Summary
In immune thrombotic thrombocytopenic purpura (iTTP), autoantibodies against the metalloprotease ADAMTS13 lead to catastrophic microvascular thrombosis. However, the potential benefits of recombinant human ADAMTS13 (rADAMTS13) in iTTP remain unknown. We report the clinical use of rADAMTS13 in iTTP. Administration of rADAMTS13 was associated with rapid suppression of disease activity and complete recovery in a critically ill patient whose condition had proven refractory to all available treatments. We show that rADAMTS13 causes immune complex formation, saturating the autoantibody and likely promoting its clearance. Our data support the role of rADAMTS13 as a novel adjunctive therapy in iTTP.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is an acute hematologic disorder characterized by thrombotic microangiopathy, end organ damage, and death if left untreated.1,2 In iTTP, autoantibodies inhibit the von Willebrand factor (vWF)-cleaving metalloprotease ADAMTS13 or induce its clearance, leading to accumulation of ultra-large vWF multimers and the formation of platelet-rich microvascular thrombi.3 The disease occurs more commonly in women of color,4,5 and patients have few if any effective options when standard treatment fails. The mainstay of therapy for iTTP is plasma exchange (PLEX), which removes the inhibitor and provides exogenous ADAMTS13.6 PLEX induces a clinical response in most patients5,7 despite repleting at best only about half of normal ADAMTS13 activity levels.8 By contrast, recombinant human ADAMTS13 (rADAMTS13) offers the possibility of greatly increased ADAMTS13 delivery and enhanced autoantibody clearance via formation of ADAMTS13-containing immune complexes. However, the benefits of rADAMTS13 in iTTP remain theoretical. We describe the clinical use of rADAMTS13 in iTTP.
Case Report
A 28-year-old woman (G1P1) of African ancestry presented 101 days post-partum with fatigue and vaginal bleeding. Laboratory studies (Table S1) revealed anemia (hemoglobin: 6.9 g/dL), elevated lactate dehydrogenase (LDH, 2089 IU/L), and profound thrombocytopenia (platelet count: 9 × 109/L). A peripheral blood smear showed abundant schistocytes (Figure 1A). The patient’s PLASMIC score9,10 (a guideline to aid the diagnosis of iTTP) was 6 (high risk: >5), leading to initiation of PLEX for presumed iTTP. The ADAMTS13 activity level returned undetectable (normal: >66.8%), with an anti-ADAMTS13 IgG titer of 49 U/ml (normal: <12 U/ml). Despite daily PLEX treatments, her severe thrombocytopenia persisted. She received adjunctive prednisone, rituximab, intravenous immunoglobulin, and bortezomib without improvement. On day 11, she received 11 mg of caplacizumab intravenously. She experienced two episodes of large-volume melena and was transfused with 4 units of red blood cells. On day 12, PLEX was increased to 1.5 plasma volumes exchanged twice daily, which failed to improve her platelet count or trough ADAMTS13 activity levels. The patient’s mental status deteriorated, and she described a sense of impending doom. She experienced ongoing gastrointestinal and vaginal hemorrhage requiring daily transfusion. She developed worsening sinus tachycardia with a heart rate of 160 beats per minute (Figure 1B), which corresponded to a worsening lactic acidosis that peaked at 6.4 mmol/L. She became acutely hypotensive and required support with intravenous vasopressors. An acute pericardial effusion with tamponade was diagnosed (Figure 1C and Supplemental Video 1). On day 14, an emergent pericardiocentesis was performed, yielding a dark, serosanguinous exudate (Supplemental Figure 1). Pathologic examination of the pericardium revealed fibrinous pericarditis with organizing fibrin on the surface and perivascular chronic inflammation composed of macrophages and lymphocytes (Figure 1D). Throughout this period, the patient’s platelet count remained below 5 × 109/L, and her LDH rose steadily to a peak of 2,234 IU/L. On the evening of day 14, she received her first dose of rADAMTS13.
Figure 1. Clinical Features of Severe Refractory iTTP.

Panel A shows the peripheral smear taken on day 0 (50X magnification) after application of a computerized algorithm for the automated detection of schistocytes (blue shaded cells). The patient’s heart rate trend (Panel B) and subcostal view on a transthoracic echocardiogram performed on day 14 (Panel C) reflect progressive pericardial tamponade, including a large circumferential pericardial effusion (red arrow) and evidence of right ventricular diastolic inversion (red arrowhead). The patient’s heart rate declined immediately following pericardiocentesis (Panel B, blue arrow). Pathologic examination of the pericardium is depicted in Panel D. At 40X magnification (left), there is fibrin on the surface of the pericardium (arrowheads). At 200X magnification (right), the fibrin (asterisks) is being organized by mononuclear cells (arrows).
