Abstract
Numerous neurological manifestations associated with COVID-19 have been reported. However, abducens nerve palsy (ANP) associated with COVID-19 is very rare and mostly related to accompanying respiratory symptoms. Here we present a 29-year-old woman with unilateral ANP manifesting with diplopia and positive SARS-CoV-2 S antibodies, which were checked later. On admission, she had signs of viral pneumonia in thorax CT without any respiratory symptoms. Her cranial neuroimaging revealed no abnormality. Following treatment with favipiravir 2x1600 mg loading dose and then 2x600mg daily maintenance, dexamethasone 8 mg/day and enoxaparin 6000 IU/day, her CT findings recovered completely whereas her ANP only partially resolved. One week after the end of COVID-19 treatment, she also developed Herpes simplex keratitis which was successfully treated with valacyclovir. It should be kept in mind that isolated abducens nerve palsy may be the only finding of COVID-19 cases without any respiratory symptoms.
Keywords: Abducens nerve palsy, COVID-19, diplopia, SARS-CoV-2
In December 2019, a new coronavirus has been reported in Wuhan, China.[1] Later COVID-19 became a pandemic. Common clinical symptoms of disease include fever, fatigue, and dry cough.[2] Neurological manifestations are seen in 36.4% of hospitalized patients and are more common in patients with severe infection (45%) in conjunction with their respiratory status.[3] In one study, one-third of the patients have had ocular abnormalities among which the conjunctivitis is the most common.[4] There is a limited number of reported cases of acute abducens nerve palsy (ANP) and Miller-Fisher syndrome in the setting of infection with the novel coronavirus SARS-CoV-2.[5,6] Besides, ANP associated with COVID-19 is mostly related to accompanying respiratory symptoms. We present a patient with unilateral abducens palsy without any respiratory or other symptoms of COVID-19 on admission.
Case Report
A 29-year-old woman was admitted to emergency room and experienced double vision for 5 days. Her husband had a positive nasopharyngeal swab polymerase chain reaction (PCR) for COVID-19 ten days ago. On the second day of her husband’s treatment, she noted occasional mild cough, high fever, left-sided moderate headache, and fatigue. Because of the close contact, favipiravir treatment was initiated but due to the increase in fatigue and nausea, she had not taken her medicines regularly and had stopped using them on the 3rd day of the treatment. She had no medical history and no smoking, vaping or alcohol usage before. Ophthalmological examination revealed binocular horizontal diplopia worse at a distance and she could not abduct her left eye. Eye movements except for a limited external gaze in the left eye were normal (Fig. 1). Other examinations including visual acuity, color vision, a visual field with confrontation method, pupils, light reflex, accommodation reflex, and fundoscopy were also normal. There were no other abnormal