patients, regardless of disease category, had higher allowed charges, higher charges per day and longer LOS for the entire acute care episode. Evaluation of the transferred patient cohort (ie, excluding non-transferred patients) demonstrated longer LOS at the accepting hospital compared with the transferring hospital across all diagnoses, with variable LOS at the transferring hospital by disease category (online supplementary appendix 2). Within most diagnoses, transferred patients had lower odds of discharge home than non-transferred patients, although differences were non-significant among patients with hip fracture/dislocation or respiratory diseases, where overall rates of discharge home were low for both transferred and non-transferred patients (figure 1).
Table 2.
Hospitalisation allowed charges and length of stay in propensity-matched transferred versus Non-transferred patients
| Disease category (n) | Allowed charges*† |
Charges per day*† |
Length of stay, days*‡ |
|||
|---|---|---|---|---|---|---|
| Transferred | Non-transferred | Transferred | Non-transferred | Transferred | Non-transferred | |
| AMI (n=25 004) | 27 230 | 9060 | 5936 | 4282 | 9.0 | 4.3 |
| CHF (n=14 708) | 27 040 | 7307 | 3947 | 2145 | 12.5 | 5.0 |
| Arrhythmia (n=13 238) | 21 903 | 6064 | 5424 | 3013 | 8.1 | 3.5 |
| Sepsis (n=13 198) | 28 572 | 12 488 | 3557 | 2507 | 14.6 | 6.8 |
| Pneumonia (n=6904) | 22 442 | 8110 | 3200 | 2013 | 12.6 | 5.5 |
| Stroke (n=6708) | 21 319 | 8453 | 3722 | 2861 | 10.7 | 4.9 |
| GI bleed (n=5810) | 18 687 | 7364 | 3413 | 2456 | 10.6 | 4.5 |
| Renal failure (n=4162) | 20 137 | 7297 | 2947 | 1969 | 12.5 | 5.3 |
| Oesophageal/GI disease (n=3706) | 19 573 | 6874 | 3207 | 2195 | 12.1 | 4.8 |
| COPD (n=3512) | 19 512 | 6817 | 3412 | 1997 | 11.0 | 4.6 |
| Hip fracture/dislocation (n=3226) | 22 339 | 10 336 | 3899 | 3259 | 10.1 | 5.0 |
| UTI (n=1774) | 16 054 | 5496 | 2830 | 1894 | 10.9 | 4.3 |
| Chest pain (n=1754) | 14 721 | 3052 | 6109 | 3296 | 5.3 | 1.8 |
| Metabolic disease (n=1626) | 17 574 | 5103 | 2963 | 1981 | 11.5 | 3.9 |
| Respiratory disease (n=1510) | 26 738 | 10 991 | 2893 | 2129 | 15.2 | 6.6 |
P< 0.001 for all comparisons.
Presented charges are in 2013 US dollars.
Length of stay encompasses the entire acute care hospitalisation.
AMI, acute myocardial infarction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; UTI, urinary tract infection.
Figure 1.
