Abstract
Introduction
Molnupiravir (MOV) is an oral antiviral for the treatment of individuals with mild-to-moderate COVID-19 and at high risk of progression to severe disease. Our objective was to conduct a systematic literature review (SLR) of evidence on the effectiveness of MOV in reducing the risk of severe COVID-19 outcomes in real-world outpatient settings.
Methods
The SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines and using pre-determined population, intervention, comparison, outcome, time, and study design inclusion criteria. Eligible studies were published between January 1, 2021, and March 10, 2023, and evaluated the real-world effectiveness of MOV compared to no treatment in reducing the risk of severe COVID-19 outcomes among outpatients ≥ 18 years of age with a laboratory-confirmed diagnosis of SARS-CoV-2 infection.
Results
Nine studies from five countries were included in the review. The size of the MOV-treated group ranged from 359 to 7818 individuals. Omicron variants of SARS-CoV-2 were dominant in all study periods. Most studies noted differences in the baseline characteristics of the MOV-treated and untreated control groups, with the treated groups generally being older and with more comorbidities. Eight studies reported that treatment with MOV was associated with a significantly reduced risk of at least one severe COVID-19 outcome in at least one age group, with greater benefits consistently observed among older age groups.
Conclusions
In this SLR study, treatment with MOV was effective in reducing the risk of severe outcomes from COVID-19 caused by Omicron variants, especially for older individuals. Differences in the ages and baseline comorbidities of the MOV-treated and control groups may have led to underestimation of the effectiveness of MOV in many observational studies. Real-world studies published to date thus provide additional evidence supporting the continued benefits of MOV in non-hospitalized adults with COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-00976-5.
Keywords: COVID-19, Lagevrio, Molnupiravir, Real-world studies, Systematic literature review
Plain Language Summary
COVID-19 continues to be a major source of morbidity and mortality. Throughout the pandemic, many countries authorized various therapies for the treatment of individuals presenting with mild-to-moderate COVID-19 and at high risk of progression to severe disease. Some of these therapies have since been rendered ineffective due to the emergence of Omicron variants in late 2021. The objective of the current study was to conduct a systematic literature review to assess real-world evidence on the effectiveness of molnupiravir, including effectiveness against COVID-19 caused by Omicron variants, to supplement the findings of the MOVe-OUT clinical trial and further inform on the potential clinical benefit and utility of this antiviral agent. Nine studies were included in the systematic literature review. We found that treatment with molnupiravir was effective in reducing the risk of severe outcomes from COVID-19 caused by Omicron variants, especially for older individuals. Differences in the ages and baseline comorbidities of the molnupiravir-treated and control groups may have led to underestimation of the effectiveness of molnupiravir in many observational studies. In summary, real-world effectiveness studies provide additional evidence supporting the continued benefits of molnupiravir in non-hospitalized adults with COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-00976-5.
Key Summary Points
| Why carry out this study? |
| Throughout the pandemic, many countries authorized various therapies for the treatment of individuals presenting with mild-to-moderate COVID-19 and at high risk of progression to severe disease, but some of these therapies have since been rendered ineffective due to the emergence of Omicron variants in late 2021. |
| Our objective was to conduct a systematic literature review of evidence on the effectiveness of molnupiravir in reducing the risk of severe COVID-19 outcomes in real-world outpatient settings. |
| What was learned from the study? |
| We identified nine real-world studies—all of which were conducted when Omicron variants of SARS-CoV-2 were dominant—that assessed the effectiveness of molnupiravir among non-hospitalized adults at high risk of progression to severe COVID-19, compared to controls who were not treated with any approved antiviral agent. |
| These real-world data provide additional clinical evidence in support of the continued benefits of molnupiravir in treating COVID-19 caused by Omicron variants of SARS-CoV-2, especially for older individuals. |
Introduction
COVID-19 continues to be a major source of morbidity and mortality: in November 2023, the World Health Organization (WHO) reported > 9400 new cases and > 120 deaths attributable to COVID-19 per week [1]. Due to decreases in testing, monitoring, and surveillance activities over time [2, 3], and especially since the WHO declared in May 2023 that the global health emergency had ended [4], both cases and deaths are likely under-reported; wastewater surveillance data suggest that clinical detection underestimates global cases 2- to 19-fold [5] and global excess mortality data suggest that the true COVID-19 mortality rate may be up to 2.7-fold higher than stated in official figures [6].
Severe COVID-19 outcomes—including hospitalization, intensive care unit (ICU) admission, invasive mechanical ventilation (IMV) use, and death—are more common among individuals ≥ 65 years of age, those who are immunocompromised or immunosuppressed, and those with certain other underlying medical conditions [7]. Many countries have authorized various therapies at different points during the pandemic for the treatment of individuals presenting with mild-to-moderate COVID-19 and at high risk of progression to severe disease, often via expedited mechanisms such as the Emergency Use Authorization status used by the US Food and Drug Administration (FDA) [8]. These therapies include several monoclonal antibodies (mAbs: casirivimab, imderimab, bamlavimab, etesevimab, and sotrovimab) that have since been rendered ineffective due to the emergence and rapid global dominance of Omicron variants of SARS-CoV-2 in late 2021 [8, 9]. The WHO recommended in May 2022 that these treatments be used only for individuals with COVID-19 caused by non-Omicron variants [8].
Several small-molecule drugs that remain effective against Omicron variants have also been approved for the treatment of mild-to-moderate COVID-19 [8]. The first to be approved by many regulatory agencies (e.g., the US FDA and the European Medicines Agency [EMA]) was remdesivir (RDV; Veklury®, Gilead Sciences, Inc.), an inhibitor of the SARS-CoV-2 RNA-dependent RNA polymerase enzyme [10, 11]. The use of this therapy has been limited because a full treatment course requires daily intravenous administration for at least 3 days, representing a logistical challenge for outpatient use [10, 11]. The viral protease inhibitor ritonavir-boosted nirmatrelvir (NRM/r; Paxlovid™, Pfizer) is an alternative treatment for COVID-19 that is taken orally and that has been approved by the FDA and EMA, among others [12, 13]. However, NRM/r is contraindicated for individuals with certain pre-existing conditions, including chronic kidney disease (CKD) and liver diseases, and also carries an FDA warning for significant drug–drug interactions caused by the ritonavir component, which can increase the blood concentration of medications that are metabolized by the cytochrome P4503A enzyme [12, 13]. There is thus still an urgent need for effective antiviral agents that can be taken orally by individuals at high risk of progression to severe disease and for whom NRM/r is contraindicated.