Methods
Recombinant Human ADAMTS13
We obtained rADAMTS13 from the manufacturer under a compassionate use protocol (FDA Emergency IND #29787 and MGB IRB Protocol #2023P001901). The drug is a purified, virally-inactivated recombinant form of human ADAMTS13 produced in Chinese Hamster Ovary (CHO) cells.11 Vials of lyophilized protein were reconstituted with sterile water to a nominal potency of either 100 IU/ml or 300 IU/ml before administration.
Patient Plasma Measurements
The ADAMTS13 activity level at diagnosis was measured in platelet-poor plasma using LC/MS-MS (Labcorp). Subsequent ADAMTS13 activity and inhibitor assays as well as vWF multimer analysis were performed at the Versiti Hemostasis Laboratory (see Supplementary Appendix). ADAMTS13 activity and inhibitor titers (in Bethesda-like units) were measured using the FRETS-VWF73 assay.12 The anti-ADAMTS13 IgG assay was performed by sandwich ELISA.13 Serial functional half-life estimates over 14 non-consecutive days were generated by fitting a one-phase exponential decay curve to ADAMTS13 activity levels measured at 0, 1, 4, 8, and 12 hours after rADAMTS13 administration. Plasma ADAMTS13 antigen was quantified by sandwich ELISA (ProteinTech product #KE00286). ADAMTS13 circulating immune complexes (CIC) were measured by ELISA assay as described previously14 and in the Supplementary Appendix. The anti-ADAMTS13 capture antibody (clone 5C11) was kindly provided by Dr. Karen Vanhoorelbeke.15 Immunoblotting of patient plasma for ADAMTS13 was performed using SDS-PAGE under reducing conditions and anti-ADAMTS13 clone 20A5 (Novus Biologicals product #NBP1–49662).
Sequencing of the ADAMTS13 Locus
Next-generation sequencing of the ADAMTS13 locus was performed at the Versiti Hematology Genetics Laboratory as described in the Supplementary Appendix.
The Harvard TMA Research Collaborative
The Harvard TMA Research Collaborative is a longitudinal, registry-based effort to study iTTP and allied disorders and features complete individual-level patient data through the on-premises electronic medical record system (EMR).9,16 For this study, data for 102 consecutive iTTP patients in the registry were extracted and analyzed.
Automated Analysis of Red Blood Cell Morphology
Computerized assessment of cell morphology on peripheral smear images was used to analyze schistocyte counts as described previously.17
Statistical Analysis
Statistical analyses were performed using Graphpad Prism version 10.1.2 and MATLAB 2023b.
Results
Molecular Exclusion of Congenital TTP
To exclude the possibility of congenital TTP (cTTP) with an alloimmune inhibitor,18 we sequenced the ADAMTS13 locus and detected no germline loss of function variants.
