Abstract
Introduction
Empowering people living with multimorbidity (multiple chronic conditions) to gain greater confidence in managing their health can enhance their quality of life. Education focused on self-management is a key tool for fostering patient empowerment and is mostly provided on an individual basis. Virtual communities of practice (VCoP) present a unique opportunity for online education in chronic condition self-management within a social context. This research aims to evaluate the effectiveness/cost-effectiveness of individualised, online self-management education compared with VCoP among middle-aged individuals living with multiple chronic conditions.
Methods and analysis
People aged 30–60, living with ≥2 chronic conditions and receiving care in primary care (PC) centres and outpatient hospital-based clinics in Madrid and Canary Islands will enrol in an 18-month parallel-design, blinded (intervention assessment and data analysts), pragmatic (adhering to the intention-to-treat principle), individually randomised trial. The trial will compare two 12-month web-based educational offers of identical content; one delivered individually (control) and the other with online social interaction (VCoP, intervention). Using repeated measures mixed linear models, with the patient as random effect and allocation groups and time per group as fixed effects, we will estimate between-arm differences in the change in Patient Activation Measure from baseline to 12 months (primary endpoint), including measurements at 6-month and 18-month follow-up. Other outcomes will include measures of depression and anxiety, treatment burden, quality of life. In addition to a process evaluation of the VCoP, we will conduct an economic evaluation estimating the relative cost-effectiveness of the VCoP from the perspectives of both the National Health System and the Community.
Ethics and dissemination
The trial was approved by Clinical Research Ethics Committees of Gregorio Marañón University Hospital in Madrid/Nuestra Señora Candelaria University Hospital in Santa Cruz de Tenerife. The results will be disseminated through workshops, policy briefs, peer-reviewed publications and local/international conferences.
Trial registration number
Keywords: Self-Management, Chronic Disease, Clinical Trial, Community-Based Participatory Research, GENERAL MEDICINE (see Internal Medicine)
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Pragmatic, multicentre design enhances the generalisability of the findings.
Comprehensive measures, including patient activation, mental health and quality of life.
Longitudinal follow-up over 18 months to assess interventions’ sustained effects.
Restricted to internet-accessible participants, impacting representativeness.
Dependent on participants’ engagement willingness in online communities.
Introduction
Multimorbidity is defined as the simultaneous presence of two or more chronic conditions in the same individual.1 Multimorbidity is becoming increasingly prevalent globally.2 While the prevalence of multimorbidity tends to rise with age,2 it is worth noting that more than 50% of individuals living with multiple chronic diseases are under the age of 65.3–5
Irrespective of age, individuals with multimorbidity tend to have a lower quality of life,6 use more healthcare services7 and die younger8 than people living with no or one chronic condition. However, how multimorbidity affects daily life may differ between middle-aged and older people.
It is in the middle age when most chronic diseases first manifest. For middle-aged individuals with multimorbidity, the challenge lies in juggling the work of self-management with professional careers, childcare, eldercare and leisure.9 Healthcare research has not adequately addressed the consequences of multimorbidity, in terms of an individual’s capacity for self-care and the significant disruptions to family life, leisure, and community and professional commitments.10 11 Comprehensive, patient-centred strategies to address both medical and psychosocial aspects of care are urgently needed for middle-aged adults living with multimorbidity.12
Empowerment is the process by which individuals gain control over managing the conditions of their daily life. Empowered individuals take actions to enhance their quality of life and possess the necessary knowledge, skills, attitudes and self-perception to adapt their behaviour and collaborate with others when required to achieve optimal well-being.13 There is a need for effective interventions that promote empowerment, self-confidence, self-esteem and the ability to cope with the profound implications of multiple chronic diseases.
According to Wenger,14 a community of practice (CoP) is a group of individuals engaged in a common activity who develop a shared identity, deepen their knowledge and expand their experiences in a particular field through ongoing interactions that strengthen their relationships. A group of people sharing the common condition of multimorbidity may benefit from an intervention where they can interact, exchange knowledge, resources, information, and receive mutual and professional support.
