Abstract
Appetitive conditioning plays an important role in the development and maintenance of pornography‐use and gaming disorders. It is assumed that primary and secondary reinforcers are involved in these processes. Despite the common use of pornography and gaming in the general population appetitive conditioning processes in this context are still not well studied. This study aims to compare appetitive conditioning processes using primary (pornographic) and secondary (monetary and gaming‐related) rewards as unconditioned stimuli (UCS) in the general population. Additionally, it investigates the conditioning processes with gaming‐related stimuli as this type of UCS was not used in previous studies. Thirty‐one subjects participated in a differential conditioning procedure in which four geometric symbols were paired with either pornographic, monetary, or gaming‐related rewards or with nothing to become conditioned stimuli (CS + porn, CS + game, CS + money, and CS−) in an functional magnetic resonance imaging study. We observed elevated arousal and valence ratings as well as skin conductance responses for each CS+ condition compared to the CS−. On the neural level, we found activations during the presentation of the CS + porn in the bilateral nucleus accumbens, right medial orbitofrontal cortex, and the right ventral anterior cingulate cortex compared to the CS−, but no significant activations during CS + money and CS + game compared to the CS−. These results indicate that different processes emerge depending on whether primary and secondary rewards are presented separately or together in the same experimental paradigm. Additionally, monetary and gaming‐related stimuli seem to have a lower appetitive value than pornographic rewards.
Keywords: anterior cingulate cortex, appetitive conditioning, fMRI, gaming, mOFC, nucleus accumbens, pornography, reward value
The results indicate that different processes emerge depending on whether primary and secondary rewards are presented separately or together in the same experimental paradigm. Additionally, the results indicate that small monetary rewards and gaming screenshots seem to have a lower appetitive value than pornographic rewards.
1. INTRODUCTION
Appetitive conditioning is a process through which new rewards and their motivational salience are learned (Martin‐Soelch et al., 2007). During this process, a neutral stimulus (CS+) becomes associated with a rewarding unconditioned stimulus (UCS) to elicit conditioned responses (CRs), such as elevated valence and arousal ratings (Blechert et al., 2016; De Houwer et al., 2001; Tapia León et al., 2018), skin conductance response (SCR) (Andreatta & Pauli, 2015) and blood‐oxygen‐level‐dependent responses (BOLD) to the CS+ (Kirsch et al., 2003). Appetitive conditioning plays an important role in the development and maintenance of internet‐use disorders, like pornography use disorder or gaming disorder (Brand et al., 2019). However, conditioning processes in the context of internet‐use disorders like pornography‐use disorder and gaming disorder are not well studied yet despite the relatively high prevalence (Grubbs et al., 2019; Mihara & Higuchi, 2017; Rissel et al., 2017) as well as the common use of pornography and gaming in the general population (Bőthe et al., 2018; Müller et al., 2015; Severo et al., 2020). Meerkerk et al. (2006) argue that pornography and gaming are the most significant activities associated with internet addiction. Therefore, it is crucial to understand the underlying neural processes of learning mechanisms in the context of internet use, such as online gaming or pornography consumption. This study aims to investigate associative learning mechanisms in the context of non‐pathological use of online gaming and pornography.
Previous research has identified the following brain areas involved in appetitive conditioning: the medial orbitofrontal cortex (mOFC), the anterior cingulate cortex (ACC, usually divided into the ventral ACC, [vACC], and the dorsal [dACC]), the anterior insula, the amygdala, the nucleus accumbens (NAcc) and the thalamus (Haber & Knutson, 2010; Martin‐Soelch et al., 2007; Stefanova et al., 2020). Specifically, studies have shown that the mOFC seems to be involved in coding the subjective value of a reward and in the mental representation of reward outcomes (Klein et al., 2020; Peters & Büchel, 2010) as well as task structure (Zhou et al., 2021). The ACC is suggested to compute a surprise signal (Alexander & Brown, 2010, 2019) and to signal salience (Alexander et al., 2015). The insula is involved in the anticipation of rewards and losses (Knutson & Greer, 2008; Samanez‐Larkin et al., 2007) and coding the salience of these events (Rutledge et al., 2010) correlating with self‐reported negative and positive arousal (Knutson & Greer, 2008). In this context, the amygdala seems to modulate the motivational salience of a CS (Metereau & Dreher, 2013; Sescousse et al., 2013; Warlow & Berridge, 2021), retrieve the outcome memories of a CS (Everitt et al., 2003; Sias et al., 2021; Wassum, 2022) and is crucial for the acquisition and expression of a CR (Everitt et al., 2003; Gallagher et al., 1990; Gore et al., 2015). The NAcc is linked to reward anticipation (Knutson & Cooper, 2005; Knutson & Greer, 2008) with increasing BOLD with increasing reward size (Knutson et al., 2001). Finally, thalamus activation during reward anticipation (Y. Chen et al., 2022; Cho et al., 2013; Knutson & Greer, 2008; Oldham et al., 2018) is thought to be a bridge for impulses between the basal ganglia and frontal cortical areas (McFarland & Haber, 2002). Additionally, these brain areas are involved in cue‐reactivity circuity (see Antons et al., 2020 and Noori et al., 2016 for an overview) since cue‐reactivity can be interpreted as a CR (Carter & Tiffany, 1999).
Several studies have been conducted comparing appetitive conditioning processes with different types of rewards within one experimental setting. For example, with monetary, erotic (but not pornographic), social, and food rewards (Barman et al., 2015; Chan et al., 2018, 2022; Distefano et al., 2018; Gola et al., 2017; Kim et al., 2011; Kirsch et al., 2003; Sescousse et al., 2013; Sescousse et al., 2015). The difference between erotic and pornographic stimuli is that erotic material is sexually suggestive and mostly shows people who are only partially naked, whereas pornographic stimuli explicitly show sexual characteristics and activity (Brand et al., 2016; Strahler et al., 2019; Voon et al., 2014). Pornographic stimuli are considered to be primary rewards due to their significance for reproduction (Gola et al., 2016; Schultz, 2015; Sescousse et al., 2010). Some studies have shown that visual pornographic material can induce attentional bias and increased distractibility compared to neutral visual material (Kagerer et al., 2014; Strahler et al., 2018; Strahler et al., 2019). In the conditioning context, cues, predicting pornographic videos (Klein et al., 2020; Markert et al., 2021), pornographic pictures (Klucken et al., 2009; Liberg et al., 2022), or both (Stark et al., 2019) constantly activated the reward brain areas in healthy male participants. Similar activation in reward circuitry has been reported in cue‐reactivity studies with pornographic material (Mitricheva et al., 2019; Poeppl et al., 2014). Additionally, Meerkerk et al. (2006) concluded that pornography carries the highest addiction potential among all internet activities. These findings confirm the highly rewarding nature of pornography.
Regarding secondary (non‐primary) rewards, Kirsch et al. (2003) compared monetary and social rewards (verbal feedback) in the same conditioning paradigm. They found a higher BOLD response in reward brain areas during anticipation of monetary reward compared to social rewards. This difference is interpreted as motivation‐dependent reactivity, with highly desirable stimuli leading to a stronger activation. This interpretation is in line with the “common neural currency” theory (Montague & Berns, 2002), which suggests that values of different rewards are compared on a single scale, resulting in increased activity in reward brain areas as the value of the reward increases (Levy & Glimcher, 2012). The abstract value of a reward is also influenced by variables like proximity to reward delivery, outcome likelihood, reward size, and others (see Kennerley et al., 2009). Meanwhile, there is support for the “common neural currency” theory for the vmPFC/mOFC, striatum, anterior insula, and ACC (Bartra et al., 2013; Elliott et al., 2008; Gu et al., 2019; Kennerley et al., 2009; Kim et al., 2011; Kobayashi & Hsu, 2019; Levy & Glimcher, 2012; Sescousse et al., 2015).
Gaming stimuli likely play a crucial role in the development and maintenance of gaming disorder. For example, studies found that preferences for visual game aesthetics were strongly associated with online game addiction (C.‐Y. Chen & Chang, 2008). Thus, these stimuli presumably play a role in appetitive learning processes, although, their exact function has not been thoroughly investigated. Some studies suggest that computer games have reinforcing features due to factors like random reward mechanisms (Nielsen & Grabarczyk, 2019) and the ability to satisfy various human needs (Przybylski et al., 2010). However, it is unclear whether gaming stimuli (e.g., screenshots of gaming scenes) can be highly rewarding and thus, have appetitive value for individuals without gaming disorder. On one hand, previous studies have shown, that gaming aesthetics are essential for enjoying in the game. Specifically, visual design of digital games has been closely associated with game enjoyment (Arı et al., 2020; Merhi, 2016; Wu et al., 2008), probably through increase of pleasure of senses and providing authentic experience (Lin et al., 2017). Thus, gaming aesthetics are connected with enjoyment and could therefore evoke wanting. Since some gaming scenes can provide visual pleasure (Niedenthal, 2009) through aesthetic effects as some works argue (Possler & Klimmt, 2021; Smuts, 2005), the gaming scenes could evoke activation in reward‐related brain areas in individuals without gaming disorder, as it is a known effect of aesthetic visual material (Di Cinzia & Vittorio, 2009). On the other hand, cue‐reactivity research with gaming pictures reports no activation in reward‐related brain areas in people without gaming disorder during the presentation of gaming stimuli (Ko et al., 2009; L. Liu et al., 2017). Therefore, further research is needed to better understand whether gaming stimuli can have an appetitive effect on the general population similar to the impact of pornographic stimuli. This could shed light on whether gaming aesthetic is a strong standalone factor in the developing of gaming disorder as suggested by C.‐Y. Chen and Chang (2008) or gaming aesthetic alone is not enough to create a strong rewarding effect, as indicated by the results of cue‐reactivity studies (Ko et al., 2009; L. Liu et al., 2017). Furthermore, comparing appetitive conditioning processes with pornographic and gaming‐related stimuli could provide more insight into their impact on the brain's reward system and their potential for addiction. This comparison could help us understand the roles of these types of stimuli for developing of pornography use and gaming disorders.
To the best of our knowledge, no studies have yet directly compared appetitive conditioning processes using nonclinical subjects with pornographic and gaming‐related stimuli in the same experimental setting. An investigation in the same experimental paradigm enables a direct comparison of the strength of the appetitive effects of these UCSs. By examining the similarities and differences in brain responses to these stimuli and to cues, that announce them, researchers can better understand the specificity and generalizability of appetitive conditioning in developing internet use disorders like pornography use disorder and gaming disorder. Additionally, we included money as another non‐primary reward, which has already been well‐studied (Y. Chen et al., 2022; Jauhar et al., 2021; Klucken et al., 2019; Knutson et al., 2000; Kruse et al., 2018; Lewis et al., 2014; Oldham et al., 2018; Tapia León et al., 2019), but has not yet been compared within the same experimental paradigm with pornographic (only with erotic) or gaming‐related stimuli. The present study compares the appetitive conditioning processes of pornographic stimuli with other stimuli, as such comparisons have only been carried out with erotic stimuli. However, we believe that pornographic stimuli better reflect the reward aspect of pornography consumption than erotic stimuli. This study examines different kinds of CR: (1) subjective ratings, (2) SCR, and (3) neural substrates of classical appetitive conditioning processes with monetary, pornographic, and gaming‐related stimuli, focusing on the anticipation phase.
