Abstract
Objectives
The impact of dietary factors on fecal occult blood (FOB) testing has been previously evaluated in cats, but the analytical sensitivity of this point-of-care test remains unexamined. The primary goal of this study was to assess the analytical sensitivity of the FOB test in cats.
Methods
Five cats were used in a repeated measures study. Following oral administration of blood, feces were collected and tested every 12 h for FOB and melena. All cats were fed an animal protein-free diet starting the week before entry into the study. Blood was administered on a milligram of hemoglobin per kilogram of body weight basis, and dosed at 1.5, 3, 15, 30 and 45 mg/kg hemoglobin in series with a wash-out period between each trial.
Results
FOB was detected in one cat at 1.5 mg/kg hemoglobin, three cats at 3 mg/kg hemoglobin and in all five cats at 15, 30 and 45 mg/kg hemoglobin. Melena was noted in one cat at 30 mg/kg and four cats at 45 mg/kg, but not at lower doses.
Conclusions and relevance
Administration of 15 mg/kg hemoglobin (equivalent to about 1.5 ml blood) was sufficient for positive results in all cats. However, detection occurred with as little as 1.5 mg/kg hemoglobin. Thus, FOB has good analytical sensitivity in cats under appropriate clinical situations.
Introduction
In veterinary medicine there is an ongoing need for cost-effective screening diagnostics (Bayer Veterinary Care Usage Study: Phase 1; Bayer Healthcare 2011). In veterinary medicine, point-of-care (POC) testing has become a routine aspect of patient assessment in many areas, including blood glucose assessment, ketoacidemia assessment and blood gas analysis.1–3 Historically, one of the earliest POC diagnostics popularized in human and veterinary medicine was designed to determine the presence of occult blood in the feces.
In human medicine, fecal occult blood (FOB) testing is widely accepted in screening of patients at risk of developing gastrointestinal cancer. 4 This has led to significant decreases in mortality associated with diseases such as colorectal cancer.5–9 Findings such as these have led to FOB testing becoming the most commonly used POC test in human colorectal cancer screening. 4 This information in human medicine highlights the potential benefit of this cheap, quick and easy diagnostic if studied further and applied to more specific situations in veterinary medicine.
Although considered by some to be an antiquated test, POC diagnostics like the FOB still have value as a diagnostic screening test when used in the correct clinical setting in veterinary medicine. For example, in previous studies, diets with animal protein, diets with high peroxidase activity and diets high in vitamin C have been shown to result in either false-positives or false-negatives.10–12 However, when diet and medications are controlled for, FOB is a reliable screening test for the presence of occult blood in humans and dogs.11–13 Given this information, over the past few decades FOB testing has been reported in a variety of veterinary species.11–18 However, currently the FOB test has had minimal validation in cats. The current extent of knowledge regarding this test in cats is centered around the effect of diet on false-positive reactions. 17 Therefore, currently, when this test is performed in clinical practice it is used without supporting evidence-based medicine. As a result, its diagnostic capability and validity are still in question in this species.
The aim of this study was to characterize the limit of detection of FOB detection in feline feces, using a commercially available testing kit. The secondary aim of this study was to determine the approximate blood volume required to cause melena.
Materials and methods
All animal use was approved by The Ohio State University Institutional Animal Care and Use Committee. Five healthy, purpose-bred cats were used in this study. All cats were 5 years of age. Cats were housed in individual cages in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All cats were acclimatized and socialized for at least 1 year before the start of experiments, with environmental enrichment provided. At the conclusion of the experiment all animals were adopted into permanent homes.
Cats were fed a balanced veterinary formula diet (APF diet) ad libitum, which contained no animal protein (Royal Canin Veterinary Diet). Daily physical examinations were performed throughout the study. Routine laboratory tests, including complete blood counts (CBC), serum chemistry, coagulation profile and urinalysis, were performed at the beginning of the acclimatization period. No treats, food additives or other oral medications were administered during the course of the study. Throughout the experiment, naturally voided feces were collected from each cat every 12 h. At each 12 h time point, the feces were immediately tested for the presence of occult bleeding and the overt visual presence of melena.