findings on her neurological examination. She had no respiratory symptoms or hypoxemia at the time of admission. Blood tests showed increased white blood cell counts 12.22x109/L, neutrophil counts 8.88x109/L, normal lymphocyte counts 2.19x109/L, increased C-reactive protein level 58.5 mg/L, elevated ferritin 513 μg/L, normal procalcitonin 0.175 μg/L, normal D-dimer 470.25 μg/L, and elevated ESR 62 mm/hour. No systematic disease was detected in her blood tests. After 3 days, her D-dimer level increased to 652 μg/L. Her nasopharyngeal swab PCR for COVID-19 was found negative. Her initial thorax CT images showed mainly bilateral, peripheral and basal, multifocal ground-glass opacities, vascular thickening, widespread and severe involvement compatible with COVID-19 pneumonia (Fig. 2). These findings are classified as CO-RADS 5 (COVID-19 Reporting and Data System). No pathology was observed in cranial CT, diffusion-weighted MR imaging (MRI), cranial MR angiography and venography. Slight thinning of the left lateral rectus muscle was detected in orbital MRI (Figs. 3, 4). In cranial MR imaging, there were non-contrast-enhanced, non-specific, hyper-intense lesions on T2/Flair sequences in periventricular and subcortical deep white matter. The lumbar puncture was performed and CSF’s (cerebrospinal fluid) opening pressure was normal (155 mm H2O). The CSF was clear, the CSF biochemistry and culture were normal. The oligo clonal band was negative and the Ig G index was normal (0.46). Her CSF PCR for CMV (Cytomegalovirus), EBV (Epstein-Barr virus), VZV (Varicella-zoster virus), HSV-1 (Herpes simplex virus-1) and HSV-2 were negative. Laboratory results for any other viral, fungal or bacterial agents revealed no abnormality. Two days later, the repeated nasopharyngeal swab PCR for COVID-19 was again negative. She was treated with favipiravir 2x1600 mg loading dose and then 2x600mg daily maintenance, dexamethasone 8 mg/day, enoxaparin 6000 IU/day. After 10 days of treatment, her thorax CT findings returned to normal (Fig. 5), and her abducens palsy recovered partially. In the follow-up, she had no fever, no respiratory complaints, no hypoxemia, her oxygen saturation was in the range of 95-100 % and she had a stable course. One week after the end of COVID-19 treatment, burning pain and redness developed in her left eye and she was consulted with an ophthalmologist. Herpes simplex keratitis was diagnosed by physical examination and valacyclovir 1000 mg 3x1 treatment was started. Later on SARS–CoV-2 S total antibodies were (>250 U/ml) positive. She was completely normal at the 2-month follow-up examination.
Figure 1.
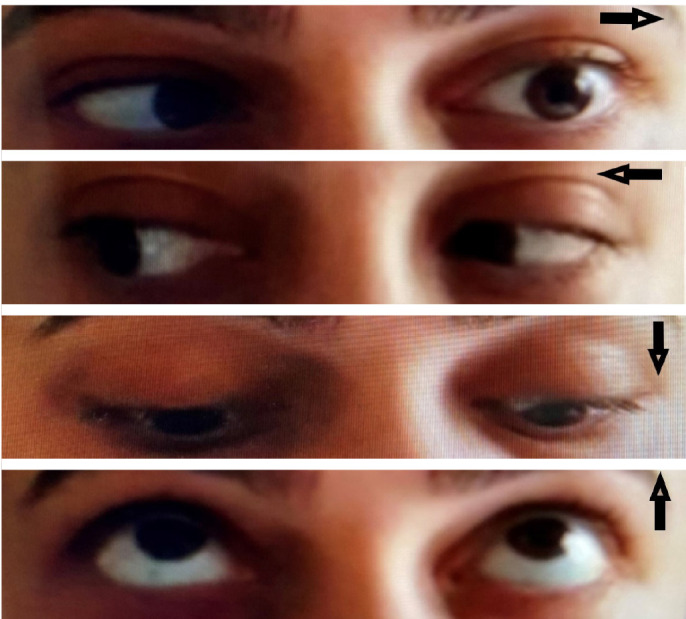
Patient’s photograph revealing her eye movements (arrows) and left abducens nerve palsy.
Figure 2.
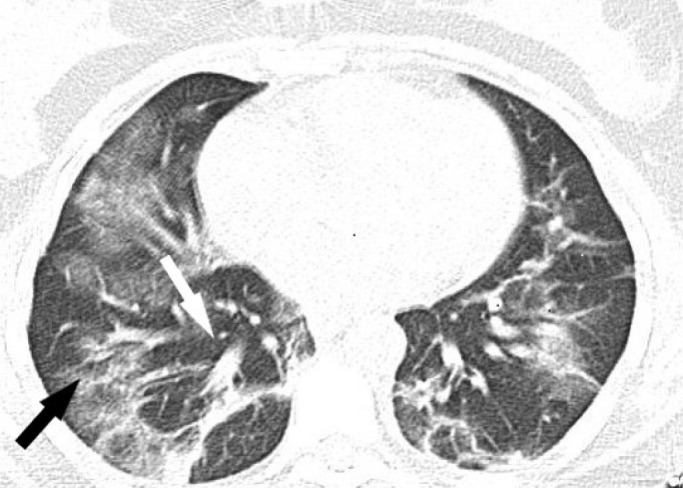
Patient’s thorax CT image showed mainly bilateral, peripheral and basal, multifocal ground-glass opacities (black arrow) and vascular thickening (white arrow) compatible with COVID-19 pneumonia.