In line with the above studies and with the common neural currency theory (Levy & Glimcher, 2012), we predict that pornographic stimuli have the highest appetitive character and will elicit the strongest appetitive response compared to monetary and gaming stimuli (non‐primary rewards) as primary rewards usually have more appetitive value than non‐primary (Sescousse et al., 2013). Therefore, we hypothesize that the greatest CR occur in the CS + porn − CS− contrast (in ratings, SCR data, and BOLD response) compared to the CS + money‐ CS− and CS + game − CS− conditions. In addition, we expect a weaker CR for CS + money compared to CS + porn because monetary rewards are secondary and in contrast to pornographic stimuli not directly delivered in the scanner. Nonetheless, their rewarding value has been well‐documented in various studies. Finally, we predict the weakest CR for gaming‐related CS+ as they may be secondary rewards for individuals with gaming disorder but are likely neutral for healthy participants.
Finally, the chosen design of the study also allowed for comparison of differences between UCSs. As this was not the primary objective of the study, we present these results in the supplement (see supplement and discussion).
2. MATERIALS AND METHODS
2.1. Participants
A total of 37 male heterosexual right‐handed participants took part in this study. Only male participants were included, as biological sex can potentially impact conditioning processes (Klucken et al., 2009; Lonsdorf et al., 2015) and sex hormones can impact memory and learning in general (Hamson et al., 2016). Moreover, the present study is part of a multicenter DFG‐funded addiction research unit (FOR2974), focusing on the affective and cognitive mechanisms of specific Internet‐use disorders (Brand et al., 2021). Our project examines pornography use and gaming disorders, comparing different samples, including a sample with pornography‐use disorder, risky pornography use, gaming disorder, risky game use and a healthy control group. This article specifically focuses on the healthy control group from this project. However, we chose to recruit only male participants, as research suggests that women tend to use pornography less frequently than males (Lewczuk et al., 2022) and men report more problematic pornography use (Tan et al., 2022), and more problematic gaming use (Fam, 2018; Mihara & Higuchi, 2017). Six participants were excluded from the analysis because they (a) did not learn the association during the acquisition phase (n = 2), (b) fell asleep during the experiment (n = 1), (c) moved their head too much in the magnetic resonance imaging (MRI) scanner (n = 1), (d) dropped out of the experiment (n = 2). Thus, we analyzed 31 participants in the final sample for the fMRI and SCR analysis. The average age in this sample was 25.9 years (SD = 4.41, range: 19–38). The analysis of valence and arousal ratings included 30 participants, as the collection of ratings from one participant was disrupted by a technical issue. All participants were German native speakers or spoke German at the native or C1 level and had a normal or corrected‐to‐normal vision. Men with a history of psychosis, schizophrenia, bipolar disorder, Parkinson's disease, epilepsy, alcohol or drug addiction (except nicotine), and reading, writing, and comprehension difficulties could not take part in the study. Regular use of psychiatric medication (except antidepressants and stimulants), acute suicidality, implants, or metallic objects in the body were also exclusion criteria. Each participant received 10 € per hour or course credits for participation in the study and an additional 5.50€ from the experimental task. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the University of Siegen. All participants gave written informed consent to participate.
2.2. Procedure
The classical conditioning procedure was part of a larger national project on internet‐use disorders (Brand et al., 2021). Before the experiment, participants completed questionnaires and various behavioral tests outside of the MRI (not reported here). Prior to entering the scanner, participants had to select their 11 most preferred pictures out of 30 per category (pornographic photographs, screenshots from computer games, and images of money), to ensure comparability in the reinforcing stimuli. The pornographic photographs and gaming screenshots were sourced from internal databases and previous studies, with the 30 images per category selected based on valence and arousal ratings in previous study samples. Images of money were obtained from copyright‐free internet image databases. Participants were instructed, that, they would receive 0.50€ for each image of money in the experimental task after leaving the scanner. All three categories of pictures were colorful images. The pornographic photographs depicted adult couples (a man and a woman or a woman with another woman) engaged in various forms of sexual intercourse, including vaginal, oral, or anal intercourse. Primary (genitals) and secondary sexual characteristics (e.g., breasts) were visible in most of the photographs. One photograph out of the 30 showed sexual intercourse between three people (two men and one woman). The selected photographs included individuals of both white and dark skin complexion. The content of these photographs was not fetish‐related. The gaming screenshots displayed different moments from popular online games like Call of Duty, Dota, Fortnite, Grand Theft Auto, League of Legends, Minecraft, World of Warcraft, Counter‐Strike: Global Offensive, Dungeon Fighter Online, and Player Unknown's Battlegrounds. These screenshots included mission fulfillment, game play without specific tasks, character creation, and also game logo presentation. Monetary photographs displayed euro banknotes or coins, with some showing parts of the hands of a person holding the money or their face. The selected 11 pictures per category served as UCSs in the experimental task, while five additional pictures were used as UCSs for the practice session but not included in the experimental task. Following the picture selection, participants rated the valence and arousal of four geometric figures, which were later used as CSs in the experimental task. A brief practice session was conducted to familiarize participants with the task before the experiment. In the practice session, other symbols for CSs and other pictures for UCSs were used compared to the experimental task.
2.3. Conditioning procedure
The acquisition phase of the classical conditioning paradigm consisted of 68 trials and lasted approximately 27 minutes. Geometric figures (three quarters of a white circle) were used as four types of CS: CS + porn, CS + game, CS + money, and CS−. While the presentation of CS+ was followed by a UCS in 62.5% of the time, the CS− was never followed by any UCS. Each trial began with the presentation of a fixation cross for 0–2.5 s. The presentation of each CS lasted 8 s, and each UCS lasted 2.5 s, followed by an intertrial interval jittered between 8.5 and 11.5 s (see Figure 1 for the order of trial events).
FIGURE 1.
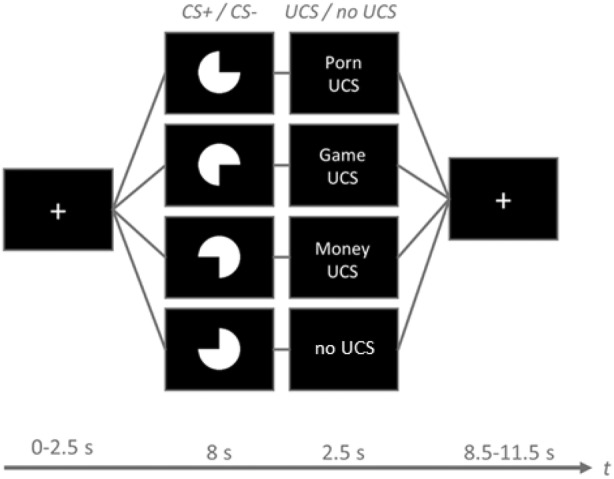
The design of the appetitive conditioning task. Each trial began with a white fixation cross (0–2.5 s), followed by one of four cues (CS + porn, CS + game, CS + money, or CS−) presented for 8 s and the presentation of a UCS (pornographic pictures, gaming pictures or money pictures) or a black screen (in the case of CS−) for 2.5 s. After the UCS had disappeared, the fixation cross was displayed again. The intertrial interval (ITI) was jittered between 8.5 and 11.5 s.
The assignment of stimuli to CS types was counterbalanced across participants. The first four trials consisted of one of each condition, with the order of trials counterbalanced across participants. Of these first four trials, all three CS+ trials were reinforced to ensure comparable initial learning across participants. The order of the CS+ and CS− trials was pseudorandomized, to ensure that no more than two trials of a CS (CS−, CS+, either reinforced or not) followed each other.
2.4. Subjective ratings
Before the practice session and after the acquisition phase, participants provided ratings for valence and arousal for each CS+, CS−, and each UCS−Set. The nine‐point Likert self‐assessment manikin scale (Bradley & Lang, 1994) was used for this purpose (valence: 1 “very unpleasant” to 9 “very pleasant”; arousal: 1 “calm and relaxed” to 9 “very aroused”). The valence and arousal ratings were examined in a 4 (CS type: CS + porn, CS + game, CS + money, CS−) × 2 (time: pre‐acquisition, post‐acquisition) analyses of variance with repeated measurements (rmANOVA), and significant effects were examined via paired t tests with SPSS 29 (SPSS 29.0 for Windows, SPSS Inc., Chicago, IL, USA). Additionally, after the acquisition phase contingency awareness was assessed. To do this, the following question was asked after the presentation of each CS: “Which type of pictures were shown after this symbol?” The answer options included: UCSporn, UCSgame, UCSmoney, “none” and “I don't know.” For the further assessment of contingency awareness, following question after the presentation of each CS was used “How likely is the presentation of a sexual/gaming/money/no picture after this symbol?” Participants used the 11‐point probability Likert‐type scale in 10%‐steps from 0 to 100% to answer this question. The last question after each CS pertained to the onset of contingency awareness: “When did you become confident that you will see a sexual/gaming/money/no picture after this symbol?” Participants rated it on an 11‐point Likert‐type scale ranging from “Beginning of experiment” to “End of experiment” with “Never” as the last option. Participants who did not become contingency aware were excluded.
2.5. Skin conductance
During the acquisition phase, SCRs were recorded at a sampling rate of 1 kHz using reusable Ag/AgCl electrodes filled with isotonic (0.05 M NaCl) electrolyte medium placed hypothenar of the left hand. The data were pre‐processed and analyzed with Ledalab 3.4.4 (Benedek & Kaernbach, 2010a). For preprocessing, the data were downsampled to 100 Hz and smoothed with a 32‐sample FWHM Gaussian kernel. The data were then analyzed with the first interval response (FIR = 1–4 s) and second interval response (SIR = 5–8 s). Using continuous decomposition analysis, the data were decomposed into continuous phasic (SCR) and tonic components and then an average phasic driver within the response window was determined (Benedek & Kaernbach, 2010b). Only responses larger than 0.01 μS were considered as SCR responses. Responses smaller than 0.01 mS were considered zero responses. All responses were log(μS + 1) transformed to correct for violation of the normal distribution of the data. Mean SCR values during the presentation of each type of CS for the whole acquisition phase were determined. The differences between SCR data in the four CS conditions were examined via paired t tests with SPSS 29 (SPSS 29.0 for Windows, SPSS Inc., Chicago, IL, USA).