Clinical Impact and Laboratory Correlates of Treatment
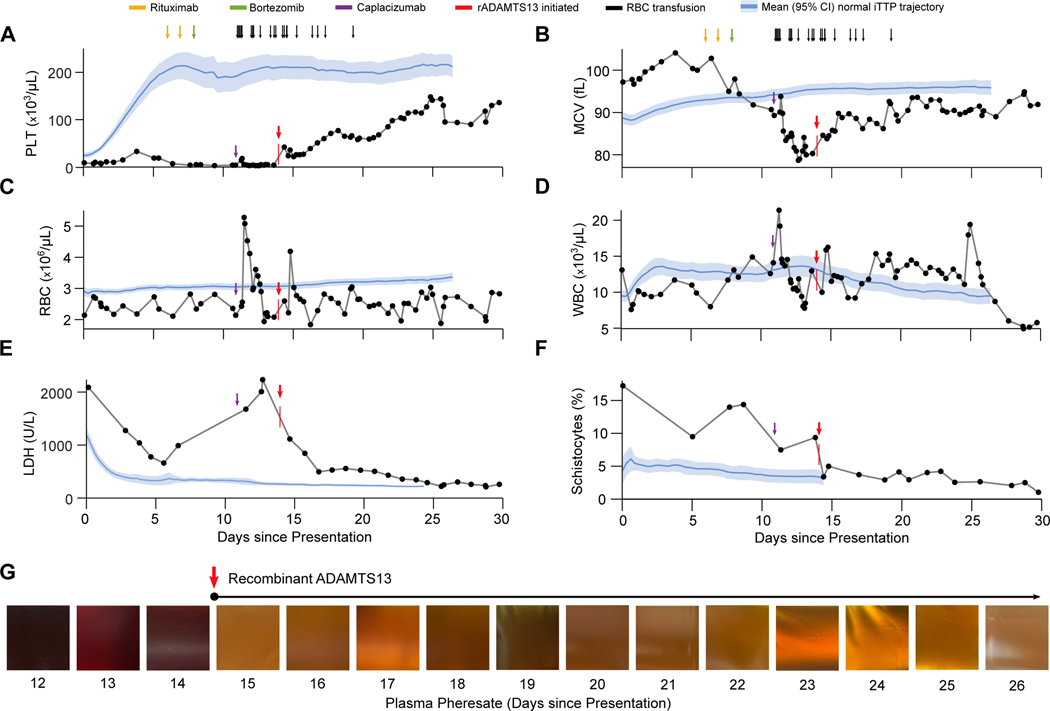
In response to progressive clinical deterioration, rADAMTS13 treatment was initiated on day 14 at a dose of 80 IU/kg intravenously every 12 hours. One dose was given each morning immediately after once-daily PLEX treatment, followed by a second dose 12 hours later. Administration of rADAMTS13 was associated with a rapid and striking improvement in our patient’s clinical status, including cessation of bleeding. We compared the trajectories of key laboratory parameters for our patient against those of 102 consecutive iTTP patients in the Harvard TMA Research Collaborative (HaTRC) registry. Whereas the vast majority of iTTP patients achieve a normal platelet count (>150 × 109/L) within the first seven days of treatment, our patient’s count remained at a mean (± SD) of 12.2 ± 7.7 × 109/L throughout this period (P<0.0001 for comparison to HaTRC) (Figure 2A). Similarly, clinical response to PLEX is almost uniformly associated with a rise in MCV, likely due to diminished sequestration of larger RBCs within microvascular thrombi and (to a lesser degree) reduced schistocyte formation.17 By contrast, our patient’s MCV declined from 104.1 fl to 78.7 fl despite daily PLEX therapy (Figure 2B) (P<0.0001 for comparison to HaTRC), a pattern that has been observed in other refractory cases (Supplemental Figure 2). Treatment with rADAMTS13 rapidly reversed this trend and was associated with a steady rise in MCV. This change was accompanied by an immediate rise in platelet count, albeit less robust than typically observed in cases that are responsive to PLEX (P<0.0001 for comparison to HaTRC) (Figure 2A). Trends in RBC count, WBC count, and creatinine are shown in Figures 2C and 2D and Supplemental Figure 3, respectively. Administration of rADAMTS13 was also associated with a large drop and sustained decline in LDH, indicating diminished disease activity (Figure 2E).
Figure 2. Laboratory Parameters Before and After Administration of Recombinant ADAMTS13.
Panels A through F depict the trajectories of key laboratory values for this patient in relation to the noted clinical events. The vertical red arrows and marks denote initiation of rADAMTS13 treatment at 80 IU/kg intravenously every 12 hours. Blue lines and shaded areas represent the corresponding mean ± 95% CI values for 102 consecutive iTTP patients in the Harvard TMA Research Collaborative dataset. The patient received daily plasma exchange treatments throughout the period shown. Panel G shows the gross appearance of the patient’s plasma pheresate at the conclusion of daily plasma exchange treatments on days 12–26. The purple arrow marks the administration of caplacizumab, while the red arrow denotes the initiation of rADAMTS13. Abbreviations: PLT, platelet count; RBC, red blood cell count; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; WBC, white blood cell count.