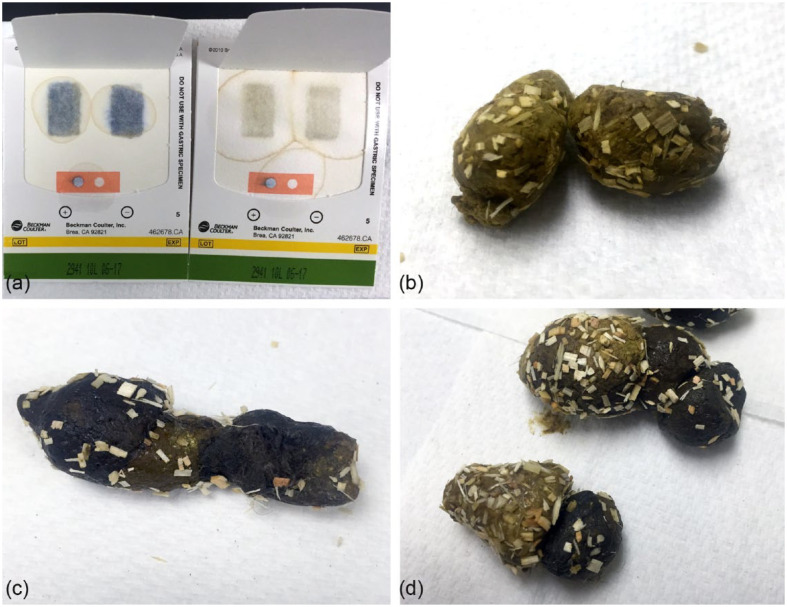
FOB testing was performed using a commercially available diagnostic kit (Hemoccult Single Slides; Beckman Coulter). The test kit was used according to the manufacturer’s instructions. In brief, an applicator stick was used to apply a thin smear of fresh feces to the two test boxes inside the test card. Once the feces were applied, the test card was closed and allowed to incubate fully for 4–5 mins at room temperature. At the completion of the 4–5 min incubation period, a developer was applied to each test spot, as well as the positive and negative controls on the test card. Results were assessed and recorded at 60 s for the fecal smears and at 10 s for the positive and negative control. Positive and negative results were determined by comparison to the positive and negative controls. If the collected fecal samples were black and positive on the occult blood test they were classified as melenic (Figure 1). All testing was performed by one author (AR).
Figure 1.
(a) Fecal occult blood test kit (Hemoccult Single Slides; Beckman Coulter). Test card on the left demonstrates positive and negative controls in orange box, as well as positive results, indicated by the blue color in the test windows. Test card on the right demonstrates positive and negative controls in orange box, as well as negative results, indicated by the lack of blue color in the test windows. (b) Normal feline feces while on the APF diet (Royal Canin Veterinary Diet). (c) Melenic feces and (d) comparison of feces before and after blood dosing at 45 mg/kg
Heterologous canine packed red blood cells were administered orally, as a single dose, to each cat on a milligram of hemoglobin per kilogram of body weight basis. Blood was obtained from the Animal Blood Bank at The Ohio State University Veterinary Medical Center. The Animal Blood Bank donors are screened based on physical examination performed by a veterinarian and normal baseline bloodwork (CBC, chemistry profile) and negative for infectious disease (ie, Dirofilaria immitis antigen, Anaplasma phagocytophilum antibody, Anaplasma platys antibody, Borrelia burgdorferi antibody, Ehrlichia canis antibody and Ehrlichia ewingii antibody). All donors are required to be ⩾1 year of age, current on vaccines and be on no medications. Blood used in the study was evaluated by CBC at the beginning of the study (day 0) (Table 1). Between trials, the blood was stored at 4°C in citrate phosphate dextrose solution with red cell preservatives (USP [CPD] BLOOD-PACK; Fenwal). This stored solution was tested independently to ensure it did not affect the results of the Hemoccult test. No positive reactions were noted during this testing (data not shown).
Table 1.
