Abstract
The increased demand and consumption of synthetic textiles have contributed to microplastic pollution in the form of microfibers. These particles are widely spread in the aquatic environment, leading to the exposure of marine biota, including edible species. The current study aimed to assess the extent of microfiber contamination in a commercially relevant fish species, Merluccius merluccius, which is considered a small-scale bioindicator for the monitoring of plastic ingestion in the Mediterranean coastal environment. The frequency of ingestion, abundance, and composition of textile microfibers isolated from the fish gut were characterized. Results showed the occurrence of microfibers in 75% of the samples, with a mean number of 10.6 microfibers/individual, of which 70% were classified as natural microfibers. The spectroscopic analyses confirmed both the visual identification of microfibers and the prevalence of cellulosic fibers. The obtained findings provided evidence of both natural/artificial and synthetic microfiber exposure in an important commercial fish species that, considering the consumption of small individuals without being eviscerated, may be a potential route of microfiber exposure in humans. Monitoring programs for fishery products from markets are needed to assess contamination levels and human health risks. In addition, measures to control microfiber pollution need to occur at multiple levels, from textile industries to international governments.
Key words: fibrous microplastics, natural microfibers, commercial fish, food safety
Introduction
Recently, textile fiber pollution has received growing attention due to its wide diffusion in the marine environment (Suaria et al., 2020; Muns-Pujadas et al., 2023; Santonicola et al., 2023). Despite much research has focused on sustainability issues related to synthetic textiles, one of the main risks, such as the microplastic pollution associated with textile fibers, has not yet been addressed effectively. During their life cycle (production, consumption, and disposal), synthetic fabrics, which are dominant in the fast fashion trend, may release microfibers that are persistent marine pollutants (Balasaraswathi and Rathinamoorthy, 2022). In recent decades, the fashion industry has contributed to 31% of total plastic pollution in the ocean (Liu et al., 2021). The available information shows that as much as 80% of microplastics in the marine environment correspond to fibers (Cesa et al., 2017), and it has been estimated that the microfiber accumulation in the environment will become more severe because of the growth in textile production and waste (Liu et al., 2021).
The domestic laundry process has also been identified as one of the major sources of microfibers in the aquatic environment (Li et al., 2022). Moreover, the degradation and fragmentation of abandoned fishing gear represent another important source of microfibers in seawater (Rathinamoorthy and Balasaraswathi, 2022a). In addition, recently, due to the rapid spread of the coronavirus, the increased consumption and disposal of single-use personal protective equipment have further increased microfiber pollution. Researchers have estimated that about 31,200 tons of microfibers were dumped into the ocean in 2020 due to the improper waste of surgical masks (Rathinamoorthy and Balasaraswathi, 2022b).
The microfibers in the marine environment can be mistaken as food by various living organisms, and the issue becomes more concerning when it can reach human beings through the food chain (Balasaraswathi and Rathinamoorthy, 2022). Indeed, several studies have highlighted the dominance of textile fibers within the digestive tract of marine biota, including commercially important fish and shellfish (Rodríguez-Romeu et al., 2020; Liu et al., 2021; Muns-Pujadas et al., 2023). The primary impact on marine biota can be physical, as the microfiber ingestion may alter food intake and growth; the secondary impact may derive from the leachability of dyes and chemicals used in the production phase (Rathinamoorthy, and Balasaraswathi, 2022a). Toxicity may also occur after these particles enter into the circulatory system with translocation from the gastrointestinal tract (GIT) into other tissues, including commonly consumed tissues, as reported in experimental studies using marine invertebrates and fish (Akoueson et al., 2020). Moreover, the edible parts of fish can be contaminated by the microplastics contained in the GIT during cleaning and preparation (Bošković et al., 2022), leading to human exposure.