Virtual communities of practice (VCoP) offer widespread access to information and opportunities for interaction among people facing similar situations, which is particularly valuable for individuals with chronic conditions. Unlike passive educational strategies, key benefits of VCoPs encompass receiving and providing information, offering social support, boosting patient optimism, improving coping skills, brightening mood, reducing anxiety and managing stress more effectively.15 16
Methods and analysis
Aim
The main objective of this study is to assess the effectiveness and cost-effectiveness of two online self-management programmes for chronic diseases. The first is delivered through a VCoP, fostering a community-based approach (intervention) while the second is provided on an individual basis (control). Other secondary objectives will be taken into account.
Trial design
We will conduct an 18-month, pragmatic, multicentre, parallel, randomised controlled trial. See online supplemental additional file 1 for Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist.
bmjopen-2024-084937supp001.pdf (3.5MB, pdf)
Study setting
Both groups will receive the intervention online as an add-on to their usual care at PC practices and outpatient hospital-based clinics in Madrid and the Canary Islands, Spain.
Eligibility criteria and study population
Patients aged 30–60 and diagnosed with two or more chronic conditions will be identified by their healthcare providers (HCPs) (PC and hospital physicians and nurses) and proposed to be screened by the research team for the following eligibility criteria:
Inclusion criteria
Age 30–60 years.
Documentation of at least two chronic diseases in the electronic medical record at the time of inclusion.
Access to the internet at home or via a smartphone.
Ability to meet the study requirements (eg, digital literacy questionnaire (online supplemental additional file 2 shows this in more details)).
Signed, written, informed consent.
bmjopen-2024-084937supp002.pdf (36.3KB, pdf)
Exclusion criteria
Institutionalised individuals.
Receiving palliative care.
Telephone/email contact information missing from clinic databases.
Recruitment and implementation strategies for HCPs in Madrid and Canary Islands
Recruitment process
HCPs from Madrid and the Canary Islands will be invited to participate in recruiting subjects for the study. To facilitate this process, the research team will conduct informative sessions with HCPs, including nurses and physicians from outpatient clinics and PC centres. These sessions will focus on detailing the project’s objectives, outlining specific recruitment guidelines and describing the responsibilities involved. Interested HCPs will then approach eligible patients, based on predefined inclusion criteria, to introduce them to the study’s aims and requirements.
Patient engagement and information dissemination
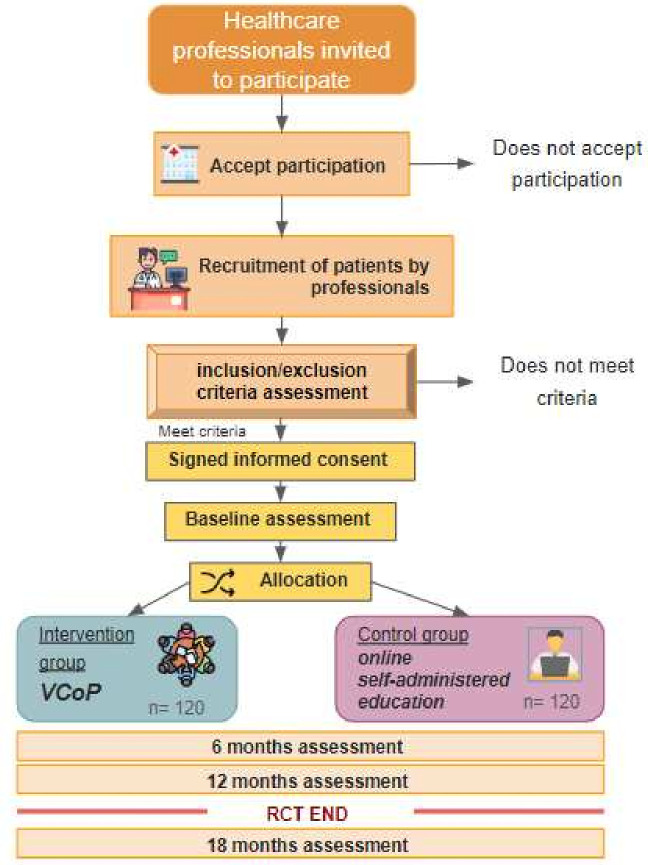
Patients expressing interest in the study will be contacted by a member of the research team. This step involves providing comprehensive information about the study, addressing any queries and assessing the patients’ familiarity with computer and internet usage. Following this, patients will gain access to a specialised web platform, designed exclusively for this project. This platform houses the informed consent document, which participants are required to understand and sign before proceeding. Subsequently, participants will complete baseline questionnaires, after which they will receive a 1-year access to their assigned implementation strategy. For data management, the patient ID will be anonymised. The study’s flow chart can be found in figure 1.
