Abstract
Introduction
Hypermucoviscous Klebsiella pneumoniae (hvKP) is related to invasive infections; however, there have been very few comprehensive reports on the clinical features and prognosis of critically ill patients with the infection.
Methods
We conducted a retrospective case series in a general intensive care unit in Japan. Patients with positive blood cultures for KP between January 1, 2020 and December 31, 2022 were included. hvKP was defined by the positivity in the string test. We analyzed the patient’s characteristics at baseline, including comorbidities, abscess formation, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation (APACHE) II score, septic shock, duration of hospitalization, 30-day mortality, and infection site.
Results
A total of 24 patients had a positive blood culture for KP; nine patients (37.5%) were positive for the string test (hvKP) while 15 (62.5%) were negative (non-hvKP). In both groups, the patients were old (mean age, hvKP 80.4 vs. non-hvKP 75.7 years) and more often male (five patients (55.6%) vs. 12 patients (80.0%)). No statistically significant difference was found between the two groups in terms of comorbidities, such as diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and malignancy. No statistical difference was seen in abscess formation (two patients [22.2%] vs. one patient (6.7%)), SOFA score (5.2±4.8 vs. 4.7±3.4), APACHE II score (19.6 (15.0-20.0) vs. 17.0 (11.2-20.8)), septic shock (five patients (55.6%) vs. four patient (26.7%)), duration of hospitalization (37.2 (12.0-51.0) vs. 32.3 (9.5-21.0)), and 30-day mortality (two patients (22.2%) vs. two patients (13.3%)). Two cases with hvKP died within 24 h. No significant difference was seen in the infection sources; respiratory infection (2 (22.2%) vs. 1 (6.7%)), hepatobiliary infection (2 (22.2%) vs. 7 (46.7%)), and genitourinary infection (1 (11.1%) vs. 5 (33.3%)).
Conclusions
Critically ill patients with hvKP infection showed characteristics similar to those reported previously. However, the disease could rapidly become severe and have a poor prognostic outcome.
Keywords: string test, klebsiella pneumoniae (kp), hypermucoviscosity, critical care medicine, antibiotic resistance, antibiotics
Introduction
Klebsiella pneumoniae (KP) is a gram-negative lactose-fermenting non-motile aerobic rod-shaped bacterium detected in the stool (5%-38%) and nasopharynx (1%-6%) samples [1]. The hypermucoviscosity phenotype is a major virulence factor of KP. A hypermucoviscous (hv) KP produces a mucoviscous exopolysaccharide web and is identified using a string test [2]. The phenotype is correlated with high serum resistance and invasive infections, such as liver abscesses, compared to that in non-hvKP [3]. Although hvKP is an important causative organism in severe invasive infections, there are only a few comprehensive reports on its clinical features and prognosis of critically ill patients with the infection. Therefore, this study aimed to examine the clinical characteristics and outcomes of patients with hvKP in a cohort admitted to the intensive care unit.
Materials and methods
Subjects
We performed a retrospective case series in the general intensive care unit (ICU) of a hospital in Japan. All patients were Japanese. This case series study included patients admitted to the ICU with a positive blood culture for KP between January 1, 2020 and December 31, 2022. Patients under 18 years old were excluded. Finally, 24 patients were included. The cases were identified based on positive blood culture results retrieved from medical microbiology databases.
The Medical Ethics Review Committee waived the need for patients’ consent owing to the retrospective nature of this study, in which the participants were not subjected to any form of action. Data are presented according to the reporting guidelines for case series [3].
Methods
The variables include age, sex, comorbidities on admission, abscess formation, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, septic shock, duration of hospitalization, 30-day mortality, and infectious source. Hypermucoviscosity was defined by a positive string test result (Figure 1).
Figure 1. Positive string test result .
Hypermucoviscous K. pneumoniae grows as sticky colonies on agar plates. A positive string test is indicated by a > 5-mm viscous string from the colony on an agar plate when stretched by a standard bacteriologic loop.
Bacterial colonies grown overnight on a blood agar plate at 37 °C were stretched by a standard bacteriological loop. If a mucoviscous string > 5 mm in length was formed, the string test was considered positive, and the isolate was identified as hvKP [4].