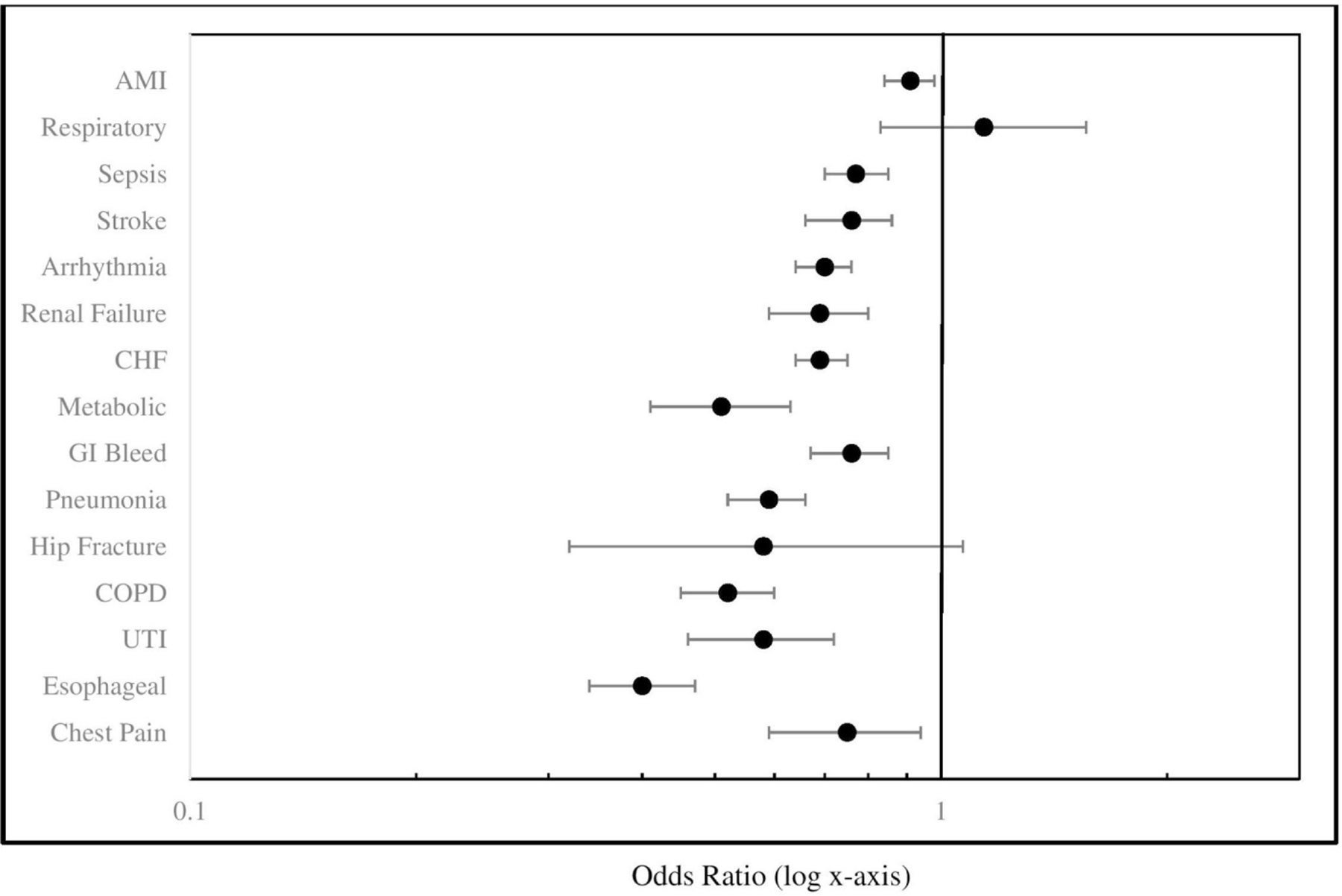
Association of inter-hospital transfer with propensity-matched odds of discharge home. AMI, acute myocardial infraction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Esophageal, oesophageal/gastrointestinal disease; GI, gastrointestinal; Respiratory, respiratory disease; UTI, urinary tract infection.
Results of 3-day and 30-day mortality demonstrated differential associations with transfer depending on disease category (figure 2). Odds of 3-day mortality was significantly lower in transferred versus non-transferred patients with the following disease categories: AMI, sepsis, stroke and respiratory diseases. Conversely, transferred patients had significantly greater odds of 3-day mortality among patients with oesophageal/GI disease. There were no significant differences in odds of 3-day mortality among patients with other disease categories (figure 2). Last, odds of 30-day mortality was significantly lower in transferred versus non-transferred patients with AMI or sepsis, but was higher among transferred patients with oesophageal/GI disease, as well as CHF, pneumonia, renal failure, COPD, hip fracture/dislocation, UTI and metabolic disorder. There were no significant differences in odds of 30-day mortality among patients with other disease categories (figure 2). There was no consistent change in the association of IHT with mortality outcomes when moving from unadjusted to adjusted analyses, that is, within some disease categories, adjusted outcomes moved closer to the null, while within others, adjusted outcomes moved further from the null or did not change (online supplementary appendix 3).