Figure 1.
Implementation strategies flow chart. RCT, randomised controlled trial; VCoP, virtual community of practice.
Implementation strategies overview
To define the interventions, we used the taxonomy of self-management interventions for chronic diseases developed by Orrego et al 17
-
Intervention group—‘e-mpoderaT’ Platform:
Platform features: A gamified virtual community of practice (VCoP), hosted on a Web 2.0 platform, will encourage the sharing of experiences and knowledge through collective learning.16 The platform will provide diverse educational and interactive content, including forums, readings, resources, videos, games and virtual sessions, all aimed at enhancing self-care and promoting knowledge exchange.
Customisation and support: Tailored to address the unique needs of people with multimorbidity, this intervention will be cocreated with patients and HCPs, leading to the development of a ‘Patient Journey Map’. A healthcare professional experienced in facilitating patient groups will moderate the VCoP, ensuring active engagement, addressing queries and fostering communication with a multidisciplinary team of experts, including general practitioners, cardiologists, psychologists and nutritionists.
Educational focus: The content emphasises patient empowerment dimensions such as health competence, behavioural change, symptom monitoring and shared decision-making, aligning with European guidelines for managing chronic diseases.16
-
Control group—standard care with educational access:
Usual care and educational resources: Participants in the control group will continue receiving standard care in line with local guidelines. Additionally, they will have access to a self-administered platform featuring the same educational content as the VCoP, minus the interactive and engagement components.
Table 1 summarizes the implementation strategy.
Table 1.
Implementation strategy
| Intervention components | Intervention group ‘Virtual community of practice’ |
Active control ‘Self-administered education’ |
| Provisioning and support methods | Provision of information Skills training Emotional support Proposal of objectives and action plans Training in self-monitoring of symptoms and monitoring of healthy behaviours Using reminders Social support by peers and professionals (key to the intervention) |
Provision of information Using reminders |
| Type of encounters | Support sessions | Self-administered intervention |
| Support modality | Remote (web based) | Remote (web based) |
| Type of platform | Web platform compatible with mobile devices | Web platform compatible with mobile devices |
| Type of communication | Synchronous (webinar-type activities, virtual meetings) and asynchronous (web) | Asynchronous (web) |
| Recipients | In a group | Individual |
| Type of providers interacting with patients | Professionals in primary and specialised care medicine, nursing, psychology. | There is no interaction with patients. |
| Setting | Primary care patients | Primary care patients |
| Content topics (examples) | Healthy life habits Clinical management of pathologies (symptom management, pathology adherence) Emotional and stress management Social management (job compatibility, social roles) |
Healthy life habits Clinical management of pathologies (symptom management, pathology adherence) Emotional and stress management Social management (job compatibility, social roles) |
| Outcomes measured | Activation, anxiety and depression, disease burden, quality of life, resource use | Activation, anxiety and depression, disease burden, quality of life, resource use |
| Type of patients | Middle-aged people with multimorbidity | Middle-aged people with multimorbidity |
| Content development | Codesigned. A multidisciplinary group of professionals prepares and reviews the contents. New contents according to the dynamics of participation and the needs of the group that participates in the community | Co-designed. A multidisciplinary group of professionals prepares and reviews the contents. |
Source: Based on the Template for intervention Description and Replication (TIDieR) guide (https://doi.org/10.1136/bmj.g1687) and Taxonomy of self‐management interventions for chronic conditions.16
Control
Description of materials and outcome measures
Primary outcome
The primary outcome will be the level of patient activation, assessed using the Patient Activation Measure (PAM) questionnaire.18 19 Higher levels of patient activation, as measured by the PAM, are linked to greater patient satisfaction, better quality of life, and enhanced physical and mental functional status.18 This questionnaire consists of 13 items that evaluate knowledge, skills and confidence for self-care in patients with chronic conditions. Responses are measured on a Likert 1–4-point scale, resulting in a total score ranging from 0 to 100, with 100 indicating the highest level of patient activation. The Spanish-translated version has been validated in patients with chronic diseases and exhibits good validity and reliability properties.20 The e-mpoderaT research team has previously employed this questionnaire in their studies (e-mpodera21 and e-mpodera222).