Molnupiravir (MOV; Lagevrio™, Merck & Co., Inc., Rahway, NJ, USA) is an oral antiviral that has been granted Emergency Use Authorization by the FDA for the treatment of mild-to-moderate COVID-19 in individuals at high risk of progression to severe disease and for whom other available antiviral therapies are not accessible or recommended, for example due to CKD or potential drug–drug interactions with NRM/r [14–16]. The drug has also been approved or authorized in many other countries [17–22]. In the MOVe-OUT phase 3 clinical trial, MOV treatment of non-hospitalized adults reduced the risk of 29-day all-cause hospitalization or death by 31% and the risk of 29-day all-cause mortality by 89% compared to placebo [23]. A 2022 meta-analysis of four studies found no evidence for significant differences between molnupiravir and placebo in terms of all adverse events, serious adverse events, or adverse events leading to death or treatment discontinuation [24].
The MOVe-OUT clinical trial enrolled participants from May to September 2021—before Omicron variants of SARS-CoV-2 became globally dominant—and included only unvaccinated participants [23]. The objective of the current research was thus to conduct a systematic literature review (SLR) to assess real-world evidence on the effectiveness of MOV, including effectiveness against COVID-19 caused by Omicron variants, to supplement the findings of the clinical trial and further inform on the potential clinical benefit and utility of this antiviral agent.
Methods
Study Inclusion Criteria and Search Strategy
The SLR was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 reporting guidelines [25]. The study used pre-determined population, intervention, comparison, outcome, time, and study design (PICOTS) inclusion criteria (Table 1) [26]. We included real-world studies published between January 1, 2021 and March 10, 2023 that evaluated the effectiveness of molnupiravir (MOV) against severe outcomes of COVID-19 (primarily hospitalization and/or death) among outpatients ≥ 18 years of age with a diagnosis of SARS-CoV-2 infection, as confirmed by nucleic acid amplification or rapid antigen test. Consistent with the MOVe-OUT clinical trial [23], eligible studies compared outcomes between individuals who received MOV and those who did not receive any authorized COVID-19 treatment. We excluded studies of populations with specific medical conditions other than SARS-CoV-2 infection.
Table 1.
Patient, intervention, comparison, outcome, time, and study design (PICOTS) criteria for study inclusion
| Study variable | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
Confirmed SARS-CoV-2 infection diagnosis (by either nucleic acid amplification test or rapid antigen test) occurring after the approval date of molnupiravir in each country included within a study Outpatients Population-based samples (non-disease specific) Age ≥ 18 years |
Populations with specific medical conditions other than SARS-CoV-2 infection diagnosis |
| Intervention | Prescription for molnupiravir | |
| Comparison | People with confirmed SARS-CoV-2 infection diagnosis who did not receive any antiviral agent, including nirmatrelvir/ritonavir, remdesivir, or any monoclonal antibody | Studies comparing MOV only to other approved antiviral agents |
| Outcome |
All-cause hospitalization All-cause death COVID-19-related hospitalization COVID-19-related death Related outcomes such as all-cause or COVID-19-related ICU admission or IMV use |
|
| Time | January 1, 2021–March 10, 2023 | |
| Study design |
Non-interventional real-world studies Estimation of a relative risk measure with corresponding confidence interval Covariate adjustment Longitudinal data analysis |
Clinical trials Single-arm studies |
ICU intensive care unit, IMV invasive mechanical ventilation
The literature search terms used to identify potentially eligible studies from the Embase and Scopus databases were developed in collaboration with a medical sciences librarian (Table S1, Supplementary Material). Additional potentially eligible COVID-19-related studies were identified via supplementary ongoing literature surveillance to capture pre-prints and other publication types that are not indexed in the study databases.
Study Selection
All studies identified in the initial literature search were imported into Rayyan, a collaborative platform for the conduct of SLR [27]. Duplicates were discarded, and the title and abstract of each unique study were reviewed independently by two epidemiologists. We excluded studies that did not meet the pre-determined PICOTS inclusion criteria based on this initial review. The full text of each remaining study was then assessed against the PICOTS criteria by the two independent reviewers, and ineligible studies were excluded. Any discrepancies between the two reviewers would have been resolved via discussion with a third independent member of the study team, but no such discrepancies arose at any stage of the study selection process.
Assessment of Risk of Bias
All included studies were assessed independently by two reviewers for risk of bias across seven domains (confounding, selection bias, intervention, deviations from intended interventions, missing data, outcome measurement, and selection of reported result) using the cohort-type study version of the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [28]. In this framework, a low risk of bias indicates that the study was comparable to a well-performed randomized controlled trial (RCT) in a given domain, while a critical risk of bias in any domain indicates that a study was not likely to provide useful evidence [28]. Per study-specific criteria, failure to control for immortal time bias due to differences in index dates and follow-up periods between the MOV-treated and control groups resulted in a moderate risk for selection bias, while studies that did not monitor adherence to MOV therapy were assigned a rating of moderate risk in the deviation from intended intervention domain. Finally, studies assessing COVID-19-related hospitalization/mortality as outcomes, or those that had the potential for outcome under-ascertainment based on the data sources used, were judged to have a moderate risk of bias in the outcome measurement domain. An overall risk of bias was then assigned based on the scores in each individual domain. Again, any discrepancies between the two reviewers would have been resolved via discussion with a third independent member of the study team, but no such discrepancies arose.
