Abstract
We describe vaginal microbiota, including Gardnerella species and sexually transmitted infections (STIs), during pregnancy and their associations with recurrent spontaneous preterm birth (sPTB). We performed a prospective cohort study in a tertiary referral centre in the Netherlands, among pregnant women with previous sPTB <34 weeks' gestation. Participants collected three vaginal swabs in the first and second trimester. Vaginal microbiota was profiled with 16S rDNA sequencing. Gardnerella species and STI's were tested with qPCR. Standard care was provided according to local protocol, including screening and treatment for bacterial vaginosis (BV), routine progesterone administration and screening for cervical length shortening. Of 154 participants, 26 (16.9 %) experienced recurrent sPTB <37 weeks' gestation. Microbiota composition was not associated with sPTB. During pregnancy, the share of Lactobacillus iners-dominated microbiota increased at the expense of diverse microbiota between the first and second trimester. This change coincided with treatment for BV, demonstrating a similar change in microbiota composition after treatment. In this cohort of high-risk women, we did not find an association between vaginal microbiota composition and recurrent sPTB. This should be interpreted with care, as these women were offered additional preventive therapies to reduce sPTB according to national guidelines including progesterone and BV treatment. The increase observed in L. iners dominated microbiota and the decrease in diverse microbiota mid-gestation was most likely mediated by BV treatment. Our findings suggest that in recurrent sPTB occurring despite several preventive therapies, the microbe-related etiologic contribution might be limited.
Keywords: Preterm birth, Recurrent spontaneous preterm birth, Vaginal microbiota, Vaginal microbiome, Lactobacillus, Gardnerella, Sexually transmitted infections
1. Introduction
Preterm birth (PTB), defined as birth before 37 completed weeks of gestation, occurs in one out of every 10 pregnancies worldwide. Prematurity is the leading cause of neonatal death during the first 28 days of life and the sequelae are the leading cause of childhood death under the age of five [1,2]. Therefore, PTB is one of the most prevalent conditions in the Global Burden of Disease analysis [3,4]. Approximately one-third of PTBs are medically indicated, while the remaining occur spontaneously after spontaneous contractions or spontaneous rupture of membranes [5]. Women with a history of spontaneous PTB (sPTB) are at high risk of recurrent sPTB. The risk of recurrence in singleton pregnancies with previous sPTB is approximately 30 %, with higher risk if the previous sPTB was more remote from term [6]. sPTB is currently seen as a syndrome attributable to multiple pathological processes, including intrauterine infection, cervical insufficiency, low levels or insufficient biological effectiveness of available progesterone, uterine overdistention and vascular disorders [7]. Bacterial vaginosis (BV), a state of dysbiosis of the vaginal microbiota with reduced presence of Lactobacillus species and proliferation of diverse other bacteria such as Gardnerella species, increases the risk for sPTB significantly [8]. However, treatment of BV with antibiotics does not significantly reduce sPTB rate [9,10].
In recent years, the use of molecular sequencing techniques has greatly increased the knowledge of vaginal bacterial communities and the association with sPTB. Several studies suggest a population-specific association between vaginal microbiota composition and sPTB and an important role for microbiota dynamics [[11], [12], [13], [14], [15], [16], [17], [18]]. Most of these studies included a variety of patients, combining nulliparous and multiparous women, with diverse risk for sPTB. These heterogeneous populations might mask associations between vaginal microbiota and sPTB that may exist in specific groups.
Recently, the genus Gardnerella was revealed to consist not of one species, but of multiple species [19]. Several studies suggested that these species have different pathogenicity and are associated with distinct risks for outcomes such as BV and sPTB, but have gone undetected by conventional diagnostic methods [13,20,21]. Sexually transmitted infections (STIs) have also been suggested to increase the risk for sPTB [[22], [23], [24]]. However, these pathogens have scarcely been studied together with microbiota composition in relation to pregnancy outcomes and thus little is known so far about their prevalence and role in microbiota dynamics in Dutch pregnant women.
Here, we aimed to describe vaginal microbiota and its dynamics, Gardnerella spp. and STIs during high-risk pregnancy. In addition, we aimed to investigate the association of vaginal microbiota with sPTB.
2. Methods
2.1. Study design
We performed a prospective cohort study conducted in two tertiary referral centres in Amsterdam, the Netherlands. Recruitment took place in Amsterdam UMC, location AMC, from December 2017 to December 2021 and Amsterdam UMC, location VUmc, from May 2019 to December 2021. Women were considered eligible if they were <16 weeks pregnant at the time of inclusion and at high risk for sPTB because of a history of sPTB with or without preterm prelabor rupture of membranes between 16 and 34 weeks. Exclusion criteria were: (i) inability to provide unassisted informed consent due to language or literacy issues and (ii) screening for BV within 10 days prior to inclusion because of possible disturbance of the vaginal microbiota due to treatment. All participants provided written informed consent. This study was not subjected to legislation for trials, as confirmed by the Institutional Review Board (registration number 2017_116#C2019447).
2.2. Routine care
Routine screening for BV was performed between 12 and 14 weeks of gestation based on Nugent score (if included at location AMC) or cervicovaginal culture (if included on location VUmc), irrespective of symptoms. Participants with a Nugent score of ≥4 or with G. vaginalis present in culture were treated with oral clindamycin 300 mg twice daily for five days according to regional protocol. Progesterone (vaginal daily or intramuscular weekly) was offered from 16 weeks of gestation as a preventive measure for sPTB. Biweekly cervical length measurements were performed between 14 and 24 weeks of gestation. In cases of cervical shortening <25 mm prior to 24 weeks of gestation, cervical cerclage or pessary (as part of a randomized controlled trial) [25] were offered. In case of three or more previous sPTB, cervical cerclage was placed around 11 weeks of gestation. For cervical cerclage braided suture material was used.