Complete blood count for donor animal
| Test | Value | Reference interval |
|---|---|---|
| RBCs (×1012/l) | 5.46 | 5.65–8.87 |
| HCT (%) | 34.1 | 37.3–61.7 |
| HGB (g/l) | 131 | 131–205 |
| MCV (fl) | 62.5 | 61.6–73.5 |
| MCH (pg) | 24.0 | 21.2–25.9 |
| MCHC (g/dl) | 38.4 | 32.0–37.9 |
| RDW (%) | 18.6 | 13.6–21.7 |
| Reticulocytes (% of RBCs) | 0.002 | |
| WBCs (×109/l) | 6.71 | 5.05–16.76 |
| Neutrophils (×109/l) | 5.14 | 2.95–11.64 |
| Lymphocytes (×109/l) | 0.88 | 1.05–5.10 |
| Monocytes (×109/l) | 0.12 | 0.16–1.12 |
| Eosinophils (×109/l) | 0.57 | 0.06–1.23 |
| Basophils (×109/l) | 0 | 0–0.10 |
| Platelets (×109/l) | 156 | 148–484 |
| MPV (fl) | 12.0 | 8.7–13.2 |
| PDW (fl) | 18.5 | 9.1–19.4 |
| PCT (%) | 0.19 | 0.14–0.46 |
RBCs = red blood cells; HCT = hematocrit; HGB = hemoglobin; MCV = mean cell volume; MCH = mean cell hemoglobin; MCHC = mean cell hemoglobin concentration; RDW = red cell distribution width; WBCs = white blood cells; MPV = mean platelet volume; PDW = platelet distribution width; PCT = plateletcrit
All cats were fed a commercial maintenance feline diet (Teklad Diets, Harlan Feline Diet #2060; Harlan Laboratories) until 1 week before entry into the study. At day −7 the diet was changed to the APF diet (Royal Canin Veterinary Diet) and FOB testing was started. Each cat was required to have six consecutive fecal tests that were negative for occult blood before the first (day 0) oral administration of blood and before each consecutive administration. Blood was dosed at 1.5, 3, 15, 30 and 45 mg/kg hemoglobin. Each dosage was administered as a single dose followed by a FOB testing period. All cats received the lowest dose first and then consecutively higher doses after each wash-out period.
Statistical analysis was performed using commercially available computer software (GraphPad Prism [GraphPad Software] and SPSS 14.0 for Mac [IBM]). Descriptive statistics were calculated separately for time to reach a positive result after blood administration, as well as the time to negative result. The data were analyzed for normality using the Shapiro–Wilk test. All data were found to be non-parametric. Friedman’s test with Dunn’s post-hoc test was therefore used to assess data. Statistical significance was set at P <0.05.
Results
Immediately after transitioning from the maintenance diet to the APF diet, one cat was FOB-negative and the other four FOB-positive. All cats became FOB-negative within 84 h (median 48 h, range 0–84 h). At the time of the first PO blood administration, all five cats were FOB-negative for at least seven consecutive evaluations (median 10, range 7–14; median 120 h, range 84–168 h).
FOB was detected in one (20%) cat at 1.5 mg/kg hemoglobin, three (60%) cats at 3 mg/kg hemoglobin, and in all five (100%) cats at 15, 30 and 45 mg/kg hemoglobin (Table 2). Melena was not detected in doses <30 mg/kg hemoglobin. At 30 mg/kg, one (20%) cat had a fecal appearance consistent with melena. At 45 mg/kg, four (80%) cats had a fecal appearance consistent with melena.
Table 2.