Data Extraction
A single reviewer extracted data on study design, setting, and outcomes from the full text of each study (including all available supplementary material) into a standardized data abstraction form. The accuracy of all abstracted information was then independently confirmed by a second reviewer; any discrepancies were resolved as above. Statistical significance was defined as a risk measure (hazard ratio [HR], odds ratio [OR], or relative risk [RR]) with a 95% CI that was entirely > 1 or entirely < 1.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Characteristics
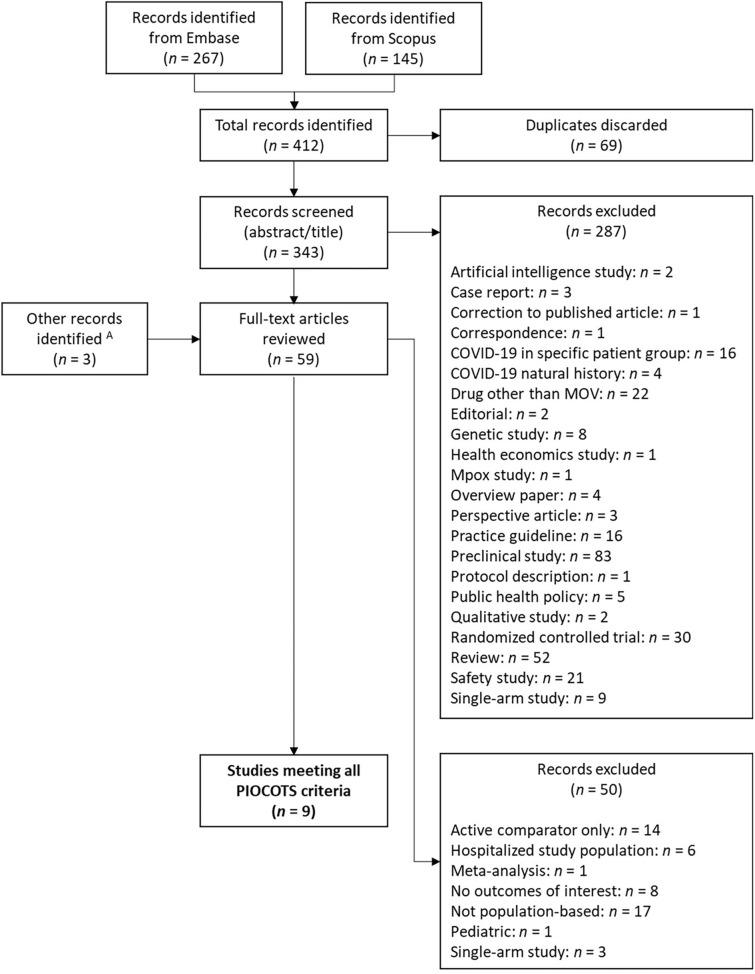
A total of 412 potentially eligible studies were identified: 267 from Embase and 145 from Scopus (Fig. 1). After excluding 69 duplicates, 343 documents underwent an initial title and abstract screen, of which 287 were excluded. The most common reasons for exclusion were preclinical study (n = 83), review article (52), RCT (30), drug other than MOV (22), and safety study (21). The remaining 56 studies, plus an additional three records identified via supplementary literature surveillance, underwent full-text review. Fifty records were excluded, with the most common reason being non-population-based study (17), active comparator only (14), and no eligible outcomes (8).
Fig. 1.
Flow chart of systematic literature review to identify, screen, and select eligible real-world studies evaluating the effectiveness of molnupiravir. PICOTS population, intervention, comparator, outcome, time, and study design. A Additional documents were identified via ongoing literature surveillance
Nine studies (six peer-reviewed studies and three pre-prints) met all PICOTS criteria and were included in the SLR (Table 2) [29–37]. Two of the three pre-prints have subsequently been published as peer-reviewed articles [38, 39]. The peer-reviewed version of Paraskevis et al. has a different title and abstract compared to the pre-print version, but the data appear to be the same [33, 39]. As the study period and results in the peer-reviewed version of Bajema et al. have been updated compared to the pre-print version, we revised the analysis to incorporate the published version of Bajema et al. rather than the pre-print [30, 38].
Table 2.
Summary of study characteristics
| Citation | Locationa | Study period | Dominant variant(s) | Relevant analysis population (N) | Risk level of study population | Prior immunity (%) | MOV treatment initiation window | Approach to confounder adjustment |
|---|---|---|---|---|---|---|---|---|
| Arbel (2022) [29]b, c | Israel [41] | 16 Jan–31 Mar 2022 | Omicron (BA.1) |
40–64 years of age MOV: 224 Control: 6075 ≥ 65 years of age MOV: 845 Control: 12,724 |
≥ 40 years of age High risk for progression to severe disease Not eligible for NMV/r due to DDI or impaired kidney function |
Documented prior infection and/or ≥ 2 vaccine doses MOV: 93 Control: 92 |
≤ 5 days after positive test | Cox proportional hazards model with time-varying covariates for treatment assignment to address immortal time bias |
| Bajema (2022) [30, 38]d | US [15] | 1 Jan–31 July 2022 | Omicron |
MOV: 3504 Control: 3504 |
≥ 18 years of age High risk for progression to severe disease |
≥ 1 vaccine dose MOV: 85.6 Control: 84.8 |
< 10 days after positive test |
Target trial emulation framework Exact and propensity score-matched cohorts Controls assigned index date same number of days after positive test as the treatment initiation date of matched MOV-treated individual to address immortal time bias |
| Evans (2023) [31]e | UK [22] | 16 Dec 2021–22 Apr 2022 | Omicron (BA.1, BA.2) |
MOV: 359 Control: 4973 |
At highest risk for hospitalization/death |
≥ 1 vaccine dose Treated: 98.2f Control: 95.6 |
≤ 7 days after positive test | Cox proportional hazards model with time-varying covariate for treatment assignment to address immortal time bias |
| Najjar-Debbiny (2023) [32]e | Israel [41] | 1 Jan–28 Feb 2022 | Omicron |
MOV: 2661 Control: 2661 |
≥ 18 years of age ≥ 1 comorbidity or condition associated with high risk for severe disease |
≥ 2 vaccine doses with most recent dose 7–180 days before diagnosis MOV: 74.6 Control: 76.1 |
≤ 5 days after positive test |
Propensity score-matched cohorts Cox proportional hazards model |
| Paraskevis (2023) [33]b, c | Greece [42] | 2 Feb–5 Mar 2022 | Omicron (BA.1, BA.2) |
MOV: 4240 Control: 4240 |
≥ 65 years of age Risk factors for progression to severe disease |
≥ 1 vaccine dose MOV: 87.4 Control: 79.7 |
≤ 3 days after symptom onset or positive test |
Matched cohorts (by age and week of SARS-CoV-2 infection diagnosis); not adjusted for comorbidities due to unavailability of data for control group |
| Wai (2023) [34]e | Hong Kong [18] | 22 Feb–31 Mar 2022 | Omicron |
MOV: 5345 Control: 23,430 |
≥ 60 years of age or < 60 years of age with ≥ 1 chronic disease | NR | ≤ 7 days after attending participating outpatient clinic |
Inverse probability of treatment weighting-adjusted Cox proportional Model Immortal time bias was not accounted for |
| Wong (2022) [35]e | Hong Kong [18] | 26 Feb–26 Jun 2022 | Omicron (BA.2.2) |
MOV: 4983 Control: 49,234 |
≥ 60 years of age or ≥ 18 years of age with risk factors for progression to severe disease |
Fully vaccinatedg MOV: 16.1 Control: 12.4 |
≤ 5 days after positive test |
Propensity score-matched cohorts with Cox proportional hazards model in retrospective cohorts Time-varying covariate for treatment assignment to address immortal time bias Conditional logistic regression for case–control sensitivity analysis |
| Xie (2023) [36]b, e | US [15] | 5 Jan–20 Oct 2022 |
Omicron (BA.1, BA.2, BA.5) |
MOV: 7818 Control: 78,180 |
≥ 1 risk factor for progression to severe disease |
≥ 1 vaccine dose MOV: 85.8 Control: 82.6 |
≤ 5 days after positive test |
Target trial emulation framework Propensity score-matched cohorts Clone method with inverse probability of censoring weighting, which also addressed immortal time bias |
| Yip (2023) [37]e | Hong Kong [18] | 16 Feb–31 Mar 2022 | Omicron |
MOV: 4798 Control: 4758 |
Elderly (not defined) or high-risk with incomplete vaccination |
Complete vaccination: population rateh MOV: 36.1 Control: 55.9 |
≤ 5 days after positive test | Cox proportional hazards model with propensity score adjustment |
DDI drug–drug interaction, MOV molnupiravir, NMV/r nirmatrelvir/ritonavir, NR not reported, UK United Kingdom, US United States
‘Control’ defined as individuals not receiving any approved treatment for COVID-19 and used as comparator group for molnupiravir-treated arm