2.6. Magnetic resonance imaging
A 3 Tesla magnetic resonance tomograph (Siemens MAGNETOM Prisma, Siemens Healthineers, Erlangen) with a 64‐channel head coil was used for the fMRI scans. The structural images were T1‐weighted and consisted of 176 sagittal slices (slice thickness 0.9 mm; FoV = 240 mm; TR = 1.58 s; TE = 2.3 s). The functional images (812 volumes per participant) were acquired with a T2*‐weighted echo‐planar imaging (EPI) with 42 slices covering the whole brain (voxel size = 3 × 3 × 3 mm; descending slice acquisition; TR = 2 s; TE = 30 ms; flip angle = 75; FoV = 222 × 222 mm; matrix size = 74 × 74; phase encoding direction: anterior–posterior). The position of the field of view was oriented relative to the AC–PC line at −30°.
Preprocessing and first and second‐level analysis were performed using SPM12 (Wellcome Department of Cognitive Neurology, 2014) implemented in MATLAB (The MathWorks Inc., 2021). For preprocessing, all EPI images underwent coregistration to an EPI template, realignment and unwarping using field maps, slice time correction, normalization to MNI standard space via segmentation of the structural T1‐image coregistered to a T1‐template and smoothing with a Gaussian kernel at 6 mm FWHM. Functional images were screened for outlying volumes using the fMRI Artifact Correction Tool (FACT). Outlier detection used a comparison of each volume with its two neighbors in a realigned time series. FACT calculated the mean squared differences between the previous and the next volume. The smaller difference was a deviation score for each volume. Using the method of Hubert and van der Veeken (2008), a threshold was determined. If the deviation score of a volume exceeded this threshold, the volume was considered an outlier. Each resulting outlying volume was included in the general linear model (GLM) as a regressor of no interest.
These experimental conditions were included in the GLM for each subject as regressors: CS + porn, CS + game, CS + money, CS−, UCSporn, noUCSporn, UCSgame, noUCSgame, UCSmoney, noUCSmoney, noUCS−. The first trial of each CS+ category and the first trial of the CS− were modeled as regressors of no interest. CS and UCS events were modeled as stick functions (0 s duration). The canonical hemodynamic response function was used for the convolution of all regressors. The six movement parameters were also included as regressors of no interest. The time series was then filtered with a high pass filter (time constant = 128 s). For each type of CS+, a CS+ − CS− contrast was defined on the participant level. Additionally, the contrasts CS + porn − CS + money, CS + porn − CS + game, CS + money − CS + game were defined on the participant level. On the group level, one sample t tests for these contrasts were performed for each region of interest (ROI) using small volume correction in SPM12 with p < .025 (FWE) to apply Bonferroni correction for two sides of the ROI masks. ROI masks (with a probability threshold of 50% and 2 mm resolution) for the amygdala, NAcc, caudate nucleus, insula, and thalamus were retrieved from the Harvard–Oxford cortical and subcortical structural atlases (Harvard Center for Morphometric Analysis). Masks for ACC were created according to the division of Vogt (2009). The mOFC mask was created with MARINA (Walter, 2003), which was done using the anatomical parcellation of the brain published by Tzourio‐Mazoyer et al. (2002). Exploratory tests on the whole brain were conducted using p < .05 (FWE).
3. RESULTS
3.1. Subjective ratings
The repeated‐measurements analysis of variance (rmANOVA) with a 4 (CS type: CS + porn, CS + game, CS + money, CS−) × 2 (time: pre‐ vs. post‐acquisition) design on valence ratings revealed a significant main effect of CS+ type and time (Table 1). There was also a significant CS type × time interaction (Table 1).
TABLE 1.
Main effects and interaction effects from 4 (CS type: CS + porn, CS + game, CS + money, CS−) × 2 (time: pre‐acquisition, post‐acquisition) rmANOVA for ratings of valence and arousal.
| Effect | df | F‐value | p‐Value | Part. η2 | |
|---|---|---|---|---|---|
| Valence | CS type | 3/87 | 5.33 | .002 | .155 |
| Time | 1/29 | 36.63 | <.001 | .558 | |
| CS type × Time interaction | 3/87 | 2.86 | .041 | .09 | |
| Arousal | CS type | 2.69/78.05 | 5.85 | .002 | .168 |
| Time | 1/29 | 4.27 | .048 | .128 | |
| CS type × Time interaction | 3/87 | 12.57 | <.001 | .302 |
Specifically, follow‐up paired t tests showed, that pre‐acquisition valence ratings were not significantly different from each other except for CS + money being rated as significantly more pleasant than CS + porn [t (29) = 2.98, p = .006, d = 0.544]. This finding may be spurious, as the examination of randomization of the CS symbols for CS + porn and CS + money using the chi‐square test showed no significant differences χ2 (3) = 1.421, p = .701. After the acquisition, the CS + porn [t (29) = 2.887, p = .004, d = 0.527], CS + game [t (29) = 3.404, p < .001, d = 0.621] and CS + money [t (29) = 3.358, p = .001, d = 0.613] were rated as more pleasant than the CS− (Figure 2). Post‐acquisition valence ratings did not differ significantly between the CS+ types (all ps > .05). We also compared valence ratings of each CS type before and after conditioning via follow‐up paired t tests. Each CS+ type was rated as more pleasant after the acquisition than before, but not CS− (see Table 2). Overall, the valence ratings indicate successful conditioning for each CS+ category.
FIGURE 2.
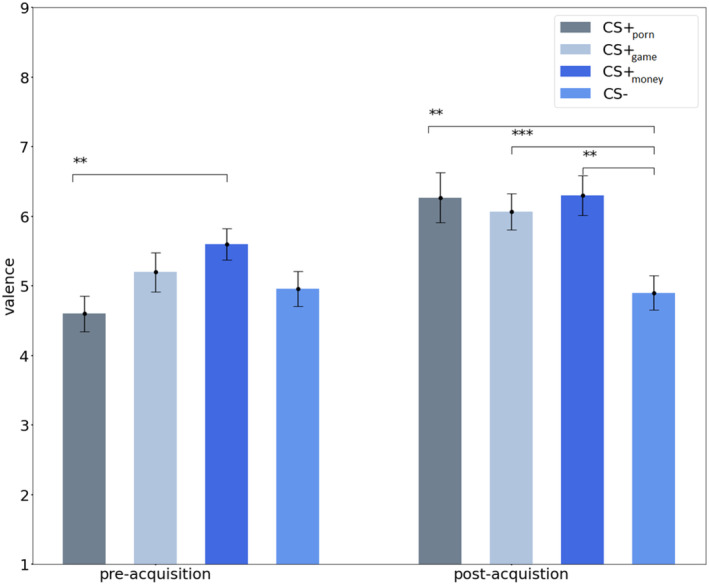
Analysis of valence ratings between CS type categories before and after the acquisition learning. The error bars represent the standard error of the mean (SEM). * indicates p < .05; ** indicates p < .01; *** indicates p < .001.
TABLE 2.
Results of the analysis via paired t tests of subjective ratings between each type of CS before and after acquisition learning.
| Comparison | t‐Value (df) | p‐Value | |
|---|---|---|---|
| Valence | CS + porn post acq > CS + porn pre‐acq | 3.85 (29) | <.001 |
| CS + money post acq > CS + money pre‐acq | 1.76 (29) | .045 | |
| CS + game post acq > CS + game pre‐acq | 2.83 (29) | .004 | |
| CS− post acq vs. CS− pre‐acq | −.17 (29) | .868 | |
| Arousal | CS + porn post acq > CS + porn pre‐acq | 3.98 (29) | <.001 |
| CS + money post acq > CS + money pre‐acq | 2.17 (29) | .019 | |
| CS + game post acq > CS + game pre‐acq | 1.57 (29) | .064 | |
| CS− post acq vs. CS− pre‐acq | −3.51 (29) | .001 |
We also conducted a post hoc comparison of valence ratings of UCS−type sets after conditioning via one‐tailed paired t tests. The set of each subject's selected pornographic pictures was rated as significantly more pleasant than the set of selected gaming‐related pictures [t = 1.88 (29), p = .035, d = 0.343]. However, we did not find significant differences between other UCS set types (all ps > .05).
Specifically, the follow‐up paired t test revealed that pre‐acquisition arousal ratings were not significantly different from each other (all ps > .05) (Figure 3). After the acquisition, the CS + porn [t (29) = 6.001, p < .001, d = 1.096], CS + game [t (29) = 3.807, p < .001, d = 0.695] and CS + money [t (29) = 4.386, p < .001, d = 0.801] were rated as more arousing than the CS− (Figure 3). Additionally, the CS + porn was rated significantly more arousing than CS + game [t (29) = 4.083, p < .001, d = 0.745.] after the acquisition. There were no significant differences in arousal ratings after the acquisition between the remaining CS+ types (all ps > .05). We also compared arousal ratings of each CS type before and after conditioning via follow‐up paired t tests. The CS + porn and the CS + money was rated as more arousing after the acquisition than before, but not CS + game (Table 2). The CS− was rated as more arousing before conditioning than after (Table 2). Overall, the arousal ratings indicate successful conditioning for each CS+ category.
FIGURE 3.
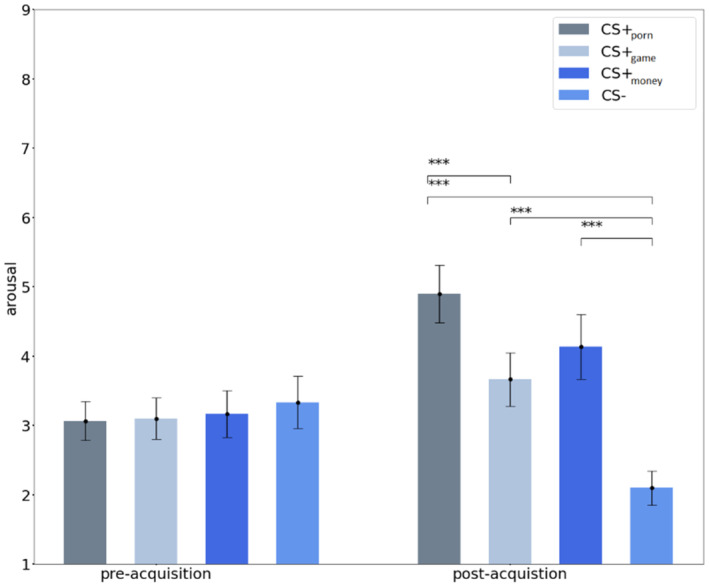
Analysis of arousal ratings between CS type categories before and after the acquisition learning. The error bars represent the SEM. * indicates p < .05; ** indicates p < .01; *** indicates p < .001.