2.3. Data collection
Three cervicovaginal swabs (eSwab, Copan Diagnostics) were collected. The first swab was taken at inclusion (before or at the same time as the swab for BV screening), the second swab was taken between 18 and 20 weeks of gestation and the third swab between 22 and 24 weeks of gestation. Cervicovaginal swabs were immediately stored at −20 °C after collecting, until transfer to the central storage facility at −80 °C within three weeks. Baseline characteristics, clinical data, preventive therapies and pregnancy outcomes were extracted from electronic health records. Data was entered in a custom Castor database. Data-entry was checked for accuracy by a second researcher in 10 % of participants.
3. Outcomes
The primary outcome measure was sPTB, defined as spontaneous preterm rupture of membranes or spontaneous preterm onset of labor. The primary outcome included both sPTB between 23 and 37 weeks of gestation and late second trimester pregnancy loss between 16 and 22 weeks of gestation. The secondary outcome measures were dynamics of vaginal microbiota, vaginal microbiota composition, presence of Gardnerella spp. and STIs during pregnancy.
3.1. Vaginal microbiota sequence analysis
DNA extraction and vaginal microbiota 16S rDNA sequence analysis was performed as previously described [26]. The code for bioinformatics analyses is available in Supplementary material. Samples with a read count <370 reads were excluded from analysis, based on the negative controls. The VALENCIA tool was used to determine vaginal Community State Types (CSTs) and divide samples into five groups (CST I, L. crispatus dominated; CST II, L. gasseri dominated; CST III, L. iners dominated; CST IV, Gardnerella, Atopobium vaginae, BVAB1 or depleted lactobacilli; CST V, L. jensenii dominated).
3.2. Gardnerella species and STI detection
Quantitative PCR (qPCR) was performed in the Laboratory Bacteriology Research (Ghent University, Ghent, Belgium) to differentiate between different Gardnerella spp. as described previously [27]. DNA for the standard dilution series and positive controls for G. leopoldii, G. piotii, G. swidsinskii and G. vaginalis were extracted from cultures of strain UGent 09.48, strain UGent 18.01T, strain GS10234 and strain LMG7832T, respectively. All these strains were cultured anaerobically at 37 °C on chocolate agar plates (Becton Dickinson) for 48 h after which DNA was extracted using the Roche High Pure PCR Template Preparation Kit (Roche).
STIs, including Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Trichomonas vaginalis, were detected using the ALINITY m STI ASSAY according to manufacturer's protocol (Abbott). These were tested retrospectively, hence no treatment was provided.
3.3. Statistical analyses
Missing variables for ethnicity and BMI were handled using single imputation. Baseline characteristics were presented as mean and standard deviation (SD) for normally distributed continuous variables, median and interquartile range (IQR) for non-normally distributed continuous variables or as absolute numbers and percentages for categorical variables. Odds ratios (ORs) and 95 % confidence intervals (CIs) were calculated using logistic regression with Firth's correction, and were corrected for ethnicity (White European/non-White European), body mass index (continuous) and smoking (no/yes or stopped during pregnancy). We defined a priori which associations to estimate and confounders to use in multivariable logistic regression. Due to the small number of events, overfitting or overestimations of associations could be an issue. To reduce overfitting, we applied Firth's correction for all ORs. Firth's correction uses penalized likelihood to shrink estimated associations that are overly optimistic [28]. The reference group consisted of all participants without sPTB, including participants with term birth, medically indicated PTB >16 weeks of gestation and pregnancy termination >16 weeks of gestation. We performed analysis with only the swabs taken <16 weeks of gestation (one swab per participant) and all available swabs (multiple swabs per participant). For determining associations between sPTB and longitudinal CST, Gardnerella spp. and STI data we used mixed model analysis and cox regression. We used MaAsLin2 for determining associations between individual taxa and sPTB [29]. This tool uses general linear models to determine associations between individual bacterial taxa and outcomes and is appropriate for longitudinal data. For this analysis, only taxa were included with a minimum relative abundance of 0.01 in at least 10 % of samples. The Mc-Nemar-Bowker test was used for paired categorical data. Statistical analyses were performed through IBM SPSS Statistics (Statistical Package for the Social Sciences, version 26.0) and R version 3.3.2 (R Core Team (2016)) with the mice, miceadds, logistf, lme4, survival and MaAsLin2 packages. P-values <0.05 were considered statistically significant.
3.4. Core outcome set
We report on all the essential items to report on in research concerning vaginal microbiota and PTB recommended by the PREBIC Biomarker Working Group [30].
3.5. Patient involvement
The research question we addressed in this paper was co-developed in collaboration with patients and clinicians in an existing James Lind Alliance priority setting, and was marked out us number one priority [31]. There was no other patient involvement.