Some studies tended to include the assessment of synthetic fibers as a particular type of microplastic (Cesa et al., 2017), while recent works consider textile microfibers separately from common microplastic particles due to their different origin and environmental abundance, as well as mitigation strategies (Avio et al., 2020; Ergas et al., 2023). This approach may prevent natural microfibers from being excluded from studies under the assumption that non-plastic fibers are readily biodegradable (Athey and Erdle, 2022). In fact, these particles, as well as man-made fibers (sometimes called “artificial fibers”), composed of processed polymers from natural materials, are widespread in the sea and oceans, where they can persist for months to decades (Suaria et al., 2020; Athey and Erdle, 2022).
In this view, the aim of the current research was to assess the extent of natural/artificial and synthetic microfiber contamination in the GIT of commercial Merluccius merluccius exemplars from the Tyrrhenian Sea. The European hake is a benthopelagic species widely diffused in the study area, and, being the main target of the commercial fishery and not able to travel long distances, it has been considered a small-scale bioindicator for the monitoring of plastic ingestion in the Mediterranean coastal environment (Giani et al., 2019; Cocci et al., 2022; Miccoli et al., 2022). In addition, the small exemplars may be used in Italian delicacies without being eviscerated, and so the anthropogenic particles contained in their gut may be ingested by the consumer (Mistri et al., 2022).
Materials and Methods
Sampling and analyses
The samples (n=20) were collected from fish markets located in the Campania region, Italy. Fish were from the same fishing area (Food and Agricultural Organization area 37, subarea 37.1, division 37.1.3), as stated on the labels. Small-sized individuals were selected because they may be employed in Italian dishes (e.g., fried fish) without being eviscerated. Thus, specimens of similar length were sorted and then wrapped in aluminum foil directly in-store. At the laboratory, after recording the weight (g), and length (cm), each fish was dissected using metal scissors, and tweezers. The GIT (including stomach and intestine) of each fish was weighed and transferred to a glass beaker, which was immediately closed with aluminum foil. Potassium hydroxide solution (10%), approximately triple the volume of the tissue, was used to digest organic matter (45°C, overnight in a static incubator), followed by density separation by adding 250 mL of NaCl hypersaline solution (density 1.2 g cm-3) to each sample, and filtration of the supernatant through cellulose nitrate membranes (8 μm pore size). To completely digest all tissue residues, a 15% H2O2 solution was added to the membranes and allowed to dry in an oven (45°C, overnight) (Santonicola et al., 2023). For the correction of potential contamination, one blank control without any tissue was performed in parallel with each sample group (5-6 individuals) analyzed on the same day. To prevent airborne contamination, the dissection of all fish was performed in a laminar flow cabinet, and samples were always covered with aluminum foil. All material was previously rinsed three times, before use and between samples, with filtered distilled water (cellulose acetate membranes, pore size 0.45 μm). Nitrile gloves and cotton lab coats were used at all times.
Microfiber identification
Microfibers isolated on the filter membranes were inspected using a light microscope (M205C, Leica, Wetzlar, Germany) with a magnification of 0.78-16× (Figure 1A), and then counted and categorized according to color. The micrographs of each microfiber were analyzed by two different operators to discriminate between synthetic and natural microfibers according to some morphological features: cross-section shape, breakages, and alterations of the fiber body, shape, and appearance of the ends (Stanton et al., 2019; Volgare et al., 2022; Santonicola et al., 2023). The morphology of natural fibers is more complex than that of synthetic ones, as they do not show a uniform diameter and are twisted upon themselves like flat ribbons with frayed edges. Meanwhile, synthetic microfibers are characterized by a uniform diameter, smooth and shiny surface, circular section, and solid edges. The microfiber length was obtained by analyzing optical micrographs using Image J (release 1.43 u).
Figure 1.

A) Optical microscope image of natural/artificial microfiber; B) μ-Fourier-transform infrared spectra.
