Abstract
Undifferentiated carcinoma with osteoclast-like giant cells (UC−OGCs) of the pancreas is a rare neoplasm that accounts for less than 1% of all pancreatic malignancies. The aim of this study was to review the literature regarding UC−OGC, and to highlight its biological behavior, clinicopathologic characteristics, prognosis, and therapeutic options. A systematic review of the literature in PubMed/Medline and Scopus databases was performed (last search October 31st, 2023) for articles concerning pancreatic UC−OGC in the adult population. Fifty-seven studies met the inclusion criteria, involving 69 patients with a male-to-female ratio of 1.1:1 and a mean age of 62.96. Main symptoms included abdominal pain (33.3%), jaundice (14.5%), weight loss (8.7%), while fourteen patients (20.3%) were asymptomatic. Surgical resection was performed in 88.4% of cases. Survival rates at one, three, and five years were 58%, 44.7%, and 37.3% respectively. Sex, age, size (cut-off of 4 cm), location, and adjuvant treatment did not significantly affect patient survival. UC−OGC of the pancreas is a rare subtype of undifferentiated pancreatic carcinoma with a better prognosis than conventional pancreatic ductal adenocarcinoma or undifferentiated carcinoma without giant cells. The establishment of a dedicated patient registry is imperative to further delineate the optimal treatment for this uncommon clinical entity.
Keywords: Pancreas, Carcinoma, Undifferentiated, Osteoclast-like giant cells
INTRODUCTION
Undifferentiated pancreatic carcinoma (UPC) is a rare and aggressive tumor with various histological appearances that include anaplastic, pleomorphic, spindle cell, sarcomatoid carcinoma, and carcinosarcoma. Despite their differences, these entities are collectively named in the current WHO Classification as undifferentiated carcinoma of the pancreas [1]. Undifferentiated carcinoma with osteoclast-like giant cells (UC−OGCs) is a distinctive UPC subtype that is considered as a variant of ductal adenocarcinoma with striking chemotaxis of osteoclastic giant cells [2].
UC−OGC is an extremely rare tumor, accounting for less than 1% of all pancreatic malignancies [3]. Histologically, UC−OGC is composed of both pleomorphic-to-spindle-shaped cells, and the hallmark feature of non-neoplastic OGCs [4]. These OGCs are positive for CD68, vimentin, and leukocyte common antigen, but negative for keratin and p53 antibodies [5]. The origin of OGCs in UC−OGC is not well understood, despite the chemotactic factors produced by cancer cells that have been found responsible so far. UC−OGC could be manifested either alone, or as part of other more common pancreatic tumors, such as pancreatic ductal adenocarcinoma or mucinous cystic neoplasm [6]. It is reportedly found in elderly females, with a mean age of presentation of approximately 63 years, and has similar clinical manifestations to other types of pancreatic tumors, such as upper abdominal pain and weight loss [7].
UC−OGC of the pancreas was initially believed to carry a poorer prognosis than invasive ductal adenocarcinoma of the pancreas [8-10], mainly due to its diagnosis at advanced stages [11], and its tendency for early recurrence, despite complete surgical removal [12,13]. However, when true UC–OGCs are carefully distinguished from other anaplastic carcinomas, it becomes clear that UC−OGCs have a more indolent behavior, especially pure UC−OGCs. Some series reported a significantly better prognosis for UC−OGC compared to conventional ductal adenocarcinoma, with a 5-year survival rate of over 50% [14].
The aim of this systematic literature review was to evaluate the available evidence on the biological behavior and prognosis of UC−OGC, as well as the role of surgical management.
MATERIALS AND METHODS
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [15]. Two investigators independently searched PubMed/Medline and Scopus databases reporting on UC−OGC (last search October 31st, 2023), using the following search algorithm: (“undifferentiated” [Title/Abstract] OR “anaplastic” [Title/Abstract]) AND “pancreas” [Title/Abstract] AND (“osteoclast” [Title/Abstract] OR “giant cells” [Title/Abstract]). Any controversy was solved by recourse to a senior investigator.
All articles written in the English language presenting case reports or case series were included in this systematic review, whereas reviews and meta-analyses were excluded. Non-English literature and articles involving children or animals were omitted. Moreover, articles with no clear diagnosis or insufficient data were excluded as irrelevant.
Data extraction of the included studies was performed independently by two of the authors (TSD and PL) using a predefined form. Information regarding sex, age, symptomatology, and medical history was compiled. Additionally, data on tumor location, treatment approach, use of neoadjuvant and adjuvant therapy, and outcome were gathered.
The study has been granted an exemption from requiring ethics approval by the Laikon General Hospital of Athens, Athens, Greece Ethics Committee.
Numerical variables were presented as the mean plus or minus standard deviation (SD), whereas categorical ones as the frequency and valid percentages. Patients included in case series were considered as unique cases, to assess the variables of interest. Several studies lacked data on some variables of interest, and therefore rates were estimated based on the available data. Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp.).