Figure 3.
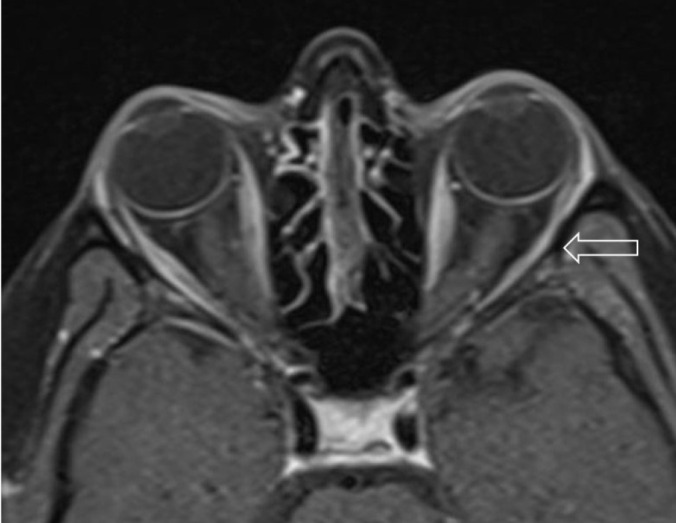
Slight thinning of the left lateral rectus muscle (arrow) on axial T1 fat-saturated post contrast orbital MRI.
Figure 5.
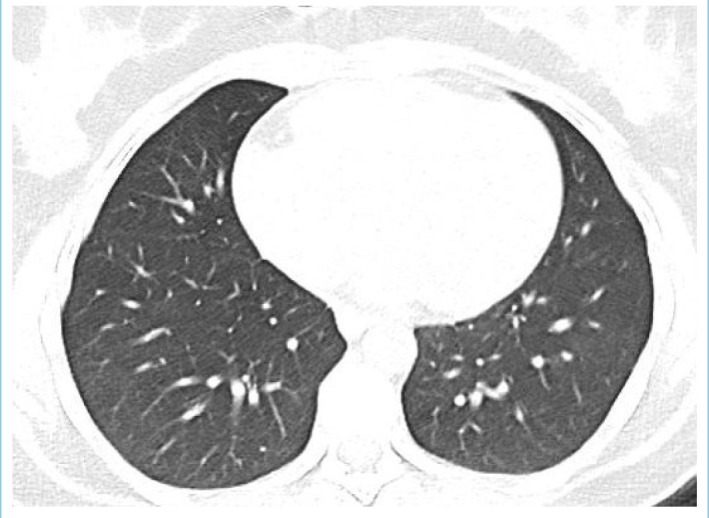
Patient’s thorax CT was normal after 10 days of treatment.
Figure 4.
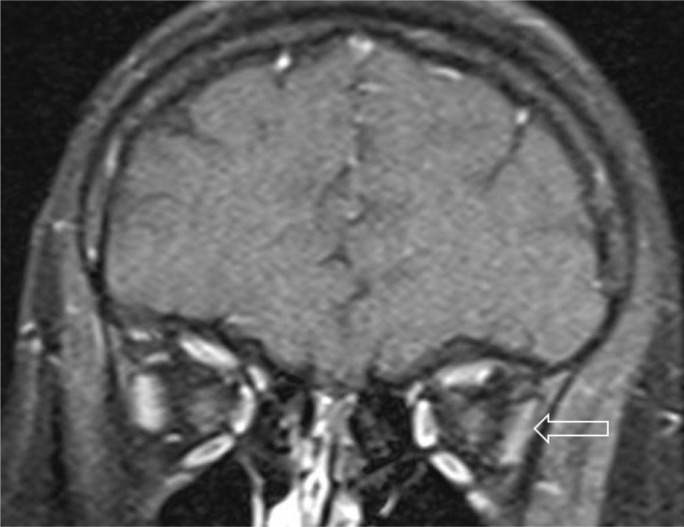
Slight thinning of the left lateral rectus muscle (arrow) on coronal T1 fat-saturated post contrast orbital MRI.