To gauge the accuracy of visual discrimination, a subset of microfibers (which correspond to 10% of isolated microfibers; Lusher et al., 2017) were chemically analyzed (Figure 1B) using a Fourier-transform infrared (FTIR) microscope (Nicolet iMX10, Thermo Fisher Scientific, Waltham, MA, USA). Due to the spectral interference caused by cellulose filters, for the FTIR analyses, microfibers were transferred to silicon membranes (MakroPor, pore size 5 µm, Thermo Fisher Scientific, Waltham, MA, USA) by washing the cellulose filters with water.
All the measures were taken in transmission mode. Following background scans, 64 scans were performed for each particle, with a resolution of 4 cm-1. OMNIC™ Specta Software (Thermo Scientific™, Waltham, MA, USA) was used for the output spectra, and the identification of polymers was performed by comparison with spectra libraries. Polymers matching the reference spectra for more than 70% were validated.
Statistical analyses
Statistical analysis of the collected data was performed using SPSS® Statistics software (IBM, Armonk, NY, USA). The data were tested for normality using the Shapiro-Wilk test and homogeneity of variance using Levene’s test. A one-way analysis of variance (ANOVA) was carried out to assess the significance of differences between the data. When the data did not comply with the assumption of normality, a post-hoc pairwise comparison was performed using the non-parametric Kruskal-Wallis test. A 5% significance level was considered for all the statistical tests (p<0.05 indicates significant differences among the data). Finally, the correlation between the data was carried out using Pearson correlation (p<0.01 indicates a significant correlation among the data).
Results
M. merluccius specimens had a total length of 18.23±2.35 cm and a body weight of 69.23±24.41 g [average ± standard deviation (SD)]. The average (±SD) weight of the GIT was 4.14±3.3 g.
Microfibers were present in 75% of the samples. Considering only the positive samples, a mean (±SD) number of 10.6±7.54 microfibers/GIT (range 0.5-29.5 microfibers/GIT) was detected, which corresponds to 5.6±6.9 microfibers/g of wet weight (w.w.) of GIT and 0.23±0.22 microfibers/g w.w. of individual. Instead, the mean number of microfibers/GIT among all the examined individuals was 7.97. Microfiber abundance was expressed as the average number of microfibers per GIT, both in individuals containing microfibers and in all individuals examined, to facilitate comparisons to the literature, according to Digka et al. (2018).
A negative correlation was observed between the number of microfibers/g w.w. of the individual and the weight and length of the samples [Pearson correlation between microfibers/g w.w. of the individual and the weight r(20)=-0.809, p=0.000, and length r(20)=-0.737, p=0.000], showing that smaller specimens contained more microfibers than the larger ones (Figure 2).
Among the isolated microfibers, 70% were classified as natural, according to the evaluation of specific morphological features. No differences were highlighted in the number of natural and synthetic microfibers detected during the microscopical analysis between hake size groups [ANOVA test between synthetic microfiber mean levels in the hake size groups r(2,17)=0.850, p=0.455; Kruskal-Wallis test between natural microfiber mean levels in the hake size groups p=0.217].
The FTIR analyses corroborated the visual identification, and the chemical composition of a subset (10%) of microfibers showed the occurrence of both cellulosic (cellulose, 50%; and regenerated cellulose, rayon, 38%) and synthetic microfibers (polyester and nylon, 12%). At the moment, it is extremely challenging to distinguish between natural and regenerated cellulose microfibers using FTIR techniques, especially when dealing with environmentally degraded polymers (Suaria et al., 2020). Therefore, we grouped natural and regenerated cellulosic microfibers together as cellulosic microfibers (88% of chemically analyzed particles).
Figure 2.

Distribution of microfibers per g wet weight according to the sample size. w.w., wet weight.
The mean lengths of natural and synthetic microfibers were, respectively, 803.76 and 856.72 µm (Figure 3).
Blue (41.7%) and black (19.8%) microfibers were the most abundant, followed by clear/transparent (17%), sky-blue (10.12%), pink (4.45%), orange (3.23%), and other (red, purple, green, and yellow; 3.7%) colored microfibers.