RESULTS
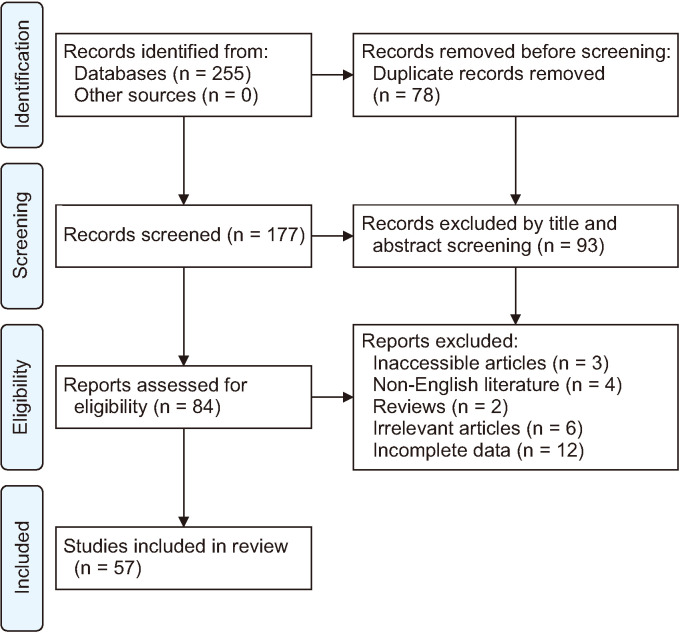
The present systematic review included 57 studies out of 176 unique articles and reports on 69 patients with UC−OGC. Fig. 1 presents the detailed article identification flow. The studies consisted of 54 case reports and 3 case series, including 36 female (52.1%) and 33 male (47.9%) patients, representing a 1.1:1 sex ratio. The mean age of the patients with UC−OGC was 62.96 ± 13.23 years (mean ± SD).
Fig. 1.
Trial flowchart of this systematic review.
Symptoms of the patients included in this review involved abdominal pain (33.3%), jaundice (14.5%), weight loss (8.7%), and bowel obstruction (4.3%). Fourteen patients (20.3%) were asymptomatic and the lesion was discovered incidentally, while 6 patients (8.7%) presented with an abdominal mass. Other clinical manifestations included complaints of fullness, upper gastrointestinal bleeding, an abnormal lung shadow on chest X-ray, urinary tract infection, and functional bowel disorders. Table 1 shows a detailed clinical presentation of the patients.
Table 1.
Clinical manifestation of pancreatic UC−OGC cases published in the literature
| Symptom | Patient (n = 69) |
|---|---|
| Abdominal pain | 23 (33.3) |
| Asymptomatic | 14 (20.3) |
| Jaundice | 10 (14.5) |
| Abdominal mass | 6 (8.7) |
| Weight loss | 6 (8.7) |
| Bowel obstruction | 3 (4.3) |
| Complaint of fullness | 2 (2.9) |
| Upper GI bleed | 2 (2.9) |
| Intra-abdominal bleeding | 1 (1.4) |
| Urinary tract infection | 1 (1.4) |
| Fatigue | 1 (1.4) |
Values are presented as number (%).
UC−OGC, undifferentiated carcinoma with osteoclast-like giant cell; GI, gastrointestinal.
The majority of the neoplasms were located in the head (36.2%), followed by the tail (27.5%) and the body (15.9%) of the pancreas. The mean size of the lesion was 74.5 ± 61.1 mm (mean ± SD), with a tumor size range, 3−280 mm. Table 2 shows the size in relation to tumor location. At the time of diagnosis, 8 patients (11.6%) presented with locally advanced disease due to the invasion of adjacent organs [16-21], vessels [22], or lymph nodes [23]. At the same time, 5 patients (7.2%) exhibited metastatic disease [24-28], primarily involving the liver [26-28], and the lungs [25].
Table 2.
Detailed location and size of UC−OGC cases published in the literature
| Location | Male | Female | Total case | Mean size (mm) |
|---|---|---|---|---|
| Head | 10 | 15 | 25 (36.2) | 49.3 |
| Tail | 9 | 10 | 19 (27.5) | 105.6 |
| Body | 6 | 5 | 11 (15.9) | 46.7 |
| Body and tail | 3 | 3 | 6 (8.7) | 158.4 |
| Head and body | 3 | 3 | 6 (8.7) | 46 |
| Unspecified | 2 | 0 | 2 (2.9) | N/A |
| Total | 33 | 36 | 69 (100) | 74.4 |
Values are presented as number only or number (%).
UC−OGC, undifferentiated carcinoma with osteoclast-like giant cell; N/A, not available.
Surgical resection of the pancreatic neoplasm was the therapeutic treatment in the majority of the patients (61 patients, 88.4%). Bypass surgery was offered to 2 patients, due to extended disease and poor performance status of the patients [23,24], while palliative stent placement was the therapeutic approach in 2 additional patients presenting with concurrent liver metastases [26,27]. Best supportive care was provided to 4 patients, based on their poor performance status [29], disease burden [28], and patient preference [24].