4. Results
4.1. Cohort characteristics
A total of 154 participants were included in this study. One participant was lost to follow up. Cohort characteristics are described in Table 1. sPTB occurred in 26 participants (16.9 %), two participants with late second trimester pregnancy loss between 16+0 and 22+6 weeks of gestation and 24 participants with sPTB between 23+0 and 36+6 weeks of gestation. Of the five participants carrying twins (3.2 %), three had a sPTB. Progesterone was initiated in 141 participants (91.6 %) at median 16 (IQR 16–16) weeks of gestation. Cervical cerclage was placed in 31 participants (20.7 %) during pregnancy at median 15 (IQR 13–18) weeks of gestation. In 14 participants (45.2 %) this was elective cervical cerclage and in 17 (54.8 %) due to cervical shortening. A cervix <25 mm was detected in 25 (17 %) participants. Several risk factors for sPTB comparing women with and without a short cervix are described in Table S1.
Table 1.
Cohort characteristics (n = 154).
| sPTB (n = 26) |
no sPTB (n = 128) |
Missing |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Gestational age at inclusion (mean, sd) | 12.5 | 1.6 | 13.0 | 2.1 | ||
| Age (mean, SD) | 32.7 | 3.9 | 32.4 | 4.5 | ||
| Ethnicity | 4 | 2.6 | ||||
| White European | 14 | 53.8 | 55 | 44.4 | ||
| African | 3 | 11.5 | 26 | 21.0 | ||
| Surinamese | 1 | 3.8 | 13 | 10.5 | ||
| Turkish | 2 | 7.7 | 4 | 3.2 | ||
| Indian | 0 | 5 | 4.0 | |||
| Other Asian | 3 | 11.5 | 2 | 1.6 | ||
| Hispanic | 1 | 3.8 | 4 | 3.2 | ||
| Mixed | 2 | 7.7 | 12 | 9.7 | ||
| Other | 0 | 3 | 2.4 | |||
| Smoking during entire pregnancy | 0 | 3 | 2.3 | |||
| BMI (median, IQR) | 24.8 | 20.5–28.8 | 24.2 | 21.8–28.6 | 3 | 1.9 |
| Previous sexually transmitted infections | ||||||
| Chlamydia trachomatis | 4 | 15.4 | 14 | 10.9 | ||
| Neisseria gonorrhoeae | 2 | 7.7 | 1 | 0.8 | ||
| Parity | ||||||
| 1 | 17 | 65.4 | 65 | 50.8 | ||
| 2 | 3 | 11.5 | 34 | 26.6 | ||
| ≥3 | 6 | 23.0 | 29 | 22.6 | ||
| Previous preterm delivery | ||||||
| <23 + 0 weeks of gestation | 5 | 19.2 | 45 | 35.2 | ||
| <28+0weeks of gestation | 10 | 38.5 | 39 | 30.5 | ||
| Interpregnancy interval in months | ||||||
| <6 months | 8 | 30.8 | 28 | 21.9 | ||
| 6–12 months | 4 | 15.4 | 25 | 19.5 | ||
| >12 months | 14 | 53.8 | 75 | 58.6 | ||
| ART current pregnancy | ||||||
| IUI | 0 | 1 | 0.8 | |||
| IVF | 3 | 11.5 | 3 | 2.3 | ||
| ICSI | 1 | 3.8 | 9 | 7.0 | ||
| Bacterial vaginosis screening | ||||||
| receiving treatment | 7 | 26.9 | 40 | 31.3 | ||
| vaginal complaints during first trimester | 1 | 3.8 | 4 | 3.1 | ||
| Preventive therapies | ||||||
| Progesterone | 24 | 92.3 | 117 | 93.6 | 3* | 1.9 |
| Cervical cerclage placed transvaginally during pregnancy | 5 | 19.2 | 26 | 20.3 | 4* | 2.6 |
| Cervical cerclage placed transabdominally pre-pregnancy | 0 | 3 | 2.3 | 4* | 2.6 | |
| Cervical pessary | 3 | 11.5 | 5 | 3.9 | ||
| Cervical length < 25 mm | 7 | 26.9 | 18 | 14.1 | 18** | 11.7 |
| Twin pregnancy | 3 | 11.5 | 2 | 1.6 | 6* | 3.8 |
| Timing of delivery | 1 | 0.6 | ||||
| Spontaneous miscarriage <16+0 weeks | 0 | 2 | 1.6 | |||
| Termination for congenital anomalies | 0 | 3 | 2.3 | |||
| Late miscarriage (16–22+6 weeks of gestation) | 2 | 7.7 | 0 | |||
| Spontaneous preterm birth (23+0–36+6 weeks of gestation) | 24 | 92.3 | 0 | |||
| medically indicated preterm birth 23+0–36+6 weeks of gestation) | 0 | 8 | 6.3 | |||
| Term birth (>37+0 weeks of gestation) | 0 | 114 | 89.8 | |||
SD: standard deviation. IQR: interquartile range. *including miscarriages <16 + 0 weeks of gestation and pregnancy terminations. **including miscarriages <16 + 0 weeks of gestation and pregnancy terminations and participants with cervical cerclage or pessary in situ before start routine cervical length measurements.