Number of animals that tested positive on fecal occult blood testing and for presence of melenic feces, stratified according to dosage
| Dose (mg/kg HGB) | Occult-positive cats (n) | Occult-negative cats (n) | Median time to positive result (h) | Melena-positive cats (n) | Melena-negative cats (n) | Median time to positive result (h) |
|---|---|---|---|---|---|---|
| 1.5 | 1 | 4 | 24 | 0 | 5 | NA |
| 3 | 3 | 2 | 24 | 0 | 5 | NA |
| 15 | 5 | 0 | 24 | 0 | 5 | NA |
| 30 | 5 | 0 | 24 | 1 | 4 | 24 |
| 45 | 5 | 0 | 24 | 4 | 1 | 24 |
HGB = hemoglobin; NA = not applicable
In all cases except one, the first FOB positive result was detected after two evaluations (24 h) after PO blood administration. In the single positive cat that was negative at 24 h, it first became positive after three evaluations (36 h). Positive results were persistent up to 108 h at the 45 mg/kg hemoglobin dosage. There was no difference in time to first positive result between dosages (P = 1.00). However, dose did affect time to negative results following positive tests (P = 0.005), with the difference between the 1.5 mg/kg dose and the 45 mg/kg dose maintaining statistical significance with post-hoc testing. All cats tested negative by 36 h after administration of 1.5 mg/kg hemoglobin, by 48 h after administration of 3 mg/kg hemoglobin, by 72 h after administration of 15 mg/kg hemoglobin (median 48 h, range 36–72 h), by 84 h (median 72 h, range 48–84 h) after administration of 30 mg/kg hemoglobin and by 108 h (median 72 h, range 72–108 h) after administration of 45 mg/kg hemoglobin.
The doses used in this study correspond to approximately 0.06, 0.12, 0.63, 1.26 and 1.89 ml (total volume) of canine blood with hemoglobin of 131 g/l. When these hemoglobin concentrations are extrapolated to typical feline blood values, the volume remains similar and corresponds to approximately 0.08, 0.17, 0.83, 1.65 and 2.48 ml (total volume) of feline blood with hemoglobin of 100 g/l. Variation in hemoglobin concentration would affect the volume necessary to cause positive results (Table 3).
Table 3.
Estimated blood volumes expected to reproduce results based on average hemoglobin concentrations
| Dose (mg/kg HGB) | Median (range) volume of canine blood (ml) (HGB 13.1) | Median (range) estimated volume of feline blood (ml) (HGB 5) | Median (range) estimated volume of feline blood (ml) (HGB 10) | Median (range) estimated volume of feline blood (ml) (HGB 15) |
|---|---|---|---|---|
| 1.5 | 0.06 (0.05–0.08) | 0.17 (0.14–0.20) | 0.08 (0.07–0.10) | 0.06 (0.05–0.07) |
| 3 | 0.12 (0.10–0.15) | 0.33 (0.28–0.41) | 0.17 (0.14–0.20) | 0.11 (0.10–0.14) |
| 15 | 0.63 (0.52–0.77) | 1.65 (1.38–2.04) | 0.83 (0.69–1.02) | 0.55 (0.46–0.68) |
| 30 | 1.26 (1.04–1.54) | 3.30 (2.76–4.08) | 1.65 (1.38–2.04) | 1.10 (0.92–1.36) |
| 45 | 1.89 (1.56–2.31) | 4.95 (4.14–6.12) | 2.48 (2.07–3.06) | 1.65 (1.38–2.04) |
HGB = hemoglobin
Discussion
This study represents the first report of the quantity of blood needed to obtain a positive test result for FOB in cats using the Hemoccult test cards. 18 Administration of 15 mg/kg hemoglobin was required to yield a positive result consistently in all cats within 24 h of administration. However, a dose as low as 1.5 mg/kg hemoglobin was detected as positive in one cat. This demonstrates that the FOB test has good sensitivity at 15 mg/kg of hemoglobin and an overall low limit of detection in appropriate clinical situations.
In dogs, based on hemoglobin content, 20 mg/kg was sufficient to cause a consistent positive on the FOB test, but 500 mg/kg was needed to cause melena. 13 In people, the lower limit of detection of a single dose of ingested blood is about 25 ml whole blood (roughly equal to 50 mg/kg of hemoglobin based on a 75 kg body weight per person and an average concentration of hemoglobin of 150 g/l). 19 In the present study, the dosage of hemoglobin necessary was similar to that used in the main canine study to cause a consistent positive result and, on rare occasions, able to detect FOB at much lower dosages. 13 However, this comparison must be interpreted with caution as diagnostic test methods and advances in test kit performance have changed since the initial studies in dogs and people were performed.