aReferences are to the guidelines for the use of molnupiravir in each location
bIdentified via supplemental literature surveillance
cPre-print
dIdentified as a pre-print and subsequently published as a peer-reviewed article with updated study period and results; the peer-reviewed version was included in the SLR
ePeer-reviewed study
fA combined prior immunity percentage was reported for all individuals receiving any treatment (molnupiravir, nirmatrelvir/ritonavir, and sotrovimab arms)
gDefined as ≥ 2 doses of BNT162b2 vaccine or ≥ 3 doses of Vero Cell or CoronaVac vaccine. The time since last vaccine dose was also measured
hDefined as ≥ 2 doses of BNT162b2 vaccine or ≥ 3 doses of CoronaVac vaccine. Vaccination data were available at a population-level; background vaccination rate was defined for each participant as the corresponding population vaccination rate on index date for individuals of the same age and gender
One of the nine included studies was conducted in Greece [33], three in Hong Kong [34, 35, 37], two in Israel [29, 32], one in the UK [31], and two in the US [30, 36, 38]. Some studies from the same country used the same data source (electronic health records from the Hong Kong Hospital Authority in three studies [34, 35, 37], the Clalit Health System in two Israeli studies [combined with another data source in one of these studies] [29, 32], and the US Veterans Health Administration COVID-19 Shared Data Resource in two studies [combined with other data sources in one of these studies]) [30, 36, 38]. However, studies using the same data source had different study periods and methodologies. The study periods were of different lengths, but all began between December 16, 2021 and February 26, 2022 and ended between February 28 and October 20, 2022; Omicron variants of SARS-CoV-2 were thus dominant during all study periods and in all study locations [29–38, 40].
The size of the MOV-treated study population ranged from 359 to 7818, and all study populations had age-related and/or other risk factors for progression to severe COVID-19 [29–38]. Prior immunity to SARS-CoV-2 (defined in various ways based on vaccination and/or previous infection) was assessed directly in seven studies [29–33, 35, 36, 38] and inferred from age- and sex-stratified population-level vaccination data in one study [37]. The proportion of the MOV-treated group with prior SARS-CoV-2 immunity ranged from 16.1% in a Hong Kong-based study [35] to 98.2% in the UK study [31]. The most common MOV treatment initiation window was ≤ 5 days after a positive test (five studies) [29, 32, 35–37]; a 3-day window was used in one study [33], a 7-day window in two studies [31, 34], and a 10-day window in one study [30, 38]. All studies used cohort designs with longitudinal data, patient-level follow-up, and robust statistical methods (e.g., multivariate regression or propensity score-based approaches) to address potential confounding [29–38].
Assessment of Study Bias
Two studies had a serious overall risk of bias, assigned in both cases due to an assessment of serious risk of bias in the confounding domain (Table 3) [31, 33]. In the case of Paraskevis et al., the serious risk of confounding was assigned because the authors were unable to obtain information on comorbidities among the untreated control group [33]. The authors attempted to address this limitation by excluding individuals < 65 years of age and by matching treated individuals to controls by age, since the number of comorbidities generally increases with age [33]. Nevertheless, comorbidities are an important risk factor for severe COVID-19 outcomes [7], and thus the study was assigned a serious risk of bias in the confounding domain. In the other study with a serious risk of confounding bias, Evans et al. did not use matching or propensity score adjustment for comparisons between the MOV-treated and control groups [31]. Although no statistical tests of differences between the treated and control groups were reported, the authors noted differences between the two groups that could potentially bias the study results in favor of MOV: the treated group were on average younger than the control group (mean age 53 versus 57 years), had fewer comorbidities (e.g., 74.6 vs. 62.8% had a Charlson comorbidity index of 0–10), and had a higher degree of prior immunity (36.3 vs. 17.6% had received ≥ 4 doses of a SARS-CoV-2 vaccine) [31]. The study reported univariate association analyses showing that younger individuals (i.e., those < 60 years of age) and those who had received ≥ 4 vaccine doses were more likely to avoid hospital admission or death within 28 days than were older or less vaccinated individuals, respectively [31]. Both studies had a low or moderate risk of bias in all other domains.
Table 3.
Risk of bias assessments
| Citation | Domain | |||||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Selection bias | Intervention | Deviations from intended intervention | Missing data | Outcome measurement | Selection of reported results | Overall assessment of bias | |
| Arbel (2022) [29] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Bajema (2022) [30, 38] | Moderate | Low | Low | Moderate | Low | Low | Low | Moderate |
| Evans (2023) [31] | Serious | Low | Low | Moderate | Low | Low | Low | Serious |
| Najjar-Debbiny (2023) [32] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Paraskevis (2023) [33] | Serious | Moderate | Low | Low | Low | Moderate | Low | Serious |
| Wai (2023) [34] | Moderate | Moderate | Low | Moderate | Low | Low | Low | Moderate |
| Wong (2022) [35] | Moderate | Moderate | Low | Moderate | Low | Moderate | Low | Moderate |
| Xie (2023) [36] | Moderate | Low | Low | Moderate | Low | Moderate | Low | Moderate |
| Yip (2023) [37] | Moderate | Moderate | Low | Moderate | Low | Low | Low | Moderate |
The risk of bias for each study was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [28]
The remaining seven studies were determined to have a moderate overall risk of bias. The domains in which these seven studies were found to have a moderate, rather than a low, risk of bias were confounding (all seven studies; moderate is the lowest possible risk of confounding bias for a non-randomized study [28]), deviations from intended intervention (five studies [30, 34–38]), outcomes measurement (four studies [29, 32, 35, 36]), and selection bias (three studies [34, 35, 37]).