Figure 2.
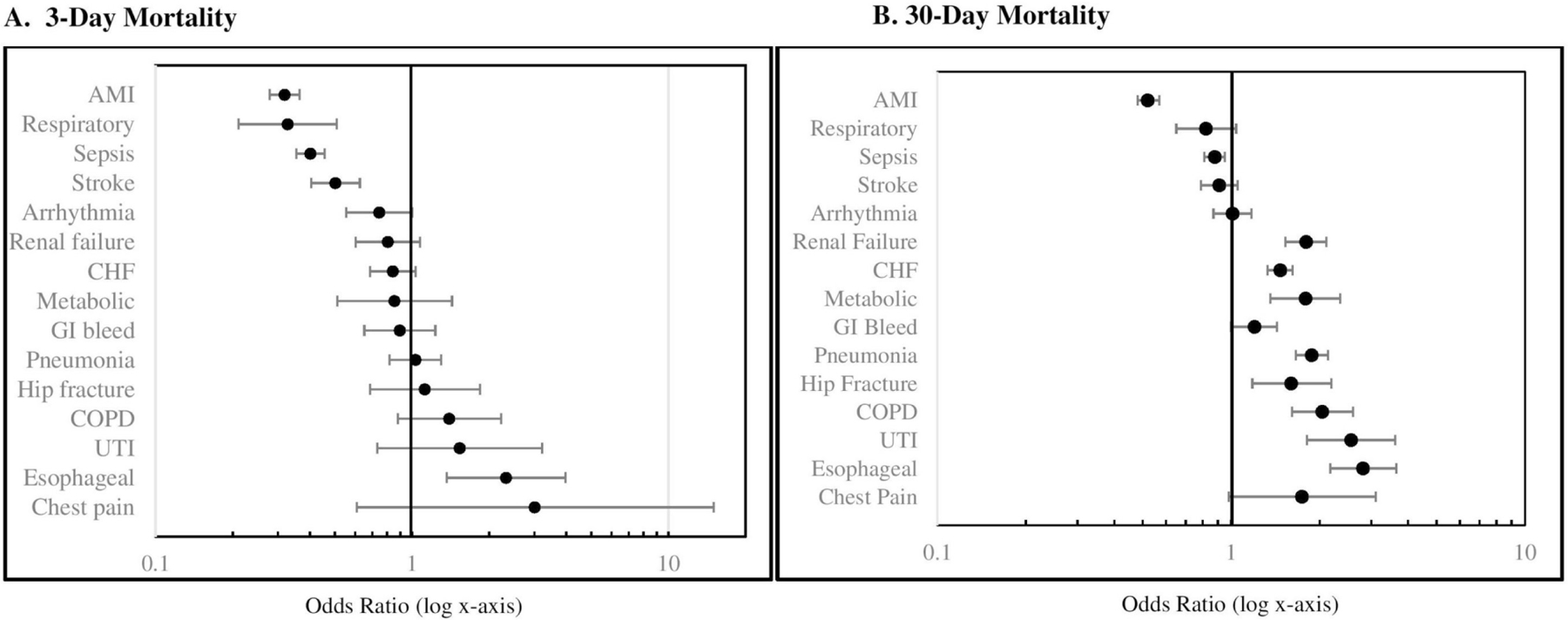
Association of inter-hospital transfer with propensity-matched odds of 3-day and 30-day mortality. AMI, acute myocardial infraction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Esophageal, oesophageal/gastrointestinal disease; GI, gastrointestinal; Respiratory, respiratory disease; UTI, urinary tract infection.
DISCUSSION
In this nationally representative study of Medicare beneficiaries, IHT was associated with higher allowed charges, higher charges per day, longer LOS and generally lower odds of discharge home, but was differentially associated with odds of early death and 30-day mortality depending on patients’ disease category. Our study adds to the existing, although limited, literature examining outcomes of transferred patients, examining IHT on a national level among patients with a variety of diagnoses while also rigorously adjusting for differences in the examined patient populations.