Statistical analysis
Statistical analyses were performed in November 2023 using R, version 4.3.1 (The R Foundation for Statistical Computing, Vienna, Austria). Differences in continuous variables between the groups were examined using an unpaired Student’s t-test after a symmetrical distribution was confirmed. Otherwise, the Mann-Whitney U test was used. Fisher’s exact test was used to analyze the frequencies of the categorical variables. Patient survival rates were estimated using the Kaplan-Meier method. Statistical significance was set at p < 0.05.
Results
A total of 24 patients were positive for KP; nine (37.5%) were positive in the string test while 15 (62.5%) were negative. The former group was defined as the hvKP-infected group, and the latter was defined as the non-hvKP-infected group. Blood cultures were collected when the patient visited the emergency department with fever or when the patient developed a fever before ICU admission. In both the groups, patients were aged (mean age, hvKP 80.4 vs. non-hvKP 75.7 years) and more often male (five patients (55.6%) vs. 12 patients (80.0%)). Baseline characteristics of the patients are presented in Table 1.
Table 1. Baseline characteristics upon ICU admission.
Continuous variables with normal distribution are expressed as mean ± SD and variables with non-normal distribution are expressed as median and IQ range (25th and 75th percentiles)
CKD; Chronic kidney disease, COPD; Chronic obstructive pulmonary disease, DM; Diabetes mellitus
| hvKP | Non-hvKP | P-value | |
| (N = 9) | (N = 15) | ||
| Age - years | 80.4±5.8 | 75.7±13.9 | 0.255 |
| Male - number (%) | 5 (55.6) | 12 (80.0) | 0.3564 |
| DM - number (%) | 3 (33.3) | 5 (33.3) | 1 |
| COPD - number (%) | 1 (11.1) | 0 (0) | 0.375 |
| CKD – number (%) | 5 (55.6) | 10 (66.7) | 0.6785 |
| Malignancy – number (%) | 2 (22.2) | 2 (13.3) | 0.6146 |
There was no statistically significant difference between the two groups. Upon ICU admission, there was no statistically significant difference in abscess formation, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, septic shock, or duration of hospitalization (Table 2).
Table 2. Severity and prognosis.
Continuous variables with normal distribution are expressed as mean ± SD and variables with non-normal distribution are expressed as median and IQ range (25th and 75th percentiles)
APACHE II; Acute physiology and chronic health evaluation II, SOFA; Sequential organ failure assessment
| hvKP | Non-hvKP | P-value | |
| (N = 9) | (N = 15) | ||
| Abscess – number (%) | 2 (22.2) | 1 (6.7) | 0.5331 |
| SOFA | 5.2±4.8 | 4.7±3.4 | 0.769 |
| APACHE II | 19.6 (15.0–20.0) | 17.0 (11.2–20.8) | 0.506 |
| Septic shock - number (%) | 5 (55.6) | 4 (26.7) | 0.2119 |
| Duration of hospitalization – days | 37.2 (12.0–51.0) | 32.3 (9.5–21.0) | 0.59 |
| 30-day mortality – number (%) | 2 (22.2) | 2 (13.3) | 0.615 |
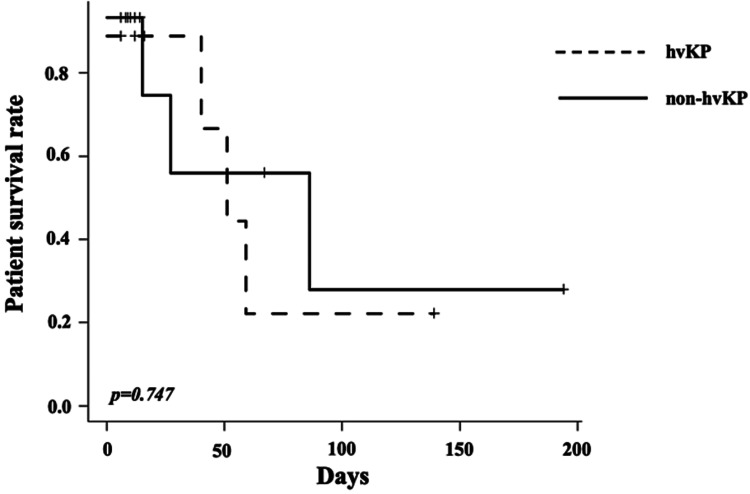
Further, no difference in life expectancy during hospitalization was seen (Figure 2).