Concerning surgical operations, distal pancreatectomy and splenectomy was the most common procedure, accounting for 31 cases (50.8%). Among these, resection of adjacent organs was performed in 11 cases, with the stomach and the transverse colon being the most common resected organs. Whipple’s procedure followed as the second most common procedure, carried out in 25 cases (40.9%). Spleen-preserving distal pancreatectomy was performed in 4 cases (6.5%), while one patient (1.6%) underwent total pancreatectomy and splenectomy. The mean length of stay for patients was 14.15 days ranging 4 to 49 days. Two postoperative complications classified as Grade 3 or higher according to the Clavien–Dindo classification were reported. The first was a case of small bowel obstruction leading to sepsis, necessitating an exploratory laparotomy, after which a satisfactory recovery of the patient was reported. The second complication was a progressively enlarging pancreatic pseudocyst, successfully addressed with a second laparotomy for pseudocyst–jejunostomy, performed three months following the initial surgical intervention. Table 3 shows the detailed surgical treatment of the patients.
Table 3.
Surgical treatment and postoperative complications of pancreatic UC−OGC patients
| Surgical operation | n | Neoadjuvant treatment | Mean length of stay (day) | Complications Clavien–Dindo Grade > II |
|---|---|---|---|---|
| Distal pancreatectomy + splenectomy | 31 | 1 | 20.5 | 1 |
| Whipple’s procedure | 25 | 2 | 15.4 | 1 |
| Spleen-preserving distal pancreatectomy | 4 | N/A | N/A | N/A |
| Total pancreatectomy + splenectomy | 1 | N/A | 14 | N/A |
UC−OGC, undifferentiated carcinoma with osteoclast-like giant cell; N/A, not available.
Pathologic staging reports were available for 42 patients, classified according to the AJCC Cancer Staging Manual (8th edition) [30]. Stage distribution among patients with UC−OGC was as follows: 2 patients (4.8%) were at stage IA (T1, N0, M0), 7 patients (16.7%) at stage IB (T2, N0, M0), 12 patients (28.6%) at stage IIA (T3, N0, M0), 2 patients (4.8%) at stage IIB (varying T1−T3, N1, M0), 14 patients (33.3%) at stage III (varying T1−T4, N2 or T4, Any N, M0), and 5 (11.9%) patients at stage IV (Any T, Any N, M1).
Following surgical resection, adjuvant chemotherapy was provided to 19 patients. The majority of patients receiving adjuvant therapy were treated with gemcitabine, or with leucovorin–fluorouracil–irinotecan–oxaliplatin (FOLFIRINOX) regimen.
Patients’ follow-up was reported in 57 cases, and the follow-up period ranged 1 month to 14 years, with a median of 20.6 months. The longest reported survival was 14 years, while 24 deaths were reported during the follow-up period. The mean survival of the entire group of patients was 14.5 months. One-year, three-year, and five-year survival rates were 58% (95% confidence interval [CI], 42.8%−73.2%), 44.7% (95% CI, 27.7%−61.7%), and 37.3% (95% CI, 17.7%−56.9%), respectively. Fig. 2 shows the cumulative survival.
Fig. 2.
Cumulative survival Kaplan–Meier curve of pancreatic undifferentiated carcinoma with osteoclast-like giant cells.
Unifactorial Cox’s proportional hazards model analysis showed that sex, age, size (setting a cut-off of 4 cm), location, and adjuvant treatment did not significantly affect patient survival (Table 4).
Table 4.
Unifactorial Cox proportional analysis regarding the overall survival of pancreatic UC−OGC patients
| Factor | Unifactorial analysis | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | p-value | |
| Female sex | 0.553 | 0.246–1.244 | 0.152 |
| Age > 60 yr | 1.489 | 0.631–3.514 | 0.363 |
| Size over 4 cm | 1.970 | 0.769–5.048 | 0.158 |
| Tumor location | |||
| Head | 1.053 | 0.453–2.446 | 0.904 |
| Body | 0.563 | 0.131–2.419 | 0.440 |
| Tail | 1.717 | 0.693–4.255 | 0.243 |
| Head-body | 0.694 | 0.089–4.994 | 0.694 |
| Body-tail | 0.550 | 0.073–4.046 | 0.550 |
| Adjuvant treatment | 1.347 | 0.546–3.325 | 0.518 |
UC−OGC, undifferentiated carcinoma with osteoclast-like giant cell; HR, hazard ratio; CI, confidence interval.
DISCUSSION
UPC is a rare type of pancreatic adenocarcinoma that usually exhibits perineural invasion, as well as infiltration of local lymph nodes and blood vessels [31]. UC−OGC is a very rare subtype of UPC, accounting for less than 1% of all pancreatic malignancies [3], and was first described by Maier and Sommers in 1987 [32]. It is characterized by the presence of undifferentiated carcinoma cells and multinucleated OGCs, resembling giant cell tumor of the bone [33]. Such cells have been reported in tumors in a variety of organs, such as the kidney, breast, heart, parotid, and skin [34].
The possible pathogenetic mechanisms of UC−OGC involve a complex interplay of genetic alterations and cellular changes. Whole exome sequencing has revealed that UC−OGC shares a similar genetic background to pancreatic ductal adenocarcinoma, including mutations in key genes, such as KRAS, CDKN2A, TP53, and SMAD4 [35]. Additional mutations in SERPINA3 [35] and GLI3 [35,36] have been detected, suggesting their role as oncogenes and drivers of UC−OGC, respectively. Furthermore, mutations in TTN, MAGEB4, and MEGF8 have also been described; thus, their functional importance remains unclear [35]. The pleiomorphic phenotype of UC−OGC cells may result from epithelial-to-mesenchymal transition (EMT), characterized by loss of E–cadherin [37], deregulation of β–catenin [38], and strong expression of vimentin and ZEB1 [39]. However, EMT activation appears to be more frequent in undifferentiated carcinoma than in UC−OGC, and its presence and significance may be affected by neoadjuvant chemotherapy [31]. Lastly, pleiomorphic tumor cells produce granulocyte colony-stimulating factor, which recruits non-neoplastic OGCs [40], playing a key role in the unique features of UC−OGC.
