Abstract
Objectives
Squamous cell carcinoma (SCC) is the most common oral tumor in cats and typically carries a poor prognosis with current treatment options. The objective of this study was to evaluate the toxicity of toceranib phosphate (Palladia; Pfizer) in cats with oral SCC in combination with other treatment modalities.
Methods
In this study, 35 cats were retrospectively evaluated to determine toxicity when treated with toceranib in combination with other treatment modalities. Cats received toceranib at a median dose of 2.75 mg/kg (range 1.9–4.17 mg/kg) 3 days a week. Cats also underwent additional therapies, including surgical excision, radiation therapy, chemotherapy and/or use of non-steroidal anti-inflammatory drugs.
Results
Toxicity was seen in six cats, with five cases of grade 1 or 2 gastrointestinal (GI) toxicity and one grade 4 metabolic toxicity. Toceranib was discontinued in one cat and two cats received dose reductions. None of the cats required treatment delays or hospitalization due to toxicity. Median toceranib treatment duration was 77 days (range 7–741 days).
Conclusions and relevance
This study revealed that toceranib was well tolerated by the majority of cats, with five cases of low-grade GI toxicity and one case of metabolic toxicity. Given the favorable toxicity profile, future studies further evaluating the safety and efficacy of toceranib for cats with oral SCC should be considered.
Introduction
Oral tumors comprise approximately 3–12% of all feline neoplasms and in one study evaluating a cohort of 371 cats, squamous cell carcinoma (SCC) was the most common, representing 61% of malignant oral tumors.1–3 The most common locations of oral SCCs in the cat are the base of the tongue, the mandible and the maxilla, and these tumors are typically locally invasive with destruction of underlying bone.3,4 These tumors have a relatively low rate of metastasis but have been reported to spread to regional lymph nodes and to lungs.4,5 Cats with oral SCC present with a variety of clinical signs, including oral pain, halitosis, ptyalism, difficulty prehending food, anorexia, weight loss, and loosening or loss of teeth. 6
Even with aggressive therapy, survival time for cats with SCC is reported to be only 2–4 months, with a 1 year survival rate of <10%. 7 More recent studies have shown some improvement but are still relatively disappointing. This poor prognosis is usually due to progression of local disease, resulting clinical signs and decreased quality of life, leading to humane euthanasia.
Currently, there is no effective standard of care for feline oral SCC. Historic treatment options for cats with oral SCCs include traditional cytotoxic chemotherapy, surgery, radiation therapy (RT), accelerated RT or some combination of these treatments. Chemotherapy alone has proven to be of little benefit. Therapy using doxorubicin in combination with cyclophosphamide yielded a median survival time (MST) of 30 days, and use of a liposomal cisplatin analog yielded an MST of 59 days.8,9 Surgery alone for the treatment of oral SCC has also shown to be of limited benefit. In a previous study, five cats with oral SCCs treated with mandibulectomy alone had an MST of 5 months, with one cat alive and tumor free at 12 months. 10 RT alone has also shown inconsistent results. Palliative RT in cats with feline oral SCCs resulted in an MST of 60 and 92 days in two studies, respectively, while accelerated RT resulted in an MST of 86 days and 174 days in two studies, respectively.11–14
Overall, combination therapies have shown slightly more encouraging results. Gemcitabine chemotherapy with palliative RT in eight cats yielded an MST of 111 days. 15 The combination of carboplatin and an accelerated RT protocol resulted in an MST of 163 days. In this study, cats with SCCs of the tonsil or cheek had a MST of 724 days. 16 Hemimandibulectomy followed by orthovoltage RT resulted in the longest MST of 420 days postsurgery in cats with mandibular oral SCCs, but this study only evaluated seven cats. 17 Three cats with sublingual SCCs treated using multimodal therapy with medical therapy, RT and surgery were still alive and in a complete remission at 759, 458 and 362 days, respectively. 18
Based on the inconsistent outcomes for these patients, even in the face of aggressive treatment, novel therapies are needed to attempt to improve tumor response and survival. Small-molecule inhibitors have been evaluated in the treatment of head and neck SCC in humans, with promising results in vitro and in vivo.19,20 Small-molecule inhibitors can have both direct antitumor effects through the disruption of molecular signaling pathways involved in tumor progression, and antiangiogenic effects through inhibition of certain growth factor receptors. The small-molecule inhibitor toceranib phosphate (Palladia; Pfizer) is a receptor tyrosine kinase inhibitor, approved for treatment of mast cell tumors in dogs, that targets several members of the split kinase family, including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), Kit, colony stimulating factor-1 receptor and Flt-3. 21 Toceranib has been shown to be well tolerated and to have activity against a broad range of tumors in dogs, including some solid tumors.21,22 Toceranib has shown modest biologic activity with one complete response, four with stable disease and three with progressive disease, and limited adverse events in 10 cats with SCCs in a 2010 abstract presented by Hohenhaus et al at the Veterinary Cancer Society conference. 23
To our knowledge, there have been no published studies evaluating toxicity of toceranib in cats with oral SCC. The objective of this study was to evaluate the toxicity of toceranib in cats with oral SCCs in combination with other treatment modalities.