Discussion
The European hake is a species of ecological importance for its trophic link between pelagic and demersal habitats, and, therefore, it has been regarded as a bioindicator of coastal marine ecosystems (Giani et al., 2019). Moreover, this species has a noticeable commercial value, being among the most targeted demersal fish species by the Mediterranean fisheries (Cocci et al., 2022; Miccoli et al., 2022).
The obtained results confirmed that M. merluccius is susceptible to microfiber ingestion, as previously observed in other areas of the Tyrrhenian Sea (Mancuso et al., 2019; Miccoli et al., 2022) and worldwide (Giani et al., 2019; Avio et al., 2020; Bošković et al., 2022; Cocci et al., 2022; Muns-Pujadas et al., 2023).
If we compare literature data on microfiber intake in the same species, the number of ingested microfibers shows high variability. 5.3 (±3.8) synthetic microfibers were detected in M. merluccius from the Adriatic Sea (Cocci et al., 2022), while M. merluccius from the coastal Northern Tyrrhenian Sea ingested 12.67 (±4.27) plastic microfibers per individual (Miccoli et al., 2022). Hakes from different localities of the Catalan coast (northwest Mediterranean) ingested a mean of 1.39 (±1.39) items/individual; among those, consistent with the current investigation, natural microfibers were predominant (77.8%) (Muns-Pujadas et al., 2023). The available data could have been influenced by several natural and anthropogenic variables, such as weather conditions, human impacts within the study areas, and waste management strategies (Giani et al., 2019; Cocci et al., 2022).
The variability among results seems to also suggest the requirement of a standardized protocol to isolate microplastics, including microfibers, in fish species. Several studies performed the screening of the stomach and gastrointestinal content directly by visual inspection (Miccoli et al., 2022; Muns-Pujadas et al., 2023), while other works reported different digestion methods to isolate microplastics from the fish gut (Cocci et al., 2022). In this light, the harmonization of sampling approaches, extraction protocols, and units for reporting plastic abundance would allow the comparison of data generated by different research teams (Giani et al., 2019). In addition, several biological factors may influence the number of ingested microfibers. For instance, the changes in the diet with the transition in juvenile hakes from an opportunistic feeding behavior to a more selective foraging behavior (Muns-Pujadas et al., 2023) could explain the higher microfiber levels in small specimens.
When comparing the microfiber levels in a different fish species (Mullus barbatus) from the same area, the results showed a slightly lower level of contamination in red mullet samples (9.2 microfibers/individual) (Santonicola et al., 2023). These findings could be due to the fact that M. merluccius, being a benthopelagic species that moves in two habitats, could have a greater possibility of ingesting litter than the benthic M. barbatus, which lives in a single habitat (Miccoli et al., 2022; Muns-Pujadas et al., 2023). Therefore, it can be inferred that the exposure to microfibers could vary depending both on environmental conditions and the species characteristics (feeding habits and habitat) (Bošković et al., 2023).
The coloration of the ingested microfibers was very similar among previously published papers. In particular, in line with our results, the dominant microfiber colors were blue and black (Giani et al., 2019; Bošković et al., 2022; Cocci et al., 2022; Miccoli et al., 2022). This prevalence of dark plastic items has been previously reported in other commercially relevant fish species (Savoca et al., 2019; Capillo et al., 2020), and one plausible explanation is that microfibers could be ingested both because they are mistaken for preys, and even purposefully chosen instead of food depending on their color (Miccoli et al., 2022).