Our findings indicate that transferred patients have greater resource utilisation, including higher allowed charges, higher charges per day and longer LOS, even after adjustment for measurable differences in transferred versus non-transferred patients, similar to other data examining IHT.10 24 Interestingly, although allowed charges were significantly greater among transferred versus non-transferred patients across all diagnoses, once LOS was accounted for via evaluation of charges per day, the differences were attenuated but remained statistically significant. There are several hypotheses that may explain these findings. First, because proposed reasons for patient transfer include access to more specialised services,18 it is possible that transferred patients reasonably require more expensive resources than their non-transferred counterparts, even after risk adjustment. However, prior data demonstrate inconsistent receipt of specialty procedural care following transfer,5 19 20 making this an incomplete explanation of our findings, unless those who received specialty care had enough increased charges and LOS to average out those transferred without receipt of these services. It is possible that our results are simply the results of greater severity of illness among transferred patients and incomplete adjustment for confounding, which we explore in greater depth below (see Limitations). Alternatively, our findings may be reflective of unnecessary redundancies that result from IHT, resulting from ambiguous and non-standardised transfer practices.1 16 17 37 For example, diagnostic studies may need to be repeated if they fail to accompany the patient at time of transfer,37 or providers and patients may require more time to effectively reconcile understanding of reason for transfer before proceeding with an agreed on plan of care.17 Last, it is also possible that, even with the most streamlined IHT processes, there will be inherent redundancies that are reasonable to expect. For example, accepting clinicians may require additional time to absorb and synthesise clinical information that accompanies the transferred patient which inherently prolongs the hospitalisation, a cognitive task not necessarily required for non-transferred patients. To what extent the transferred patient’s prolonged LOS would be considered ‘appropriate’ (ie, under ideal transfer conditions) remains unclear.
Similarly, across most disease categories examined, we found that transferred patients were less likely to be discharged home than their non-transferred counterparts. It is possible that due to IHT, transferred patients may end up hospitalised far from their home and/or in a hospital not affiliated with their home care providers,18 hindering the discharge coordination process, resulting in greater likelihood that patients are discharged to a rehabilitation or skilled nursing facility. In addition, prolonged LOS may also lead to greater deconditioning and loss of physical function,38 39 leading to greater need for discharge to a subacute facility. Alternatively, these results could be reflective of greater in-hospital mortality for transferred versus non-transferred patients (ie, if patients died in the hospital they were less likely to be discharged home). However, examination of in-hospital mortality did not demonstrate greater mortality for transferred versus non-transferred patients across the same diagnoses where lower odds of discharge home was observed. Additionally, mortality rates were much lower than rates of discharge home across all diagnoses. Thus, this explanation of our findings is less likely. Last, patients with respiratory disease or hip fracture/dislocation demonstrated no significant difference in discharge home between transferred and non-transferred patients, likely reflective of the overall low percentages of discharge home in patients with these diagnoses.
Intriguingly, we found differences in the association between IHT and both early death and 30-day mortality depending on patients’ disease category. To our knowledge, only two contemporary studies have examined the association between IHT and patient outcomes across many diagnoses and services, both of which demonstrated worse outcomes in transferred versus non-transferred patients, including longer ICU time, higher inpatient mortality and greater risk of inpatient adverse events.10 24 Both studies included some form of risk-adjustment in their analyses, though neither used propensity matched analysis, arguably more effective at creating more comparable exposure groups and accounting for confounding by indication.28 Additionally, both studies combined all patient diagnoses in their analyses and therefore were unable to examine for differential mortality by disease category.