Secondary outcomes
Depression: The Patient Health Questionnaire-923 will be used to detect depression, characterise its severity24 and support follow-up.25 Validated in Spanish,26 it consists of nine items that assess the presence of depressive symptoms in the last 2 weeks. Each item has a severity index: 0=‘never’, 1=‘some days’, 2=‘more than half the days’ and 3=‘almost every day’. A score between 0 and 4 indicates no depressive symptoms, 5–9 mild depressive symptoms, 10–14 moderate depressive symptoms, 15–19 moderately severe depressive symptoms and 20–27 severe depressive symptoms.
Anxiety: The self-administered Hospital Anxiety and Depression Scale subscale27 is a seven-item questionnaire, validated in Spanish and used in PC.28–30 Items are scored from 0 to 3, with a score of 8 indicating possible and >10 probable anxiety with good specificity and predictive value.31
Mutimorbidity Treatment Burden (MTB): Based on the self-administered Treatment Burden Questionnaire.32 A 10-item version was validated in PC in the UK in patients with multimorbidity.33 It uses a Likert scale that ranges from 0 (not difficult/does not apply) to 4 (extremely difficult) to assess the burden related to taking medication, self-care, medical appointments and the need for organisation. We will translate and adapt the MTB Questionnaire using the forward and back-translation procedure.
Health-related quality of life: We will assess this construct using the self-administered EuroQol-5Dimension-5Level (EQ-5D-5L)34 validated in Spanish and used in PC.35 It enables the calculation of quality-adjusted life-years (QALYs). The EQ-5D-5L descriptive system comprises five dimensions (mobility, personal care, daily activities, pain/discomfort and anxiety/depression).
Explanatory and adjustment variables
Sociodemographic: Age (years), gender, nationality, whether they live in Madrid or Canarias, marital status (married/partner, single, separated, divorced, widowed), the number of living children, whether they have caregiving duties for parents (yes/no), educational level (incomplete primary studies, complete primary studies, secondary education, university studies or equivalent) and current occupation (ie, unemployed, employed, self-employed, sick leave and another situation).
Morbidity: Number and description of concomitant chronic diseases. This information will be collected by collaborating professionals coinciding with the baseline evaluation of each patient. An additional file of the O’Halloran list shows this in more detail (see online supplemental additional file 3).36
Treatment: We will record the quantity and details of medications prescribed for long term (ie, at least 3 months), continuous use for each patient. This information will be meticulously collected by our team of collaborating HCPs at the time of each patient’s baseline assessment, ensuring accurate and comprehensive medication data.
Use of resources: PC visits, visits to the emergency department, visits to specialists, the number of hospitalisations, lengths of stay.
Loss of productivity: Self-administered questionnaire about work absences related to the illness.
Use of VCoP: VCoP use data will be collected through the platform database.
Unintended consequences of the interventions will be monitored along the duration of the study.37
bmjopen-2024-084937supp003.pdf (234KB, pdf)
All the outcome measures will be collected online from a patient self-reported questionnaire that the research team will elaborate. VCoP use data will be collected through the platform database.
See table 2 for more details.
Table 2.