We also conducted a post hoc comparison of arousal ratings of UCS−type sets after conditioning via one‐tailed paired t tests. The set of each subject selected pornographic pictures was rated as significantly more arousing than set of selected gaming pictures [t (29) = 5.79, p < .001, d = 1.057] and set of selected money pictures [t (29) = 3.27, p = .001, d = 0.597]. There were no significant differences between arousal ratings of set of gaming pictures and set of money pictures [t (29) = .47, p = .321].
3.2. Skin conductance responses
The paired t tests revealed significant differences between CS + porn and CS− [t(30) = 2.53, p = .009, d = 0.454], CS + money, and CS− [t(30) = 2.02, p = .026, d = 0.363] and between CS + game and CS− [t(30) = 1.81, p = .04, d = 0.325] in the FIR during appetitive conditioning (Figure 4). The results of the FIR analysis suggest that appetitive conditioning with CS + porn and CS + money was successful. There were no significant differences between the types of CS+ in FIR (all ps > .05). The paired t tests for SIR analysis found no significant differences between CS types (all ps > .05).
FIGURE 4.
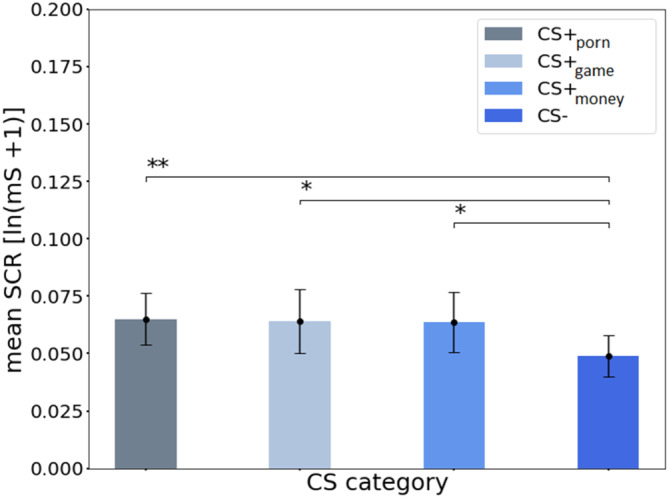
SCRs (mS log‐transformed) during the acquisition for each type of CS in FIR (first interval response). The error bars represent the SEM (standard error of mean). * indicates p < .05; ** indicates p < .01.
3.3. Functional MRI
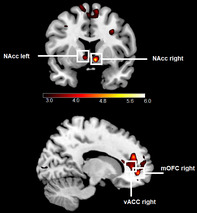
The main effects of appetitive conditioning for CS + porn (CS + porn − CS−) revealed increased BOLD responses in both sides of the NAcc, right mOFC, and the right vACC (refer to Table 3 and, Figure 5). No significant results were found on the whole brain level for this contrast (all ps > .05). There were also no significant main effects of conditioning on the whole brain level (all ps > .05) and in our ROIs for CS + game − CS− and for CS + money − CS− (all ps > .025). For a comprehensive overview of all results, including non‐significant findings, please refer to the supplementary materials.
TABLE 3.
Significant ROI activations during the acquisition phase for CS + porn − CS− contrast.
| Structure | Side | k | x | y | z | z max | T max | p FWE‐corr |
|---|---|---|---|---|---|---|---|---|
| NAcc | L | 30 | −6 | 8 | −4 | 3.42 | 3.82 | .005 |
| NAcc | R | 56 | 8 | 8 | −6 | 4.64 | 5.67 | <.001 |
| mOFC | R | 310 | 14 | 50 | −4 | 4.21 | 4.97 | .004 |
| vACC | R | 854 | 12 | 42 | 6 | 3.80 | 4.35 | .012 |
Note: The threshold for NAcc and mOFC was p < .025 (family‐wise‐error [FWE] and Bonferroni corrected). The threshold for vACC was p < .0125 (family‐wise‐error [FWE] and Bonferroni corrected). All coordinates are given in MNI space. L: left hemisphere, R: right hemisphere.
FIGURE 5.
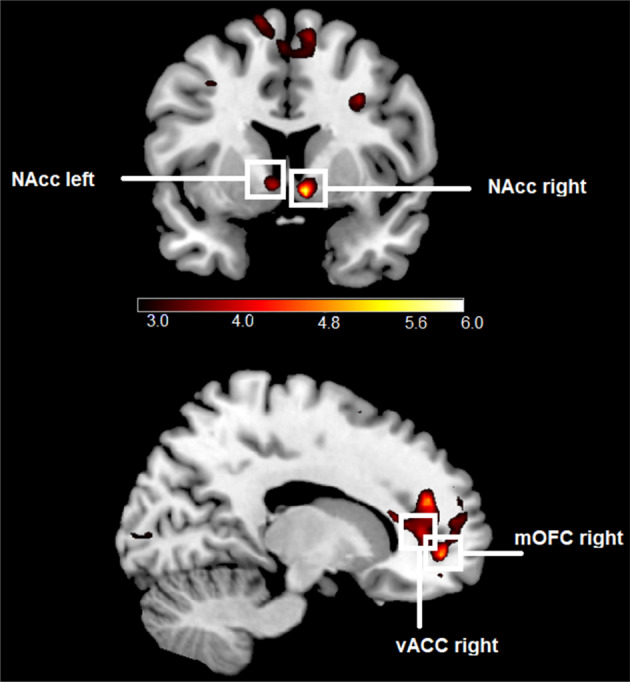
Significant ROI activations during the presentation of CS + porn in comparison to CS−. Displayed t‐values are thresholded between t > 3 and t < 6.
There were significant differences between the presentation of CS + porn and CS + money in bilateral NAcc and dACC (Table 4). The BOLD responses were also significantly higher during the presentation of CS + porn than CS + game in the left insula, left NAcc, bilateral dACC, and bilateral thalamus (Table 4). There were no significant results for the contrast CS + money − CS + game (all ps > .025). No significant results were found on the whole brain level for all three contrasts (all ps > .05).
TABLE 4.
Significant differences in ROIs during the acquisition phase between CS+ categories.
| Contrast | Structure | Side | k | x | y | z | z max | T max | p FWE‐corr |
|---|---|---|---|---|---|---|---|---|---|
| CS + porn − CS + money | NAcc | L | 40 | −10 | 14 | −4 | 3.00 | 3.28 | .016 |
| NAcc | R | 26 | 6 | 8 | −4 | 3.00 | 3.27 | .014 | |
| dACC | L | 709 | −8 | 24 | 26 | 3.92 | 4.53 | .007 | |
| dACC | R | 765 | 10 | 22 | 28 | 3.65 | 4.13 | .016 | |
| CS + porn − CS + game | Insula | L | 185 | −32 | 16 | −6 | 4.20 | 4.96 | .002 |
| NAcc | L | 38 | −8 | 12 | −4 | 3.31 | 3.67 | .007 | |
| dACC | L | 790 | −8 | 24 | 26 | 4.74 | 5.85 | <.001 | |
| dACC | R | 801 | 10 | 20 | 32 | 3.92 | 4.53 | .007 | |
| Thalamus | L | 338 | −20 | −28 | 12 | 3.84 | 4.41 | .012 | |
| Thalamus | R | 288 | 6 | −10 | 16 | 3.64 | 4.12 | .023 |
Note: The threshold was p < .025 (family‐wise‐error [FWE] and Bonferroni corrected). The threshold for vACC was p < .0125 (family‐wise‐error [FWE] and Bonferroni corrected). All coordinates are given in MNI space. L: left hemisphere, R: right hemisphere.
3.4. Sensitivity analysis
Since we did not find any significant results in CS + money − CS− and CS + game‐ CS− contrast, we conducted a post hoc sensitivity analysis using G‐power software (Faul et al., 2007). The post hoc conducted sensitivity analysis with a power of 0.8, n = 31, and α = .025, revealed that the smallest effect detectable was d z = 0.52.
4. DISCUSSION
This study aims to compare conditioning processes of pornographic, monetary, and gaming‐related rewards in the context of non‐pathological pornography and gaming use. It also investigates, whether gaming screenshots can evoke CR due to conditioning learning and thus be rewarding for participants without problematic gaming use.
Regarding valence and arousal ratings, we confirmed successful appetitive conditioning for all CS+ types, as indicated by increased post‐acquisition valence and arousal ratings compared to the CS−. Additionally, the CS + porn was rated as more arousing than the CS + game after the acquisition, possibly indicating greater excitement for pornographic pictures than gaming screenshots. Regarding SCRs, we found higher SCR during the presentation of each CS+ type relative to the CS− in FIR. This finding also indicates the successful acquisition of conditioning with all types of stimuli.
In addition, we found increased neural activations in the bilateral NAcc, right mOFC and right vACC during the presentation of CS + porn compared to CS−. No significant differences were observed during the presentation of the CS + money compared to the CS− or the CS + game compared to the CS−. During the presentation of CS + porn compared to CS + money stronger BOLD‐response in bilateral NAcc and dACC were found. Comparing CS + porn to CS + game stronger activations in the left insula, left NAcc, and both sides of dACC, and thalamus could be identified. Overall, these results are mostly consistent with our hypotheses. We will discuss these results, considering our main research questions.
4.1. Pornographic stimuli
The results of the present study confirm and extend findings of previous studies (Klucken et al., 2009; Liberg et al., 2022; Markert et al., 2021; Stark et al., 2019), showing activation in reward circuitry (in bilateral NAcc, right mOFC, and right vACC) during conditioning processes with pornographic rewards. They also align with previous findings about the involvement of NAcc and insula in reward anticipation (Knutson & Greer, 2008) and the presumed function of ACC and the insula in salience coding (Alexander et al., 2015; Rutledge et al., 2010), as pornography is expected to be more salient than a 50 cents reward and gaming screenshots. Similar to the study by Stark et al. (2019) a higher BOLD response was observed in the right mOFC during the presentation of CS + porn relative to the control stimulus. This result supports the assumption about the importance of mOFC in the mental representation of reward outcomes (Peters & Büchel, 2010). Given the evidence that activation in the striatum, anterior insula, ACC, and mOFC/ vmPFC follows the “common currency theory” (Bartra et al., 2013; Elliott et al., 2008; Gu et al., 2019; Kim et al., 2011; Kobayashi & Hsu, 2019; Levy & Glimcher, 2012; Sescousse et al., 2015), the present results could mean that pornographic rewards are preferred to small monetary rewards and gaming stimuli. The fact that CS + porn was rated in our study as more pleasurable and arousing than CS− is consistent with studies by Klucken et al. (2009, 2016). Finally, our skin conductance results were similar to those of Klucken et al. (2009, 2016). Since the SCR data are a physiological measure of arousal (Khalfa et al., 2002), both SCR data and arousal ratings indicate that expecting pornographic pictures induced arousal in participants. Overall, the results from our study regarding all three types of CRs confirm the highly rewarding properties of pornographic stimuli in affective learning processes.