In contrast to these studies, we found that IHT is associated with lower odds of early death or 30-day mortality in patients with AMI and sepsis (associated with both lower odds of 3-day and 30-day mortality), stroke and respiratory disease. It is possible that these findings reflect mortality benefit of transfer for patients with these diagnoses, supported by prior data that demonstrate patients with AMI experience lower mortality with early intervention, including early transfer to receive such care,40–42 and critically ill patients (ie, with sepsis) benefit from transfer to hospitals with more resources.2 Prior data examining outcomes of transferred patients with stroke, though hampered by improving ‘door to treatment’ times,43 also largely reflect the benefit of transfer when patients are managed at specialised stroke centres.44 45 Though no prior data has examined outcomes of patients transferred with respiratory disease, arguably these patients are similar to the critically ill patient population (ie, requiring external ventilation), which have been shown to benefit from transfer.2 Collectively, these select diagnoses tend to have defined pathways directing rapid diagnostic and therapeutic management at specialised care centres and standard guidelines directing IHT practices,46 47 possibly explaining the observed association between transfer and lower mortality.
Conversely, we found that transfer was associated with greater odds of early death or 30-day mortality among patients with diagnoses of oesophageal/GI disease (associated with both higher odds of 3-day and 30-day mortality) and CHF, pneumonia, renal failure, COPD, hip fracture/dislocation, UTI and metabolic disorder, findings more consistent with existent data examining IHT and patient outcomes.10 24 In contrast to the diagnoses associated with lower mortality, these diagnoses include conditions that are arguably more heterogenous (online supplementary appendix 1) and therefore, the underlying explanation of these findings is less clear. It is possible that, due to the heterogeneity of these diagnoses, there are less explicit guidelines directing management and non-standardised IHT practices,16 making these patients more vulnerable to risks associated with suboptimal aspects of the transfer process without offsetting benefits. Suboptimal transfer practices may include factors such as inappropriate delays in placing transfer requests, delays in transferring the patient once accepted, mismanagement of information exchange or communication with transfer and transfer of patients that should not have been transferred, among others. This may also explain why no mortality differences were seen between transferred and non-transferred patients with diagnoses of arrhythmia, GI bleed and chest pain; that is, any potential benefits from transfer may be equally matched by associated risks. Conceivably, given advances in the safety of other types of care transitions, including patient discharge48 49 and intra-hospital patient handoffs,50 suboptimal aspects of the transfer process can identified and improved,37 51 shifting this balance in the future where benefit outweighs risk. It is also possible that transferred patients with these diagnoses are more medically complex than we are able to capture with our data, as reflected by their prolonged LOS and thus the increased observed mortality is reflective of their greater illness severity or chronic complexity (see Limitations, below). Indeed, transferred and non-transferred patients may be different from each other for a variety of reasons, including need for specialised services, differences in patient/family goals, values or preferences and desire for a second opinion. Last, regarding the specific finding of greater 3-day mortality in patients with oesophageal/GI disease, it is possible that this is not an indication that IHT was inappropriate but rather that the patient was not transferred early enough.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included non-transferred patients within our transferred cohort if they were discharged from one hospital (ie, to home) and then admitted to a different hospital within 1 day, though quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.1 Second, as our results and prior data demonstrate,1 10 18 transferred patients are inherently ‘sicker’ than non-transferred patients. Thus, though we used propensity matching methodology,28 it is still possible that there are unmeasured differences between these patient populations that account for our observed results. However, our findings demonstrate different outcomes depending on patients’ disease category. Therefore, we would have to assume differential effect of unmeasured confounders based on patients’ disease category, that is, we would have to assume that patients with demonstrated lower mortality from transfer possessed unmeasured variables associated with being less ‘sick’, where patients with demonstrated greater mortality from transfer possessed unmeasured variables associated with being more ‘sick’. This is certainly possible: for example, patients with high-mortality conditions (eg, sepsis) must be considered healthy enough to survive and benefit from transfer, possibly selecting for less ‘sick’ patients, while the same may not be true for lower mortality conditions. The correlation between high mortality conditions and benefit from transfer is partial (including respiratory disease, sepsis and AMI), but incomplete across all observed results. Moreover, if this differential effect were the case, one might expect that the observed ORs would move closer to the null when moving from unadjusted to adjusted analyses for each condition, indicating that on further adjustment the effect size would disappear, but this was not observed with our analyses (online supplementary appendix 3). Finally, if patients with diagnoses associated with lower mortality possessed unmeasured variables associated with being less ‘sick’, one may also expect these patients to have lower cost, lower LOS and lower odds of discharge home, which was not observed. Thus, though it remains possible that unmeasured confounders explain our observed findings, this explanation is less likely. Last, we limited our study to include only the top 15 diagnoses on transfer and only hospitalised patients (ie, by definition, excluding any transfers through the emergency department); thus, our findings may not be generalisable to other diagnoses of transferred patients or locations of care.