Using a validated, open-source image analysis algorithm to quantify schistocytosis on peripheral blood smears,17 we found that our patient’s presenting schistocyte count was 17.2%, compared to a mean (± SD) of 5.9 ± 3.2% in the HaTRC dataset (Figure 1A). After gradually declining schistocytosis over the first 13 days, administration of rADAMTS13 was associated with a sharp drop and sustained suppression of her schistocyte burden (Figure 2F). Improved laboratory parameters corresponded to a marked change in the gross appearance of our patient’s plasma from port-wine to straw-colored (Figure 2G). She continued receiving rADAMTS13 and daily PLEX throughout her hospitalization. She experienced complete recovery and was discharged home on day 39. Because the patient was clinically stable and we wished to transition her from an experimental approach to standard of care therapy, we discontinued rADATMS13 on day 46. In total, the patient received 53 doses of rADAMTS13 (80 IU/kg) over 32 days, including six once-daily doses as an outpatient.
Characterization of Recombinant ADAMTS13 Function in vivo
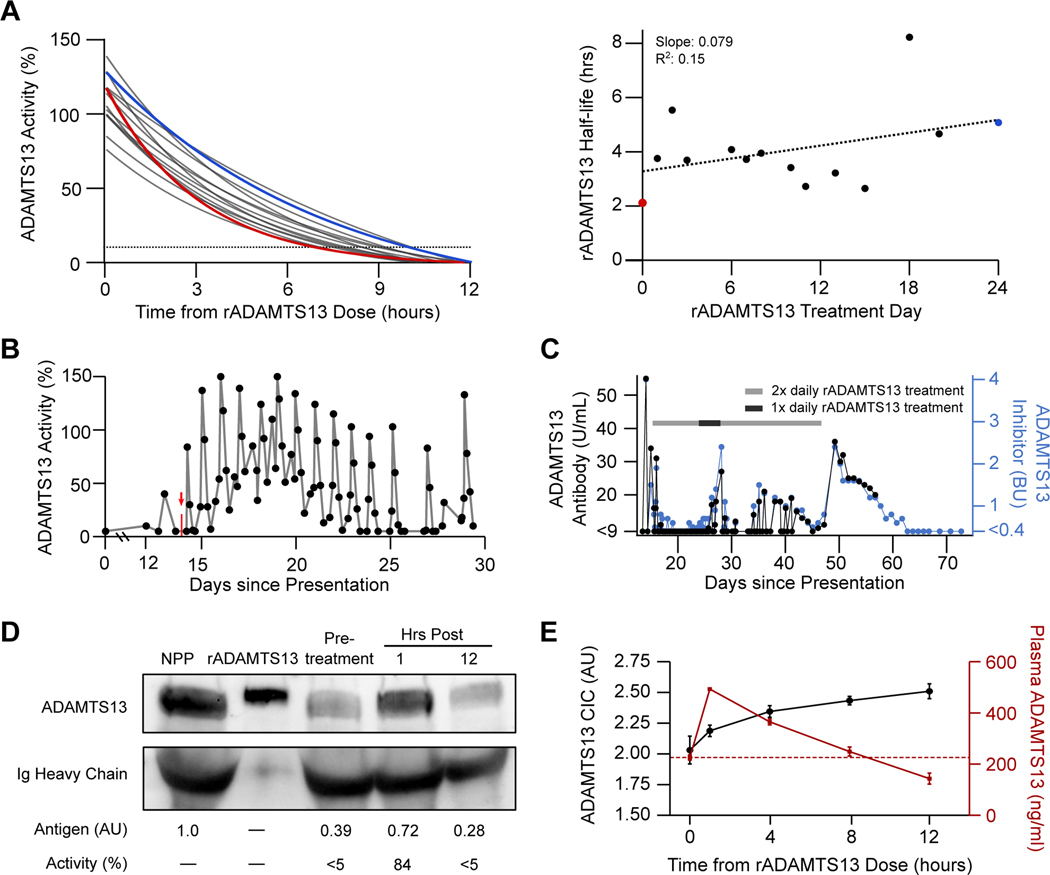
One hour after first receiving rADAMTS13, the patient’s plasma ADAMTS13 activity level climbed from undetectable to 84%, followed by a gradual decline over 12 hours. The functional half-life of rADAMTS13 in the presence of the patient’s autoantibody was estimated to be 2.11 hours (expected: 36.5–66.9 hours19). With subsequent doses, we observed a modest prolongation in half-life, and the patient maintained an ADAMTS13 activity level of >10% for approximately 6–9 hours of each 12-hour dosing cycle (Figure 3A). Administration of rADAMTS13 immediately after daily PLEX resulted in a sawtooth pattern for ADAMTS13 activity levels (Figure 3B). Taken together, these findings suggest that a significant portion of the infused rADAMTS13 retained in vivo function for a considerable period despite the patient’s inhibitor. The patient’s mean (± SD) vWF activity to antigen ratio was 0.86 ± 0.19 while she received rADAMTS13 and rose to 1.02 ± 0.04 after discontinuing therapy (Supplemental Figure 4). In order to assess whether rADAMTS13 was causing loss of high-molecular-weight (HMW) vWF multimers, we analyzed the patient’s plasma at selected time points (Supplemental Figure 5). We identified a relative decrease in HMW multimers that correlated with periods of high iTTP disease activity and largely resolved together with the patient’s clinical improvement. By contrast, final discontinuation of rADAMTS13 was associated with only a modest rise in HMW forms.