Materials and methods
This study was designed as a multi-institutional retrospective study. Medical records from The Veterinary Cancer Center (Norwalk, CT, USA), Veterinary Referral and Emergency Center of Westbury (Westbury, NY, USA), Pacific Veterinary Specialists (Capitola, CA, USA), Katonah Bedford Veterinary Center (Bedford Hills, NY, USA), Angell Animal Medical Center (Boston, MA, USA) and Veterinary Healthcare Associates (Winter Haven, FL, USA) were retrospectively evaluated for cats treated for oral SCC from October 2009 to August 2013. To be included in the study cats had to have histologically confirmed oral SCC and have been treated with toceranib as either a sole therapy or in conjunction with other treatment modalities. Patients were excluded from the study if they did not have a confirmed histologic diagnosis of oral SCC, they were not treated with toceranib, or they did not have toxicity or follow-up data for at least 4 weeks after starting toceranib. None of the cases reviewed were excluded if they developed toxicity prior to the 4 week time point. Information was collected regarding signalment, tumor location, clinical signs, initial surgical procedure, initial toceranib dose (mg/kg), toceranib dose after any dose reductions (mg/kg), toceranib treatment duration, concurrent medications (including non-steroidal anti-inflammatory drugs) and other treatment modalities such as RT and cytotoxic chemotherapy. Results of staging tests were reviewed, including complete blood count (CBC), chemistry panel, urinalysis, lymph node cytology and thoracic radiographs, when available. Initial size of the oral mass was recorded, when available, from medical records. If possible, cats were staged using the clinical staging system for oral tumors in dogs and cats. Records were also reviewed for re-staging and response data when available. Hematologic and gastrointestinal (GI) toxicity was graded using the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events. 24
Statistical analyses
The Wilcoxon rank-sum test was used to evaluate the relationship between initial toceranib dose (mg/kg) and the development of any toxicity. A P value <0.05 was considered statistically significant. The Kaplan–Meier product limit method was used to calculate median duration of toceranib therapy and to generate a curve for duration of treatment. Cats that were still on toceranib at the time of data collection or lost to follow-up were censored for analysis of duration of treatment. Statistical analyses and generation of curves were performed using commercially available software (STATA version 11.2; Stata Corp).
Results
Thirty-five cats met the inclusion criteria and were included in the study. The cats in this study were evaluated from October 2009 until the last follow-up date of August 2013. Median age was 14 years (range 5–20 years). There were 19 female and 16 male cats. Thirty-four cats were altered and one was an intact female. There were four purebreds, including one Bengal, one Maine Coon, one Norwegian Forest Cat and one Abyssinian. The other 31 cats were domestic shorthairs, domestic mediumhairs and domestic longhairs. The median body weight was 4.5 kg (range 2.4–5.9 kg). Eighteen cats had tumors located on the mandible, eight on the maxilla, seven sublingual or lingual, one was on the left lip commissure and the exact location of one oral tumor was not specified. Eight tumors were located rostral to the third premolar (PM3) within the oral cavity, 10 were located caudal to PM3 and in the remaining 17 the tumor location was not specified.
The most common presenting clinical signs were facial swelling in 10 cats (29%), ptyalism in eight cats (23%), decreased appetite in seven cats (20%), dysphagia in five cats (14%), weight loss in five cats (14%), halitosis in four cats (11%) and pawing at the mouth in four cats (11%). Six of the cats (17%) had their tumor identified during a routine dental cleaning and two of the cats (6%) were presented for oral bleeding.