Taken as a whole, our findings have several potential explanations, including billing/administrative data that do not adequately capture complexity of illness or reasons for transfer; inappropriate delays in placing transfer requests or in transfers being completed once accepted; suboptimal care between acceptance and arrival at the accepting hospital; transfer of patients that should not have been transferred and hazards due to discontinuity of care, including communication deficits, resulting in increased risks to patients.
In summary, in this national study of IHT and patient outcomes across a variety of patient diagnoses, we found that IHT is associated with greater resource utilisation (higher allowed charges, longer LOS, lower rates of discharge home) across all examined diagnoses. It remains unclear whether these findings reflect a reasonable response to the inherent discontinuity in care associated with IHT, or whether resource utilisation is inappropriately increased due to poor quality and/or inefficiencies of this care transition (or some combination of both). We additionally demonstrated that IHT was associated with differential mortality outcomes dependent on patients’ disease category, with suggestion of mortality benefit of transfer among patients with diseases that carry clear guidelines directing management (AMI, sepsis, stroke and respiratory disease). We also demonstrated an association between IHT and increased mortality among patients with more heterogenous disease categories, requiring further research to tease out the underlying aetiologies that may explain these findings, with an ultimate goal of developing targeted quality improvement initiatives to create safer hospital transfers among all patients. Collectively, these results present a nuanced assessment of this complex care transition across a variety of patient diagnoses, raising awareness that there is heterogeneity among transferred patients depending on the diagnosis and highlighting the possibility that not all patients may benefit from transfer.
Supplementary Material
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/bmjqs-2018-008087).
Competing interests None declared.
Patient consent Not required.
Ethics approval Our study protocol (ID 2015P000421) was approved by the Partners Healthcare Human Subjects Review Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Mueller SK, Zheng J, Orav EJ. Rates, predictors and variability of interhospital transfers: a national Evaluation. J Hosp Med 2017;12:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Christie JD, Moody J, et al. The structure of critical care transfer networks. Med Care 2009;47:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RH, Stalhandske EJ, McCargar PA, et al. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J 1999;138(4 Pt 1):688–95. [DOI] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Kahn JM, Hayward RA, et al. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes 2010;3:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J 2008;156:185–92. [DOI] [PubMed] [Google Scholar]
- 6.Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003;138:161–7. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care 2010;19:493–7. [DOI] [PubMed] [Google Scholar]
- 8.Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med 2009;84:1775–87. [DOI] [PubMed] [Google Scholar]
- 9.Arora V, Johnson J, Lovinger D, et al. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care 2005;14:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol-Hessner L, White AA, Davis KF, et al. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med 2016;11:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard AM, Hayward RA, Rosevear J, et al. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med 1996;71:262–6. [DOI] [PubMed] [Google Scholar]
- 12.Golestanian E, Scruggs JE, Gangnon RE, et al. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med 2007;35:1470–6. [DOI] [PubMed] [Google Scholar]
- 13.Durairaj L, Will JG, Torner JC, et al. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med 2003;31:1981–6. [DOI] [PubMed] [Google Scholar]
- 14.Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA 1989;262:70–3. [PubMed] [Google Scholar]
- 15.Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med 1989;17:418–22. [DOI] [PubMed] [Google Scholar]
- 16.Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care 2011;49:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care 2013;28:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med 2012;40:2470–8. [DOI] [PubMed] [Google Scholar]
- 19.Mueller SK, Zheng J, Orav J. Interhospital transfer and receipt of specialty procedures. J Hosp Med 2018;13:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med 2014;174:213–22. [DOI] [PubMed] [Google Scholar]