4.2. Vaginal microbiota dynamics
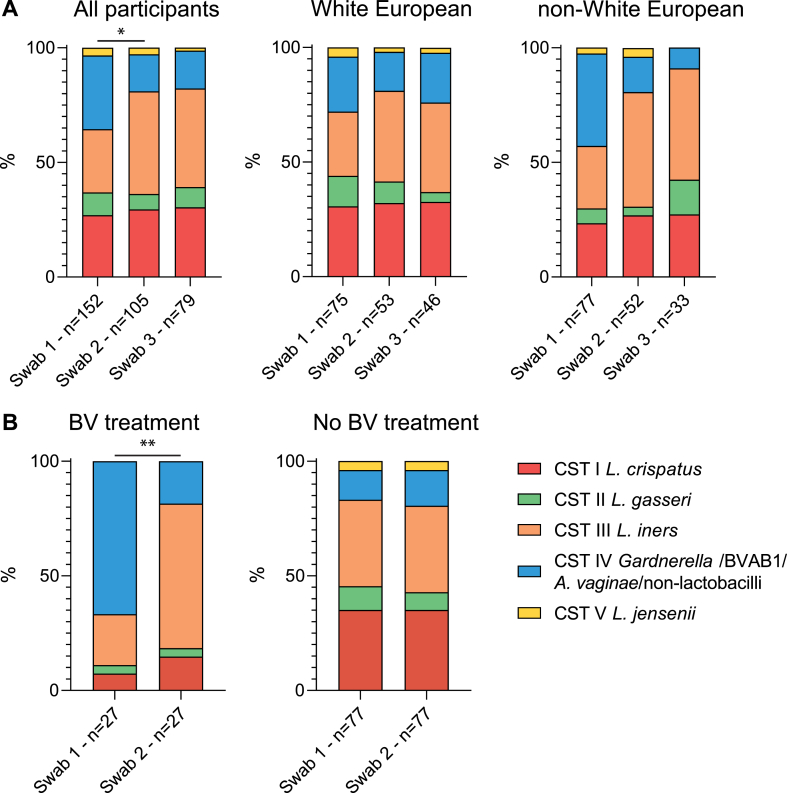
We collected 348 swabs in total. From 39 participants (25.3 %) one swab was collected, from 36 (23.4 %) two swabs were collected and from 79 (51.3 %) three swabs were collected. The first swab was taken at a mean of 12.7 weeks of gestation (standard deviation (SD) 1.6), the second swab at a mean of 19.0 weeks of gestation (SD 1.2) and the third swab at a mean of 23.2 weeks of gestation (SD 2.1). Based on 16S rDNA sequencing, 12 samples were excluded from vaginal microbiota composition analysis due to low read count. The read counts with assigned bacteria and CST and subCST are described in Table S2. At the collection of the first swab, the most common vaginal microbiota profile was CST IV (32.2 %), Fig. 1A. Later in pregnancy, at collection of the second swab, a statistically significant increase in participants with CST III (44.8 %) and a decrease of participants with CST IV (16.2 %) was observed (p = 0.04). The distribution of CSTs was similar at collection of the second and third swab. The increase in CST III at collection of the second swab was most prominent in non-White European participants, although not statistically significant in subgroup analysis, see Fig. 1A. The majority of participants had the same microbiota profile at collection of all samples (76.1 %), Fig. 2. Screening and treatment for BV occurred between the first and second swab for the majority of participants. In participants who were treated for BV, a change in microbiota profile from mainly CST-IV to mainly CST-III was observed after treatment (p < 0.01), Fig. 1B. Among the participants that were not treated for BV no statistically significant change in microbiota profile was observed, Fig. 1B.
Fig. 1.
Community State Type dynamics. Swab 1 taken at mean 12.8 weeks of gestation, swab 2 at mean 19.3 weeks of gestation and swab 3 at mean 23.3 weeks of gestation. A) Distribution of community state types (CSTs) at different sampling moments during pregnancy, for all participants, White European participants and non-White European participants. B) On the left bars depicting the distribution of CSTs of swab 1 and 2 of participants that were treated for bacterial vaginosis (BV) and on the right participants that were not treated for BV. BV treatment was given to participants between swab 1 and 2 if Nugent score ≥4 or G. vaginalis was present in cervicovaginal culture in a separate sample. *P < 0.05, **P < 0.01.
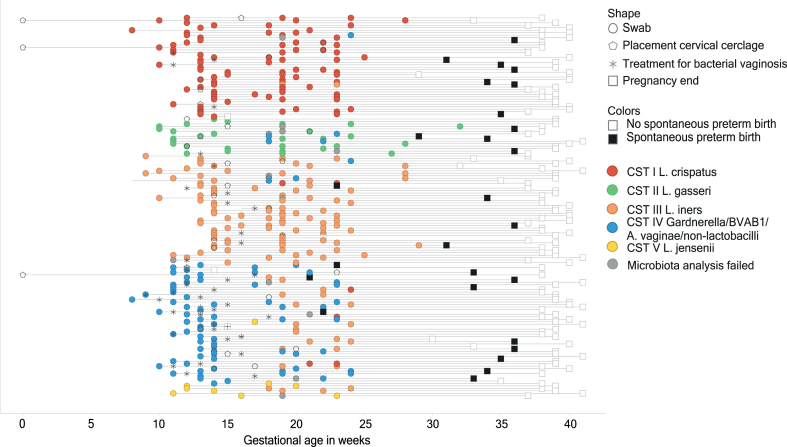
Fig. 2.
Course of pregnancy. Each line represents a participant. Each circle represents a swab taken for the study. The other shapes represent an event occurring in routine care. Events at 0 weeks of gestation took place pre-pregnancy.