The diagnosis of UC−OGC is made through a combination of clinical, imaging, and histopathological evaluation. Depending on the location of the tumor, patients may present with abdominal pain, jaundice, weight loss, or bowel obstruction. The findings of the present study suggest that approximately one in six patients with UC−OGC remains asymptomatic until incidentally diagnosed.
The imaging characteristics of UC–OGC are not well established, due to the rarity of the disease. It typically appears as a well-defined lesion with or without a cystic component, often including areas of hemorrhage and/or necrosis [41] and causing bile duct or pancreatic duct dilatation [42,43]. On contrast-enhanced computed tomography (CT), the tumor appears as a heterogeneous well-defined hypervascular mass, possibly related to its rapid growth of UC−OGC, or the associated inflammatory reaction. In contrast, the typical ductal pancreatic adenocarcinoma appears hypovascular on contrast CT scans. On magnetic resonance imaging, UC−OGC typically displays low-to-dark signal intensity on T1- and T2-weighted images. This characteristic is attributed to multifocal hemorrhage within the lesion, or the formation of bony and cartilaginous tissue by recruited osteoclasts [44]. Differentiating it from other cystic diseases of the pancreas can be challenging, due to the wide variety of features observed in UC−OGC [41]. Endoscopic ultrasound with fine needle aspiration or biopsy can provide cytological or histological material for the definitive diagnosis of UC−OGC. However, giant cells may also be present in cases of pancreatitis, thus representing a challenge for differential diagnosis [45]. Fig. 3, 4 present typical radiological images of UC−OGC.
Fig. 3.
Enhanced computed tomography (CT). The arterial phase (47 seconds after contrast injection) of the CT image showed (A) a low-attenuated mass located at the pancreatic uncinate process (arrow), (B) continuously invading the duodenum with marginal enhancement (small arrow). (C) The tumor was slightly enhanced at the late phase (3 minutes), especially at the margin (small arrows). Cited from the article of Matsubayashi et al. (Intern Med 2019;58: 3545-3550) [20].
Fig. 4.
Magnetic resonance imaging. A low-signal-intensity mass was seen on (A) T1-weighted, (B) T2-weighted, and (C) diffusion-weighted images. Cited from the article of Matsubayashi et al. (Intern Med 2019;58: 3545-3550) [20].
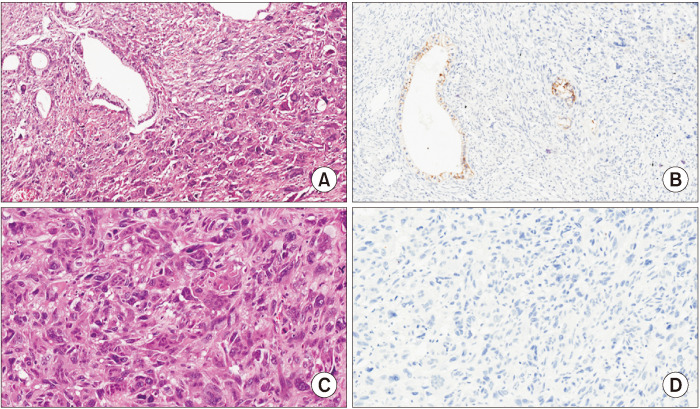
As previously mentioned, the histopathological appearance of UC−OGC is characterized by the coexistence of three distinct cell populations: non-neoplastic osteoclast-like multinucleated giant cells, mononuclear histiocytes, and neoplastic mononuclear cells [1]. The OGCs are often found contiguous to hemorrhage or necrosis, and may also be accompanied by osteochondroid differentiation, osteoid development, and bone formation [29,46,47]. Along with mononuclear histiocytes, giant cells are positive for histiocytic markers and negative for keratin staining, which differentiates them from the bizarre-appearing giant neoplastic cells seen in anaplastic undifferentiated carcinomas [1,48]. The neoplastic cells in UC−OGC range in morphology from spindle to epithelioid, and can be very large and pleomorphic [1]. Most of them have a high Ki–67 proliferation index and express vimentin, while they can show keratin and p53 positivity [1,9]. Additionally, in UC−OGC, the cancer cells typically lack cohesive organization, and instead present as individual cells, or in loose aggregates. These cells are frequently observed within the cytoplasm of OGCs. This differs markedly from the glandular, duct-like structures commonly seen in conventional ductal adenocarcinomas [1,49]. In general, UC−OGC can be pure, or may arise in association with conventional ductal adenocarcinoma or its precursors, such as intraductal papillary mucinous neoplasm and mucinous cystic neoplasm of the pancreas [12,14,50]. Fig. 5 presents representative histological images of UC−OGC.