Figure 2. Patient survival curve.
There was no difference in prognosis between the hvKP group and the non-hvKP group.
hvKP; Hypermucoviscous Klebsiella pneumoniae
Two patients with hvKP died within 24 h. No significant difference was seen in infectious sources: respiratory infection (2 (22.2%) vs. 1 (6.7%)), hepatobiliary infection (2 (22.2%) vs. 7 (46.7%)), and genitourinary infection (1 (11.1%) vs. 5 (33.3%)). The infectious sources are presented in Table 3. All patients initially received intravenous antibiotic therapy for bacteriological sensitivity.
Table 3. Site of infection.
| hvKP | Non-hvKP | |
| (N = 9) | (N = 15) | |
| Respiratory - number (%) | 2 (22.2) | 1 (6.7) |
| Hepatobiliary - number (%) | 2 (22.2) | 7 (46.7) |
| Genitourinary - number (%) | 1 (11.1) | 5 (33.3) |
| Other - number (%) | 4 (44.4) | 2 (13.3) |
Discussion
To the best of our knowledge, this is one of the first case series of hvKP infection in a critically ill patient. It confirmed the characteristics of hvKP in critical care settings.
KP has five major virulence factors, namely capsular serotype, hypermucoviscosity phenotype, lipopolysaccharide, siderophores, and pili [1]. The mucoviscous exopolysaccharide web produced by hvKP inhibits complement-mediated clearance and is considered a major virulence factor of hvKP [5]. This ability is associated with serum resistance and invasive infections, including abscess formation [6,7]. In a study in Taiwan, of the 51 cases with hvKP, 31 formed abscesses [8]. Based on these features, hypervirulent KP strains are defined as those with a hypermucoviscosity phenotype [9].
The prevalence of hvKP was 37.5% in our study, which is similar to the 38% prevalence reported previously [9]. The rate is generally higher for community-acquired infections than for nosocomial infections. All cases of hvKP were diagnosed as hvKP infection by blood cultures obtained from the emergency department in our study, suggesting that community-acquired infections were common even in critically ill patients.
Several factors that impair host immune responses, such as diabetes mellitus, alcoholism, malignancy, chronic obstructive pulmonary disease, renal failure, and glucocorticoid therapy, have been reported in KP infections [10,11]. However, obvious risks for hvKP infection have not been detected yet, and baseline characteristics are not reported to be different between patients with hv strains and those with non-hv strains [12]. A study on patients with KP liver abscess suggested that diabetes mellitus is a risk factor for hvKP infection; however, some patients developed KP liver abscess without any comorbidity [13]. In our study, the incidence of diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, and malignancy did not differ between the groups. However, in both groups, approximately one-third of the patients had diabetes mellitus, which has been proven to be a risk factor for KP infection, regardless of the hypermucoviscosity phenotype. Specific factors involved in hvKP infection in critically ill patients were not identified in our study, and diabetes mellitus was suggested as a common risk factor for KP infection.
Although hvKP was initially reported as the causative organism of pyogenic liver abscesses [14], previous studies have shown that hvKP can cause disseminated infections as well [15], and the prognosis of hvKP infection is generally worse than that of non-hv phenotypes. Even in patients without comorbidities, the mortality rate is reportedly 3%-42% [16]. In our study, 30-day all-cause mortality was statistically similar in both groups. However, two fatal cases of hvKP died within 24 hours of ICU admission, suggesting that patients with hvKP infection could deteriorate rapidly and have a poorer prognosis. The relationship between SOFA or APACHE II scores and KP infection has not been reported yet. Our current study revealed no statistically significant difference in these scores; however, the number of cases was limited, and the two fatal cases suggested that some cases of hvKP infection were more severe than those of non-hvKP infection. On the other hand, 30-day mortality was not different in our study, which also suggested that mortality can be evitable by an appropriate therapy, including drainage procedures and antibiotic therapies, even in critical cases with hvKP.
In our study, there was no tendency towards the infection site. We focused only on blood culture samples, and all the included patients had disseminated infections. According to previous studies, abscess formation is strongly associated with the hv phenotype [17], and hvKP developed abscesses not only in the liver but also at other sites with the spread of metastasis. In our study, the frequency of abscess formation did not differ between hv and non-hv phenotypes. However, the number of patients was limited, and further analyses would be required in this regard.