4.3. Gardnerella species
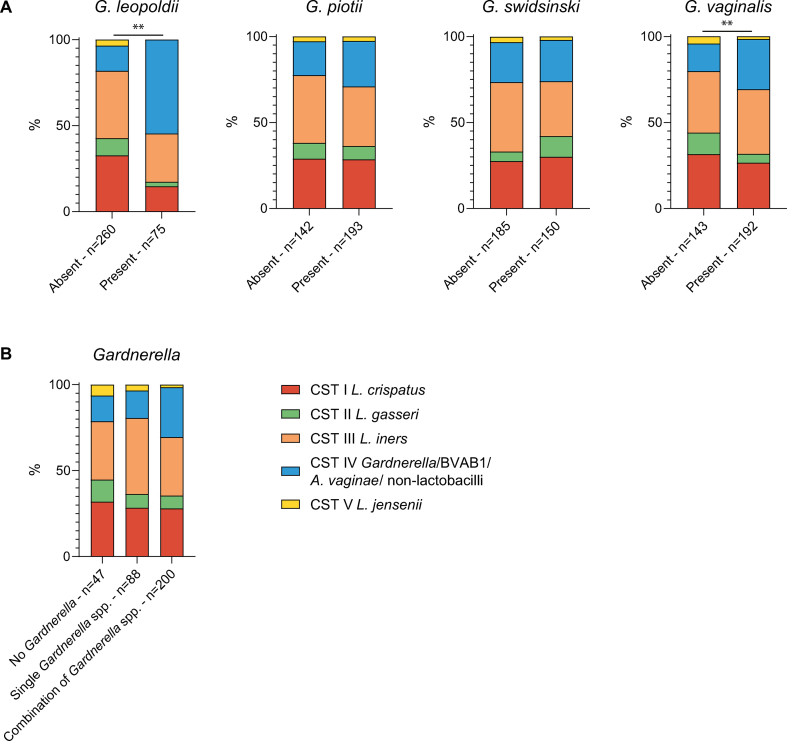
Our results were further refined by investigating the presence of four Gardnerella spp.: G. leopoldii, G. piotii, G. swidsinskii and G. vaginalis. Of the 348 total samples, one did not contain enough material for further analysis. In the majority of samples, at least one Gardnerella spp. was detected (86.2 %). G. leopoldii was the least abundant species, being detected in 22.2 % of samples, followed by G. swidsinskii, which was detected in 44.4 % of samples. G. vaginalis and G. piotii had a similar prevalence, of 57.1 % and 57.3 % respectively. In 27.4 % of the 347 samples, a single Gardnerella spp. was present (G. leopoldii 2.3 %, G. piotii 9.5 %, G. swidsinskii 5.2 % and G. vaginalis 10.4 %), and in 58.8 % a combination of species. In the majority of participants, the distribution of Gardnerella spp. changed over time (91.3 %). In the samples with G. leopoldii or G. vaginalis (as single species or in combination with other Gardnerella spp.), CST IV was statistically significantly more abundant compared to participants without these species (p < 0.001 and p = 0.007, respectively), Fig. S1A. Samples with a combination of Gardnerella spp. displayed more CST IV compared to samples without any or with a single Gardnerella spp., but this difference was not statistically significant, Fig. S1B.
4.4. Sexually transmitted infections
Of the 348 available samples, analysis of STIs failed in five samples. N. gonorrhoea was not detected in any sample. In 16 samples from 11 participants, STIs were detected, Table S3. Three participants had samples that were positive for more than one STI, one with both M. genitalium and T. vaginalis and two with C. trachomatis, M. genitalium and T. vaginalis. Two participants had STIs in subsequent samples, that were not detected in the first sample, showing incident infection during the course of pregnancy. The first participant had T. vaginalis only in the first sample and C. trachomatis, M. genitalium and T. vaginalis in the second sample, the second participant had no STIs in the first sample and M. genitalium in the second sample. Of the samples in which an STI was detected, this was most often accompanied by CST IV (50.0 %, compared to 28.6 % with CST I and 21.4 % with CST III).
4.5. Associations with spontaneous preterm birth
We found no statistically significant associations with any of the CSTs in univariate or multivariate analysis using only samples taken <16 weeks of gestation or all available samples, Table 2. Due to the limited study size, inconsistent results were observed using mixed model and Cox regression analysis. The unstable estimations that these models provided, were similar to the results of the logistic regression models presented. Neither did we find statistically significant associations between individual taxa and sPTB.
Table 2.
Associations with spontaneous preterm birth.
| only samples <16 weeks of gestation |
all samples |
|||||||
|---|---|---|---|---|---|---|---|---|
| univariate |
multivariatea |
univariate |
multivariatea |
|||||
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Microbiota | ||||||||
| CST I | ref | Ref | ||||||
| CST II | 1.8 | 0.4–6.8 | 1.6 | 0.4–6.1 | 2.3 | 0.8–5.9 | 2.1 | 0.8–5.6 |
| CST III | 0.5 | 0.1–1.8 | 0.5 | 0.1–1.8 | 1.0 | 0.5–2.1 | 1.0 | 0.5–2.2 |
| CST IV | 1.4 | 0.5–4.0 | 1.4 | 0.5–4.0 | 1.7 | 0.8–3.8 | 1.8 | 0.8–3.9 |
| CST V | 0.4 | <0.1–4.4 | 0.4 | <0.1–4.0 | 0.3 | <0.1–2.6 | 0.3 | <0.1–2.6 |
| Gardnerella species detection | ||||||||
| G. leopoldii | 2.4 | 1.0–5.7 | 2.8 | 1.1–6.9 | 2.0 | 1.0–3.7 | 2.2 | 1.2–4.2 |
| G. piotii | 0.8 | 0.3–1.9 | 0.8 | 0.3–1.8 | 0.6 | 0.3–1.0 | 0.6 | 0.3–1.0 |
| G. swidsinskii | 0.6 | 0.2–1.4 | 0.6 | 0.2–1.4 | 0.7 | 0.4–1.2 | 0.7 | 0.4–1.2 |
| G. vaginalis | 0.8 | 0.4–1.9 | 0.8 | 0.4–1.9 | 1.3 | 0.7–2.3 | 1.2 | 0.7–2.3 |
| Any STI | 1.3 | 0.2–5.4 | 1.5 | 0.2–6.4 | 0.9 | 0.2–3.2 | 1.2 | 0.2–4.5 |
All logistic regression analyses with Firth'ss correction adjusted for gestational age. Only including participants with a pregnancy that progressed beyond 16 weeks of gestation. a Multivariate analyses are adjusted for ethnicity (White European/non-White European), body mass index (continuous) and smoking (no/yes or stopped during pregnancy). Statistically significant data (p < 0.05) are presented in bold.