Fig. 5.
(A, B) A representative case of the undifferentiated carcinoma of the pancreas with osteoclast-like giant cells, with an associated ductal adenocarcinoma, is shown. (A) Hematoxylin–eosin staining reveals an undifferentiated carcinoma with atypical cells and the presence of multinucleated osteoclast-like giant cells; neoplastic glands of the associated ductal adenocarcinoma are also evident (original magnification, × 20). (B) E–cadherin expression is lost by undifferentiated neoplastic cells, while it is retained by neoplastic glands (original magnification, × 20). (C, D) A representative case of undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (without an associated ductal adenocarcinoma) is shown. (C) Hematoxylin–eosin staining reveals an undifferentiated carcinoma with atypical cells and the presence of multinucleated osteoclast-like giant cells (original magnification, × 20). (D) E–cadherin expression is lost by undifferentiated neoplastic cells (original magnification, × 20). Cited from the article of Mattiolo et al. (Virchows Arch 2021;478:319-326) [31].
Treatment approach for UC−OGC mainly involves en bloc surgical excision of the tumor, in accordance with the principles of pancreatic adenocarcinoma treatment. The role of neo-adjuvant chemotherapy is not yet determined, considering that out of the 67 patients, the use of neoadjuvant chemotherapy was reported in only three cases (4.4%). As far as adjuvant therapy is concerned, treatment regiments similar to those applied for pancreatic adenocarcinoma are offered, using FOLFIRINOX schemes in fit patients, and gemcitabine-based regiments in frailer patients. In contrast to the limited use of neoadjuvant chemotherapy, adjuvant chemotherapy was offered to 39% of the reported patients. In addition to the chemotherapy, Obayashi et al. [25] reported a case of UC−OGC with distant metastasis, where the use of anti-PD−1 antibody therapy was effective against a lung metastasis, reflecting the potential benefit of immunotherapy in these tumors.
Surgical resection is considered to carry the best chance for cure, but the extent of resection required is not well established, due to the rarity of the disease, and the limited data available [4]. In general, the location of the primary lesion dictates the type and extent of surgical resection. For tumors located in the head of the pancreas, traditional or pylorus-preserving pancreatoduodenectomy is performed, whereas distal pancreatectomy is conducted for carcinomas of the pancreatic body or tail. In some cases, UC−OGC can invade adjacent organs, such as the stomach, jejunum, colon, left kidney, and diaphragm, and composite resection may be necessary [51].
UC−OGC is a specific type of pancreatic cancer that presents with unique clinicopathological characteristics and varying prognosis. Recent research has shown that patients with UC−OGC tend to have a better prognosis, compared to those with conventional pancreatic ductal adenocarcinoma, or those with UPC with pleomorphic/sarcomatoid giant cell tumors [52]. The improved survival of patients with pure UC−OGC may suggest that its unique morphology is a result of the immune response to the classical pancreatic ductal adenocarcinoma, potentially leading to the elimination of the pancreatic ductal adenocarcinoma component via a strong immune response [35].
While generally displaying a slower metastatic progression and a reduced rate of lymph node metastasis, compared to carcinoma without giant cells [14], cases of UC−OGC exhibiting aggressive behavior are not uncommon, reported mean survival varies among studies, with documented durations of 13 months [53], 19.6 months [41], and 20.4 months [51]. Several demographic and clinical factors have been associated with poorer prognosis, including the presence of a concurrent ductal adenocarcinoma component, older age, male sex, expression of PD−L1, and positive lymph node metastasis [24,27,28,53,54]. Conversely, indications of a more favorable prognosis have been associated with the identification of pure UC−OGC histology and surgical intervention for tumor removal [2,53]. In the present study, it was demonstrated that the median survival rate of patients with UC−OGC was 14.5 months, with one-, three-, and five-year survival rates of 58%, 44.7%, and 37.3%, respectively. The hazards model analysis of the present work revealed that sex, age, tumor size (using a cutoff of 4 cm), location, and adjuvant treatment did not significantly affect patient survival.
To the best of the authors’ knowledge, this is the first systematic review of the literature concerning the epidemiology, clinical manifestation, treatment approach, and prognosis of patients with pancreatic UC−OGC. However, the present study presents some limitations. First, the data presented in this study were obtained from case reports and case series, which implies that the accuracy and completeness of the data depend on the authors of those reports. Additionally, the data came from various surgical centers with different diagnostic, treatment, and follow-up algorithms, leading to heterogeneity and potential biases in the present analysis. Furthermore, this study may have been affected by methodological limitations, such as the small sample size, and the unavailability of full data. Another possible limitation of the present work could be the infrequent reporting of neoadjuvant and adjuvant therapies, and the use of a variety of therapeutic regimens, which may have impacted the prognosis of the cases under analysis. Lastly, there is a potential for publication bias, where cases with poorer outcomes may have been underreported, leading to a potential limitation in the generalizability of our findings.