Trial outcomes
| Variables | Name | Type of variable | Measures |
| Primary | Patient Activation Measure | Ordinal qualitative | Likert scale: 0–100, where 100 indicates highest level of activation |
| Secondary | Patient Health Questionnaire | Ordinal qualitative | Likert scale: Depression intervals: 0–4, 5–9, 10–14, 15–19, 20–27 |
| Hospital Anxiety and Depression Scale: Subscale of Anxiety | Ordinal qualitative | Likert scale: Scored each item 0–3. ≥8 indicates possible cases | |
| Treatment Burden Questionnaire | Ordinal qualitative | Likert scale: 0–20, where 20 indicates significant problem | |
| Health Related Quality of Life | Ordinal qualitative | Likert scale: never-very often | |
| Sociodemographic | Age | Discrete quantitative | years |
| Sex (gender) | Categorical qualitative | 4 categories: 1-male, 2- female, 3-other, 4-refused to answer | |
| Nationality | Nominal | Open question | |
| Autonomous community of residence | Categorical qualitative | 2 categories: 1-Madrid, 2-Canary Islands | |
| Marital status | Categorical qualitative | 5 categories: 1-married/partner, 2-single, 3-separated, 4-divorced, 5-widowed | |
| Have children | Dichotomous qualitative | Yes/no | |
| Number of children | Discrete | Open question (number/units) | |
| Caring parents | Dichotomous qualitative | Yes/no | |
| Educational level | Categorical qualitative | 4 categories: 1-incomplete primary studies, 2-complete primary studies, 3-secondary education, 4-university studies or equivalent | |
| Current occupation | Nominal | Open question | |
| Multimorbidity | Number and description of concomitant chronic diseases | Discrete/nominal | O’Halloran list |
| Treatment | Number and description of chronic treatments | Discrete/nominal | Open question (number of treatments in electronic medical record) |
Timeline
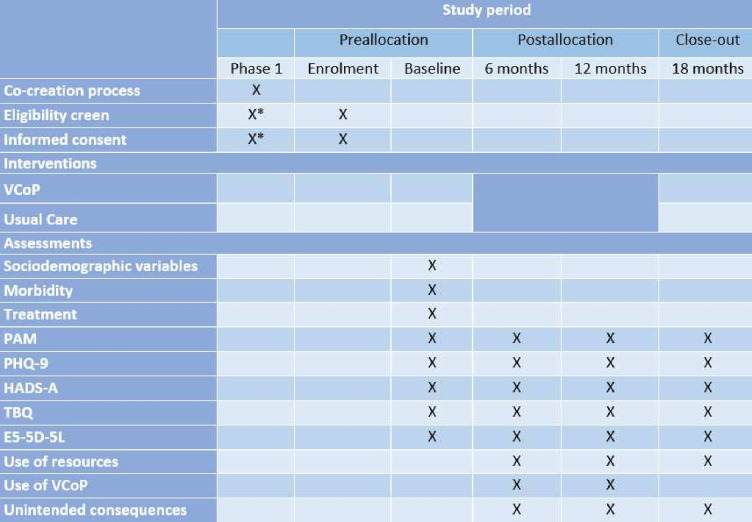
The primary outcome of our study (PAM) will be evaluated over a period of 12 months, starting from baseline. To ensure a thorough understanding of the PAM’s progression, we will also conduct additional assessments at 6 and 18 months. Secondary outcome measures will be collected before the start of the VCoP intervention and at 6, 12 and 18 months. This information is shown in more details in figure 2.
Figure 2.
Schedule of enrolment, interventions and assessments. *The eligibility screen and informed consent of the co-creation phase are like the RCT phase. EQ-5D-5L, EuroQol 5 Dimension 5 Level; HADS, Hospital Anxiety and Depression Scale; PAM, Patient Activation Measure; PHQ -9, Patient Health Questionnaire; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials; TBQ, Treatment Burden Questionnaire; VCoP, Virtual Community of Practice. RCT, randomised controlled trial.
Data monitoring
The data will be monitored by the research team throughout the research process. Special attention will be paid to their quality and their correct collection. Primary analyses will be conducted following completion of the 6, 12 and 18 months assessment questionnaires.
Randomisation and blinding
The STATA V.17.0 software will generate a random sequence used by an investigator to allocate participants to different platform groups and notify them via email after they have been provided written consent. The intervention allocation will be blinded to participants, clinicians and data analysts.
Statistical analysis
Sociodemographic and clinical baseline variables of both groups will be analysed by descriptive methods according to the type of variable (mean (SD), median (range), n (%)). The VCoP effect on the primary and secondary outcomes will be examined by means of repeated measures mixed linear models, with the intervention, time point (0, 6, 12 and 18 months) and their interaction as fixed effects (along with other potential covariates), random intercepts for patients and clinicians, and unstructured covariance to account for within-subject correlations. We will also analyse the three-way interaction intervention×time×centre since usual care could vary between centres, leading to differential intervention effects. We expect to recruit enough clinicians to allow their inclusion in the model as a random intercept, but we will perform a sensitivity analysis as well as excluding this component. Between-group differences at each time point will be compared by means of Wald’s χ2 test. We will perform the analyses on an intention-to-treat basis (a sensitivity analysis on the per-protocol population will also be performed). Multiple imputations will be used for missing data, if applicable (Markov Chain Monte Carlo multivariate imputation algorithm, with 10 imputations per variable). Analyses will be carried out with the statistical software R V.4.0.2 (http://www.R-project.org/).