Ten cats (29%) had no reported prior or concurrent medical conditions. Eleven cats (31%) had a heart murmur, five (14%) were hyperthyroid, three (9%) had chronic renal disease based on long-term azotemia and isosthenuria, and the following were seen in one cat (3%) each: hypertrophic cardiomyopathy, history of allergies and presumptive feline asthma, feline immunodeficiency virus, a brain tumor presumed to be a meningioma based on the presence of a mass on computed tomography (CT) scan, a history of multiple mammary tumor excisions (one adenocarcinoma and two adenomas) and small cell intestinal lymphoma. Eight cats had more than one prior or concurrent medical condition.
Staging tests varied for each cat. Thirty-four of the 35 cats (97%) had CBCs performed. In 31 of these cats (91%) the CBC was within normal limits, and three cats (9%) had mild anemia. Thirty-four cats (97%) had chemistry panels. In 25 of these cats (74%) the chemistry panel revealed no significant findings. Two cats (6%) had an elevated blood urea nitrogen (BUN) with normal creatinine (55 mg/dl and 42 mg/dl, respectively; BUN range 14–36 mg/dl), one cat (3%) had an elevated creatinine with normal BUN (2.5 mg/dl; creatinine range 0.6–2.4 mg/dl) and three cats (9%) were listed as having azotemia in their medical record, but BUN/creatinine values were unavailable. One cat (3%) had hypercalcemia (12.5 mg/dl; range 8.6–10.6 mg/dl), one cat (3%) with a history of severe stomatitis had hyperglobulinemia (6.3 g/dl; range 2.3–5.9 g/dl), and one cat (3%) had mildly elevated alanine transaminase (ALT) (153 U/l; range 10–100 U/l) and hypercalcemia (11 mg/dl; range 8.6–10.6 mg/dl).
Twenty-eight cats (80%) were staged with thoracic radiographs and 26 (93%) of these showed no significant findings. One cat had a solitary pulmonary nodule that had been present for >6 months prior to the diagnosis of oral SCC. The other cat had a solitary left cranial lung lobe mass, which was interpreted as either a primary lung tumor or a metastatic lesion. No further diagnostic testing was performed on either of these cats to determine the cause for these pulmonary lesions.
Four of the 35 cats (11%) had a mandibular lymph node aspirate performed. In two of these cats (6% of the total study population) the cytology was consistent with metastatic SCC, and in the other two, cytology revealed a reactive lymph node.
One of the 35 cats (3%) had an abdominal ultrasound that revealed changes consistent with chronic kidney disease and signs of potential pancreatitis. Two of the 35 cats (6%) had skull radiographs and two of the 35 cats (6%) had a CT scan (one of the thoracic cavity and one of the skull).
Twenty-five of the cats (71%) had incisional biopsy of their tumor only. Ten cats (29%) underwent an initial surgical procedure, with seven of these (70%) having debulking of their tumors, one of these (10%) having an incomplete excision, one of these (10%) having incomplete excision with a third of the tongue excised for primary tongue SCC, and one of these (10%) having a complete but narrow excision with 1–2 mm margins reported on the histopathology report.
Thirty-four cats (97%) received toceranib as the initial therapy, while one cat (3%) received a single dose of intravenous (IV) carboplatin prior to starting toceranib. All cats in the study were treated on a Monday/Wednesday/Friday schedule (this dose schedule was based on that used in dogs). The median toceranib dose received was 2.75 mg/kg (range 1.9–4.17 mg/kg). The median toceranib treatment duration was 77 days (range 7–741 days).
Nine of the 35 cats (26%) received palliative RT as part of their treatment. The RT protocols varied based on clinician discretion, with four cats (44%) receiving four treatments of 8 Gy each, two cats (22%) receiving three treatments of 8 Gy each, one cat (11%) receiving six treatments of 6 Gy, one cat (11%) receiving one treatment of 12 Gy and one cat (11%) receiving six treatments of 5 Gy. Two of these cats (22%) had CT scans performed for radiation planning, three (33%) did not have CT scans for radiation planning and this information was unavailable for the remaining four cats (44%). Specific dosing information, other than prescribed dose, was not available for the radiation plans.