The visual approach showed a prevalence of natural microfibers, while only 30% was represented by synthetic fibers. These results agree with those reported in different commercial fish species (Boops boops, M. barbatus, Trigla lyra, Galeus melastomus) (Savoca et al., 2019; Capillo et al., 2020), as well as in seawater samples collected worldwide (Suaria et al., 2020). The chemical analyses corroborated the visual identification, allowing us to identify both non-plastic (cellulose, 88%) and synthetic (polyester and nylon, 12%) polymers. As the composition of 10% of total fibers has been confirmed by micro-FTIR, results and interpretations must be taken with care. However, considering the documented abundance of cellulosic microfibers in marine environments (Suaria et al., 2020), the composition of the fibers in the fish gut could reflect the high distribution of these fibers in the surrounding environment (Ergas et al., 2023). Despite synthetic polymers have dominated the textile market during the last decades, natural/artificial cellulosic microfibers are reported as the most abundant in marine habitats, probably because these fibers are more damageable, shedding from clothes and other textiles into the environment (Liu et al., 2023). One of the limitations of the present study could be the small sample size; however, considering the limited knowledge of microfiber ingestion in commercial fish species and the high incidence of microfiber exposure in the selected species, the sample sizes may be sufficient for providing potentially useful data (Cocci et al., 2022). In addition, the visual classification of natural and synthetic microfibers may be criticized for its susceptibility to human error. In this light, the analyses of the fiber micrographs by two different operators, the use of micrographs of some natural and synthetic microfibers as references during the observation under the microscope (Volgare et al., 2022), and the FTIR analyses of a subsample of particles may help correctly identify microfiber types. The effort to implement a fast and easy method for the evaluation of microfiber contamination is linked to the difficulties in obtaining clear FTIR spectra from the small, often curved, surfaces of textile microfibers (Stanton et al., 2019). Due to methodological issues, microfibers have received little attention in the reporting of microplastic pollution. Therefore, a thorough and accurate evaluation of the extent of microfiber exposure in fish species may help in understanding the threats that textile fibers pose. The main concern linked to microfiber pollution is that it may be transferred through the food chain with detrimental consequences for humans (Li et al., 2022). Recent evidence has shown the occurrence of microfibers in the human placenta, and the exposure could also be attributed to the consumption of contaminated food (Zhu et al., 2023). However, as discussed before, microfibers are underestimated in the literature, and further studies based on consistent sampling and analysis methods of seafood from markets, with a particular focus on those eaten whole, are needed to assess the contamination levels and human health risks (Santonicola et al., 2023). In addition, governments should encourage the development of a more environmentally friendly textile and fashion industry by designing sustainable approaches and innovative solutions to control the microfibers entering the oceans (European Parliament, 2023).
Figure 3.
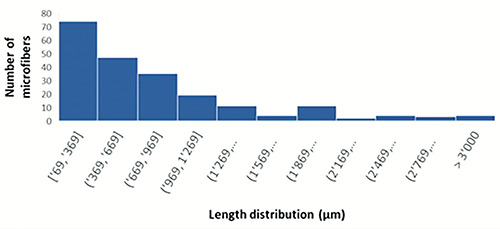
Microfiber length distribution in Merluccius merluccius.
Conclusions
During the last decades, the increased production and consumption of synthetic textiles, due to fast fashion trends, have been reported as the key accelerators of microplastic pollution. The results of this study confirmed the exposure to natural/artificial and synthetic microfibers in a relevant commercial fish species (M. merluccius) from the Tyrrhenian Sea. At the moment, studies focused on contamination in fishery products sold for human consumption are limited, and the available data are difficult to compare due to several natural and anthropogenic variables and because natural microfibers are frequently excluded. Our results confirmed the wide distribution of natural/artificial cellulosic microfibers, pointing to the need to better understand the food safety implications and the potential harm to consumer health. Considering that the consumer could be exposed to microfibers through the ingestion of fish consumed as a whole or due to the contamination of the edible parts by the microfibers in the GIT during cleaning and preparation of fish, the issue of microfiber pollution should receive more attention, with further efforts to standardize analyses and data collection methods. The small size of these particles, the presence of dyes, and the low spectral signal intensities of natural materials may hinder chemical identification using spectroscopic techniques. In this context, morphological analysis could be an alternative approach to identifying natural and synthetic microfibers. In addition, future research should also focus on the potential sources throughout the entire textile chain to mitigate the emissions and environmental impact of microfibers.