Figure 3. Characterization of Recombinant ADAMTS13 Function in vivo.
Panel A shows serial half-life modeling for rADAMTS13 over 14 non-consecutive days using fitted one-phase exponential decay curves based on ADAMTS13 activity levels measured at 0, 1, 4, 8, and 12 hours after administration (left) as well as the computed ADAMTS13 activity half-life by treatment day (right). The red line and data point represent the first dose, while the blue line and data point represent the 41st dose. The dotted line in the left panel represents 10% ADAMTS13 activity level, the diagnostic cutoff for severe deficiency. The results of simple linear regression analysis are shown in the right panel. Panel B depicts serial ADAMTS13 activity measurements between days 0 and 30. The vertical red arrow and mark denote initiation of intravenous rADAMTS13 treatment. Similarly, ADAMTS13 autoantibody was measured serially via two independent methods (Panel C), including titer in Bethesda-like units (BU) evaluated by FRETS-VWF73 assay (blue) and plasma anti-ADAMTS13 antibody concentration, measured by sandwich ELISA (black). The gray bar denotes periods during which rADAMTS13 (80 IU/kg) was dosed every 12 hours, while the black bar represents dosing every 24 hours. Immunoblotting of patient plasma for ADAMTS13 at 3 timepoints relative to the first dose of rADAMTS13 is shown Panel D alongside normal pooled plasma (NPP) and purified rADAMTS13 in buffer. Corresponding levels of ADAMTS13 antigen (assessed by densitometry) and activity (assessed by FRETS-VWF73 assay) are noted below the image. Assessments of total ADAMTS13 antigen and autoantibody-bound plasma ADAMTS13 levels are shown in Panel E. Sandwich ELISA assays were performed on patient plasma at the indicated times after rADAMTS13 administration to detect total plasma ADAMTS13 (red) and autoantibody-bound ADAMTS13 in the form of circulating immune complexes (CIC) (black). ADAMTS13 CIC concentration is expressed in arbitrary units (AU), while total plasma ADAMTS13 is expressed in ng/ml. Time 0 samples were drawn immediately after daily plasma exchange treatment and prior to administration of rADAMTS13. Data represents the mean (± SD) of four consecutive dosing cycles. The horizontal red dotted line represents the mean baseline plasma ADAMTS13 antigen level at time 0 (225.9 ng/ml).
The formation of ADAMTS13-containing circulating immune complexes (CIC) during rADAMTS13 treatment in iTTP could be central to its mechanism of action but has never been evaluated. We found that the ADAMTS13 inhibitor titer and anti-ADAMTS13 IgG level remained suppressed while our patient received twice daily rADAMTS13 but rebounded during a brief period of reduced dosing (days 24–28) and after final discontinuation (day 46) (Figure 3C). Extended data on the patient’s course including relationships between ADAMTS13 activity and inhibitor levels, platelet count, and key iTTP therapies are shown in Supplemental Figure 6. Western blot analysis showed that at baseline the patient retained a significant quantity of circulating ADAMTS13 antigen compared to normal pooled plasma, though the enzyme exhibited minimal activity (Figure 3D). One hour after the first dose of rADAMTS13, we detected an 85% rise in plasma ADAMTS13 antigen (0.39 AU to 0.72 AU), which corresponded to approximately 80% increased ADAMTS13 proteolytic activity. By 12 hours, the circulating ADAMTS13 antigen had declined to 0.28 AU, with <5% activity detected. We next evaluated steady-state plasma ADAMTS13 antigen and ADAMTS13 CIC levels14 (Figure 3E). After each rADAMTS13 administration, the plasma ADAMTS13 antigen concentration demonstrated a spike followed by gradual decline, whereas CIC levels rose steadily throughout the dosing interval. Taken together, these data suggest that rADAMTS13 at 80 IU/kg was sufficient to saturate the anti-ADAMTS13 autoantibody and likely contributed to autoantibody clearance via the formation of CICs.