Given the rare nature and distinctive features of UC−OGC, it is prudent to establish a dedicated patient registry to comprehensively document cases, thereby facilitating a collaborative and detailed analysis. Furthermore, research into the molecular foundations of these tumors is crucial to elucidate the exact mechanisms underlying their development. Importantly, investigations into UC−OGC promise significant advancements in understanding and treating this specific cancer type, while also potentially providing vital insights into pancreatic adenocarcinoma. It is hypothesized that the enhanced survival rates observed in UC−OGC cases may be indicative of an immune response that is capable of effectively targeting cells associated with classical pancreatic ductal adenocarcinoma, potentially orchestrating their elimination through a potent immune response [35].
From a surgical perspective, the notably better prognosis associated with UC−OGC, compared to conventional pancreatic ductal adenocarcinoma, strongly advocates pursuing surgical resection whenever feasible. Simultaneously, it is imperative for the oncology community to pioneer innovative treatment strategies, aiming to identify the most efficacious therapies for these tumors. A flexible approach, possibly modifying the treatment plans based on the presence or absence of a ductal component, might pave the way for more personalized therapies, potentially enhancing patient outcomes and extending survival rates.
CONCLUSION
UC−OGC of the pancreas is a rare subtype of UPC characterized by the presence of non-neoplastic OGCs. Depending on the location of the tumor, patients can present with abdominal pain, jaundice, weight loss, or remain asymptomatic until the diagnosis is made incidentally. Surgical resection of the malignancy along with involved adjacent organs constitutes the mainstay of treatment. UC−OGC appears to convey a better prognosis than conventional pancreatic ductal adenocarcinoma or Undifferentiated Carcinoma without giant cells. Further research on pathogenesis, as well as the establishment of a patient registry, are required to investigate potential prognostic factors for this tumor type; the exploration of possible therapeutic approaches that may improve patient outcomes is also needed. The identification and understanding of the unique characteristics of UC−OGC are crucial for appropriate clinical management, and for the development of potential targeted therapies.
Funding Statement
FUNDING None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: AM, KGT, DS. Data curation: NM. Methodology: MF. Visualization: PL. Writing - original draft: AM, DT. Writing - review & editing: TSD, MF, NM, KGT, DS.
References
- 1.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:1821188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demetter P, Maréchal R, Puleo F, Delhaye M, Debroux S, Charara F, et al. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: what do we know so far? Front Oncol. 2021;11:630086. doi: 10.3389/fonc.2021.630086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP. 2011;12:170–176. [PubMed] [Google Scholar]
- 4.Gao HQ, Yang YM, Zhuang Y, Liu P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J Gastroenterol. 2015;21:694–698. doi: 10.3748/wjg.v21.i2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loya AC, Ratnakar KS, Shastry RA. Combined osteoclastic giant cell and pleomorphic giant cell tumor of the pancreas: a rarity. An immunohistochemical analysis and review of the literature. JOP. 2004;5:220–224. [PubMed] [Google Scholar]
- 6.Ashfaq A, Thalambedu N, Atiq MU. A rare case of pancreatic cancer: undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. Cureus. 2022;14:e25118. doi: 10.7759/cureus.25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo YL, Ruan LT, Wang QP, Lian J. Undifferentiated carcinoma with osteoclast-like giant cells of pancreas: a case report with review of the computed tomography findings. Medicine (Baltimore) 2018;97:e13516. doi: 10.1097/MD.0000000000013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou XP, Yu ZL, Li ZS, Zhou GZ. Clinicopathological features of giant cell carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2004;3:300–302. [PubMed] [Google Scholar]
- 9.Lukás Z, Dvorák K, Kroupová I, Valásková I, Habanec B. Immunohistochemical and genetic analysis of osteoclastic giant cell tumor of the pancreas. Pancreas. 2006;32:325–329. doi: 10.1097/01.mpa.0000202951.10612.fa. [DOI] [PubMed] [Google Scholar]
- 10.Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann F, Esposito I, Michalski CW, Herpel E, Friess H, Schirmacher P. Early undifferentiated pancreatic carcinoma with osteoclastlike giant cells: direct evidence for ductal evolution. Am J Surg Pathol. 2007;31:1919–1925. doi: 10.1097/PAS.0b013e318067bca8. [DOI] [PubMed] [Google Scholar]
- 12.Wada T, Itano O, Oshima G, Chiba N, Ishikawa H, Koyama Y, et al. A male case of an undifferentiated carcinoma with osteoclast-like giant cells originating in an indeterminate mucin-producing cystic neoplasm of the pancreas. A case report and review of the literature. World J Surg Oncol. 2011;9:100. doi: 10.1186/1477-7819-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano H, Morita K, Tachibana S, Okimura A, Fujisawa T, Ouchi S, et al. Undifferentiated carcinoma with osteoclast-like giant cells arising in a mucinous cystic neoplasm of the pancreas. Pathol Int. 2008;58:383–389. doi: 10.1111/j.1440-1827.2008.02240.x. [DOI] [PubMed] [Google Scholar]