4.2. Monetary and gaming stimuli
The valence and arousal ratings as well as the SCR data in the FIR indicate that acquisition of conditioning with monetary rewards and gaming stimuli was successful. These results with monetary rewards are in line with previous studies, that found similar effects on ratings (Klucken et al., 2019; Kruse et al., 2018; Tapia León et al., 2019). The arousal ratings and SCR data both support the assumption that expecting monetary and gaming stimuli constantly induced arousal. It is possible that this arousal conditioning effect occurred because the presentation of monetary and gaming pictures is more exciting than the presentation of a black screen (in the case of CS−). The higher valence ratings of CS + money and CS + game compared to CS− could indicate the appetitive value of monetary and gaming rewards. Notably, there were no increased activations in brain areas, usually involved in appetitive conditioning during the presentation of CS + money and CS + game compared to CS−. These fMRI results of CS + money compared to CS− contradict previous studies, which show activation in reward brain areas during the presentation of a CS, which predicted monetary reward compared to a control stimulus (Y. Chen et al., 2022; Jauhar et al., 2021; Klucken et al., 2019; Kruse et al., 2018; Tapia León et al., 2019). The fMRI results of CS + game compared to CS− align with previous cue‐reactivity studies, which found no activation in reward circuitry in the sample without problematic gaming (Ko et al., 2009; L. Liu et al., 2017). This also contradicts the assumption that CS + game could evoke such activation in a sample without problematic gaming due to the aesthetic properties of gaming screenshots.
The following section discusses the reasons and implications for these findings. Since the conditioning in the present study was done with pornographic, monetary, and gaming rewards in the same experimental paradigm, this could mean that CS + money and CS + game are associated with secondary rewards, but also represent an omission of a pornographic reward. An important property of the organism is to detect exactly which reward is announced by which stimuli, but also by which stimuli an alternative reward or its omission can be obtained. This could mean that these announcement stimuli are processed ambivalently and therefore do not activate the reward network unambiguously. Additionally, overshadowing effects of monetary and gaming rewards (and, respectively, from CS + money and CS + game) may have occurred towards pornographic rewards (and, respectively, towards CS + porn) due to their presentation in the same experimental paradigm and possibly because pornographic rewards have greater reward value than monetary and gaming rewards. This could be explained by the shift in attention from secondary to primary amplifiers.
To gain more clarity regarding this interpretation, a post hoc comparison of activation in ROIs during each type of UCS presentation (see supplementary materials) was conducted. Thus, we compared the BOLD response in the ROIs during UCSporn − UCSgame contrast and found significant higher BOLD response in the insula, NAcc, mOFC, vACC, dACC, thalamus, and amygdala (all ROIs bilaterally). Since all these regions are not only involved in reward anticipation but also reward consumption (Dillon et al., 2008; Knutson et al., 2003; X. Liu et al., 2011; Oldham et al., 2018; Rademacher et al., 2010; Sescousse et al., 2013), this post hoc analysis supports the proposition that pornographic stimuli hold more reward value than gaming stimuli. A reverse contrast UCSgame − UCSporn revealed no significant differences in the BOLD response in the ROIs. We also compared post hoc the activation in the ROIs during UCSporn − UCSmoney contrast and found a significantly higher BOLD response in the right NAcc (see supplementary materials). The reversed comparison UCSmoney − UCSporn revealed no significant results in the ROIs. These results support the interpretation of a higher reward value of pornography compared to a small monetary reward. Finally, we observed a significantly higher BOLD‐response in both sides of anterior insula, NAcc and in right mOFC during the UCSmoney compared to UCSgame. These results are also in line with our hypothesis, that monetary gains should hold greater reward value for healthy people than gaming screenshots. Furthermore, we conducted post hoc analyses of UCS ratings on arousal and valence scales after conditioning with paired t tests. The results also support our interpretation of the main results since the set of each subject selected pornographic pictures was rated as significantly more arousing than a set of selected gaming pictures and a set of selected money pictures. The valence ratings evaluation results of the UCS categories after conditioning with paired t tests only partially support our interpretations. Thus, the set of each subject's selected pornographic pictures was rated as significantly more pleasant than the set of selected gaming‐related pictures. We found the expected difference between the valence ratings of the UCSporn set and the UCSmoney set at the descriptive level; however, this difference was not significant. Therefore, these explanations illustrate that affective learning processes are very sensitive and specific, integrating many different pieces of information and the context of the information. Furthermore, both processes (reward omission and overshadowing) are not mutually exclusive but can occur together.
Interestingly, we found significant differences between CS + money and CS + game and CS− in valence and arousal ratings and in SCR data, but no relevant differences between these stimuli were found at the neural level, even though some of the ROIs (e.g., the anterior insula and NAcc) are thought to be involved in arousal‐related salience coding (Knutson & Greer, 2008). One possible explanation for this discrepancy in results is that the existing sample demonstrated sufficient test power to detect differences in ratings and SCR data but not in MRI data, since the smallest effect, which is possible to detect with our tests, is estimated to be d z = 0.52. Therefore, the non‐significant results in CS + money– CS− and CS + game − CS− should not necessarily lead to the conclusion that there are no activations in the ROIs during these contrasts. Another explanation comes from fear conditioning studies, which presume, that different outcome measures may represent slightly different aspects of learning (Jambazova, 2021; Lonsdorf et al., 2017; Sevenster et al., 2014), which may also be possible in the case of appetitive conditioning. Indeed some appetitive conditioning studies have also shown different results in different outcome measures of CR (Klucken et al., 2009).
4.3. General discussion, limitations, and future directions
Overall, the results of the present study indicate that pornographic stimuli (primary rewards) enable appetitive affective learning processes and strong conditioning effects in human subjects. Thus, appetitive learning with primary reinforcers appears to be less impaired when secondary reinforcers are simultaneously presented, underscoring the ecological role of primary reinforcers. This explanation is in line with the assumption that primary rewards typically hold more appetitive value than non‐primary rewards (Sescousse et al., 2013). In this sense, omission and overshadowing effects could influence appetitive learning processes with secondary reinforces more strongly. Future studies could delve deeper into the exact mechanisms of these effects. In other words, these results suggest that primary and secondary reinforcers are evaluated and processed differently depending on whether they are presented together or separately in an experiment. This explanation aligns with the “common neural currency” theory (Montague & Berns, 2002), which proposes that values of different rewards are compared on a single scale, leading to increased activity in reward‐related brain areas as the reward value increases (Levy & Glimcher, 2012).
However, it is important to note that the results of the present study do not allow for a general conclusion about processing all types of primary and secondary rewards, but only for the types of stimuli used in this study. This limitation may arise from the fact that such rewards can vary greatly, and their value can also be influenced by factors such as the size of the reward and the conditions of the subjects (hunger, socioeconomic status, etc.) (Kennerley et al., 2009). Future studies might consider comparing other types of primary and secondary rewards in a conditioning paradigm to gain a better understanding on the differences and similarities between these two types of reward.
The results of the present study suggest that gaming screenshots hold a lower reward value for healthy men compared to pornographic material. In the same experimental paradigm with pornographic rewards, conditioning with gaming screenshots evoked only CRs on the subjective and SCR‐levels but not on the neural level. This interpretation was also supported by post hoc comparisons of activations during the presentation of UCSporn and UCSgame (see supplementary materials) and of subjective ratings of UCSporn and UCSgame. This finding is in line with the conclusions from Meerkerk et al. (2006) about pornography having the highest addictive potential among all internet activities. However, it is worth to compare the conditioning processes using video sequences from different video games instead of gaming screenshots as it may provide a more immersive experience of being in the game world. However, we cannot definitely state, that gaming screenshots were not rewarding at all, as conditioning with gaming stimuli was successful, evoking pronounced CR in SCR data and subjective ratings. It would be crucial to investigate conditioning processes with gaming‐related stimuli in a healthy sample without other rewards in the same experimental paradigm to determine if CS + game would evoke CR on the neural level.
Finally, we observed, that different outcome measures in the conditioning task did not always yield consistent results. For pornographic stimuli, significant effects were found in all three outcome measures (BOLD‐response, subjective ratings, and SCR data). However, for monetary and gaming‐related stimuli, effects were only observed in ratings and SCR data. These results suggest potential differences in effect size across various reward conditions. Indeed, this possible explanation would be in line with our interpretation of results, since a smaller effect size in monetary and gaming compared to pornography condition would also speak for greater reward value of pornographic stimuli. Alternatively, these results could also suggest that different outcome measures represent slightly different aspects of learning (Jambazova, 2021; Lonsdorf et al., 2017; Sevenster et al., 2014), which is consistent with the findings of another appetitive conditioning study (Klucken et al., 2009). Future studies with larger sample sizes would be necessary to validate our interpretations.
One limitation of this study is the sample, which only included male participants. The results of previous research have been inconclusive. For example, one study showed no differences at the neural level in the conditioning processes with pornographic material between sexes (Stark et al., 2019), while another study found differences (Klucken et al., 2009). It is unclear if the brain responses would be different in a female sample using a paradigm with different stimuli besides pornographic ones. It is possible that anticipating pornographic material in females does not lead to overshadowing effects compared to other rewards, which could possibly explain why women seem to use pornography less often than males (Lewczuk et al., 2022; Petersen & Hyde, 2010; Solano et al., 2020) and why men report more problematic pornography use (Tan et al., 2022). However, this is one possible speculation, that requires further exploration. It should also be borne in mind that there are other explanations for the fact that women seem to consume less pornography. For example, some studies have shown that other factors seem to be important for women when viewing sexual material than for men and that women and men may perceive different stimuli as sexually arousing (Rupp & Wallen, 2008). It could be that pornographic material is currently designed to evoke sexual arousal in men rather than women. It should also be taken into account in future studies choosing appropriate sexual material for investigating conditioning processes in samples with women.
Previous studies have shown that nicotine consumption (and nicotine dependence) could impact the processing of other types of rewards (e.g., monetary reward) (Lin et al., 2020; Peters et al., 2011). Unfortunately, the measure of nicotine consumption and the potential cessation of nicotine were not included as a covariate in this study. We recommend taking this into account in future studies to eliminate or control the potential influence of nicotine consumption.
Finally, another potential area for future studies could be exploring the functional connectivity within reward‐related brain areas. An example of a possible research question would be the investigation whether the regions of interest are also more strongly functionally connected when anticipating pornographic rewards compared to small monetary rewards or gaming stimuli. If this were the case, it would support the interpretation of the high addictive potential of pornography.
4.4. Conclusions
The results of the present study demonstrate successful conditioning with pornographic rewards. In line with previous studies, activations were observed during the anticipation of a pornographic reward in bilateral NAcc, the right mOFC, and right vACC compared to the control condition. Additionally, we found higher activation during the anticipation of a pornographic reward compared to a monetary reward in bilateral NAcc and dACC and higher activation in left NAcc and insula as well as bilateral dACC and thalamus compared to the anticipation of gaming screenshots. These findings suggest show that different processes emerge depending on whether primary and secondary stimuli are presented separately or together. Future research should keep it in mind whether they want to examine primary and secondary reinforcers together in one experiment.