We will conduct a cost-effectiveness analysis of the VCoP over 18 months, comparing it to standard care with educational access for middle-aged patients with multimorbidity. This analysis will include both direct healthcare costs and indirect costs like productivity losses. Costs for each patient will be calculated using healthcare resources and the indirect costs will be assessed based on productivity impacts. The study will also include the initial costs of developing and implementing the VCoP, and any costs incurred during the follow-up period.
The primary measure will be the cost per QALY gained. We will derive QALY estimates from the EQ-5D-5L questionnaire completed by patients at the study’s start and each follow-up. The results will be presented as the incremental cost-effectiveness ratio (ICER), which compares cost and health outcomes differences between the VCoP and standard care. We will use robust statistical methods to ensure reliable ICER estimates and conduct sensitivity analyses to evaluate the effects of various factors on the results. The analysis will help determine whether the VCoP is a cost-effective option within our health system.
Sample size
To detect a mean difference of 4 points (SD=10) in the PAM between the intervention and control groups, with individual randomisation, 100 patients per group are required. This threshold of 4 points (SD=10) was selected to capture clinically meaningful changes in patient activation.18 For this calculation, an alpha error of 0.05 and a power of 80% are assumed. This size is increased by the estimate of 20% loss, making a total of 240 patients.
Patient and public involvement
This protocol was developed without patient or public involvement. A group of middle-aged patients with multimorbidity will actively participate in a content-design previous stage using a cocreation methodology with virtual activities.
Ethics and dissemination
Informed consent (online supplemental file 4) will be obtained from each participant before randomisation. The project has been approved by the local Ethics Committees of each participating Autonomous Community: Clinical Research Ethics Committee of Gregorio Marañón University Hospital in Madrid (PI22/01124) and Nuestra Señora de Candelaria University Hospital in Santa Cruz de Tenerife (CHUNSC_2023_06) (online supplemental files 5 and 6). Patients will be personally informed by their physicians or nurses about the study and the possibility to participate during a programmed consultation. They will receive written information of the proposed research project, regarding its aims, the duration of their involvement, the expected benefits for them and the procedures involved in the participation. Recruiters will emphasise that enrolment in the study is voluntary, that participants can withdraw at any moment of the project, and that any decision they take in this respect will have no bearing on the medical care received. Once patients have signed the written informed consent, a researcher from the ‘e-mpoderaT’ team will contact them via phone and/or email to provide further information along with the necessary data (username and password) to login into the online platform. Additionally, recruiters will highlight that information generated by the study will be published, but no identification details will be divulged. Patients and healthcare professionals will be informed of whom to contact in case of any query, and research staff will be available to answer questions. We will prepare presentations to disseminate the study findings to healthcare stakeholders and patients, and at relevant national and international conferences. We aim to publish the results of the trial in peer-reviewed journals and try to grant public access to the full manuscripts.
bmjopen-2024-084937supp004.pdf (100.2KB, pdf)
bmjopen-2024-084937supp005.pdf (75.4KB, pdf)
Trial status
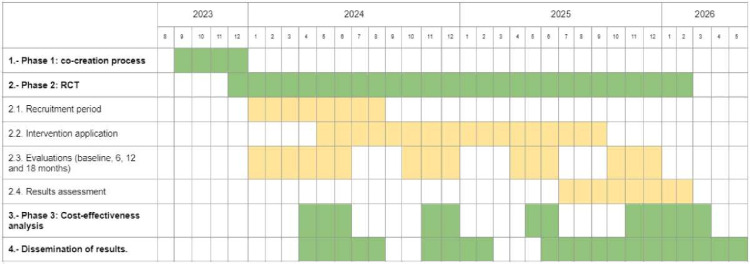
The recruitment of patients in each region will start in January–February 2024. The estimated end date of the recruitment for this study is June 2024. This information is shown in more detail in figure 3.
Figure 3.
Project timeline. RCT, randomised controlled trial.
Supplementary Material
Acknowledgments
Avedis Donabedian Research Institute has been actively engaged in this area of research right from the beginning. We sincerely appreciate their immense efforts and support, as this research would not be possible without their valuable contributions.
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the projects "PI22/01124" and "PI22/00691" and co-funded by the European Union.
Footnotes
AC, IG-L, VR-G and DK contributed equally.