Seven studies were appropriately designed to accurately classify all time at risk of the outcome for both the MOV-treated and the control groups, and thereby mitigated the risk of immortal time bias. However, two of the Hong Kong-based studies did not account for immortal time bias due to differences in index dates and follow-up periods between the MOV-treated and control groups [34, 37]. Six studies accounted for other key confounders (e.g., age, prior immunity, and time since last vaccine dose). One of the three exceptions was Paraskevis et al., where (as noted above) the authors were not able to adjust for any differences in comorbidity distribution between the treated and control groups [33]. In addition, two of the three studies conducted in Hong Kong either did not report SARS-CoV-2 vaccination status [34] or inferred the proportion of each group who were fully vaccinated from age-, sex-, and index-date-matched population-level data, rather than from individual vaccination status data [37].
Effectiveness of Molnupiravir in Real-World Studies
Most studies noted differences in the baseline characteristics of the unmatched MOV-treated and untreated control groups. The treated group was on average older than the unmatched control group in five studies [29, 32, 34, 36, 37], younger in two studies [31, 35], and of similar age in two studies [30, 33, 38]. The treated group had more baseline comorbidities than the control group in six studies [29, 32, 34–37], fewer comorbidities in one study [31], and a similar baseline comorbidity distribution in one study [30, 38]; baseline comorbidity comparisons were not reported in one study due to a lack of data for the control group [33].
Seven studies reported the effectiveness of MOV in reducing the risk of hospitalization [29, 30, 33–38], seven reported effectiveness in reducing the risk of mortality [29, 30, 32–36, 38], four reported composite hospitalization/mortality outcomes [30, 31, 33, 36, 38], and four reported other outcomes such as severe disease, ICU admission, IMV use, and/or other composite outcomes [30, 32, 35, 37, 38] (Table 4). Three studies reported COVID-19-related outcomes [29, 32, 33], five reported all-cause outcomes [30, 31, 34, 36–38], and one reported a mixture of both [35]. The outcomes measurement period generally ranged from 28 to 35 days, with the exception of a 10-day COVID-19-related hospitalization outcome in one study [33].
Table 4.
Real-world effectiveness of molnupiravir in reducing the risk of hospitalization, mortality, and/or other outcomes
| Citation | Hospitalization outcome: risk measure (95% CI) | Mortality outcome: risk measure (95% CI) | Hospitalization/mortality composite outcome: risk measure (95% CI) | Other outcome: risk measure (95% CI) |
|---|---|---|---|---|
| Arbel (2022) [29] |
35-day COVID-19-related hospitalization 40–64 years of age: HR 1.80 (0.86–3.77) ≥ 65 years of age: HR 0.55 (0.34–0.88) |
35-day COVID-19-related mortality 40–64 years of age: HR 12.82 (3.41–48.17) ≥ 65 years of age: HR 0.26 (0.10–0.73) |
NR | NR |
| Bajema (2022) [30, 38] |
30-day all-cause hospitalization RR 0.98 (0.81–1.18) |
30-day all-cause mortality RR 0.23 (0.13–0.43) |
30-day all-cause hospitalization/mortality Overall: RR 0.82 (0.68–0.98) 18–64 years of age: RR 0.33 (0.04–2.92) ≥ 65 years: RR 0.85 (0.71–1.02) |
30-day all-cause ICU admission RR 1.06 (0.66–1.68) 30-day all-cause IMV use RR 0.93 (0.47–1.83) |
| Evans (2023) [31] | NR | NR |
28-day all-cause hospitalization/mortality HR 0.49 (0.29–0.83) |
NR |
| Najjar-Debbiny (2023) [32] | NR |
28-day COVID-19-related mortality HR 0.81 (0.46–1.43) |
NR |
28-day severe COVID-19a HR 0.75 (0.51–1.12) 28-day COVID-19-related mortality/severe COVID-19b composite outcome Overall: HR 0.83 (0.57–1.21) ≤ 75 years of age: HR 2.46 (1.13–5.33) > 75 years of age: HR 0.54 (0.34–0.86) |
| Paraskevis (2023) [33] |
10-day COVID-19-related hospitalization Overall: OR 0.40 (0.32–0.48) 65–69 years of age: OR 1.05 (0.60–1.82) 70–74 years of age: OR 0.65 (0.38–1.08) 75–79 years of age: OR 0.42 (0.26–0.68) ≥ 80 years of age: OR 0.29 (0.22–0.39) |
35-day COVID-19-related mortality OR 0.31 (0.22–0.43) |
35-day COVID-19-related hospitalization/mortality OR 0.40 (0.34–0.48) |
NR |
| Wai (2023) [34] |
28-day all-cause hospitalization OR 0.72 (0.52–0.98) |
28-day all-cause mortality HR 0.31 (0.24–0.40) |
NR | NR |
| Wong (2022) [35] |
28-day COVID-related hospitalization Overall: HR 0.98 (0.89–1.06) ≤ 60 years of age: HR 1.15 (0.84–1.59) > 60 years of age: HR 0.89 (0.81–0.97) |
28-day all-cause mortality Overall: HR 0.76 (0.61–0.95) ≤ 60 years of age: HR 2.31 (0.77–6.90) > 60 years of age: HR 0.75 (0.60–0.93) |
NR |
In-hospital disease progressionb Overall: HR 0.57 (0.43–0.76) ≤ 60 years of age: HR 1.18 (0.35–3.98) > 60 years of age: HR 0.55 (0.42–0.73) |
| Xie (2023) [36] |
30-day all-cause hospitalization RR 0.80 (0.71–0.90) |
30-day all-cause mortality RR 0.35 (0.24–0.49) |
30-day all-cause hospitalization/mortality RR 0.72 (0.64–0.79) |
NR |
| Yip (2023) [37] |
30-day all-cause hospitalization Overall: HR 1.17 (0.99–1.39) < 70 years of age: HR 1.07 (0.73–1.56) ≥ 70 years of age: HR 1.15 (0.95–1.39) ≥ 60 years of age or with comorbidity: HR 1.07 (0.90–1.26) |
NR | NR |
30-day all-cause mortality/ICU admission/IMV composite outcome Overall: HR 1.12 (0.68–1.82) < 70 years of age: HR 0.97 (0.26–3.66) ≥ 70 years of age: HR 1.08 (0.65–1.82) ≥ 60 years or with comorbidity: HR 1.04 (0.63–1.73) |
CI confidence interval, HR hazard ratio, ICU intensive care unit, IMV invasive mechanical ventilation, NR not recorded, OR odds ratio, RR relative risk
Statistically significant differences shown in bold
aDefined as oxygen saturation < 94% on room air, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen < 300 mmHg, or respiratory rate > 30 breaths per minute
bDefined as in-hospital mortality, invasive mechanical ventilation, or intensive care unit admission
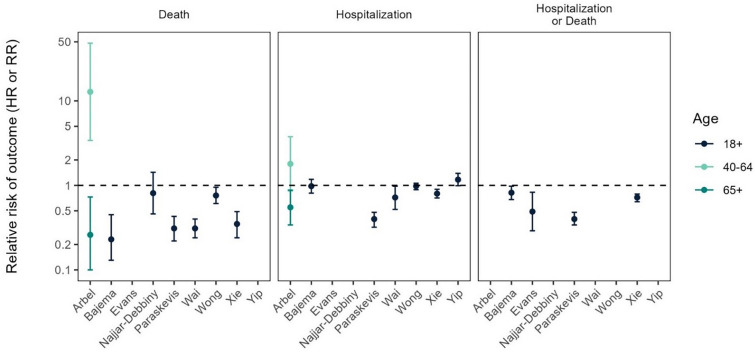
