Abstract
Background
Intravenous (IV) infusion therapy is commonly used in health care settings. However, IV therapy at home can be challenging because it relies on the patient's ability to manage multiple medications correctly, which may lead to decreased treatment adherence.
Objective
We aimed to assess the usability and accuracy of the IVsight monitor in capturing IV infusion data compared to manual recording.
Methods
A prospective, single-center, usability study involving patients receiving IV infusion therapy at one of the BJC's Home Infusion suites was conducted to evaluate the accuracy, performance, and acceptability of the device IVsight as a monitor for IV infusions.
Results
Of the 15 participants, the median (IQR) age was 46 years (36–55), 8 (53%) were female, and 13 (87%) were non-Hispanic white. Each participant received a median (IQR) of 4 (4–5) infusions during the study, and 68 infusions were observed overall. The intraclass correlation coefficient between the IVsight measurement and manual recording of infusion duration in seconds was excellent (ICC 0.97, 95% confidence interval 0.96–0.98). The Bland–Altman plot visually showed an acceptable limit of agreement for the 2 measurements, and the linear regression analysis revealed no significant proportional bias between the 2 methods for measuring the IV infusion time. None of the participants thought that IVsight was difficult to hold, use, clean, or store. Only one participant was concerned that the device could interfere with the IV infusion, and all participants felt comfortable with the device transmitting data to their health care providers.
Conclusions
The IVsight infusion monitoring device showed near-perfect agreement on the recorded IV infusion duration compared with manual recording, and good acceptability among the study participants.
Key words: Device, Infusion, Intravenous, Monitoring
Introduction
Intravenous (IV) infusion therapy is commonly provided in health care settings under the supervision of trained staff. However, the home health industry is rapidly expanding as home health agencies are able to provide antimicrobials, total parenteral nutrition, and other services for chronic conditions.1 Outpatient parenteral antimicrobial therapy is one of the main uses of IV therapy at home, as it has been shown to be cost-saving, well accepted by patients, and leads to good treatment outcomes.2, 3, 4 Although IV therapy at home is cost-saving compared to inpatient care, it often relies on the patient's ability to correctly manage multiple medications, maintain a sanitary environment, and self-administer medications at the right time, often for several weeks, without direct health care supervision.5 This can be difficult for some patients and may lead to poor adherence to treatment and unplanned readmissions.6 Therefore, interventions to better monitor and share the status of IV infusions to improve infusion adherence are needed, a process that can be made more efficient using an automated digital solution.
HIVE's IVsight is a patent-pending, smart, external IV infusion monitoring device with a simple embedded mechano-electrical sensor that detects when an infusion connection is established. IVsight neither interferes with any part of the IV fluid pathway nor touches any fluid in this pathway; hence, it is categorized as a nonsignificant risk device by the Food and Drug Administration's 21 CFR Part 812.7 IVsight can immediately send this information wirelessly to the electronic medical record through a gateway device (HIVE AWS/similar system), enabling clinicians to use this information for patient care. Data transmission from the gateway device to the electronic medical record is linked through a device serial number, and personal health information is not stored in IVsight or the gateway; hence, the risk of confidentially breach is minimal. The purpose of this study was to assess the accuracy of data capture measured during IV infusion administration using IVsight compared to manual recording. The secondary aim was to evaluate the patient's acceptability of IVsight for IV infusion monitoring.