Contributors: VR-G, DK, JB-C, SG-E, HV-R, AD-D, CMartín, MvdA, CO, LP-P and AIG-G contributed to the design of the study. AIG-G and LP-P are the guarantors. AC and IG-L wrote the first draft of the manuscript. VR-G, DK, AS-Á, JB-C, SG-R, AC-L, PC, CC-S, SD-C, FJG-G, SG-E, BGdL, JR-V, CMartín, CS-F, PP-C, EFV-R, PQ-C, ABR-P, MR-L, M-ET-B, ES-G, BU-A, HV-R, AD-D, AA-S, AH-Y, AT-C, YA-P, CMuth, MvdA, VMM, CO, LP-P and AIG-G contributed to the manuscript. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by Instituto de Salud Carlos III (ISCIII), grant number PI22/01124 and PI22/00691 and cofunded by the European Union. Funding has been provided as well from the RICORS, code RD21/0016/00028 (Redes de Investigación Cooperativa Orientadas a Resultados en Salud) networks.
Disclaimer: The funders have no role in the study design.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract Enero De 1996;2:65–70. 10.3109/13814789609162146 [DOI] [Google Scholar]
- 2. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 3. Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS ONE 2014;9:e102149. 10.1371/journal.pone.0102149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, et al. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014;67:254–66. 10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 5. Nicholson K, Terry AL, Fortin M, et al. Prevalence, characteristics, and patterns of patients with multimorbidity in primary care: a retrospective cohort analysis in Canada. Br J Gen Pract 2019;69:e647–56. 10.3399/bjgp19X704657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. N’Goran AA, Déruaz-Luyet A, Haller DM, et al. Comparing the self-perceived quality of life of multimorbid patients and the general population using the EQ-5D-3L. PLOS ONE 2017;12:e0188499. 10.1371/journal.pone.0188499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopman P, Heins MJ, Rijken M, et al. Health care utilization of patients with multiple chronic diseases in the Netherlands: differences and underlying factors. Eur J Intern Med 2015;26:190–6. 10.1016/j.ejim.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 8. Willadsen TG, Siersma V, Nicolaisdóttir DR, et al. Multimorbidity and mortality: a 15-year longitudinal registry-based nationwide Danish population study. J Comorb 2018;8. 10.1177/2235042X18804063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ko D, Bratzke LC, Roberts T. Self-management assessment in multiple chronic conditions: a narrative review of literature. Int J Nurs Stud 2018;83:83–90. 10.1016/j.ijnurstu.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 10. Lachman ME, Teshale S, Agrigoroaei S. Midlife as a pivotal period in the life course: balancing growth and decline at the crossroads of youth and old age. Int J Behav Dev. enero de 2015;39:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leppin A, Montori V, Gionfriddo M. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare n.d.;3:50–63. 10.3390/healthcare3010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dinh TS, Brünn R, Schwarz C, et al. How do middle-aged patients and their healthcare providers manage multimorbidity? Results of a qualitative study. PLOS ONE 2023;18:e0291065. 10.1371/journal.pone.0291065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. EMPATHiE Consortium . Final summary report: Empathie, empowering patients in the management of chronic diseases. 2014.