FUNDING INFORMATION
The work on this article was carried out in the context of the Research Unit ACSID, FOR2974, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—411232260 (DFG grant numbers: KL 2500/10‐1; STA 475/22‐1).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
DATA S1 Supporting Information.
ACKNOWLEDGMENTS
The study was carried out in the Research Unit ACSID, FOR2974, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—411232260. Open Access funding enabled and organized by Projekt DEAL.
Krikova, K. , Klein, S. , Kampa, M. , Walter, B. , Stark, R. , & Klucken, T. (2024). Appetitive conditioning with pornographic stimuli elicits stronger activation in reward regions than monetary and gaming‐related stimuli. Human Brain Mapping, 45(8), e26711. 10.1002/hbm.26711
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alexander, W. H. , & Brown, J. W. (2010). Computational models of performance monitoring and cognitive control. Topics in Cognitive Science, 2(4), 658–677. 10.1111/j.1756-8765.2010.01085.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, W. H. , & Brown, J. W. (2019). The role of the anterior cingulate cortex in prediction error and signaling surprise. Topics in Cognitive Science, 11(1), 119–135. 10.1111/tops.12307 [DOI] [PubMed] [Google Scholar]
- Alexander, W. H. , Fukunaga, R. , Finn, P. , & Brown, J. W. (2015). Reward salience and risk aversion underlie differential ACC activity in substance dependence. NeuroImage: Clinical, 8, 59–71. 10.1016/j.nicl.2015.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta, M. , & Pauli, P. (2015). Appetitive vs. aversive conditioning in humans. Frontiers in Behavioral Neuroscience, 9, 128. 10.3389/fnbeh.2015.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antons, S. , Brand, M. , & Potenza, M. N. (2020). Neurobiology of cue‐reactivity, craving, and inhibitory control in non‐substance addictive behaviors. Journal of the Neurological Sciences, 415, 116952. 10.1016/j.jns.2020.116952 [DOI] [PubMed] [Google Scholar]
- Arı, E. , Yılmaz, V. , & Elmastas Dikec, B. (2020). An extensive structural model proposal to explain online gaming behaviors. Entertainment Computing, 34, 100340. 10.1016/j.entcom.2020.100340 [DOI] [Google Scholar]
- Barman, A. , Richter, S. , Soch, J. , Deibele, A. , Richter, A. , Assmann, A. , Wüstenberg, T. , Walter, H. , Seidenbecher, C. I. , & Schott, B. H. (2015). Gender‐specific modulation of neural mechanisms underlying social reward processing by autism quotient. Social Cognitive and Affective Neuroscience, 10(11), 1537–1547. 10.1093/scan/nsv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra, O. , McGuire, J. T. , & Kable, J. W. (2013). The valuation system: A coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek, M. , & Kaernbach, C. (2010a). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190(1), 80–91. 10.1016/j.jneumeth.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek, M. , & Kaernbach, C. (2010b). Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology, 47(4), 647–658. 10.1111/j.1469-8986.2009.00972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert, J. , Testa, G. , Georgii, C. , Klimesch, W. , & Wilhelm, F. H. (2016). The Pavlovian craver: Neural and experiential correlates of single trial naturalistic food conditioning in humans. Physiology & Behavior, 158, 18–25. 10.1016/j.physbeh.2016.02.028 [DOI] [PubMed] [Google Scholar]
- Bradley, M. M. , & Lang, P. J. (1994). Measuring emotion: The self‐assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Brand, M. , Müller, A. , Stark, R. , Steins‐Loeber, S. , Klucken, T. , Montag, C. , Diers, M. , Wolf, O. T. , Rumpf, H.‐J. , Wölfling, K. , & Wegmann, E. (2021). Addiction research unit: Affective and cognitive mechanisms of specific internet‐use disorders. Addiction Biology, 26(6), e13087. 10.1111/adb.13087 [DOI] [PubMed] [Google Scholar]
- Brand, M. , Snagowski, J. , Laier, C. , & Maderwald, S. (2016). Ventral striatum activity when watching preferred pornographic pictures is correlated with symptoms of internet pornography addiction. NeuroImage, 129, 224–232. 10.1016/j.neuroimage.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Brand, M., Wegmann, E., Stark, R., Müller, A., Wölfling, K., Robbins, T. W., & Potenza, M. N. (2019). The Interaction of Person‐Affect‐Cognition‐Execution (I‐PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet‐use disorders, and specification of the process character of addictive behaviors. Neuroscience & Biobehavioral Reviews, 104, 1–10. 10.1016/j.neubiorev.2019.06.032 [DOI] [PubMed] [Google Scholar]
- Bőthe, B. , Tóth‐Király, I. , Zsila, Á. , Griffiths, M. D. , Demetrovics, Z. , & Orosz, G. (2018). The development of the problematic pornography consumption scale (PPCS). Journal of Sex Research, 55(3), 395–406. 10.1080/00224499.2017.1291798 [DOI] [PubMed] [Google Scholar]
- Carter, B. L. , & Tiffany, S. T. (1999). Meta‐analysis of cue‐reactivity in addiction research. Addiction, 94(3), 327–340. 10.1046/j.1360-0443.1999.9433273.x [DOI] [PubMed] [Google Scholar]
- Chan, Y.‐C. , Hsu, W.‐C. , & Chou, T.‐L. (2018). Dissociation between the processing of humorous and monetary rewards in the ‘motivation’ and ‘hedonic’ brains. Scientific Reports, 8(1), 15425. 10.1038/s41598-018-33623-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Y.‐C. , Hsu, W.‐C. , & Chou, T.‐L. (2022). Differential neural substrates for responding to monetary, sexual humor, and erotic rewards. Biological Psychology, 172, 108385. 10.1016/j.biopsycho.2022.108385 [DOI] [PubMed] [Google Scholar]
- Chen, C.‐Y. , & Chang, S.‐L. (2008). An exploration of the tendency to online game addiction due to user's liking of design features. Asian Journal of Health and Information Sciences, 3(1–4), 38–51. Retrieved from https://www.researchgate.net/profile/chi-ying-chen/publication/255590068_an_exploration_of_the_tendency_to_online_game_addiction_due_to_user's_liking_of_design_features [Google Scholar]
- Chen, Y. , Chaudhary, S. , & Li, C.‐S. R. (2022). Shared and distinct neural activity during anticipation and outcome of win and loss: A meta‐analysis of the monetary incentive delay task. NeuroImage, 264, 119764. 10.1016/j.neuroimage.2022.119764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. T. , Fromm, S. , Guyer, A. E. , Detloff, A. , Pine, D. S. , Fudge, J. L. , & Ernst, M. (2013). Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage, 66, 508–521. 10.1016/j.neuroimage.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer, J. , Thomas, S. , & Baeyens, F. (2001). Associative learning of likes and dislikes: A review of 25 years of research on human evaluative conditioning. Psychological Bulletin, 127(6), 853–869. 10.1037/0033-2909.127.6.853 [DOI] [PubMed] [Google Scholar]
- Di Cinzia, D. , & Vittorio, G. (2009). Neuroaesthetics: A review. Current Opinion in Neurobiology, 19(6), 682–687. 10.1016/j.conb.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Dillon, D. G. , Holmes, A. J. , Jahn, A. L. , Bogdan, R. , Wald, L. L. , & Pizzagalli, D. A. (2008). Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology, 45(1), 36–49. 10.1111/j.1469-8986.2007.00594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano, A. , Jackson, F. , Levinson, A. R. , Infantolino, Z. P. , Jarcho, J. M. , & Nelson, B. D. (2018). A comparison of the electrocortical response to monetary and social reward. Social Cognitive and Affective Neuroscience, 13(3), 247–255. 10.1093/scan/nsy006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, R. , Agnew, Z. , & Deakin, J. F. W. (2008). Medial orbitofrontal cortex codes relative rather than absolute value of financial rewards in humans. The European Journal of Neuroscience, 27(9), 2213–2218. 10.1111/j.1460-9568.2008.06202.x [DOI] [PubMed] [Google Scholar]
- Everitt, B. J. , Cardinal, R. N. , Parkinson, J. A. , & Robbins, T. W. (2003). Appetitive behavior. Annals of the New York Academy of Sciences, 985(1), 233–250. 10.1111/j.1749-6632.2003.tb07085.x [DOI] [PubMed] [Google Scholar]
- Fam, J. Y. (2018). Prevalence of internet gaming disorder in adolescents: A meta‐analysis across three decades. Scandinavian Journal of Psychology, 59(5), 524–531. 10.1111/sjop.12459 [DOI] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A.‐G. , & Buchner, A. (2007). G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Gallagher, M. , Graham, P. W. , & Holland, P. C. (1990). The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. Journal of Neuroscience, 10(6), 1906–1911. 10.1523/JNEUROSCI.10-06-01906.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola, M. , Wordecha, M. , Marchewka, A. , & Sescousse, G. (2016). Visual sexual stimuli‐cue or reward? A perspective for interpreting brain imaging findings on human sexual behaviors. Frontiers in Human Neuroscience, 10, 402. 10.3389/fnhum.2016.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola, M. , Wordecha, M. , Sescousse, G. , Lew‐Starowicz, M. , Kossowski, B. , Wypych, M. , Makeig, S. , Potenza, M. N. , & Marchewka, A. (2017). Can pornography be addictive? An fMRI study of men seeking treatment for problematic pornography use. Neuropsychopharmacology, 42(10), 2021–2031. 10.1038/npp.2017.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore, F. , Schwartz, E. C. , Brangers, B. C. , Aladi, S. , Stujenske, J. M. , Likhtik, E. , Russo, M. J. , Gordon, J. A. , Salzman, C. D. , & Axel, R. (2015). Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell, 162(1), 134–145. 10.1016/j.cell.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs, J. B. , Kraus, S. W. , & Perry, S. L. (2019). Self‐reported addiction to pornography in a nationally representative sample: The roles of use habits, religiousness, and moral incongruence. Journal of Behavioral Addictions, 8(1), 88–93. 10.1556/2006.7.2018.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, R. , Huang, W. , Camilleri, J. , Xu, P. , Wei, P. , Eickhoff, S. B. , & Feng, C. (2019). Love is analogous to money in human brain: Coordinate‐based and functional connectivity meta‐analyses of social and monetary reward anticipation. Neuroscience and Biobehavioral Reviews, 100, 108–128. 10.1016/j.neubiorev.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, S. N. , & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson, D. K. , Roes, M. M. , & Galea, L. A. M. (2016). Sex hormones and cognition: Neuroendocrine influences on memory and learning. Comprehensive Physiology, 6(3), 1295–1337. Retrieved from https://open.library.ubc.ca/soa/circle/collections/52383/items/1.0369058 [DOI] [PubMed] [Google Scholar]
- Hubert, M. , & van der Veeken, S. (2008). Outlier detection for skewed data. Journal of Chemometrics, 22(3–4), 235–246. 10.1002/cem.1123 [DOI] [Google Scholar]
- Jambazova, A. A. (2021). Studying the behavioural, physiological, and neural indices of associative learning in multi‐trial paradigms: Methodological and analytical considerations [University of Glasgow]. DataCite.