We found a statistically significant association between G. leopoldii and sPTB (aOR 2.8 95 % CI 1.1–6.9 for only samples <16 weeks of gestation, before antibiotic or progesterone treatment, and OR 2.0 95 % CI 1.0–3.7, aOR 2.2 95 % CI 1.2–4.2 for all samples). The other Gardnerella spp. did not have statistically significant associations with sPTB. We found no associations between the presence of STIs and sPTB.
5. Discussion
5.1. Main findings
In this descriptive study of Dutch pregnant women with a history of sPTB, we observed no association between microbiota composition and recurrent sPTB. Our study is one of the few reporting on vaginal microbiota composition specifically among women with a history of sPTB. As these women received different preventive therapies such a routine administration of progesterone and treatment of bacterial vaginosis with a 5-day course of oral clindamycin, our findings may suggest that the aetiology of the remaining recurrent sPTB in our study population might be underpinned by other mechanisms than disturbed vaginal microbiota.
5.2. Interpretation
Existing literature has not shown a consistent picture of the role of the vaginal microbiome in recurrent sPTB. While several studies draw conclusions based on a mixed population, including participants with low and high risk for sPTB, data on recurrent sPTB specifically is limited. One study reported a protective effect of L. crispatus dominance against recurrent sPTB, whereas L. iners was associated with increased risk of recurrent sPTB [11]. Three other studies did not find a statistically significant association between vaginal microbiota composition and recurrent sPTB [13,32,33]. However, none of these studies reported on antibiotic use during pregnancy. Three of the studies are performed in the United Kingdom and one in the United States of America, both countries having very different obstetric care systems compared to The Netherlands. Also, the ethnic background of the participants in the different studies is very diverse, which makes generalizing these results to the Dutch population difficult. And only one the studies adjusted their analyses for ethnicity [11]. Many other studies with mixed population reported associations between lactobacilli-depleted microbiota composition and sPTB [12,13,15,17,[34], [35], [36]]. However, antibiotic BV treatment has thus far not been proven to reduce (recurrent) sPTB [9,10]. Microbe-related inflammation could be involved in a spectrum of obstetric syndromes, including dysfunctional placentation associated with pre-eclampsia [37]. The absence of an association with recurrent sPTB in our and other studies could indicate that vaginal microbiota attributes little to recurrent sPTB within this specific population, taking all the other preventive therapies into account. Microbe-related sPTB may be more involved in sporadic pregnancy loss, while in women with recurrent sPTB other risk factors, like cervical insufficiency, are more important.
We observed a decrease in diverse microbiota and increase in L. iners-dominated microbiota during pregnancy, which coincided with initiation of progesterone therapy and treatment of BV. Previous studies have investigated the dynamics during pregnancy. Serrano et al. performed a study among pregnant women without progesterone use, sampled in first, second and third trimester and demonstrated similar dynamics of vaginal microbiota [38]. However, this study did not mention BV treatment between the first and second trimester. Two other studies did not find obvious changes in vaginal microbiota composition during pregnancy, nor an effect of progesterone, but both studies started sampling at the start of the second trimester [11,32]. In our study, the treatment of BV most likely explains the change in microbiota profile during pregnancy. A larger randomized study could evaluate the effect of progesterone, BV treatment and the natural course of pregnancy on vaginal microbiota in relation with recurrent sPTB.
While the association between G. leopoldii and recurrent sPTB is interesting, it should be interpreted with caution. It might be a reflection of small sample size and unknown interrelations between G. leopoldii, confounders and the outcome. However, other studies also found associations between different species of Gardnerella and sPTB [13,39]. A recent study made an contrasting observation in a group of Zambian women, displaying less G. leopoldii in women with PTB [39]. One study suggested the distribution of Gardnerella spp. is dependent on ethnicity, as is common in vaginal microbiome composition and other vaginal species [38]. Little is known on the topographic divergence and the pathogenic potential of different Gardnerella spp., while this could further refine the association between vaginal microbiota and sPTB. Future studies should further explore the role of different Gardnerella spp. and sPTB, and especially G. leopoldii, to confirm our results.
We observed that multiple participants had one or more STIs, or contracted an STI during pregnancy. Each of these STIs have been consistently linked to sPTB in meta-analyses, all with ORs <2.0 [22,40,41]. We most likely did not find a statistically significant association between sPTB and the STIs tested, due to the small expected effect size and limited sample size. Despite the limited attribution to sPTB, the high rates of STIs in this population raises the question whether we should screen and treat all women or specific populations for STIs before or during pregnancy.