Discussion
The ANP is the most commonly affected nerve among ocular motor nerves.[7] The aetiologies include vascular, idiopathic, neoplastic, traumatic, aneurysm, intracranial inflammation, infection, Multiple sclerosis, Guillain-Barre syndrome, and Benign intracranial hypertension.[8]
Neurological manifestations of SARS-CoV-2 such as cerebrovascular diseases, infections of the central nervous system, cranial nerve disturbances (hyposmia, anosmia, vision impairment, dysgeusia, facial pain, dysphagia, ophthalmoparesis), and autoimmune and inflammatory syndromes (Guillain-Barre syndrome, Miller-Fisher syndrome and polyneuritis cranialis) have been reported.[9]
The SARS-CoV-2 virus enters the brain via a hematogenous route or trans-neuronally via the olfactory system. The virus utilizes the Angiotensin-converting enzyme 2 receptor for entry into endothelial cells. Severe infection predisposes to coagulopathy.[10]
In a single case report of a patient with ANP and acute hypoxemic respiratory failure due to COVID-19, the authors hypothesized that ANP is due to a direct or indirect virally mediated insult occurring along the path of the abducens nerve.[6]
A woman with HT, COVID-19 pneumonia with cough, fever and hypoxemia is also reported to exhibit ANP. They proposed that the presence of optic nerve sheath enhancement of the involved eye could reflect viral leptomeningeal invasion. The same authors presented an oculomotor palsy and bilateral ANP in a man with presumed Miller-Fisher syndrome due to COVID-19. MRI revealed enhancement and enlargement of the oculomotor nerve consistent with cranial nerve inflammation.[5]
Some authors stated that the pathogenesis of Miller-Fisher syndrome and polyneuritis cranialis in SARS-CoV-2 infection may include immune mechanisms or direct viral neuropathogenic effects and the presence of antibodies to gangliosides supporting the hypothesis of immune-mediated injury.[11]
In addition, 2 cases of COVID-19-associated neurologic manifestations with abducens palsy, headache, encephalopathy and intraparenchymal MRI findings have been reported.[12]
One case with ANP, headache and positive nasopharyngeal PCR for SARS-CoV-2 has been described. In the absence of an alternative underlying cause or risk factors identified, her etiology was presumed to be microvascular thrombotic and potentially related to the viral infection. The presence of SARS-CoV-2 in the CSF favors direct viral involvement of CNS. It is known that SARS-CoV-2 presence in CSF depends on the severity of the systemic disease. They hypothesized that the normal routine CSF constituents in their otherwise asymptomatic case are less likely to detect SARS-CoV-2 in the CSF.[13] Although it can be coincidental, the combination of ophthalmoparesis with typical thorax CT findings for COVID-19 pneumonia in our patient suggests that her ANP may be associated with this viral infection. Positive SARS-CoV-2 S antibodies tested later support the diagnosis. We could not find any other aetiological factor for this occurrence. Our patient’s chest CT signs are typical for COVID-19 pneumonia without respiratory symptoms at the time of admission, because she was in a period after the acute stage of the respiratory infection and her only examination sign was isolated abducens palsy. We have not observed any contrast enhancement on MRI. We could not evaluate SARS-CoV-2 in the CSF because it was not available in our laboratory and we did not analyze our case for anti-ganglioside antibodies and cytokines. These are our limitations.