The overall pattern among the outcomes of the included studies was that MOV was effective in reducing the risk of severe COVID-19 outcomes, particularly among older age groups: eight of the nine studies reported a statistically significantly reduced risk of hospitalization, death, or composite outcomes in ≥ 1 age group [29–36, 38] (Table 4, Fig. 2). Of the six studies that reported hospitalization outcomes in the overall study population, three reported that MOV was associated with a significantly reduced risk of all-cause or COVID-19-related hospitalization compared to controls; two others reported a numerically (i.e., non-statistically significantly) lower risk [30, 33–36, 38]. Three other studies that reported hospitalization outcomes stratified by age group found that MOV was associated with a significantly reduced risk of hospitalization in older age groups (≥ 65 years of age in Arbel et al.; 75–79 and ≥ 80 years of age in Paraskevis et al.; and > 60 years of age in Wong et al.) but a numerically higher risk of hospitalization among younger age groups [29, 33, 35]. However, Yip et al. reported a numerically higher risk of 30-day all-cause hospitalization among the overall MOV-treated group compared to the control group [37].
Fig. 2.
Summary of the real-world effectiveness of molnupiravir in reducing the risk of hospitalization and/or mortality, stratified by age group (in years)
Five of the six studies that assessed mortality outcomes for the overall study population reported that treatment with MOV was associated with significantly decreased risk of all-cause or COVID-19-related death compared to controls, while the other study observed a numerically lower risk [30, 32–36, 38]. Two studies reported mortality outcomes stratified by age group; as with the hospitalization outcomes, treatment with MOV was associated with a significantly reduced risk among older age groups (≥ 65 years of age in Arbel et al. and > 60 years of age in Wong et al.), but a significantly (Arbel) or numerically (Wong) increased risk among younger individuals [29, 35]. Treatment with MOV was also associated with a significantly reduced risk of a composite hospitalization/mortality outcome in four studies [30, 31, 33, 36, 38]. One of these studies also reported this composite outcome stratified by age group and observed a numerically reduced risk of all-cause hospitalization or mortality compared to controls in both the 18–64 years and the ≥ 65 years age groups [30, 38].
Four studies reported outcomes other than hospitalization or mortality. Bajema et al. found that in the overall study population, MOV treatment was associated with a numerically higher risk of all-cause ICU admission and a numerically lower risk of all-cause IMV use compared to controls [30, 38]. Yip et al. reported a composite outcome combining all-cause mortality, ICU admission, and IMV use, and found that overall, in individuals ≥ 70 years of age, and in those ≥ 60 years of age with comorbidity, treatment with MOV was associated with a numerically higher risk; in contrast, individuals < 70 years of age and treated with MOV had a slightly numerically lower risk of this outcome compared to controls [37]. In Najjar-Debbiny et al., the risk of both severe COVID-19 and a severe COVID-19/COVID-19-related mortality composite outcome was numerically lower among all MOV-treated individuals than among controls; for the composite outcome, MOV treatment was associated with a significantly higher risk among individuals ≤ 75 years of age and a significantly lower risk among those > 75 years of age [32]. Finally, Wong et al. found that treatment with MOV was associated with a significantly lower risk of in-hospital disease progression in the overall population and among those > 60 years of age, but a numerically higher risk among those ≤ 60 years of age [35].
Discussion
In this SLR, we identified nine real-world studies that assessed the effectiveness of MOV among non-hospitalized adults at high risk of progression to severe COVID-19, compared to controls who were not treated with any approved antiviral agent. The studies were conducted in multiple locations with populations at different levels of baseline risk for severe COVID-19 outcomes and with different levels of prior immunity. All studies took place when Omicron variants of SARS-CoV-2 were dominant worldwide [40]. Overall, the evidence from eight of the nine included real-world, Omicron-era studies was consistent with the conclusion of the MOVe-OUT clinical trial that MOV is effective in reducing the risk of the most severe consequences of COVID-19 among non-hospitalized adults, particularly among older age groups.
All of the included real-world study populations had potentially relevant differences to the MOVe-OUT clinical trial population that may have affected the estimation of MOV effectiveness [23]. For instance, in contrast to the clinical trial (from which vaccinated individuals were excluded), the study populations in all nine of the included manuscripts had some (highly variable) degree of prior immunity to SARS-CoV-2, via vaccination and/or previous infection. The MOVe-OUT inclusion criteria also specified laboratory confirmation of SARS-CoV-2 infection and onset of symptoms ≤ 5 days before randomization [23]. In contrast, the treatment initiation window in all nine of the included real-world studies was based on date of positive SARS-CoV-2 test rather than date of onset of symptoms; the length of the window varied between studies, from ≤ 3 to < 10 days following a positive test. Studies with longer treatment initiation windows may have included participants who were not treated with MOV in accordance with the drug’s FDA approval, which states that treatment should begin ‘as soon as possible after a diagnosis of COVID-19 has been made, and within 5 days of symptom onset’ [15].
Our risk of bias assessment identified several other factors that may also have affected the estimation of the effectiveness of MOV in the reviewed studies, including potential baseline differences between treated and control groups. In most studies that compared the baseline characteristics of the unmatched MOV-treated and control groups, the MOV-treated group was generally older and/or had more comorbidities than the respective control group [29, 32, 34–37]. These studies, as well as a study that was not able to compare baseline comorbidities between the MOV-treated and control groups [33], may therefore have underestimated the effectiveness of MOV. Indeed, Yip et al. (who reported non-significant increases in risk associated with MOV treatment) noted that the effectiveness of MOV may have been underestimated because local treatment guidelines at the time of the study restricted the use of MOV to individuals at the highest level of risk for severe COVID-19 outcomes [37].