Three of the 35 cats (9%) received cytotoxic chemotherapy as part of their treatment. One cat (33%) received two treatments with carboplatin at 190 mg/m2 IV every 3 weeks after failing toceranib. One cat (33%) received one treatment with carboplatin at 200 mg/m2 IV followed by one treatment of mitoxantrone (Novantrone; Pfizer) at 5 mg/m2 IV, both after failing toceranib. Toceranib was completely discontinued in both of these cats prior to starting cytotoxic chemotherapy. One cat (33%) received one treatment with carboplatin at 200 mg/m2 IV prior to starting toceranib. One cat was being treated for small cell intestinal lymphoma with chlorambucil (Leukeran; GlaxoSmithKline) at 0.4 mg/kg orally every 14 days for 1 year prior to diagnosis with oral SCC, but the chlorambucil was discontinued prior to administration of toceranib. One cat (3%) was treated with one dose of Pamidronate (Aredia; Novartis) at 2 mg/kg IV while on toceranib.
Nineteen of the 35 cats (54%) received a non-steroidal anti-inflammatory drug (NSAID) concurrently with toceranib. In all cases where it was used concurrently with toceranib, the NSAID was given on the days when toceranib was not given. Eighteen out of 19 cats received meloxicam (Metacam; Boehringer Ingelheim) and the dose varied based on clinician discretion. Information on dose was available for 13/18 cats that received meloxicam. Median meloxicam dose was 0.1 mg/kg (mean dose 0.078 mg/kg; range 0.025–0.200 mg/kg). Meloxicam was given 2–4 days per week, depending on clinician preference. Dosing information was unavailable for 5/18 cats that received meloxicam. The remaining cat received piroxicam (Feldene; Pfizer) 0.3 mg/kg on a Tuesday/Thursday/Saturday schedule. Six of the 35 cats (17%) received prednisolone concurrently with the toceranib at varied doses based on clinician discretion. Doses varied from 1 mg/kg to 2 mg/kg once to twice daily on days when toceranib was not given. None of the cats received prednisolone and an NSAID concurrently.
By reviewing medical records, all 35 cats were evaluated for toxicity. All cats had bloodwork rechecked on a regular basis while receiving toceranib. Seven cats (20%) experienced toxicity. Six of these (86%) experienced toxicity attributed to toceranib (Table 1). One of these seven (14%) experienced vomiting the day after receiving carboplatin (cat was started on carboplatin after toceranib was discontinued) but had no reported toxicity while receiving toceranib. Of the six cats with toxicity while on toceranib, there were two cases of grade 2 vomiting (33%), two cases of grade 2 anorexia (33%), one case of grade 1 anorexia (17%) and one case of grade 4 metabolic toxicity (17%), with elevations in ALT of 1130 (reference interval [RI] 10–100 U/l) and aspartate aminotransferase (AST) of 1047 (RI 10–100 U/l). Toceranib and meloxicam were permanently discontinued in the cat with metabolic toxicity and the cat was started on 90 mg S-adenosylmethionine plus 9 mg silybin A+B (Denamarin; Nutramax) once daily. This cat’s ALT and AST levels both returned to normal (with an ALT of 62 U/l and AST of 36 U/l) 21 days after discontinuing the toceranib and meloxicam and starting S-adenosylmethionine with silybin. Toceranib was not discontinued in any other cats secondary to toxicity. Two of the cats (6%) received toceranib dose reductions. One of the cats with grade 2 anorexia received a transient toceranib dose reduction of 26% for five doses (this cat was not on an NSAID or steroid) and was then re-started on the full dose. One cat with grade 2 anorexia received a permanent dose reduction of 10%. This cat was also receiving meloxicam at 0.026 mg/kg every second or third day on toceranib off days. All dose reductions and drug discontinuations were at the discretion of the clinician. There were no treatment delays and no cats required hospitalization due to toxicity.
Table 1.