- Jauhar, S. , Fortea, L. , Solanes, A. , Albajes‐Eizagirre, A. , McKenna, P. J. , & Radua, J. (2021). Brain activations associated with anticipation and delivery of monetary reward: A systematic review and meta‐analysis of fMRI studies. PLoS One, 16(8), e0255292. 10.1371/journal.pone.0255292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer, S. , Wehrum‐Osinsky, S. , Klucken, T. , Walter, B. , Vaitl, D. , & Stark, R. (2014). Sex attracts: Investigating individual differences in attentional bias to sexual stimuli. PLoS One, 9(9), e107795. 10.1371/journal.pone.0107795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley, S. W. , Dahmubed, A. F. , Lara, A. H. , & Wallis, J. D. (2009). Neurons in the frontal lobe encode the value of multiple decision variables. Journal of Cognitive Neuroscience, 21(6), 1162–1178. 10.1162/jocn.2009.21100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalfa, S. , Isabelle, P. , Jean‐Pierre, B. , & Manon, R. (2002). Event‐related skin conductance responses to musical emotions in humans. Neuroscience Letters, 328(2), 145–149. 10.1016/S0304-3940(02)00462-7 [DOI] [PubMed] [Google Scholar]
- Kim, H. , Shimojo, S. , & O'Doherty, J. P. (2011). Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex, 21(4), 769–776. 10.1093/cercor/bhq145 [DOI] [PubMed] [Google Scholar]
- Kirsch, P. , Schienle, A. , Stark, R. , Sammer, G. , Blecker, C. , Walter, B. , Ott, U. , Burkart, J. , & Vaitl, D. (2003). Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system. NeuroImage, 20(2), 1086–1095. 10.1016/S1053-8119(03)00381-1 [DOI] [PubMed] [Google Scholar]
- Klein, S. , Kruse, O. , Markert, C. , Tapia León, I. , Strahler, J. , & Stark, R. (2020). Subjective reward value of visual sexual stimuli is coded in human striatum and orbitofrontal cortex. Behavioural Brain Research, 393, 112792. 10.1016/j.bbr.2020.112792 [DOI] [PubMed] [Google Scholar]
- Klucken, T. , Kruse, O. , Klein, S. , Kampa, M. , Tapia León, I. , & Stark, R. (2019). The relationship between neuroticism and appetitive conditioning. Neurobiology of Learning and Memory, 164, 107068. 10.1016/j.nlm.2019.107068 [DOI] [PubMed] [Google Scholar]
- Klucken, T. , Schweckendiek, J. , Merz, C. J. , Tabbert, K. , Walter, B. , Kagerer, S. , Vaitl, D. , & Stark, R. (2009). Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. The Journal of Sexual Medicine, 6(11), 3071–3085. 10.1111/j.1743-6109.2009.01405.x [DOI] [PubMed] [Google Scholar]
- Klucken, T. , Wehrum‐Osinsky, S. , Schweckendiek, J. , Kruse, O. , & Stark, R. (2016). Altered appetitive conditioning and neural connectivity in subjects with compulsive sexual behavior. The Journal of Sexual Medicine, 13(4), 627–636. 10.1016/j.jsxm.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Knutson, B. , & Cooper, J. C. (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18(4), 411–417. 10.1097/01.wco.0000173463.24758.f6 [DOI] [PubMed] [Google Scholar]
- Knutson, B. , & Greer, S. M. (2008). Anticipatory affect: Neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1511), 3771–3786. 10.1098/rstb.2008.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , Adams, C. M. , Fong, G. W. , & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159. 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , Fong, G. W. , Bennett, S. M. , Adams, C. M. , & Hommer, D. (2003). A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event‐related fMRI, 18(2), 263–272. 10.1016/S1053-8119(02)00057-5 [DOI] [PubMed] [Google Scholar]
- Knutson, B. , Westdorp, A. , Kaiser, E. , & Hommer, D. (2000). Fmri visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- Ko, C.‐H. , Liu, G.‐C. , Hsiao, S. , Yen, J.‐Y. , Yang, M.‐J. , Lin, W.‐C. , Yen, C.‐F. , & Chen, C.‐S. (2009). Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatric Research, 43(7), 739–747. 10.1016/j.jpsychires.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Kobayashi, K. , & Hsu, M. (2019). Common neural code for reward and information value. Proceedings of the National Academy of Sciences, 116(26), 13061–13066. 10.1073/pnas.1820145116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, O. , Tapia León, I. , Stalder, T. , Stark, R. , & Klucken, T. (2018). Altered reward learning and hippocampal connectivity following psychosocial stress. NeuroImage, 171, 15–25. 10.1016/j.neuroimage.2017.12.076 [DOI] [PubMed] [Google Scholar]
- Levy, D. J. , & Glimcher, P. W. (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. 10.1016/j.conb.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk, K. , Wójcik, A. , & Gola, M. (2022). Increase in the prevalence of online pornography use: Objective data analysis from the period between 2004 and 2016 in Poland. Archives of Sexual Behavior, 51(2), 1157–1171. 10.1007/s10508-021-02090-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, A. H. , Porcelli, A. J. , & Delgado, M. R. (2014). The effects of acute stress exposure on striatal activity during Pavlovian conditioning with monetary gains and losses. Frontiers in Behavioral Neuroscience, 8, 179. 10.3389/fnbeh.2014.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg, B. , Görts‐Öberg, K. , Jokinen, J. , Savard, J. , Dhejne, C. , Arver, S. , Fuss, J. , Ingvar, M. , & Abé, C. (2022). Neural and behavioral correlates of sexual stimuli anticipation point to addiction‐like mechanisms in compulsive sexual behavior disorder. Journal of Behavioral Addictions, 11(2), 520–532. 10.1556/2006.2022.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Deng, J. , Shi, L. , Wang, Q. , Li, P. [. P.]. , Li, H. , Liu, J. , Que, J. , Chang, S. , Bao, Y. , Shi, J. , Weinberger, D. R. , Wu, P. , & Lu, L. (2020). Neural substrates of smoking and reward cue reactivity in smokers: A meta‐analysis of fMRI studies. Translational Psychiatry, 10(1), 97. 10.1038/s41398-020-0775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. ‑L., Lin, H. ‑W., & Yang, Y. ‑T. (2017). Players’ Value Structure in Digital Games. Games and Culture, 12(1), 72–99. 10.1177/1555412015581710 [DOI] [Google Scholar]
- Liu, L. , Yip, S. W. , Zhang, J.‐T. , Wang, L.‐J. , Shen, Z.‐J. , Liu, B. , Ma, S.‐S. , Yao, Y.‐W. , & Fang, X.‐Y. (2017). Activation of the ventral and dorsal striatum during cue reactivity in internet gaming disorder. Addiction Biology, 22(3), 791–801. 10.1111/adb.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Hairston, J. , Schrier, M. , & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–1236. 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf, T. B. , Haaker, J. , Schümann, D. , Sommer, T. , Bayer, J. , Brassen, S. , Bunzeck, N. , Gamer, M. , & Kalisch, R. (2015). Sex differences in conditioned stimulus discrimination during context‐dependent fear learning and its retrieval in humans: The role of biological sex, contraceptives and menstrual cycle phases. Journal of Psychiatry & Neuroscience, 40(6), 368–375. 10.1503/140336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf, T. B. , Menz, M. M. , Andreatta, M. , Fullana, M. A. , Golkar, A. , Haaker, J. , Heitland, I. , Hermann, A. , Kuhn, M. , Kruse, O. , Meir Drexler, S. , Meulders, A. , Nees, F. , Pittig, A. , Richter, J. , Römer, S. , Shiban, Y. , Schmitz, A. , Straube, B. , … Merz, C. J. (2017). Don't fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience and Biobehavioral Reviews, 77, 247–285. 10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Markert, C. , Klein, S. , Strahler, J. , Kruse, O. , & Stark, R. (2021). Sexual incentive delay in the scanner: Sexual cue and reward processing, and links to problematic porn consumption and sexual motivation. Journal of Behavioral Addictions, 10(1), 65–76. 10.1556/2006.2021.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Soelch, C. , Linthicum, J. , & Ernst, M. (2007). Appetitive conditioning: Neural bases and implications for psychopathology. Neuroscience & Biobehavioral Reviews, 31(3), 426–440. 10.1016/j.neubiorev.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland, N. R. , & Haber, S. N. (2002). Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. Journal of Neuroscience, 22(18), 8117–8132. 10.1523/JNEUROSCI.22-18-08117.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerkerk, G.‐J. , van den Eijnden, R. J. J. M. , & Garretsen, H. F. L. (2006). Predicting compulsive internet use: It's all about sex! Cyberpsychology & Behavior, 9(1), 95–103. 10.1089/cpb.2006.9.95 [DOI] [PubMed] [Google Scholar]
- Merhi, M. I. (2016). Towards a framework for online game adoption. Computers in Human Behavior, 60, 253–263. 10.1016/j.chb.2016.02.072 [DOI] [Google Scholar]
- Metereau, E. , & Dreher, J.‐C. (2013). Cerebral correlates of salient prediction error for different rewards and punishments. Cerebral Cortex, 23(2), 477–487. 10.1093/cercor/bhs037 [DOI] [PubMed] [Google Scholar]
- Mihara, S. , & Higuchi, S. (2017). Cross‐sectional and longitudinal epidemiological studies of internet gaming disorder: A systematic review of the literature. Psychiatry and Clinical Neurosciences, 71(7), 425–444. 10.1111/pcn.12532 [DOI] [PubMed] [Google Scholar]
- Mitricheva, E. , Kimura, R. , Logothetis, N. K. , & Noori, H. R. (2019). Neural substrates of sexual arousal are not sex dependent. Proceedings of the National Academy of Sciences, 116(31), 15671–15676. 10.1073/pnas.1904975116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague, P. , & Berns, G. S. (2002). Neural economics and the biological substrates of valuation. Neuron, 36(2), 265–284. 10.1016/S0896-6273(02)00974-1 [DOI] [PubMed] [Google Scholar]
- Müller, K. W. , Janikian, M. , Dreier, M. , Wölfling, K. , Beutel, M. E. , Tzavara, C. , Richardson, C. , & Tsitsika, A. (2015). Regular gaming behavior and internet gaming disorder in European adolescents: Results from a cross‐national representative survey of prevalence, predictors, and psychopathological correlates. European Child & Adolescent Psychiatry, 24(5), 565–574. 10.1007/s00787-014-0611-2 [DOI] [PubMed] [Google Scholar]
- Niedenthal, S. (2009). What We Talk About When We Talk About Game Aesthetics. Presented at the Digital Games Research Association (DiGRA), London, UK (2009). Retrieved from https://urn.kb.se/resolve?urn=urn:nbn:se:mau:diva-11023 [Google Scholar]
- Nielsen, R. K. L. , & Grabarczyk, P. (2019). Are loot boxes gambling? Random reward mechanisms in video games. Transactions of the Digital Games Research Association, 4(3), 171–207. 10.26503/todigra.v4i3.104 [DOI] [Google Scholar]
- Noori, H. R. , Cosa Linan, A. , & Spanagel, R. (2016). Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta‐analysis. European Neuropsychopharmacology, 26(9), 1419–1430. 10.1016/j.euroneuro.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Oldham, S. , Murawski, C. , Fornito, A. , Youssef, G. , Yücel, M. , & Lorenzetti, V. (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta‐analysis of the monetary incentive delay task. Human Brain Mapping, 39(8), 3398–3418. 10.1002/hbm.24184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, J. , & Büchel, C. (2010). Neural representations of subjective reward value. Behavioural Brain Research, 213(2), 135–141. 10.1016/j.bbr.2010.04.031 [DOI] [PubMed] [Google Scholar]
- Peters, J. , Bromberg, U. , Schneider, S. , Brassen, S. , Menz, M. , Banaschewski, T. , Conrod, P. J. , Flor, H. , Gallinat, J. , Garavan, H. , Heinz, A. , Itterman, B. , Lathrop, M. , Martinot, J.‐L. , Paus, T. , Poline, J.‐B. , Robbins, T. W. , Rietschel, M. , Smolka, M. , … Büchel, C. (2011). Lower ventral striatal activation during reward anticipation in adolescent smokers. The American Journal of Psychiatry, 168(5), 540–549. 10.1176/appi.ajp.2010.10071024 [DOI] [PubMed] [Google Scholar]
- Petersen, J. L. , & Hyde, J. S. (2010). A meta‐analytic review of research on gender differences in sexuality, 1993‐2007. Psychological Bulletin, 136(1), 21–38. 10.1037/a0017504 [DOI] [PubMed] [Google Scholar]
- Poeppl, T. B. , Langguth, B. , Laird, A. R. , & Eickhoff, S. B. (2014). The functional neuroanatomy of male psychosexual and physiosexual arousal: A quantitative meta‐analysis. Human Brain Mapping, 35(4), 1404–1421. 10.1002/hbm.22262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possler, D. , & Klimmt, C. (2021). The entertaining appeal of video game aesthetics: Full Paper Presentation at the Annual Conference of the International Communication Association (ICA), Game Studies Division, Denver, CO, USA.