5.3. Strengths and limitations
The major strength of our study is the specific population, including only women with a history of one or more sPTB. This allows the investigation of specific mechanisms in recurrent sPTB. Our cohort is reflective of the population eligible for preventive therapies. Also, the ethnic diversity in our population reflects the urban area in which the study took place and helps to generalise our results. We also included participants pregnant with twins. This enhances representability of pregnant women at risk for recurrent sPTB, but could have had a limited effect on the association with microbe related recurrent preterm birth. Another strength is the broad dataset on other risk factors for preterm birth, including STIs.
The foremost limitation of our study is the limited sample size. Due to the limited number of sPTB cases, we were not able to investigate associations with subgroups such as sPTB at lower gestational ages at less than 28 or 34 weeks of gestation. Infection as a cause for sPTB is mainly present at lower gestational ages. In this sense our study was similar to existing cohorts on this topic [12,13,17,32]. However, we performed additional statistical corrections to account for this, but longitudinal regression models were not reliable. With the sample size of our study, we would have been able to demonstrate a clinically relevant OR ≥ 2.5. The fact that we did not find a statistically significant association between vaginal microbiota and sPTB means that in our population, any association we did not pick up is small, if present at all, and clinical relevance would be doubtful. Systematically aggregating the available evidence for an association between vaginal microbiota and recurrent sPTB would provide more insight, but has not been considered in existing meta-analyses to date [30,42]. Small sample size was further exacerbated by the fact that several participants collected fewer than three swabs and there were slight differences in the gestational ages at which the swabs were collected, which could have contributed to statistical uncertainty. An additional major limitation is the fact that this cohort of women at high risk for recurrent sPTB was treated according to national guidelines for this specific population, including screening and antibiotic treatment of BV, progesterone between 16 and 36 weeks of gestation and cervical cerclage if indicated. The antibiotic treatment of BV has influenced the vaginal microbiota composition, as described in Fig. 1. The preventive therapies may have influenced recurrent sPTB rates and thus have limited our ability to assess an association between vaginal microbiota and sPTB <37 weeks of gestation. Another limitation is the lack of information on several factors associated with vaginal microbiota and/or sPTB, such as vaginal douching and sexual practices.
6. Conclusion
In conclusion, we did not find an association between vaginal microbiota and recurrent sPTB at less than 37 weeks of gestation in this population of women with a history of sPTB. Although vaginal microbiota have been robustly shown to play a role among sPTB in general, our data suggests that other pathophysiological phenomena may play a larger role in the aetiology of recurrent sPTB in a population treated with vaginal progesterone and clindamycin for bacterial vaginosis. Future meta-analyses could shed further light on this topic by considering women with a history of sPTB separately, particularly because our study's size was insufficient to rule out a small effects of vaginal microbiota on recurrent sPTB.
Funding
Amsterdam Reproduction and Development (AR&D 2016).
Ethics statement
This study was reviewed and approved by the Biobank Review Committee of the Academic Medical Centre, with registration number: 2017_116. This study was not subjected to legislation for trials, as confirmed by the Institutional Review Board of the Academic Medical Centre, registration number 2017_116#C2019447. All participants provided written informed consent to participate in the study.
Data availability statement
The data that has been used in this study is confidential and has not been deposited into a publicly available repository. Individual participant data, after de-identification, will be made available upon reasonable request, to researchers who provide a methodologically sound proposal, immediately following publication. Proposals should be directed at the corresponding author; to gain access, data requestors will need to sign a data access agreement according to national legislation.
CRediT authorship contribution statement
Heleen J. Schuster: Writing – original draft, Visualization, Project administration, Methodology, Investigation, Formal analysis, Data curation. Anouk M. Bos: Writing – review & editing, Formal analysis, Data curation. Lisa Himschoot: Writing – review & editing, Formal analysis. Rik van Eekelen: Writing – review & editing, Software, Methodology, Formal analysis. Sébastien P.F. Matamoros: Writing – review & editing, Software, Methodology, Formal analysis. Marjon A. de Boer: Writing – review & editing, Investigation. Martijn A. Oudijk: Writing – review & editing, Investigation. Carrie Ris-Stalpers: Writing – review & editing, Resources, Project administration. Piet Cools: Writing – review & editing, Supervision, Resources, Formal analysis. Paul H.M. Savelkoul: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Conceptualization. Rebecca C. Painter: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Robin van Houdt: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:R.C. Painter, P.H.M. Savelkoul reports financial support was provided by Amsterdam Reproduction and Development. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the participants and the staff of the outpatients clinic for their assistance in the execution and logistics of the study. Funding for this study was provided by Amsterdam Reproduction and Development (AR&D 2016). The funder had no influence on the execution of the study and the content of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30685.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
References
- 1.Liu L., et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Liu L., et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison M.S., Goldenberg R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2015;21(2):74–79. doi: 10.1016/j.siny.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Naghavi M., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips C., Velji Z., Hanly C., Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. Br. Med. J. 2017;7(6) doi: 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R., Dey S., Fisher S. Preterm labor: one syndrome many causes. Sci. J. 2014;345(6198):760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitich H., et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 2003;189(1):139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 9.Brocklehurst P., Gordon A., Heatley E., Milan S.J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 2013;(1) doi: 10.1002/14651858.CD000262.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subtil D., et al. Early clindamycin for bacterial vaginosis in pregnancy (PREMEVA): a multicentre, double-blind, randomised controlled trial. Lancet. 2018;392(10160):2171–2179. doi: 10.1016/S0140-6736(18)31617-9. [DOI] [PubMed] [Google Scholar]