- 14. Wenger E. Communities of practice and social learning systems. Organization 2000;7:225–46. 10.1177/135050840072002 [DOI] [Google Scholar]
- 15. Rodgers S, Chen Q. Internet community group participation: psychosocial benefits for women with breast cancer. J Comput Mediat Commun 2005;10. 10.1111/j.1083-6101.2005.tb00268.x [DOI] [Google Scholar]
- 16. Koatz D, Torres-Castaño A, Salrach-Arnau C, et al. Exploring value creation in a virtual community of practice: a framework analysis for knowledge and skills development among primary care professionals. BMC Med Educ 2024;24:121. 10.1186/s12909-024-05061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orrego C, Ballester M, Heymans M, et al. Talking the same language on patient empowerment: development and content validation of a taxonomy of self‐management interventions for chronic conditions. Health Expect 2021;24:1626–38. 10.1111/hex.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions J Ambul Care Manage 2007;30:21–9. 10.1097/00004479-200701000-00005 [DOI] [PubMed] [Google Scholar]
- 19. Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; delivery systems should know their patients scores. Health Affairs 2013;32:216–22:216. 10.1377/hlthaff.2012.1064 [DOI] [PubMed] [Google Scholar]
- 20. Moreno-Chico C, González-de Paz L, Monforte-Royo C, et al. Adaptation to European Spanish and psychometric properties of the patient activation measure 13 in patients with chronic diseases. Fam Pract 2017;34:627–34. 10.1093/fampra/cmx022 [DOI] [PubMed] [Google Scholar]
- 21. Orrego C, Perestelo-Pérez L, González-González AI, et al. A virtual community of practice to improve primary health care professionals’ attitudes toward patient empowerment (e-MPODERA): A cluster randomized trial. Ann Fam Med 2022;20:204–10. 10.1370/afm.2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koatz D, Torres Castaño A, Ramos García V, et al. A virtual community of practice (VCoP) for people with ischemic heart disease: the implementation process. Int J Integr Care n.d.;22:404. 10.5334/ijic.ICIC22208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 24. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Löwe B, Kroenke K, Herzog W, et al. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the patient health questionnaire (PHQ-9). J Affect Disord 2004;81:61–6. 10.1016/S0165-0327(03)00198-8 [DOI] [PubMed] [Google Scholar]
- 26. Diez-Quevedo C, Rangil T, Sanchez-Planell L, et al. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients: psychosom MED. Psychosomatic Medicine 2001;63:679–86. 10.1097/00006842-200107000-00021 10.1097/00006842-200107000-00021 [DOI] [PubMed] [Google Scholar]
- 27. Quintana JM, Padierna A, Esteban C, et al. Evaluation of the psychometric characteristics of the Spanish version of the hospital anxiety and depression scale. Acta Psychiatr Scand 2003;107:216–21. 10.1034/j.1600-0447.2003.00062.x [DOI] [PubMed] [Google Scholar]
- 28. Herrero MJ, Blanch J, Peri JM, et al. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry 2003;25:277–83. 10.1016/s0163-8343(03)00043-4 [DOI] [PubMed] [Google Scholar]
- 29. Terol-Cantero MC, Cabrera-Perona V, Martín-Aragón M. Revisión de estudios de la escala de ansiedad y depresión hospitalaria (HAD) en Muestras Españolas. Analesps n.d.;31:494. 10.6018/analesps.31.2.172701 [DOI] [Google Scholar]
- 30. Moryś JM, Bellwon J, Adamczyk K, et al. Depression and anxiety in patients with coronary artery disease, measured by means of self-report measures and clinician-rated instrument. Kardiol Pol 2016;74:53–60. 10.5603/KP.a2015.0116 [DOI] [PubMed] [Google Scholar]
- 31. Bunevicius A, Staniute M, Brozaitiene J, et al. Screening for anxiety disorders in patients with coronary artery disease. Health Qual Life Outcomes 2013;11:37. 10.1186/1477-7525-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tran VT, Harrington M, Montori VM, et al. Adaptation and validation of the treatment burden questionnaire (TBQ) in English using an Internet platform. BMC Med 2014;12:109. 10.1186/1741-7015-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duncan P, Murphy M, Man M-S, et al. Development and validation of the multimorbidity treatment burden questionnaire (MTBQ). BMJ Open 2020;8:e019413. 10.1136/bmjopen-2017-019413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herdman M, Badia X, Berra S. El Euroqol-5D: una alternativa sencilla para la medición de la calidad de vida relacionada con la salud en atención primaria. Atención Primaria 2001;28:425–9. 10.1016/S0212-6567(01)70406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Halloran J, Miller GC, Britt H. Defining chronic conditions for primary care with ICPC-2. Fam Pract 2004;21:381–6. 10.1093/fampra/cmh407 [DOI] [PubMed] [Google Scholar]
- 37. Ziebland S, Hyde E, Powell J. Power, paradox and pessimism: on the unintended consequences of digital health technologies in primary care. Soc Sci Med 2021;289:114419. 10.1016/j.socscimed.2021.114419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-084937supp001.pdf (3.5MB, pdf)
bmjopen-2024-084937supp002.pdf (36.3KB, pdf)
bmjopen-2024-084937supp003.pdf (234KB, pdf)
bmjopen-2024-084937supp004.pdf (100.2KB, pdf)
bmjopen-2024-084937supp005.pdf (75.4KB, pdf)