Funding Statement
Funding: none.
References
- Akoueson F, Sheldo LM, Danopoulos E, Morris S, Hotten J, Chapman E, Rotchell JM, 2020. A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ Pollut 263:114452. [DOI] [PubMed] [Google Scholar]
- Athey SN, Erdle LM, 2022. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ Toxicol Chem 41:822-37. [DOI] [PubMed] [Google Scholar]
- Avio CG, Pittura L, d’Errico G, Abel S, Amorello S, Marino G, Regoli F, 2020. Distribution and characterization of microplastic particles and textile microfibers in Adriatic food webs: general insights for biomonitoring strategies. Environ Pollut 258:113766. [DOI] [PubMed] [Google Scholar]
- Balasaraswathi SR, Rathinamoorthy R, 2022. Synthetic textile and microplastic pollution: an analysis on environmental and health impact. In: Muthu SS, ed. Sustainable approaches in textiles and fashion: circular economy and microplastic pollution. Springer, Singapore, pp 1-20. [Google Scholar]
- Bošković N, Joksimović D, Bajt O, 2022. Microplastics in fish and sediments from the Montenegrin coast (Adriatic Sea): similarities in accumulation. Sci Total Environ 850:158074. [DOI] [PubMed] [Google Scholar]
- Capillo G, Savoca S, Panarello G, Mancuso M, Branca C, Romano V, Spanò N, 2020. Quali-quantitative analysis of plastics and synthetic microfibers found in demersal species from Southern Tyrrhenian Sea (Central Mediterranean). Mar Pollut Bull 150:110596. [DOI] [PubMed] [Google Scholar]
- Cesa FS, Turra A, Baruque-Ramos J, 2017. Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci Total Environ 598:1116-29. [DOI] [PubMed] [Google Scholar]
- Cocci P, Gabrielli S, Pastore G, Minicucci M, Mosconi G, Palermo FA, 2022. Microplastics accumulation in gastrointestinal tracts of Mullus barbatus and Merluccius merluccius is associated with increased cytokine production and signaling. Chemosphere 307:135813. [DOI] [PubMed] [Google Scholar]
- Digka N, Tsangaris C, Torre M, Anastasopoulou A, Zeri C, 2018. Microplastics in mussels and fish from the Northern Ionian Sea. Mar Pollut Bull 135:30-40. [DOI] [PubMed] [Google Scholar]
- Ergas M, Figueroa D, Paschke K, Urbina MA, Navarro JM, Vargas-Chacoff L, 2023. Cellulosic and microplastic fibers in the Antarctic fish Harpagifer antarcticus and Sub-Antarctic Harpagifer bispinis. Mar Pollut Bull 194:115380. [DOI] [PubMed] [Google Scholar]
- European Parliament, 2023. EU strategy for sustainable and circular textiles. Available from: https://www.europarl. europa.eu/doceo/document/TA-9-2023-0215_EN.pdf. Accessed on: 31/10/2023. [Google Scholar]
- Giani D, Baini M, Galli M, Casini S, Fossi MC, 2019. Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea. Mar Pollut Bull 140:129-37. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu Q, Xing Y, Liu K, Ling W, Yang J, Zhao D, 2022. Review of research on migration, distribution, biological effects, and analytical methods of microfibers in the environment. Sci Total Environ 855:158922. [DOI] [PubMed] [Google Scholar]
- Liu J, Liang J, Ding J, Zhang G, Zeng X, Yang Q, Gao W, 2021. Microfiber pollution: an ongoing major environmental issue related to the sustainable development of textile and clothing industry. Environ Dev Sustain 23:11240-56. [Google Scholar]
- Liu J, Zhu B, An L, Ding J, Xu Y, 2023. Atmospheric microfibers dominated by natural and regenerated cellulosic fibers: explanations from the textile engineering perspective. Environ Pollut 317:120771. [DOI] [PubMed] [Google Scholar]