Discussion
We describe the use of rADAMTS13 as a novel salvage therapy in a patient with iTTP with multiorgan dysfunction and clinical deterioration despite daily PLEX. The administration of rADAMTS13 was followed by prompt improvement of our patient’s clinical picture and the achievement of a full recovery.
Recombinant ADAMTS13 was recently approved as replacement therapy for patients with cTTP,8,11 where germline ADAMTS13 loss of function causes lifelong severe deficiency of the protease.8,19 Unknown is whether rADAMTS13 could be effective in iTTP given the presence of inhibitory anti-ADAMTS13 autoantibodies. Here, rADAMTS13 allowed us to isovolemically deliver the equivalent enzyme content of approximately 7 L (28 units) of plasma per day, far exceeding the capacity of PLEX. Our data indicate that infused rADAMTS13 was able to functionally overwhelm the autoantibody inhibitor during a critical window of approximately 12–18 hours each day, sufficient to promote marked clinical improvement. This impact was observed almost immediately upon administration of rADAMTS13 and after daily high-volume PLEX over two weeks had previously failed to induce remission. Conversely, an established advantage of PLEX is its ability to mechanically remove autoantibody, whereas the contribution of rADAMTS13 to autoantibody clearance remains uncertain. Between doses, we observed a gradual increase in ADAMTS13 CIC levels and decrease in total ADAMTS13 antigen levels as free rADAMTS13 became bound by autoantibody, and receipt of twice daily rADAMTS13 corresponded with suppression of the patient’s inhibitor titer. These data raise the intriguing possibility that in at least some cases high-dose rADAMTS13 may possess autoantibody clearance properties that are independent of PLEX. A Phase 2b randomized clinical trial of rADAMTS13 in iTTP (NCT05714969) was recently initiated and may provide additional insights.
During her acute illness, our patient displayed a decreased vWF activity to antigen ratio, suggestive of a mild acquired vWF abnormality. While other work has shown that rADAMTS13 may cause a temporary decline in HMW multimers when given to cTTP patients during remission,19 we found here that the relative loss of plasma HMW vWF multimers was likely due primarily to the known sequestration of HMW forms in the microcirculation during acute iTTP.20 Accordingly, rADAMTS13 proved safe under the conditions we tested, and initiation of rADAMTS13 was associated with platelet count recovery and cessation of our patient’s clinically significant vaginal and gastrointestinal hemorrhages. The apparent safety of rADAMTS13 may be due to its relative selectivity for the open form of vWF unraveled under shear stress,21 conformational regulation of ADAMTS13 function,22 and the blunting of ADAMTS13 activity by autoantibody.
In summary, we obtained clinical, pharmacokinetic, and pharmacodynamic proof of concept that rADAMTS13 can add significant value in PLEX-refractory iTTP despite the presence of anti-ADAMTS13 autoantibodies. Building on these results, well-designed randomized controlled trials will be required to determine whether rADAMTS13 should serve as an adjunct to, or replacement for, current standard of care with PLEX in patients with iTTP.
Supplementary Material
Acknowledgements:
The authors wish to thank Dr. Karen Vanhoorelbeke for the kind gift of anti-ADAMTS13 antibody clone 5C11; the employees of Takeda Pharmaceuticals and the FDA for facilitating compassionate use of recombinant human ADAMTS13; and the patient and her family.