Demographics, toceranib dose information, concurrent medications and other therapies for the six cats with toxicity
| Cat | Age (years) |
Sex | Tumor location | Toxicity | Initial toceranib dose (mg/kg) |
Dose reduction (yes/no) |
Dose after dose reduction (mg/kg) |
Permanent dose reduction (yes/no) |
Toceranib discontinued (yes/no) |
Concurrent meloxicam (yes/no, dose in mg/kg) |
Concurrent prednisolone (yes/no and dose) |
RT (yes/no) |
Other chemotherapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
15 | MN | Mandible | Grade 4 metabolic: elevated ALT and AST |
3.2 | Yes | Yes: once every 3 days on toceranib off days (dose NA) | No | No | No | |||
| 2 | 17 | MN | Maxilla | Grade 2 vomiting | 2.8 | No | No | No | No | No | No | ||
| 3 | 5 | F | Mandible | Grade 2 anorexia | 4.17 | Yes | 3.1 | No: five doses only | No | No | No | No | No |
| 4 | 15 | FS | Sublingual | Grade 2 vomiting | 2.1 | No | No | No | No | Yes: 8 Gy three times concurrent with toceranib | Carboplatin: two doses after failing toceranib | ||
| 5 | 16 | FS | Mandible | Grade 2 anorexia | 3.3 | Yes | 2.97 | Yes | No | Yes: 0.026 every 2–3 days on toceranib off days | No | No | No |
| 6 | 14 | FS | Sublingual | Grade 1 anorexia | 2.2 | No | No | No | Yes: 1 mg/kg q24h | No | No |
RT = radiation therapy; MN = male neutered; ALT = alanine transaminase; AST = aspartate aminotransferase; NA = not available; F = female; FS = female spayed
At the end of the study period, three cats (9%) were still alive (at 38, 85 and 741 days, respectively), 29 cats (83%) died or were humanely euthanized due to progression of their oral SCC, one cat (3%) was humanely euthanized due to complications of chronic renal failure and two cats (6%) were lost to follow-up. The cat with the longest follow-up time in this study (alive at the end of the study period at 741 days) was a cat with a small (<0.5 cm) mass on the left rostral mandible that was surgically excised and treated with toceranib therapy for 741 days. Median toceranib treatment duration was 77 days (range 7–741 days) (Figure 1). There was no association between initial toceranib dose and toxicity (P = 0.2079).
Figure 1.
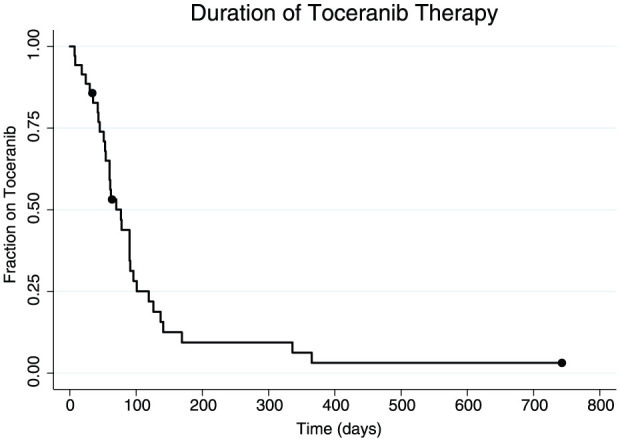
Kaplan–Meier curve for duration of toceranib therapy. Dots represent censors. Median toceranib treatment duration was 77 days (range 7–741 days)
Discussion
The objective of this study was to evaluate the toxicity of toceranib in cats with feline oral SCC in combination with other treatment modalities. Toceranib was well tolerated by the majority of cats in this study with only 6/35 cats (17%) experiencing toxicity. This toxicity included five cats with grade 1–2 GI toxicity and one grade 4 metabolic toxicity. Two cats with GI toxicity had dose reductions at the discretion of the attending clinician. None of the cats with GI toxicity required treatment discontinuation or hospitalization. One cat with metabolic toxicity developed severe elevations of ALT and AST. This cat had no reported prior or concurrent medical conditions. This cat was being treated with toceranib and meloxicam concurrently but was on no other medications. To our knowledge there have been no other published reports showing liver enzyme elevations in cats secondary to toceranib; however, studies evaluating the toxicity of toceranib in cats are limited. An abstract presented at the 2010 Veterinary Cancer Society conference by Hohenhaus et al evaluated the biological activity and adverse event profile in cats treated with toceranib. 23 This abstract included details of 29 cats treated with toceranib for several different tumor types. The cats in this abstract tolerated toceranib well, with only 10 cats having adverse events, one of which had elevated liver enzymes. Studies evaluating the toxicity of two other tyrosine kinase inhibitors, imatinib mesylate and masitinib mesylate, revealed only one cat (out of a total of 29) that had a grade 1 AST elevation and a grade 4 ALT elevation.25,26 This cat was part of the imatinib mesylate study and the study authors noted that they were unable to confirm that these elevations were secondary to the imatinib. The cat in the current study was receiving meloxicam concurrently with toceranib so it is possible that the liver enzyme elevations were secondary to the meloxicam alone, the toceranib alone, a combination of the two drugs or an unrelated issue. The toceranib and meloxicam were both permanently discontinued in this cat, S-adenosylmethionine plus silybin was started and the liver enzymes were normal 21 days later. In the phase 1 dose escalating study of toceranib in dogs, two dogs developed transient elevations in ALT, which resolved spontaneously without discontinuation of therapy. The cause for these elevations was not known but were not believed to be related to the toceranib administration. 22