- Lusher AL, Welden NA, Sobral P, Cole M, 2017. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Methods 9:1346-60. [Google Scholar]
- Mancuso M, Savoca S, Bottari T, 2019. First record of microplastics ingestion by European hake Merluccius merluccius from the Tyrrhenian Sicilian coast (Central Mediterranean Sea). J Fish Biol 94:517-9. [DOI] [PubMed] [Google Scholar]
- Miccoli A, Mancini E, Saraceni PR, Della Ventura G, Scapigliati G, Picchietti S, 2022. First evidence of in vitro cytotoxic effects of marine microlitter on Merluccius merluccius and Mullus barbatus, two Mediterranean commercial fish species. Sci Total Environ 813:152618. [DOI] [PubMed] [Google Scholar]
- Mistri M, Sfriso AA, Casoni E, Nicoli M, Vaccaro C, Munari C, 2022. Microplastic accumulation in commercial fish from the Adriatic Sea. Mar Pollut Bull 174:113279. [DOI] [PubMed] [Google Scholar]
- Muns-Pujadas L, Dallarés S, Constenla M, Padrós F, Carreras-Colom E, Grelaud M, Soler-Membrives A, 2023. Revealing the capability of the European hake to cope with micro-litter environmental exposure and its inferred potential health impact in the NW Mediterranean Sea. Mar Environ Res 186:105921. [DOI] [PubMed] [Google Scholar]
- Rathinamoorthy R, Balasaraswathi SR, 2022a. Microfiber shedding from textile materials other than apparels. In: Rathinamoorthy R, Balasaraswathi SR, eds. Microfiber pollution. Springer Nature, Berlin, Germany, pp 52-67. [Google Scholar]
- Rathinamoorthy R, Balasaraswathi SR, 2022b. Impact of coronavirus pandemic litters on microfiber pollution - effect of personal protective equipment and disposable face masks. Int J Environ Sci Technol 20:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Romeu O, Constenla M, Carrassón M, Campoy-Quiles M, Soler-Membrives A, 2020. Are anthropogenic fibres a real problem for red mullets (Mullus barbatus) from the NW Mediterranean?. Sci Total Environ 733:139336. [DOI] [PubMed] [Google Scholar]
- Santonicola S, Volgare M, Cocca M, Dorigato G, Giaccone V, Colavita G, 2023. Impact of fibrous microplastic pollution on commercial seafood and consumer health: a review. Animals 13:1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santonicola S, Volgare M, Di Pace E, Mercogliano R, Cocca M, Raimo G, Colavita G, 2023. Research and characterization of fibrous microplastics and natural microfibers in pelagic and benthic fish species of commercial interest. Ital J Food Saf 12:11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca S, Capillo G, Mancuso M, Faggio C, Panarello G, Crupi R, Spanò N, 2019. Detection of artificial cellulose microfibers in Boops boops from the northern coasts of Sicily (Central Mediterranean). Sci Total Environ 691:455-65. [DOI] [PubMed] [Google Scholar]
- Stanton T, Johnson M, Nathanail P, MacNaughtan W, Gomes RL, 2019. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci Total Environ 666:377-89 [DOI] [PubMed] [Google Scholar]
- Suaria G, Achtypi A, Perold V, Lee JR, Pierucci A, Bornman TG, Aliani S, Ryan PG, 2020. Microfibers in oceanic surface waters: a global characterization. Sci Adv 6:eaay8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgare M, Santonicola S, Cocca M, Avolio R, Castaldo R, Errico ME, Gentile G, Raimo G, Gasperi M, Colavita G, 2022. A versatile approach to evaluate the occurrence of microfibers in mussels Mytilus galloprovincialis. Sci Rep 12:21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhu J, Zuo R, Xu Q, Qian Y, Lihui AN, 2023. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci Total Environ 856:159060. [DOI] [PubMed] [Google Scholar]