- 14.Muraki T, Reid MD, Basturk O, Jang KT, Bedolla G, Bagci P, et al. Undifferentiated carcinoma with osteoclastic giant cells of the pancreas: clinicopathologic analysis of 38 cases highlights a more protracted clinical course than currently appreciated. Am J Surg Pathol. 2016;40:1203–1216. doi: 10.1097/PAS.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazawa T, Watanabe A, Araki K, Segawa A, Hirai K, Kubo N, et al. Complete resection of a huge pancreatic undifferentiated carcinoma with osteoclast-like giant cells. Int Cancer Conf J. 2017;6:193–196. doi: 10.1007/s13691-017-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olayinka O, Kaur G, Gupta G. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells and associated ductal adenocarcinoma with focal signet-ring features. Cureus. 2021;13:e14988. doi: 10.7759/cureus.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur YH, Kim HH, Seoung JS, Seo KW, Kim JW, Jeong YY, et al. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc. 2011;81:146–150. doi: 10.4174/jkss.2011.81.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vnuk K, Pavić I, Brletić D, Zovak M, Krušlin B. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells: report of two cases. Lib Oncol. 2022;50:39–43. doi: 10.20471/LO.2022.50.01.07. [DOI] [Google Scholar]
- 20.Matsubayashi H, Kaneko J, Sato J, Satoh T, Ishiwatari H, Sugiura T, et al. Osteoclast-like giant cell-type pancreatic anaplastic carcinoma presenting with a duodenal polypoid lesion. Intern Med. 2019;58:3545–3550. doi: 10.2169/internalmedicine.3271-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osaka H, Yashiro M, Nishino H, Nakata B, Ohira M, Hirakawa K. A case of osteoclast-type giant cell tumor of the pancreas with high-frequency microsatellite instability. Pancreas. 2004;29:239–241. doi: 10.1097/00006676-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi Y, Gocho T, Taniai T, Uwagawa T, Hamura R, Shirai Y, et al. Conversion surgery for undifferentiated carcinoma with osteoclast-like giant cells of the pancreas: a case report. Surg Case Rep. 2022;8:42. doi: 10.1186/s40792-022-01385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharkhach A, Bouhout T, Serji B, El Harroudi T. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: a review and case report analysis. J Gastrointest Cancer. 2021;52:1106–1113. doi: 10.1007/s12029-021-00583-4. [DOI] [PubMed] [Google Scholar]
- 24.Lahiff C, Swan N, Conlon K, Malone D, Maguire D, Hoti E, et al. Osteoclastic-type giant cell tumours of the pancreas: a homogenous series of rare tumours diagnosed by endoscopic ultrasound. Dig Surg. 2016;33:401–405. doi: 10.1159/000445303. [DOI] [PubMed] [Google Scholar]
- 25.Obayashi M, Shibasaki Y, Koakutsu T, Hayashi Y, Shoji T, Hirayama K, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells curatively resected after pembrolizumab therapy for lung metastases: a case report. BMC Gastroenterol. 2020;20:220. doi: 10.1186/s12876-020-01362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speisky D, Villarroel M, Vigovich F, Iotti A, García TA, Quero LB, et al. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas diagnosed by endoscopic ultrasound guided biopsy. Ecancermedicalscience. 2020;14:1072. doi: 10.3332/ecancer.2020.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swaid MB, Vitale E, Alatassi N, Siddiqui H, Yazdani H. Metastatic undifferentiated osteoclast-like giant cell pancreatic carcinoma. Cureus. 2022;14:e27586. doi: 10.7759/cureus.27586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pop RM, Diaconu CI, Rimbaş M, Mateescu RB, Rouhani F, Popp C, et al. EUS-guided fine needle biopsy is able to provide diagnosis in rare osteoclast-like giant cells undifferentiated carcinoma of the pancreas: report of two cases. Rom J Intern Med. 2023;61:116–124. doi: 10.2478/rjim-2023-0008. [DOI] [PubMed] [Google Scholar]
- 29.Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1279::AID-CNCR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more 'personalized' approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 31.Mattiolo P, Fiadone G, Paolino G, Chatterjee D, Bernasconi R, Piccoli P, et al. Epithelial-mesenchymal transition in undifferentiated carcinoma of the pancreas with and without osteoclast-like giant cells. Virchows Arch. 2021;478:319–326. doi: 10.1007/s00428-020-02889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier HC, Sommers SC. Recurrent and metastatic pulmonary fibrous histiocytoma/plasma cell granuloma in a child. Cancer. 1987;60:1073–1076. doi: 10.1002/1097-0142(19870901)60:5<1073::AID-CNCR2820600524>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Jo S. Huge undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. World J Gastroenterol. 2014;20:2725–2730. doi: 10.3748/wjg.v20.i10.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakhi R, Hamza A, Khurram MS, Ibrar W, Mazzara P. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells reported in an asymptomatic patient: a rare case and literature review. Autops Case Rep. 2017;7:51–57. doi: 10.4322/acr.2017.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luchini C, Pea A, Lionheart G, Mafficini A, Nottegar A, Veronese N, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol. 2017;243:148–154. doi: 10.1002/path.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matissek SJ, Elsawa SF. GLI3: a mediator of genetic diseases, development and cancer. Cell Commun Signal. 2020;18:54. doi: 10.1186/s12964-020-00540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yonemasu H, Takashima M, Nishiyama KI, Ueki T, Yao T, Tanaka M, et al. Phenotypical characteristics of undifferentiated carcinoma of the pancreas: a comparison with pancreatic ductal adenocarcinoma and relevance of E-cadherin, alpha catenin and beta catenin expression. Oncol Rep. 2001;8:745–752. doi: 10.3892/or.8.4.745. [DOI] [PubMed] [Google Scholar]