Toceranib can have both direct antitumor benefit through disruption of molecular proliferation pathways, and an indirect antitumor benefit through inhibition of growth factor receptors leading to antiangiogenic affects. Toceranib is a multitargeted receptor tyrosine kinase inhibitor active against several members of the split-kinase family, including VEGFR, PDGFR and Kit. 21 Toceranib was primarily developed not only for its Kit inhibition, but also as an antiangiogenic agent, owing to its inhibition of VEGFR and PDGFR. In the phase 1 study of toceranib in dogs, partial responses and stable disease were noted in a variety of cancers.21,22 These data suggest that toceranib has additional antitumor activity that is not Kit dependent. This study also showed that the toceranib was well tolerated and safe at doses up to 3.25 mg/kg given as alternate daily dosing. Most dogs experienced toxicities that were mild in severity and amenable to minimal supportive care at that dose and dose schedule.21,22
While toceranib has activity against the split-kinase family, including VEGFR, PDGFR and Kit, tyrosine kinase inhibitors with activity against different growth factor receptors have been evaluated for the treatment of SCCs of the head and neck in humans. In particular, erlotinib hydrochloride (Tarceva; Genentech), which has activity against the epidermal growth factor receptor (EGFR), provided disease stabilization in people with recurrent or metastatic SCCs of the head and neck. 27 Interestingly, two recent studies evaluating the expression of EGFR in feline oral SCC revealed that EGFR is expressed in some (69% in one study) feline oral SCC lesions and thus use of EGFR inhibitors in the future may provide clinical benefit when used alone or in conjunction with conventional therapies.28,29
Survival was not an objective of this study and was not evaluated due to the lack of response data, lack of protocol standardization and use of multiple therapies in this retrospective study, which made treatment benefits inconclusive. Tumor response data is an important factor as it more accurately allows one to evaluate the biological response to therapy. Objective response data, based on measurements or imaging, are also much less susceptible to bias. Response data are particularly important in the evaluation of targeted chemotherapy agents as stabilization of disease may be considered an appropriate endpoint with this form of therapy.30–33 This is in contrast to traditional cytotoxic chemotherapy agents where the desired endpoint is typically disease reduction. Also, the lack of a control group for comparison made it impossible for this study to fully evaluate the true safety and biological effectiveness of toceranib. Future studies should be designed to evaluate therapies like toceranib using blinding and randomization. Prospective studies would also allow for standardization of dose, dose reductions and drug discontinuation. The use of multiple treatment modalities was also a limitation of this study as it made it difficult to fully evaluate the toxicity secondary to just toceranib without the bias of other concurrent therapies. Small sample size was another study limitation and may have affected the ability of the study to identify other patient or treatment associations with toxicity. Owner compliance may also have been a concern as it can be difficult to administer oral medications to cats, especially cats with oral tumors, as they may have associated oral pain from their tumor. The concern is that not all cats may have been receiving the oral toceranib as prescribed and given the retrospective nature of this study, owner compliance could not be evaluated.
The cat with the longest survival time in this study (alive 741 days at the end of the study period) was a cat with a small (<0.5 cm) mass on the left rostral mandible that was surgically excised and followed with toceranib therapy. The mass was incompletely excised leaving behind microscopic disease only. It is likely that the small size of this mass and the rostral location (that made it amenable to surgical excision) were factors that played a role in this cat’s survival time. Multimodal therapy has been shown to be effective for many tumor types in pets and in people. It is possible that the best patient outcomes will include a combination of locoregional control via surgery and/or RT plus chemotherapy with targeted chemotherapy agents such as toceranib and/or cytotoxic chemotherapeutics.