Methods
Study design
We performed a prospective, nonrandomized study of patients who received IV infusions at BJC Home Care infusion suite in December 2022. BJC Home Care is a health care company that provides services including infusion, home medical equipment, hospice, and home health care in the St. Louis metropolitan area, Missouri, USA. All patients scheduled for IV infusion at the BJC home infusion suite were deemed eligible if they were ≥18 years of age and were able to complete a written survey at the end of the infusion. We excluded patients who were unable to provide their own informed consent had no functional vascular access to receive the IV infusion, and were non-English speakers as the informed consent and other materials were only available in English. If patients agreed to participate, they were asked to briefly recall information about the trial's aim and the potential risk of participating. Participants were eligible patients who provided informed consent and were included in the study. This study was approved by the Institutional Review Board of Washington University. Data were collected and managed using Research Electronic Data Capture (REDCap), a secure, web-based software platform electronic data capture tool hosted at Washington University in St. Louis, Missouri, USA, and protected by its server's firewalls.8
Study procedures
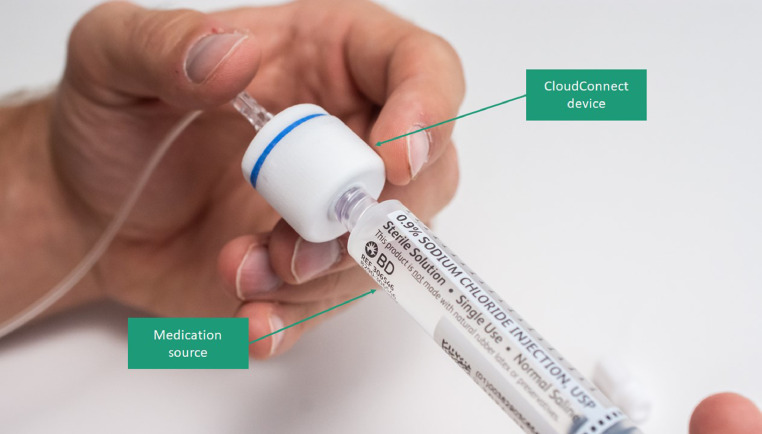
The device was prepackaged and provided to the study team by HIVE Medical. The study nurse used one device package per participant (Figure 1) and assembled the IVsight components according to the manufacturer's instructions. Once assembled, the research nurse plugged the gateway into an electricity outlet, turned the device on, plugged the gateway into an electricity outlet to transmit the data into REDCap, and then proceeded to provide IV infusion as per the standard of care. All the steps were performed in front of the participants, and they were explained to them. We captured all infusions, including standard saline flushes used to clear the IV lines before and after medication administration.
Figure 1.
The IVsight device attached to a saline syringe.
Participant information was recorded using the data collection form (Supplementary Material Form 1) and entered directly into the electronic case report form in REDCap by the research coordinator. The start and end times of infusion were recorded manually to calculate the infusion duration. To ensure privacy, the device and standardized questionnaire were given to the participants after the IV infusion was completed.
Study outcomes
The primary outcome was to determine the accuracy of the IV infusion duration recorded by the IVsight compared with manual recording. Manual recording refers to notes taken by the research coordinator to establish the start and end time of each infusion. The secondary outcome was participant acceptance of the IVsight device using a standardized questionnaire (Supplementary Material Form 2).
Statistical analysis
We performed descriptive statistics on the patient population and survey responses. We used the intraclass correlation coefficient (ICC) and Bland–Altman plot with linear regression analysis to assess the agreement and fixed bias between the measurements between measurement of the IV infusion time in minutes captured by the IVsight compared to the time recorded, manually by a study team member (i.e., the gold standard, respectively). For missing values on the infusion recording by the device, we opted not to perform mean-value imputation because it artificially reduces the variation in the data set and defaults the infusion duration to zero in keeping with the absence of the IV infusion duration. All statistical analyses were performed using SPSS Statistics for Windows, Version 26.0 (IBM SPSS Statistics for Windows, Version 26.0, IBM Corp, Armonk, New York).
Results
Demographic characteristics
Of 17 eligible patients, 15 participants were analyzed (Supplementary Figure 1). The median (IQR) age of the participants was 46 years (36–55), 8 (53%) were females, and 13 (87%) were non-Hispanic white. The main indication for IV therapy was inflammatory bowel disease immunomodulator therapy (n = 7, 46%), and the majority (13, 86%) of the participants were on long-term monthly IV infusions. The medications infused included infliximab (n = 5), ocrelizumab (n = 4), vedolizumab (n = 3), natalizumab (n = 1), eculizumab (n = 1), and methylprednisolone (n = 1). Most participants had received a prior infusion (13, 86%), with over half receiving regular IV infusions for >1 year (8, 53%). The median planned duration of infusion was 90 minutes (49–150).