- 38.Sano M, Homma T, Hayashi E, Noda H, Amano Y, Tsujimura R, et al. Clinicopathological characteristics of anaplastic carcinoma of the pancreas with rhabdoid features. Virchows Arch. 2014;465:531–538. doi: 10.1007/s00428-014-1631-5. [DOI] [PubMed] [Google Scholar]
- 39.Naito Y, Kinoshita H, Okabe Y, Arikawa S, Higaki K, Morimitsu Y, et al. Pathomorphologic study of undifferentiated carcinoma in seven cases: relationship between tumor and pancreatic duct epithelium. J Hepatobiliary Pancreat Surg. 2009;16:478–484. doi: 10.1007/s00534-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 40.Kubota N, Naito Y, Kawahara A, Taira T, Yamaguchi T, Yoshida T, et al. Granulocyte colony-stimulating factor-producing pancreatic anaplastic carcinoma in ascitic fluid at initial diagnosis: a case report. Diagn Cytopathol. 2017;45:463–467. doi: 10.1002/dc.23682. [DOI] [PubMed] [Google Scholar]
- 41.Togawa Y, Tonouchi A, Chiku T, Sano W, Doki T, Yano K, et al. A case report of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and literature review. Clin J Gastroenterol. 2010;3:195–203. doi: 10.1007/s12328-010-0160-2. [DOI] [PubMed] [Google Scholar]
- 42.Fukukura Y, Kumagae Y, Hirahara M, Hakamada H, Nagano H, Nakajo M, et al. CT and MRI features of undifferentiated carcinomas with osteoclast-like giant cells of the pancreas: a case series. Abdom Radiol (NY) 2019;44:1246–1255. doi: 10.1007/s00261-019-01958-9. [DOI] [PubMed] [Google Scholar]
- 43.Shindoh N, Ozaki Y, Kyogoku S, Nakanishi A, Sumi Y, Katayama H. Osteoclast-type giant cell tumor of the pancreas: helical CT scans. AJR Am J Roentgenol. 1998;170:653–654. doi: 10.2214/ajr.170.3.9490947. [DOI] [PubMed] [Google Scholar]
- 44.Yang KY, Choi JI, Choi MH, Park MY, Rha SE, Byun JY, et al. Magnetic resonance imaging findings of undifferentiated carcinoma with osteoclast-like giant cells of pancreas. Clinical Imaging. 2015;40:148–151. doi: 10.1016/j.clinimag.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Brosens LA, Leguit RJ, Vleggaar FP, Veldhuis WB, van Leeuwen MS, Offerhaus GJA. EUS-guided FNA cytology diagnosis of paraduodenal pancreatitis (groove pancreatitis) with numerous giant cells: conservative management allowed by cytological and radiological correlation. Cytopathology. 2015;26:122125. doi: 10.1111/cyt.12140. [DOI] [PubMed] [Google Scholar]
- 46.Luchini C. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells: a challenging cancer with new horizons? Virchows Archiv. 2021;478:595–596. doi: 10.1007/s00428-021-03021-9. [DOI] [PubMed] [Google Scholar]
- 47.Manduch M, Dexter DF, Jalink DW, Vanner SJ, Hurlbut DJ. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: report of a case with osteochondroid differentiation. Pathol Res Pract. 2009;205:353–359. doi: 10.1016/j.prp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Oymaci E, Yakan S, Yildirim M, Argon A, Namdaroglu O. Anaplastic carcinoma of the pancreas: a rare clinical entity. Cureus. 2017;9:e1782. doi: 10.7759/cureus.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren B, Liu X, Suriawinata AA. Pancreatic ductal adenocarcinoma and its precursor lesions: histopathology, cytopathology, and molecular pathology. Am J Pathol. 2019;189:9–21. doi: 10.1016/j.ajpath.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Jang KT, Park SM, Basturk O, Bagci P, Bandyopadhyay S, Stelow EB, et al. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: implications for management and prognosis. Am J Surg Pathol. 2015;39:179–187. doi: 10.1097/PAS.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, et al. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. 2002;7:376–380. doi: 10.1007/s101470200059. [DOI] [PubMed] [Google Scholar]
- 52.Strobel O, Hartwig W, Bergmann F, Hinz U, Hackert T, Grenacher L, et al. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery. 2011;149:200–208. doi: 10.1016/j.surg.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 53.Xu M, Chen W, Wang D, Nie M. Clinical characteristics and prognosis of osteoclast-like giant cell tumors of the pancreas compared with pancreatic adenocarcinomas: a population-based study. Med Sci Monit. 2020;26:e922585. doi: 10.12659/MSM.922585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hrudka J, Lawrie K, Waldauf P, Ciprová V, Moravcová J, Matěj R. Negative prognostic impact of PD-L1 expression in tumor cells of undifferentiated (anaplastic) carcinoma with osteoclast-like giant cells of the pancreas: study of 13 cases comparing ductal pancreatic carcinoma and review of the literature. Virchows Arch. 2020;477:687–696. doi: 10.1007/s00428-020-02830-8. [DOI] [PubMed] [Google Scholar]