Further, although MOV is indicated for individuals with mild-to-moderate COVID-19, none of the included studies required that members of both the MOV-treated and the control groups have mild-to-moderate illness on the index date, and none adjusted for baseline severity of illness. The direction of any resulting bias is unknown, although studies where the MOV-treated group included a higher proportion of individuals with symptomatic or more severe cases of COVID-19 would likely underestimate the effectiveness of MOV. In addition, two of the Hong Kong-based studies did not account for immortal time bias; differences in index dates and follow-up periods between the MOV-treated and control groups may therefore have resulted in overestimation of the effectiveness of MOV in these studies [34, 37].
The use of potentially heterogeneous and/or subjective outcome measures may also have affected the effectiveness estimates of some studies. For instance, Yip et al. noted that non-mortality outcomes may have been subject to surveillance bias, with MOV-treated patients being monitored more closely than controls [37]. Similar issues may have occurred in other studies that reported non-mortality outcomes, since the criteria for decisions regarding hospitalization, ICU admission, and IMV use can vary depending on factors such as local treatment guidelines, individual patients’ comorbidities and general medical history, individual physician discretion, and the availability of hospital/ICU beds and IMV equipment. Even among studies that reported COVID-19-specific mortality outcomes, the determination of a COVID-19-related death may vary depending on local guidelines, the criteria used by specific study protocols, and other factors such as individual physician or coroner discretion. All-cause mortality outcomes are not subject to these potential biases, but could still result in an underestimation of the effectiveness of MOV in those studies where the treated group had more comorbidities than the control group and therefore a presumably higher risk of non-COVID-19-related death.
The overall generalizability of the findings of the SLR is unknown for several reasons, including small numbers of outcome events in some studies. For example, one of the two studies that reported a significantly increased risk of severe COVID-19 outcomes among younger age groups treated with MOV reported a small number (n = 4) of COVID-19-related deaths in the younger age group [29]; the absolute number of events was not reported for subgroup analyses in the other study [32]. In addition, we excluded studies of the effectiveness of MOV in populations with specific medical conditions other than SARS-CoV-2 infection. However, in several jurisdictions (including the US) the use of MOV is recommended for populations with an increased risk of progression to severe COVID-19 outcomes, for example due to CKD, immunocompromised status, or other medical conditions [7]. Although some individuals with these conditions were recruited into the included studies, their outcomes were not assessed separately. Filling this knowledge gap should be a priority area for future research, to provide evidence on how best to protect high-risk individuals with underlying medical conditions from COVID-19-related hospitalization, death, and other severe clinical outcomes. Finally, the heterogenous designs and populations of the included studies, including differences in the level of prior immunity through vaccination and/or infection, precluded any direct comparisons of their outcomes; a meta-analysis was considered but deemed to be infeasible due to the lack of overlapping estimates for the study outcomes within distinct study populations. Future research should include rigorous network meta-analyses to aid in the ongoing assessment of the real-world effectiveness of MOV.
Conclusions
In conclusion, this SLR identified and assessed nine geographically diverse studies of the effectiveness of MOV in real-world populations and during a different phase of the pandemic compared to the MOVe-OUT clinical trial, with Omicron variants of SARS-CoV-2 dominant in all studies. In general, the included studies found that MOV was effective at reducing the risk of the most serious consequences of COVID-19 in real-world settings, particularly for older populations. While several factors may have influenced each study’s estimation of MOV effectiveness, including the level of prior immunity among the study population, the clearest pattern was that the treated groups in many studies were older and had more baseline comorbidities than the untreated control groups, which may have resulted in underestimation of the effectiveness of MOV. Overall, these real-world data provide additional clinical evidence in support of the continued benefits of MOV in treating COVID-19 caused by Omicron variants of SARS-CoV-2, especially for older individuals.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing/Editorial Assistance.
Medical writing assistance was provided by Cath Ennis, PhD, in collaboration with ScribCo, and funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author Contributions
Conceptualization: Julia Richmond DiBello, Xinyue Liu, Amy Puenpatom, and Deanna D. Hill. Literature review: Julia Richmond DiBello, Xinyue Liu, Amy Puenpatom, Kathryn Peebles, Nazleen Khan, and Deanna D. Hill. Data analysis: Julia Richmond DiBello and Xinyue Liu. Writing- original draft preparation: Julia Richmond DiBello, Valerie T. Raziano, Xinyue Liu, Amy Puenpatom, and Deanna D. Hill. Writing- review and editing: Julia Richmond DiBello, Valerie T. Raziano, Xinyue Liu, Amy Puenpatom, Kathryn Peebles, Nazleen Khan, and Deanna D. Hill. All authors read and approved the final manuscript.
Funding
This study, including the journal’s Rapid Service Fee, was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Data Availability
No new data were generated during this study.
Declarations
Conflict of Interest
Julia Richmond DiBello, Valerie T. Raziano, Xinyue Liu, Amy Puenpatom, Kathryn Peebles, Nazleen F. Khan, and Deanna D. Hill are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and shareholders of Merck & Co., Inc., Rahway, NJ, USA.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Available from: https://covid19.who.int/. Accessed 1 Dec 2023.
- 2.Eales O, Plank MJ, Cowling BJ, Howden BP, Kucharski AJ, Sullivan SG, et al. Key challenges for respiratory virus surveillance while transitioning out of acute phase of COVID-19 pandemic. Emerg Infect Dis. 2024;30(2):1–9. doi: 10.3201/eid3002.230768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO policy brief: COVID-19 surveillance 2023 [Available from: https://iris.who.int/bitstream/handle/10665/366741/WHO-2019-nCoV-Policy-Brief-Surveillance-2023.1-eng.pdf. Accessed 1 Dec 2023.
- 4.World Health Organization. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic 2023 [Available from: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed 1 Dec 2023.
- 5.World Health Organization. COVID-19 Epidemiological Update 2024 [updated February 16, 2024. Edition 164:[Available from: https://www.who.int/publications/m/item/covid-19-epidemiological-update-16-february-2024. Accessed 1 Dec 2023.
- 6.Msemburi W, Karlinsky A, Knutson V, Aleshin-Guendel S, Chatterji S, Wakefield J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature. 2023;613(7942):130–137. doi: 10.1038/s41586-022-05522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control and Prevention. People with Certain Medical Conditions [Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 1 Dec 2023.