Infusion times recording
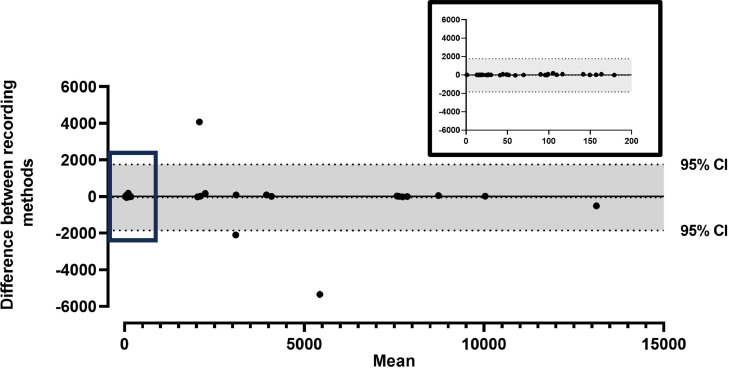
Fifteen participants received a median (IQR) of 4 (4–5) infusion connections during the study, and 68 infusion durations were observed to compare the agreement between the IVsight and manually recorded times. Infusion times were not captured by the device in eight infusions (Supplementary Table 1). Overall, the mean (±SD) duration of IV infusion in seconds captured by IVsight was 1369.04 ± 2862.1, compared to the manually recorded duration of 1394.03 ± 2985.7, and the t-test for the mean difference between the 2 measurement techniques was not statistically significant (−45.55, 95% confidence interval [CI] −257.5 to 166.4, P = 0.67). The ICC between the IVsight measurement and manual recording of the infusion duration in seconds was excellent (ICC 0.97, 95% CI 0.96–0.98). This did not change when analysis excluded missing values (ICC 0.97, 95% CI 0.96–0.98). The Bland–Altman plot visually showed an acceptable limit of agreement for the 2 measurements (Figure 2), and the linear regression analysis revealed no significant proportional bias between the 2 methods for measuring the IV infusion time. In one instance, there was an 8-minute difference between manual recording (longer) and the device data capture.
Figure 2.
Bland–Altman plot for the duration of the intravenous infusion in seconds measured by the IVsight device compared to manual recording. Inlay in the right upper corner highlights 52 measurement clustered within the box denoted in the main graphic.
Acceptability questionnaire results
None of the participants thought that IVsight was difficult to hold, use, clean, or store. Although none thought IVsight may make the infusion process more complicated, one participant (7%) was concerned the device could interfere with the IV infusion, one participant said it interfered with the ability to cover the peripheral catheter with a sleeve because the device was too bulky; however, none found IVsight offensive to the touch. All participants felt comfortable with the device transmitting data to their health care providers, ranking the level of comfort with data transmission ≥8 on a 10-point ordinal scale. Similarly, all participants ranked ≥7/10 their openness to potential increased communication with their health care providers as a result of the data transferred by the device.
The main suggestions provided by the participants to improve the IVsight device included making it smaller and adding sound notifications when a successful connection was established and when an infusion was completed.
Discussion
The IVsight infusion monitoring device showed near-perfect agreement with the manually recorded IV infusion duration. Although there were some outliers in the infusion times, mainly due to missing recording by the device, the overall findings suggest that the device accuracy was high and warrants further evaluation in future studies. No safety issues were observed during the study, and IVsight was well-accepted by the participants. Furthermore, the measurement of the infusion might have varied by the way the initiation time was recorded, as direct observation would only start counting the infusion time when the IV fluid was started, and the IVsight sensor would register the moment the needless connector was locked into the IV line.
The issues with missing data on some infusions by IVsight were related to the saline flushes commonly used to clear the catheter line before or after the administration of medication. The short duration of saline flushes may have been blocked by debouncing or missed due to a pre-established window period of approximately ten seconds where a recording could be missed. However, this time window can be modified and shortened in future trials. Other missed instances occurred because of inconsistencies in the physical case. Owing to the small number of cases in this trial, the device cases were produced with 3D printing, which is less accurate than the injection molding standards that will be used in the future. The tolerance of the sensor to detect a connection is small, and the limitations of 3D printing might have led to a few devices overshooting this tolerance window, thus missing the times. Finally, in one case, some of the infusion time was missed when a patient left to use the bathroom, potentially due to the device being jostled. Future versions of the device will have tighter cases owing to injection molding, which will prevent any accidental connections or disconnections.
Although not explored in this pilot trial, the goal of the IVsight device is to provide remote monitoring of at-home IV infusions to enable direct and timely interventions, given its capability to transmit live information to a third party (e.g., electronic medical record system) as it is being captured. Previous studies have shown the impact of remote monitoring on reducing negative outcomes such as unplanned readmissions. In a study by Hale et al.,9 a remote medication monitoring system was able to reduce all-cause hospitalization for heart failure patients by 80% and decreased the average length of stay. In another study by Logan et al.,10 a remote blood pressure monitoring system was shown to improve ambulatory blood pressure control among patients with diabetes and hypertension, and importantly, patient's acceptability and perception of effectiveness on blood pressure control was high. Similarly, we aim to explore the impact of live monitoring IV infusions being provided at home in a future randomized trial, given that in this study all participants thought that IVsight was easy to use and clean, and they felt comfortable about the device transmitting data to their health care providers.