- Przybylski, A. K. , Rigby, C. S. , & Ryan, R. M. (2010). A motivational model of video game engagement. Review of General Psychology, 14(2), 154–166. 10.1037/a0019440 [DOI] [Google Scholar]
- Rademacher, L. , Krach, S. , Kohls, G. , Irmak, A. , Gründer, G. , & Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage, 49(4), 3276–3285. 10.1016/j.neuroimage.2009.10.089 [DOI] [PubMed] [Google Scholar]
- Rissel, C. , Richters, J. , de Visser, R. O. , McKee, A. , Yeung, A. , & Caruana, T. (2017). A profile of pornography users in Australia: Findings from the second Australian study of health and relationships. Journal of Sex Research, 54(2), 227–240. 10.1080/00224499.2016.1191597 [DOI] [PubMed] [Google Scholar]
- Rupp, H. A. , & Wallen, K. (2008). Sex differences in response to visual sexual stimuli: A review. Archives of Sexual Behavior, 37(2), 206–218. 10.1007/s10508-007-9217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge, R. B. , Dean, M. , Caplin, A. , & Glimcher, P. W. (2010). Testing the reward prediction error hypothesis with an axiomatic model. Journal of Neuroscience, 30(40), 13525–13536. 10.1523/JNEUROSCI.1747-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez‐Larkin, G. R. , Gibbs, S. E. B. , Khanna, K. , Nielsen, L. , Carstensen, L. L. , & Knutson, B. (2007). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–791. 10.1038/nn1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, W. (2015). Neuronal reward and decision signals: From theories to data. Physiological Reviews, 95(3), 853–951. 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G. , Caldú, X. , Segura, B. , & Dreher, J.‐C. (2013). Processing of primary and secondary rewards: A quantitative meta‐analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(4), 681–696. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Sescousse, G. , Li, Y. , & Dreher, J.‐C. (2015). A common currency for the computation of motivational values in the human striatum. Social Cognitive and Affective Neuroscience, 10(4), 467–473. 10.1093/scan/nsu074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G. , Redouté, J. , & Dreher, J.‐C. (2010). The architecture of reward value coding in the human orbitofrontal cortex. Journal of Neuroscience, 30(39), 13095–13104. 10.1523/JNEUROSCI.3501-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster, D. , Beckers, T. , & Kindt, M. (2014). Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety. Frontiers in Behavioral Neuroscience, 8, 32. 10.3389/fnbeh.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severo, R. B. , Soares, J. M. , Affonso, J. P. , Giusti, D. A. , de Souza Junior, A. A. , de Figueiredo, V. L. , Pinheiro, K. A. , & Pontes, H. M. (2020). Prevalence and risk factors for internet gaming disorder. Revista Brasileira De Psiquiatria, 42(5), 532–535. 10.1590/1516-4446-2019-0760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sias, A. C. , Morse, A. K. , Wang, S. , Greenfield, V. Y. , Goodpaster, C. M. , Wrenn, T. M. , Wikenheiser, A. M. , Holley, S. M. , Cepeda, C. , Levine, M. S. , & Wassum, K. M. (2021). A bidirectional corticoamygdala circuit for the encoding and retrieval of detailed reward memories. eLife, 10, e68617. 10.7554/eLife.68617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts, A. (2005). Are video games art? Contemporary Aesthetics, 3 Retrieved from https://digitalcommons.risd.edu/liberalarts_contempaesthetics/vol3/iss1/6/ [Google Scholar]
- Solano, I. , Eaton, N. R. , & O'Leary, K. D. (2020). Pornography consumption, modality and function in a large internet sample. Journal of Sex Research, 57(1), 92–103. 10.1080/00224499.2018.1532488 [DOI] [PubMed] [Google Scholar]
- Stark, R. , Klein, S. , Kruse, O. , Weygandt, M. , Leufgens, L. K. , Schweckendiek, J. , & Strahler, J. (2019). No sex difference found: Cues of sexual stimuli activate the reward system in both sexes. Neuroscience, 416, 63–73. 10.1016/j.neuroscience.2019.07.049 [DOI] [PubMed] [Google Scholar]
- Stefanova, E. , Dubljević, O. , Herbert, C. , Fairfield, B. , Schroeter, M. L. , Stern, E. R. , Urben, S. , Derntl, B. , Wiebking, C. , Brown, C. , Drach‐Zahavy, A. , Kathrin Loeffler, L. A. , Albrecht, F. , Palumbo, R. , Boutros, S. W. , Raber, J. , & Lowe, L. (2020). Anticipatory feelings: Neural correlates and linguistic markers. Neuroscience and Biobehavioral Reviews, 113, 308–324. 10.1016/j.neubiorev.2020.02.015 [DOI] [PubMed] [Google Scholar]
- Strahler, J. , Baranowski, A. M. , Walter, B. , Huebner, N. , & Stark, R. (2019). Attentional bias toward and distractibility by sexual cues: A meta‐analytic integration. Neuroscience and Biobehavioral Reviews, 105, 276–287. 10.1016/j.neubiorev.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Strahler, J. , Kruse, O. , Wehrum‐Osinsky, S. , Klucken, T. , & Stark, R. (2018). Neural correlates of gender differences in distractibility by sexual stimuli. NeuroImage, 176, 499–509. 10.1016/j.neuroimage.2018.04.072 [DOI] [PubMed] [Google Scholar]
- Tan, S.‐A. , Goh, Y. S. , Mohd Zaharim, N. , Gan, S. W. , Yap, C. C. , Nainee, S. , & Lee, L. K. (2022). Problematic internet pornography use and psychological distress among emerging adults in Malaysia: Gender as a moderator. International Journal of Environmental Research and Public Health, 19(6), 3682. 10.3390/ijerph19063682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia León, I. , Kruse, O. , Stalder, T. , Stark, R. , & Klucken, T. (2018). Neural correlates of subjective CS/UCS association in appetitive conditioning. Human Brain Mapping, 39(4), 1637–1646. 10.1002/hbm.23940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia León, I. , Kruse, O. , Stark, R. , & Klucken, T. (2019). Relationship of sensation seeking with the neural correlates of appetitive conditioning. Social Cognitive and Affective Neuroscience, 14(7), 769–775. 10.1093/scan/nsz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , Mazoyer, B. , & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Vogt, B. A. (2009). Regions and subregions of the cingulate cortex. In Vogt B. A. (Ed.), Cingulate neurobiology and disease (pp. 3–30). Oxford University Press. 10.1093/oso/9780198566960.003.0001 [DOI] [Google Scholar]
- Voon, V. , Mole, T. B. , Banca, P. , Porter, L. , Morris, L. , Mitchell, S. , Lapa, T. R. , Karr, J. , Harrison, N. A. , Potenza, M. N. , & Irvine, M. (2014). Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLoS One, 9(7), e102419. 10.1371/journal.pone.0102419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, B. (2003). MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. The 9th International Conference on Functional Mapping of the Human Brain, June 19‐22, 2003, New York, NY . Retrieved from https://ci.nii.ac.jp/naid/10026248034/
- Warlow, S. M. , & Berridge, K. C. (2021). Incentive motivation: ‘Wanting’ roles of central amygdala circuitry. Behavioural Brain Research, 411, 113376. 10.1016/j.bbr.2021.113376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum, K. M. (2022). Amygdala‐cortical collaboration in reward learning and decision making. eLife, 11, e80926. 10.7554/eLife.80926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Li, P. [. P.]. , & Rao, S. (2008). Why they enjoy virtual game worlds? An empirical investigation. Journal of Electronic Commerce Research, 9(3), 219–230. [Google Scholar]
- Zhou, J. , Gardner, M. P. H. , & Schoenbaum, G. (2021). Is the core function of orbitofrontal cortex to signal values or make predictions? Current Opinion in Behavioral Sciences, 41, 1–9. 10.1016/j.cobeha.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