- 11.Kindinger L.M., et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5(1):6. doi: 10.1186/s40168-016-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiGiulio D.B., et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. U.S.A. 2015;112(35):11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan B.J., et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. U.S.A. 2017;114(37):9966–9971. doi: 10.1073/pnas.1705899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stout M.J., et al. Early pregnancy vaginal microbiome trends and preterm birth. Am. J. Obstet. Gynecol. 2017;217(3) doi: 10.1016/j.ajog.2017.05.030. 356 e1-356 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elovitz M.A., et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 2019;10(1):1305. doi: 10.1038/s41467-019-09285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruski P., et al. Direct on-swab metabolic profiling of vaginal microbiome host interactions during pregnancy and preterm birth. Nat. Commun. 2021;12(1):5967. doi: 10.1038/s41467-021-26215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fettweis J.M., et al. The vaginal microbiome and preterm birth. Nat. Med. 2019;25(6):1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown R.G., et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16(1):9. doi: 10.1186/s12916-017-0999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaneechoutte M., et al. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019;69(3):679–687. doi: 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 20.Balashov S.V., Mordechai E., Adelson M.E., Gygax S.E. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J. Med. Microbiol. 2014;63(Pt 2):162–175. doi: 10.1099/jmm.0.066407-0. [DOI] [PubMed] [Google Scholar]
- 21.Janulaitiene M., et al. Phenotypic characterization of Gardnerella vaginalis subgroups suggests differences in their virulence potential. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenzer C., et al. Adverse pregnancy and perinatal outcomes associated with Mycoplasma genitalium: systematic review and meta-analysis. Sex. Transm. Infect. 2022;98(3):222–227. doi: 10.1136/sextrans-2021-055352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao R., et al. Association of maternal sexually transmitted infections with risk of preterm birth in the United States. JAMA Netw. Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.33413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klebanoff M.A., et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N. Engl. J. Med. 2001;345(7):487–493. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 25.Koullali B., et al. A multi-centre, non-inferiority, randomised controlled trial to compare a cervical pessary with a cervical cerclage in the prevention of preterm delivery in women with short cervical length and a history of preterm birth - PC study. BMC Pregnancy Childbirth. 2017;17(1):215. doi: 10.1186/s12884-017-1393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuster H.J., et al., The interrelation between microbial immunoglobulin coating, vaginal microbiota, ethnicity and preterm birth (2022), accepted for publication. [DOI] [PMC free article] [PubMed]
- 27.Latka A., et al. Optimization of propidium monoazide qPCR (viability-qPCR) to quantify the killing by the gardnerella-specific endolysin PM-477, directly in vaginal samples from women with bacterial vaginosis. Antibiotics (Basel) 2022;11(1) doi: 10.3390/antibiotics11010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X. Firth logistic regression for rare variant association tests. Front. Genet. 2014;5:187. doi: 10.3389/fgene.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallick H., et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021;17(11) doi: 10.1371/journal.pcbi.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peelen M.J., et al. The influence of the vaginal microbiota on preterm birth: a systematic review and recommendations for a minimum dataset for future research. Placenta. 2019;79:30–39. doi: 10.1016/j.placenta.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Alliance J.L. Preterm Birth Top. 2022;10 https://www.jla.nihr.ac.uk/priority-setting-partnerships/preterm-birth/top-10-priorities/ [cited 2022 18-12-2022]; Available from: [Google Scholar]
- 32.Chan D., et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022;13(1):975. doi: 10.1038/s41467-022-28620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodfellow L., et al. Vaginal bacterial load in the second trimester is associated with early preterm birth recurrence: a nested case-control study. BJOG. 2021;128(13):2061–2072. doi: 10.1111/1471-0528.16816. [DOI] [PubMed] [Google Scholar]
- 34.Romero R., Hassan S.S., Gajer P. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome Journal. 2014 doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun S., et al. Race, the vaginal microbiome, and spontaneous preterm birth. mSystems. 2022 doi: 10.1128/msystems.00017-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudnadottir U., et al. The vaginal microbiome and the risk of preterm birth: a systematic review and network meta-analysis. Sci. Rep. 2022;12(1):7926. doi: 10.1038/s41598-022-12007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giorgione V., et al. Routine first-trimester pre-eclampsia screening and risk of preterm birth. Ultrasound Obstet. Gynecol. 2022;60(2):185–191. doi: 10.1002/uog.24915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano M.G., et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019;25(6):1001–1011. doi: 10.1038/s41591-019-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price J.T., et al. HIV-associated vaginal microbiome and inflammation predict spontaneous preterm birth in Zambia. Sci. Rep. 2022;12(1):8573. doi: 10.1038/s41598-022-12424-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He W., et al. Effect of Chlamydia trachomatis on adverse pregnancy outcomes: a meta-analysis. Arch. Gynecol. Obstet. 2020;302(3):553–567. doi: 10.1007/s00404-020-05664-6. [DOI] [PubMed] [Google Scholar]
- 41.Van Gerwen O.T., et al. Trichomoniasis and adverse birth outcomes: a systematic review and meta-analysis. BJOG. 2021;128(12):1907–1915. doi: 10.1111/1471-0528.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosti I., et al. Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front. Microbiol. 2020;11:476. doi: 10.3389/fmicb.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used in this study is confidential and has not been deposited into a publicly available repository. Individual participant data, after de-identification, will be made available upon reasonable request, to researchers who provide a methodologically sound proposal, immediately following publication. Proposals should be directed at the corresponding author; to gain access, data requestors will need to sign a data access agreement according to national legislation.