Our study had several limitations. First, the retrospective design of our study created a risk of information bias. Although we comprehensively assessed all patient records, possible inaccuracies in the provided data cannot be ruled out. Second, overlapping infections with bacteria other than KP could not be ruled out, because we analyzed only blood cultures. Third, the number of patients included in the study was limited.
Conclusions
In this case series, we found that critically ill patients with hvKP infection showed characteristics similar to those reported previously, in terms of patient background, comorbidities, and prognosis. In contrast, a review of two fatal cases of hvKP infection showed that the disease could rapidly become severe and have a poor prognostic outcome. Therefore, further case studies and analyses would be required in future.
Acknowledgments
We thank Editage (www.editage.com) for the English language editing.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Daichi Yomogida, Hiroyuki Kuwano, Tatsuya Miyakoshi, Shiori Mizuta, Shinjiro Horikawa, Yosinao Koshida
Acquisition, analysis, or interpretation of data: Daichi Yomogida, Shinjiro Horikawa, Yosinao Koshida
Drafting of the manuscript: Daichi Yomogida, Yosinao Koshida
Critical review of the manuscript for important intellectual content: Daichi Yomogida, Hiroyuki Kuwano, Tatsuya Miyakoshi, Shiori Mizuta, Shinjiro Horikawa, Yosinao Koshida
Supervision: Yosinao Koshida
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Podschun R, Ullmann U. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hypermucoviscosity: an extremely sticky phenotype of Klebsiella pneumoniae associated with emerging destructive tissue abscess syndrome. Kawai T. Clin Infect Dis. 2006;42:1359–1361. doi: 10.1086/503429. [DOI] [PubMed] [Google Scholar]
- 3.Appropriate use and reporting of uncontrolled case series in the medical literature. Kempen JH. Am J Ophthalmol. 2011;151:7–10. doi: 10.1016/j.ajo.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. J Exp Med. 2004;199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Alvarez D, Merino S, Tomás JM, Benedí VJ, Albertí S. Infect Immun. 2000;68:953–955. doi: 10.1128/iai.68.2.953-955.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. Lee HC, Chuang YC, Yu WL, et al. J Intern Med. 2006;259:606–614. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Invasive Klebsiella pneumoniae syndrome in North America. Nadasy KA, Domiati-Saad R, Tribble MA. Clin Infect Dis. 2007;45:0–8. doi: 10.1086/519424. [DOI] [PubMed] [Google Scholar]
- 8.Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Yu WL, Ko WC, Cheng KC, et al. Clin Infect Dis. 2006;42:1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 9.Antimicrobial resistance pattern, pathogenicity and molecular properties of Hypervirulent Klebsiella pneumonia (hvKp) among hospital-acquired infections in the Intensive Care Unit (ICU) Alharbi MT, Almuhayawi MS, Nagshabandi MK, et al. Microorganisms. 2023;11:661. doi: 10.3390/microorganisms11030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Ko WC, Paterson DL, Sagnimeni AJ, et al. Emerg Infect Dis. 2002;8:160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. Kang CI, Kim SH, Bang JW, et al. J Korean Med Sci. 2006;21:816–822. doi: 10.3346/jkms.2006.21.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mucoviscous characteristics of Klebsiella pneumoniae strains: a factor of clinical severity? Fauvet T, Tarantola A, Colot J, Merlet A, Goarant C, Marot B, Série M. Med Mal Infect. 2020;50:500–506. doi: 10.1016/j.medmal.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Klebsiella pneumoniae liver abscess: a new invasive syndrome. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Pomakova DK, Hsiao CB, Beanan JM, Olson R, MacDonald U, Keynan Y, Russo TA. Eur J Clin Microbiol Infect Dis. 2012;31:981–989. doi: 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 15.Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. Choby JE, Howard-Anderson J, Weiss DS. J Intern Med. 2020;287:283–300. doi: 10.1111/joim.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Shon AS, Bajwa RP, Russo TA. Virulence. 2013;4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hypervirulent Klebsiella pneumoniae. Patel PK, Russo TA, Karchmer AW. Open Forum Infect Dis. 2014;1:0. doi: 10.1093/ofid/ofu028. [DOI] [PMC free article] [PubMed] [Google Scholar]