Conclusions
Toceranib at a median dose of 2.75 mg/kg (range 1.9–4.17 mg/kg) given on a Monday/Wednesday/Friday schedule was tolerated well by the majority of cats in this study when used in combination with other treatment modalities for the treatment of oral SCC in cats.
Acknowledgments
We would like to acknowledge and thank the following doctors, staff and institutions who contributed cases to this study: Theresa Arteaga DVM, DACVIM (Oncology), Pacific Veterinary Specialists, Capitola, CA, USA; Virginia Gill DVM, DACVIM (Oncology), Katonah Bedford Veterinary Center, Bedford Hills, NY, USA; Philip Bergman DVM, MS, PhD, DACVIM (Oncology), Katonah Bedford Veterinary Center, Bedford Hills, NY, USA; Carrie Wood DVM, DACVIM (Oncology), Angell Animal Medical Center, Boston, MA, USA; Christine Anderson DVM, MS, DACVIM (Oncology), DACVR (Radiation Oncology), Angell Animal Medical Center, Boston, MA, USA; Karri Barabas Miller DVM, MS, DACVIM (Oncology), Veterinary Healthcare Associates, Winter Haven, FL, USA.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 16 February 2016
References
- 1. Stebbins KE, Morse CC, Goldschmidt MH. Feline oral neoplasia: a ten-year survey. Vet Pathol 1989; 26: 121–128. [DOI] [PubMed] [Google Scholar]
- 2. Dorn CR. Epidemiology of canine and feline tumors. J Am Anim Hosp Assoc 1976; 12: 307–310. [Google Scholar]
- 3. Tannehill-Gregg SH, Levine AL, Rosol TJ. Feline head and neck squamous cell carcinoma: a natural model for the human disease and development of as mouse model. Vet Comp Oncol 2006; 4: 84–97. [DOI] [PubMed] [Google Scholar]
- 4. Snyder LA, Bertone ER, Jakowski RM, et al. P53 expression and environmental tobacco smoke exposure in feline oral squamous cell carcinoma. Vet Pathol 2004; 41: 209–214. [DOI] [PubMed] [Google Scholar]
- 5. Moore AS, Ogilvie GK. Tumors of the alimentary tract. In: Feline oncology. Trenton: Veterinary Learning Systems, 2001, pp 271–291. [Google Scholar]
- 6. Bertone ER, Snyder LA, Moore AS. Environmental and lifestyle risk factors for oral squamous cell carcinoma in domestic cats. J Vet Intern Med 2003; 17: 557–562. [PubMed] [Google Scholar]
- 7. Fulmer AK, Mauldin GE, Mauldin GN. Evaluation of plasma folate and homocysteine concentrations in cats with and without oral squamous cell carcinoma. Vet Comp Oncol 2008; 6: 248–256. [DOI] [PubMed] [Google Scholar]
- 8. Mauldin GN, Matus RE, Patnaik AK, et al. Efficacy and toxicity of doxorubicin and cyclophosphamide used in the treatment of selected malignant tumors in 23 cats. J Vet Intern Med 1988; 2: 60–65. [DOI] [PubMed] [Google Scholar]
- 9. Fox LE, Rosenthal RC, King RR, et al. Use of cis-bis-neodecanoato-trans-R,R-1, 2-diaminocyclohexane platinum (II), a liposomal cisplatin analogue, in cats with oral squamous cell carcinoma. Am J Vet Res 2000; 61: 791–795. [DOI] [PubMed] [Google Scholar]
- 10. Bradley RL, MacEwen EG, Loar AS. Mandibular resection for removal of oral tumors in 30 dogs and 6 cats. J Am Vet Med Assoc 1984; 184: 460–463. [PubMed] [Google Scholar]
- 11. Bregazzi VS, LaRue SM, Powers BE, et al. Response of feline oral squamous cell carcinoma to palliative radiation therapy. Vet Radiol Ultrasound 2001; 42: 77–79. [DOI] [PubMed] [Google Scholar]
- 12. Sabhlok A, Ayl R. Palliative radiation therapy outcomes for cats with oral squamous cell carcinoma (1999–2005). Vet Radiol Ultrasound 2014; 55: 565–570. [DOI] [PubMed] [Google Scholar]