Our study has several limitations. First, it is a single-center usability trial that examined a very small population that might not be representative of the general population in different health care settings. Given the small number of participants in this exploratory trial, the study might have been underpowered to identify differences in the accuracy of the data capture. However, we used infusion measurements instead of individual participants in order to increase the number of measurements. Second, some infusions were not captured by IVsight during the trial. However, these were all saline flushes, and the issues identified around them can be easily addressed to avoid them in future trials. Finally, given the exploratory nature of the study, we cannot make a definitive conclusion regarding patient acceptability based on the questionnaire administered.
In conclusion, the IVsight device is a simple, noninterfering, mechano-electrical sensor outside the fluid pathway for monitoring IV infusions, with excellent accuracy and good acceptability among users. Larger clinical trials in diverse health care settings are warranted to confirm these encouraging findings and determine their best use as a real time monitoring device for those receiving IV infusion outside the hospital setting.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
CM-C reports a Centers for Disease Control and Prevention subaward, a vendor/ individual agreement with Wayne State University, and serves as Associate Editor for Open Forum Infectious Diseases. CM-C also reports research grants from the CDC and INSMED. CM-C, and BH report serving as external advisor for HIVE Medical Inc.; both ad honorem. JB, GK, JE, and SD are all part of the HIVE Medical Inc. executive team that is the sponsor of this study.
Acknowledgments
The authors wish to thank Dorothy Sinclair for her help in developing the REDCap database for this study.
Funding: This study was financially sponsored by HIVE Medical Inc. The sponsor provided the devices for the trial, the institutional research fees, helped with data collection, reviewed the report, and approved the decision to submit the article for publication.
Author Contributions: All authors are responsible for the accuracy of the data. Study concept and design: Mejia-Chew and Heuring. Acquisition, analysis, or interpretation of data: Mejia-Chew, Salmons, Eshelman, and Dodda. Drafting of the manuscript: Mejia-Chew, Heuring, Salmons, Weilmuester, Beggs, Kleinschmidt, Eshelman, and Dodda.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2024.100747.
Appendix. Supplementary materials
References
- 1.Monk-Tutor MR. The U.S. home infusion market. Am J Health Syst Pharm. 1998;55(19):2019–2025. doi: 10.1093/ajhp/55.19.2019. [DOI] [PubMed] [Google Scholar]
- 2.Chapman AL, Dixon S, Andrews D, et al. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother. 2009;64(6):1316–1324. doi: 10.1093/jac/dkp343. [DOI] [PubMed] [Google Scholar]
- 3.Saillen L, Arensdorff L, Moulin E, et al. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis. 2017;36(8):1387–1392. doi: 10.1007/s10096-017-2944-5. [DOI] [PubMed] [Google Scholar]
- 4.Loesch GH, Cruz JAW, Gasparetto J, et al. Cost minimization analysis of outpatient parenteral/oral antibiotic therapy at a trauma hospital: public health system. Infect Control Hosp Epidemiol. 2021;42(12):1445–1450. doi: 10.1017/ice.2021.22. [DOI] [PubMed] [Google Scholar]
- 5.Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007;60(2):356–362. doi: 10.1093/jac/dkm210. [DOI] [PubMed] [Google Scholar]
- 6.Ng N, Bailey P, Pryor R, et al. Experiences in outpatient parenteral antimicrobial therapy (OPAT): barriers and challenges from the front lines. Antimicrob Steward Healthc Epidemiol. 2021;1(1):e42. doi: 10.1017/ash.2021.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820. Accessed 11 January 2024.
- 8.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale TM, Jethwani K, Kandola MS, et al. A remote medication monitoring system for chronic heart failure patients to reduce readmissions: a two-arm randomized pilot study. J Med Internet Res. 2016;18(5):e91. doi: 10.2196/jmir.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan AG, McIsaac WJ, Tisler A, et al. Mobile phone-based remote patient monitoring system for management of hypertension in diabetic patients. Am J Hypertens. 2007;20(9):942–948. doi: 10.1016/j.amjhyper.2007.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.