- 21.Data ADA, 2013. American hospital association annual survey database. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/ (accessed 1 Jul 2013). [Google Scholar]
- 22.U.S. Department of Health and Human Services (HRSA), 2016. What are critical access hospitalis (CAH)?. http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html (accessed 9 Jun 2016). [Google Scholar]
- 23.Centers for Medicare &Medicaid Services, 2013. CMS standardization methodology for allowed amount. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/Downloads/Geo_Var_PUF_Technical_Supplement.pdf. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/Downloads/Geo_Var_PUF_Technical_Supplement.pdf. (accessed 1 Dec 2017). [Google Scholar]
- 24.Hernandez-Boussard T, Davies S, McDonald K. Interhospital facility transfers in the united states: a nationwide outcomes study. J Patient Saf 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med 2006;166:2511–7. [DOI] [PubMed] [Google Scholar]
- 26.Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care 2013;51:567–74. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res 2010;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127(8 Pt 2):757–63. [DOI] [PubMed] [Google Scholar]
- 29.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick AR, Schneeweiss S, Brookhart MA, et al. The implications of propensity score variable selection strategies in pharmacoepidemiology: an empirical illustration. Pharmacoepidemiol Drug Saf 2011;20:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons L Reducing Bias in a propensity score matched-pair sample using greedy matching techniques.Proceedings of the twenty-sixth annual SAS (Users Group International Conference). http://www2.sas.com/proceedings/sugi26/p214-26.pdf [Google Scholar]
- 33.Herzig SJ, Howell MD, Ngo LH, et al. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009;301:2120–8. [DOI] [PubMed] [Google Scholar]
- 34.López L, Hicks LS, Cohen AP, et al. Hospitalists and the quality of care in hospitals. Arch Intern Med 2009;169:1389–94. [DOI] [PubMed] [Google Scholar]
- 35.Borzecki AM, Christiansen CL, Chew P, et al. Comparison of in-hospital versus 30-day mortality assessments for selected medical conditions. Med Care 2010;48:1117–21. [DOI] [PubMed] [Google Scholar]
- 36.Sokol-Hessner L, White A, Feldman K. Patients transferred from outside hospitals to academic hospitalists and general internists have higher mortality and costs than patients from the ed. Washington D.C: Paper presented at: Society of Hospital Medicine National Conference, 2013. [Google Scholar]
- 37.Mueller SK, Schnipper JL. Physician perspectives on interhospital transfers. J Patient Saf 2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, et al. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs 2007;16:46–57. [DOI] [PubMed] [Google Scholar]
- 39.Zisberg A, Shadmi E, Sinoff G, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc 2011;59:266–73. [DOI] [PubMed] [Google Scholar]
- 40.Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009;360:2705–18. [DOI] [PubMed] [Google Scholar]
- 41.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation 2006;113:1683–92. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs AK. Regional systems of care for patients with ST-elevation myocardial infarction: being at the right place at the right time. Circulation 2007;116:689–92. [DOI] [PubMed] [Google Scholar]
- 43.Sun CH, Nogueira RG, Glenn BA, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation 2013;127:1139–48. [DOI] [PubMed] [Google Scholar]
- 44.Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke 2005;36:1597–616. [DOI] [PubMed] [Google Scholar]
- 45.Gupta R, Horev A, Nguyen T, et al. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg 2013;5:294–7. [DOI] [PubMed] [Google Scholar]
- 46.Guidelines for the transfer of critically ill patients. Guidelines committee of the american college of critical care medicine; society of critical care medicine and american association of critical-care nurses transfer guidelines task force. Crit Care Med 1993;21:931–7. [PubMed] [Google Scholar]
- 47.Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 guidelines for the management of patients with unstable Angina/Non-ST-Elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;123:e426–579. [DOI] [PubMed] [Google Scholar]
- 48.Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med 2014;89:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwald JL, Jack BW. Preventing the preventable: reducing rehospitalizations through coordinated, patient-centered discharge processes. Prof Case Manag 2009;14:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med 2014;371:1803–12. [DOI] [PubMed] [Google Scholar]
- 51.Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med 2015;10:415–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