Hemoccult (Hemoccult Single Slides; Beckman Coulter) blood testing is based on detection of the heme portion of the hemoglobin molecule. 20 Heme has peroxidase activity, which catalyzes the oxidation of alpha (α)-guaiaconic acid (the active component of the guaiac paper) by hydrogen peroxide (the active component of the developer) to form a highly conjugated blue quinone compound. Guaiac tests vary in their performance characteristics, and more recent test kits, including the Hemoccult, reportedly result in a more intense blue color reaction translating to improved overall readability and precision, according to the manufacturer (Beckman Coulter). However, as mentioned before, these variations can result in different testing results between kits. In the initial reports of occult blood testing using older guaiac acid-based tests, their sensitivity was reduced compared with other tests. 21 This variability and recent advancements, which are different between test kits, may explain the improved analytical sensitivity in the Hemoccult test that was used in this study. However, we are unable to determine if this is the definitive cause as species differences, testing condition or other variables may be playing a role.
When considering the hemoglobin molecule, it is typically viewed as a tetramer comprised of four globulins (two α and two beta (β) subunits) with a single heme molecule bound in the center. There is well-conserved homology across mammalian species in the heme, while genetic and structural variation is commonly seen in the globulin components. 22 Therefore, species differences are unlikely to affect the reaction and differences in the globin are irrelevant. As a result, in this study we chose to utilize canine blood products, owing to their ease of availability. However, this represents a theoretical limitation of the study.
In humans, it is also known that FOB testing can be altered by other patient-related factors including diet, collection method and medications.23–30 Specifically, the effect of diet on FOB detection has been reported in cats. 17 However, the amount of hemoglobin associated with blood detection (occult or clinical) was never assessed in that study. The animals used in the present study were in a highly controlled environment and represent an ideal situation in terms of clinical use and minimized patient-based factors. Therefore, effects of diet on the FOB test sensitivity was minimized for false-positive results and optimized for clinical performance. Importantly, these data cannot be extrapolated to other diets or clinical scenarios. As a result, it could limit the clinical utility of this test if the cat will not eat the test diet. Additionally, as all cats had naturally voided fecal samples and were on no medications at the time of the study, it is assumed that these factors are not altering the presented data.
Conclusions
For clinical application of the results reported in this study, we stress the importance of utilizing this screening test in the recommended manner. Most importantly, ensuring the animal is on a diet devoid of animal protein and does not have a medication history, which could result in false-positive results in healthy patients, is imperative. Also, gastrointestinal hemorrhage below the limit of detection may occur, albeit at very small volume, causing a false-negative result. Because both false-positive and false-negative results are possible in a clinical situation, the FOB test should remain a screening test utilized in strict clinical conditions, including a controlled diet, adequate washout period prior to testing and no medication, which could interfere with results.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 10 March 2016
References
- 1. Zini E, Moretti S, Tschuor F, et al. Evaluation of a new portable glucose meter designed for the use in cats. Schweiz Arch Tierheilkd 2009; 151: 448–451. [DOI] [PubMed] [Google Scholar]
- 2. Zeugswetter FK, Rebuzzi L. Point-of-care β-hydroxybutyrate measurement for the diagnosis of feline diabetic ketoacidaemia. J Small Anim Pract 2012; 53: 328–331. [DOI] [PubMed] [Google Scholar]
- 3. Irizarry R, Reiss A. Arterial and venous blood gases: indications, interpretations and clinical applications. Compend Contin Educ Vet 2009; 31: E1–E7. [PubMed] [Google Scholar]