- 13. Fidel JL, Sellon RK, Houston RK, et al. A nine-day accelerated radiation protocol for feline squamous cell carcinoma. Vet Radiol Ultrasound 2007; 48: 482–485. [DOI] [PubMed] [Google Scholar]
- 14. Poirier VJ, Kaser-Hotz B, Vail DM, et al. Efficacy and toxicity of an accelerated hypofractionated radiation therapy protocol in cats with oral squamous cell carcinoma. Vet Radiol Ultrasound 2013; 54: 81–88. [DOI] [PubMed] [Google Scholar]
- 15. Jones PD, de Lorimier LP, Kitchell BE, et al. Gemcitabine as a radiosensitizer for non-resectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc 2003; 39: 463–467. [DOI] [PubMed] [Google Scholar]
- 16. Fidel J, Tripp LC, Houston R, et al. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J Vet Intern Med 2011; 25: 504–510. [DOI] [PubMed] [Google Scholar]
- 17. Hutson CA, Willauer CC, Walder EJ, et al. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987–1989). J Am Vet Med Assoc 1992; 201: 777–781. [PubMed] [Google Scholar]
- 18. Marconato L, Buchholz J, Keller M, et al. Multimodal therapeutic approach and interdisciplinary challenge for the treatment of unresectable head and neck squamous cell carcinoma in six cats: a pilot study. Vet Comp Oncol 2012; 11: 101–112. [DOI] [PubMed] [Google Scholar]
- 19. Johnson FM, Saigal B, Talpaz M, et al. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res 2005; 11: 6924–6932. [DOI] [PubMed] [Google Scholar]
- 20. Soulieres D, Senzer NN, Vokes EE, et al. Multicenter Phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004; 22: 77–85. [DOI] [PubMed] [Google Scholar]
- 21. London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia) in solid tumors. Vet Comp Oncol 2011; 10: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res 2003; 9: 2755–2768. [PubMed] [Google Scholar]
- 23. Hohenhaus A, Henry C, Greene S, et al. Biological activity and adverse event profile in cats treated with toceranib phosphate [abstract]. Vet Comp Oncol 2011; 9: 20–21. [Google Scholar]
- 24. Veterinary Cooperative Oncology Group. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004; 2: 194–213. [DOI] [PubMed] [Google Scholar]
- 25. Daly M, Sheppard S, Cohen N, et al. Safety of masitinib mesylate in healthy cats. J Vet Intern Med 2011; 25: 297–302. [DOI] [PubMed] [Google Scholar]
- 26. Lachowicz JL, Post GS, Brodsky E. A Phase I clinical trial evaluating imatinib mesylate (Gleevec) in tumor-bearing cats. J Vet Intern Med 2005; 19: 860–864. [DOI] [PubMed] [Google Scholar]
- 27. Soulieres D, Senzer NN, Vokes EE, et al. Multicenter Phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004; 22: 177–185. [DOI] [PubMed] [Google Scholar]
- 28. Looper JS, Malarkey DE, Ruslander D, et al. Epidermal growth factor receptor expression in feline oral squamous cell carcinomas. Vet Comp Oncol 2006; 4: 33–40. [DOI] [PubMed] [Google Scholar]
- 29. Bergkvist GT, Argyle DJ, Morrison L, et al. Expression of epidermal growth factor receptor (EGFR) and Ki67 in feline oral squamous cell carcinomas (FOSCC). Vet Comp Oncol 2011; 9: 106–117. [DOI] [PubMed] [Google Scholar]
- 30. Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol 2002; 20: 3815–3825. [DOI] [PubMed] [Google Scholar]
- 31. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 32. Rasmussen F. RECIST and targeted therapy. Acta Radiol 2009; 50: 835–836. [DOI] [PubMed] [Google Scholar]
- 33. Rosen MA. Use of modified RECIST criteria to improve response assessment in targeted therapies: challenges and opportunities. Cancer Biol Ther 2010; 9: 20–22. [DOI] [PubMed] [Google Scholar]