A cytokine storm has been reported to be responsible for all complications of severe COVID-19 infection.[10]
Our case had no severe dyspnea and hypoxemia and was otherwise asymptomatic. We did not detect an albumin-cytological dissociation in CSF and she had no ataxia and areflexia. Therefore, she was not clinically compatible with Miller-Fisher syndrome. Hence, we suggest that isolated abducens nerve palsy in our patient has been developed due to nerve vascular microiscemia initiated by the viral infection. Complete recovery may be due to partial denervation.
Herpes simplex keratitis has been reported in patients with COVID-19.[14] In our patient, keratitis may have developed due to facilitated virus reactivation after COVID-19 infection or the drugs we use in the treatment of COVID-19.
It should be reminded that the cause of isolated cranial neuropathy in otherwise asymptomatic patients may be COVID-19 disease. It is not known why COVID-19 causes different neurological manifestations in patients, but it should be considered and investigated in patients presenting with cranial neuropathy.
Footnotes
Please cite this article as ”Tata G, Isik S, Diktas H, Genc G, Bulut S. Abducens Nerve Palsy in a Patient with COVID-19: A Case Report. Med Bull Sisli Etfal Hosp 2024;58(1):131–134”.
Disclosures
Informed consent
Written informed consent was obtained from the patient for the publication of the case report and the accompanying images.
Peer-review
Externally peer-reviewed.
Conflict of Interest
None declared.
Authorship Contributions
Concept – G.T., S.I., H.D., G.G., S.B.; Design – G.T., S.I., H.D., G.G., S.B.; Supervision – G.T., S.I., H.D., G.G., S.B.; Materials – G.T.; Data collection &/or processing – G.T., S.I., H.D., G.G., S.B.; Analysis and/or interpretation – G.T., S.I., H.D., G.G., S.B.; Literature search – G.T., G.G., S.B.; Writing – G.T., S.I., G.G., S.B.; Critical review – G.T., S.I., H.D., G.G., S.B.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. Erratum JAMA 2021 325 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95:221–3. doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 6.Falcone MM, Rong AJ, Salazar H, Redick DW, Falcone S, Cavuoto KM. Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID-19) J AAPOS. 2020;24:216–7. doi: 10.1016/j.jaapos.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park UC, Kim SJ, Hwang JM, Yu YS. Clinical features and natural history of acquired third, fourth, and sixth cranial nerve palsy. Eye (Lond) 2008;22:691–6. doi: 10.1038/sj.eye.6702720. [DOI] [PubMed] [Google Scholar]
- 8.Jung EH, Kim SJ, Lee JY, Cho BJ. The incidence and etiology of sixth cranial nerve palsy in Koreans: a 10-year nationwide cohort study. Sci Rep. 2019;9:18419. doi: 10.1038/s41598-019-54975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonck K, Garrez I, De Herdt V, Hemelsoet D, Laureys G, Raedt R, et al. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020;27:1578–87. doi: 10.1111/ene.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg RK. Spectrum of neurological manifestations in Covid-19: a review. Neurol India. 2020;68:560–72. doi: 10.4103/0028-3886.289000. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 12.Pascual-Goñi E, Fortea J, Martínez-Domeño A, Rabella N, Tecame M, Gómez-Oliva C, et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurol Neuroimmunol Neuroinflamm. 2020;7:e823. doi: 10.1212/NXI.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anilkumar A, Tan E, Cleaver J, Morrison HD. Isolated abducens nerve palsy in a patient with asymptomatic SARS-CoV-2 infection. J Clin Neurosci. 2021;89:65–7. doi: 10.1016/j.jocn.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majtanova N, Kriskova P, Keri P, Fellner Z, Majtan J, Kolar P. Herpes simplex keratitis in patients with SARS-CoV-2 infection: a series of five cases. Medicina (Kaunas) 2021;57:412. doi: 10.3390/medicina57050412. [DOI] [PMC free article] [PubMed] [Google Scholar]