Funding
Supported by the National Institutes of Health (1R01HL166246 to PKB) and the Luick Family Fund of the Department of Pathology at Massachusetts General Hospital.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Cuker A, Cataland SR, Coppo P, et al. Redefining outcomes in immune TTP: an international working group consensus report. Blood 2021;137(14):1855–1861. (In eng). DOI: 10.1182/blood.2020009150. [DOI] [PubMed] [Google Scholar]
- 2.Mancini I, Pontiggia S, Palla R, et al. Clinical and Laboratory Features of Patients with Acquired Thrombotic Thrombocytopenic Purpura: Fourteen Years of the Milan TTP Registry. Thromb Haemost 2019;119(5):695–704. DOI: 10.1055/s-0039-1679907. [DOI] [PubMed] [Google Scholar]
- 3.George JN, Nester CM. Syndromes of thrombotic microangiopathy. The New England journal of medicine 2014;371(7):654–66. (In eng). DOI: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 4.Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. The Lancet Haematology 2016;3(5):e237–45. (In eng). DOI: 10.1016/s2352-3026(16)30018-7. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi S, Antun AG, Farland AM, et al. Race, rituximab, and relapse in TTP. Blood 2022;140(12):1335–1344. DOI: 10.1182/blood.2022016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. Journal of thrombosis and haemostasis : JTH 2020;18(10):2496–2502. (In eng). DOI: 10.1111/jth.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colling M, Sun L, Upadhyay V, et al. Deaths and complications associated with the management of acute immune thrombotic thrombocytopenic purpura. Transfusion 2020;60(4):841–846. (In eng). DOI: 10.1111/trf.15721. [DOI] [PubMed] [Google Scholar]
- 8.Asmis LM, Serra A, Krafft A, et al. Recombinant ADAMTS13 for Hereditary Thrombotic Thrombocytopenic Purpura. The New England journal of medicine 2022;387(25):2356–2361. (In eng). DOI: 10.1056/NEJMoa2211113. [DOI] [PubMed] [Google Scholar]
- 9.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. The Lancet Haematology 2017. DOI: 10.1016/S2352-3026(17)30026-1. [DOI] [PubMed] [Google Scholar]
- 10.Li A, Khalighi PR, Wu Q, Garcia DA. External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. Journal of thrombosis and haemostasis : JTH 2018;16(1):164–169. (In eng). DOI: 10.1111/jth.13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ADZYNMA (ADAMTS13, recombinant-krhn) [package insert]. Lexington, MA; Takeda Pharmaceuticals; 2023. [Google Scholar]
- 12.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. British journal of haematology 2005;129(1):93–100. (In eng). DOI: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 13.Froehlich-Zahnd R, George JN, Vesely SK, et al. Evidence for a role of anti-ADAMTS13 autoantibodies despite normal ADAMTS13 activity in recurrent thrombotic thrombocytopenic purpura. Haematologica 2012;97(2):297–303. DOI: 10.3324/haematol.2011.051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotta LA, Valsecchi C, Pontiggia S, et al. Measurement and prevalence of circulating ADAMTS13-specific immune complexes in autoimmune thrombotic thrombocytopenic purpura. Journal of thrombosis and haemostasis : JTH 2014;12(3):329–36. DOI: 10.1111/jth.12494. [DOI] [PubMed] [Google Scholar]
- 15.Deforche L, Roose E, Vandenbulcke A, et al. Linker regions and flexibility around the metalloprotease domain account for conformational activation of ADAMTS-13. Journal of thrombosis and haemostasis : JTH 2015;13(11):2063–75. DOI: 10.1111/jth.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendapudi PK, Li A, Hamdan A, et al. Impact of severe ADAMTS13 deficiency on clinical presentation and outcomes in patients with thrombotic microangiopathies: the experience of the Harvard TMA Research Collaborative. British journal of haematology 2015;171(5):836–44. (In eng). DOI: 10.1111/bjh.13658. [DOI] [PubMed] [Google Scholar]
- 17.Foy B, Stefely J, Bendapudi PK, et al. Computer vision quantitation of erythrocyte shape abnormalities provides diagnostic, prognostic, and mechanistic insight. Blood advances 2023. (In eng). DOI: 10.1182/bloodadvances.2022008967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhaliwal G, Mojtahed A, Fogerty AE, Kadauke S, Mack JP. Case 36–2017. New England Journal of Medicine 2017;377(21):2074–2083. DOI: 10.1056/NEJMcpc1710565. [DOI] [PubMed] [Google Scholar]
- 19.Scully M, Knöbl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017;130(19):2055–2063. (In eng). DOI: 10.1182/blood-2017-06-788026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. The New England journal of medicine 1982;307(23):1432–5. DOI: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 21.Crawley JT, Scully MA. Thrombotic thrombocytopenic purpura: basic pathophysiology and therapeutic strategies. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 2013;2013:292–9. (In eng). DOI: 10.1182/asheducation-2013.1.292. [DOI] [PubMed] [Google Scholar]
- 22.Ercig B, Arfman T, Hrdinova J, et al. Conformational plasticity of ADAMTS13 in hemostasis and autoimmunity. The Journal of biological chemistry 2021;297(4):101132. DOI: 10.1016/j.jbc.2021.101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.