- 4. Benson VS, Patnick J, Davies AK, et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer 2008; 122: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 5. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 6. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 7. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 8. Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecel occult blood screening for colorectal cancer. Br J Surg 2008; 95: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 9. Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999; 91: 434–437. [DOI] [PubMed] [Google Scholar]
- 10. Bond JH. Fecal occult blood tests in occult gastrointestinal bleeding. Sem Gastrointest Dis 1999; 10: 48–52. [PubMed] [Google Scholar]
- 11. Cook AK, Gilson SD, Fischer WD, et al. Effects of diet on the results obtained by the use of two commercial test kits for the detection of occult blood in feces of dogs. Am J Vet Res 1992; 53: 1749–1751. [PubMed] [Google Scholar]
- 12. Rice JE, Ihle SL. Effects of diet on fecal occult blood testing in healthy dogs. Can J Vet Res 1994; 58: 134–137. [PMC free article] [PubMed] [Google Scholar]
- 13. Gilson SD, Parker BB, Twedt DC. Evaluation of two commercial test kits for detection of occult blood in feces of dogs. Am J Vet Res 1990; 51: 1385–1387. [PubMed] [Google Scholar]
- 14. Jinbo T, Shinmura R, Shida T, et al. Experimental detection of canine haemoglobin (occult blood) in canine faeces by reversed passive latex agglutination. Vet Res Comm 1997; 21: 347–353. [DOI] [PubMed] [Google Scholar]
- 15. Payton AJ, Glickman LT. Fecal occult blood in cattle. Am J Vet Res 1980; 41: 918–921. [PubMed] [Google Scholar]
- 16. Boulay JP, Lipowitz AJ, Klausner JS, et al. Evaluation of a flourometric method for the quantitative assay of fecal hemoglobin in the dog. Am J Vet Res 1986; 47: 1292–1295. [PubMed] [Google Scholar]
- 17. Tuffli SP, Gaschen F, Neiger R. Effect of dietary factors on the detection of fecal occult blood in cats. J Vet Diagn Invest 2001; 13: 177–179. [DOI] [PubMed] [Google Scholar]
- 18. Smith BB, McKim JM, Jr, Pearson EG, et al. Fecal occult blood in the horse: quantitation and interpretation using the hemoglobin peroxidase test [abstract]. Proc Conf Res Workers Anim Dis 1985; 66: 24. [Google Scholar]
- 19. Ross G, Gray CH, de Silva S, et al. Assessment of routine tests for occult blood in faeces. Br Med J 1964; 1: 1351–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kratochvil JF, Burris RH, Seikel MK, et al. Isolation and characterization of alpha guaiaconic acid and the nature of guaiacum blue. Phytochemistry 1971; 10: 2529. [Google Scholar]
- 21. Van Dam L, Kuipers EJ, van Leerdam ME. Performance improvements of stool-based screening tests. Best Pract Res Clin Gastroenterol 2010; 24: 479–492. [DOI] [PubMed] [Google Scholar]
- 22. Hardison R. Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J Exp Biol 1998; 201: 1099–1117. [DOI] [PubMed] [Google Scholar]
- 23. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US-Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134: 1570–1595. [DOI] [PubMed] [Google Scholar]
- 24. Sinatra MA, St John DJ, Young GP. Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clin Chem 1999; 45: 123–126. [PubMed] [Google Scholar]
- 25. Pignone M, Campbell MK, Carr C, et al. Meta-analysis of dietary restriction during fecal occult blood testing. Eff Clin Pract 2001; 4: 150–156. [PubMed] [Google Scholar]
- 26. Greenberg PD, Cello JP, Rockey DC. Relationship of low-dose aspirin to GI injury and occult bleeding: a pilot study. Gastrointest Endosc 1999; 50: 618–622. [DOI] [PubMed] [Google Scholar]
- 27. Kahi CJ, Imperiale TF. Do aspirin and nonsteroidal anti-inflammatory drugs cause false-positive fecal occult blood test results? A prospective study in a cohort of veterans. Am J Med 2004; 1: 837–841. [DOI] [PubMed] [Google Scholar]
- 28. European Colorectal Cancer Screening Guidelines Working Group. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013; 45: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collins JF, Lieberman DA, Durbin TE, et al. Accuracy of screening for fecal occult blood on a single stool sample obtained by digital rectal examination: a comparison with recommended sampling practice. Ann Intern Med 2005; 142: 81–85. [DOI] [PubMed] [Google Scholar]
- 30. Sawhney MS, McDougall H, Nelson DB, et al. Fecal occult blood test in patients on low-dose aspirin, warfarin, clopidogrel, or non-steroidal anti-inflammatory drugs. Dig Dis Sci 2010; 55: 1637–1642. [DOI] [PubMed] [Google Scholar]