- 8.World Health Organization. Therapeutics and COVID-19: living guideline, 14 July 2022. 2022. [PubMed]
- 9.Li M, Lou F, Fan H. SARS-CoV-2 variant Omicron: currently the most complete "escapee" from neutralization by antibodies and vaccines. Signal Transduct Target Ther. 2022;7(1):28. doi: 10.1038/s41392-022-00880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US National Institutes of Health. Remdesivir 2023 [Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/remdesivir/. Accessed 1 Dec 2023.
- 11.US Food and Drug Administration. Remdesivir (Veklury) package insert 2023 [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214787s019lbl.pdf. Accessed 1 Dec 2023.
- 12.US Food and Drug Administration. Fact sheet for healthcare providers: Emergency Use Authorization for Paxlovid 2023 [Available from: https://www.fda.gov/media/155050/download. Accessed 1 Dec 2023.
- 13.US National Institutes of Health. Ritonavir-Boosted Nirmatrelvir (Paxlovid) 2023 [Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid. Accessed 1 Dec 2023.
- 14.US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults 2021 [Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain. Accessed 1 Dec 2023.
- 15.US Food and Drug Administration. Fact sheet for healthcare providers: Emergency Use Authorization for Lagevrio (molnupiravir) capsules. 2023 [Available from: https://www.fda.gov/media/155054/download. Accessed 1 Dec 2023.
- 16.US National Institutes of Health. Molnupiravir 2023 [Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/molnupiravir/. Accessed 1 Dec 2023.
- 17.Gobierno de México. Cofepris autoriza tratamiento oral para COVID-19 en uso de emergencia controlada 2022 [Available from: https://www.gob.mx/cofepris/articulos/cofepris-autoriza-tratamiento-oral-para-covid-19-en-uso-de-emergencia-controlada. Accessed 1 Dec 2023.
- 18.Hong Kong Free Press. Hong Kong’s Hospital Authority expands use of Covid-19 oral drugs 2022 [Available from: https://hongkongfp.com/2022/03/22/hong-kongs-hospital-authority-expands-use-of-covid-19-oral-drugs/. Accessed 1 Dec 2023.
- 19.Ministry of Health Labour and Welfare. Changes in approval conditions of Molnupiravir (LAGEVRIO®) capsules [in Japanese] 2023 [Available from: https://www.mhlw.go.jp/content/001090926.pdf. Accessed 1 Dec 2023.
- 20.Syed YY. Molnupiravir: First Approval. Drugs. 2022;82(4):455–460. doi: 10.1007/s40265-022-01684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Medicines and Healthcare products Regulatory Agency. First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA 2021 [August 4]. Available from: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra. Accessed 1 Dec 2023.
- 22.United Kingdom National Health Service. Who can and cannot take molnupiravir [Available from: https://www.nhs.uk/medicines/molnupiravir/who-can-and-cannot-take-molnupiravir/. Accessed 1 Dec 2023.
- 23.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386(6):509–20. [DOI] [PMC free article] [PubMed]
- 24.Amani B, Zareei S, Amani B. Rapid review and meta-analysis of adverse events associated with molnupiravir in patients with COVID-19. Br J Clin Pharmacol. 2022;88(10):4403–4411. doi: 10.1111/bcp.15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riva JJ, Malik KM, Burnie SJ, Endicott AR, Busse JW. What is your research question? An introduction to the PICOT format for clinicians. J Can Chiropr Assoc. 2012;56(3):167–171. [PMC free article] [PubMed] [Google Scholar]
- 27.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbel R, Sagy YW, Battat E, Lavie G, Sergienko R, Friger M, et al. Molnupiravir use and severe COVID-19 outcomes during the omicron surge. Research Square. 2022;PREPRINT (Version 1). 10.21203/rs.3.rs-2115769/v1
- 30.Bajema KL, Berry K, Streja E, Rajeevan N, Li Y, Yan L, et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes. medRxiv. 2022.12.05.22283134. 10.1101/2022.12.05.22283134 [DOI] [PMC free article] [PubMed]
- 31.Evans A, Qi C, Adebayo JO, Underwood J, Coulson J, Bailey R, et al. Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: a retrospective cohort study. J Infect. 2023;86(4):352–360. doi: 10.1016/j.jinf.2023.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of molnupiravir in high-risk patients: a propensity score matched analysis. Clin Infect Dis. 2023;76(3):453–460. doi: 10.1093/cid/ciac781. [DOI] [PubMed] [Google Scholar]
- 33.Paraskevis D, Gkova M, Mellou K, Gerolymatos G, Psalida P, Gkolfinopoulou K, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 community, highly vaccinated patients with high risk for severe disease: Evidence that both antivirals reduce the risk for disease progression and death. medRxiv. 2023;2023.02.09.23285737. 10.1101/2023.02.09.23285737
- 34.Wai AK, Chan CY, Cheung AW, Wang K, Chan SC, Lee TT, et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac. 2023;30:100602. doi: 10.1016/j.lanwpc.2022.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–1222. doi: 10.1016/S0140-6736(22)01586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y, Bowe B, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with COVID-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;380:e072705. doi: 10.1136/bmj-2022-072705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip TC, Lui GC, Lai MS, Wong VW, Tse YK, Ma BH, et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19) Clin Infect Dis. 2023;76(3):e26–e33. doi: 10.1093/cid/ciac687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajema KL, Berry K, Streja E, Rajeevan N, Li Y, Mutalik P, et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes. Ann Intern Med. 2023;176(6):807–816. doi: 10.7326/M22-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paraskevis D, Gkova M, Mellou K, Gerolymatos G, Psalida N, Gkolfinopoulou K, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in high-risk patients. J Infect Dis. 2023;228(12):1667–1674. doi: 10.1093/infdis/jiad324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodcroft EB. CoVariants: SARS-CoV-2 Mutations and Variants of Interest 2021 [Available from: https://covariants.org/. Accessed 1 Dec 2023.
- 41.Israeli Ministry of Health. Authorization for emergency use to Molnupiravir under regulation 29 for the treatment of Corona 2019 disease (COVID-19) [Available from: https://www.gov.il/BlobFolder/policy/molnupiravir/he/files_regulation_MOLNUPIRAVIR_Molnupiravir_patient_info-Jan22_ENG.pdf. Accessed 1 Dec 2023.
- 42.European Medicines Agency. Conditions of use, conditions for distribution and patients targeted and conditions for safety monitoring addressed to member states for unauthorised product Lagevrio (molnupiravir) 2021 [Available from: https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-conditions-use-conditions_en.pdf. Accessed 1 Dec 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated during this study.