Abstract
Practical relevance:
Many veterinary practices have invested in quality automated hematology instruments for use in-house. However, regardless of their specific choice of analyzer, there are important hematology findings that can only be determined by microscopic examination of stained blood films. For this reason, and also for the purpose of quality control for the analyzer, a quick blood film review should be performed alongside every automated complete blood count. Even those practices that submit their blood samples to outside diagnostic laboratories for evaluation, still require the capability to examine stained blood films in emergency situations.
Series outline:
This is the second of a two-part article series that aims to familiarize the practitioner with normal findings on feline blood films, with a particular focus on unique features in the cat, as well as to assist with interpretation of common abnormalities. Part 2 focuses on the morphology of feline leukocytes and platelets in health and disease.
Evidence base:
The information and guidance offered is based on the published literature and the author’s own extensive clinical pathology research.
Leukocyte morphology – normal and abnormal
Neutrophils
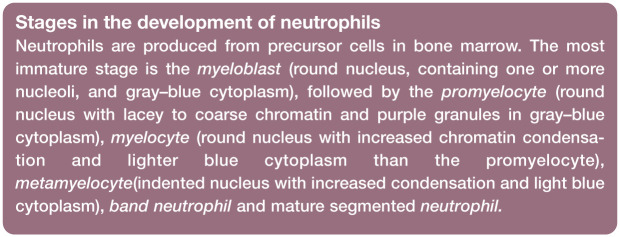
Cat neutrophils are similar in appearance to those of other domestic mammals. The chromatin of the nucleus is condensed (dark-staining clumped areas separated by lighter-staining areas) and segmented (lobulated), and stains purple to blue (Figure 1a). Nuclear lobes may be joined by fine filaments, but often there is simply a narrowing of the nucleus between lobes without true filament formation. The cytoplasm of neutrophils often appears colorless, but may appear pale pink or faintly blue.
Figure 1.

Morphology of feline neutrophils. (a) Normal neutrophil. (b) Band neutrophil. (c) Neutrophil from a female domestic medium hair cat. A Barr body (sex chromatin lobe or drumstick) is present (black arrow). Three nuclear vesicular appendages (blebs) are also present (one is marked by a red arrow); this cat was febrile and had suspected infection. Blood film courtesy of Matthew Williams. (d) Purple granules in the cytoplasm of an apparently healthy Siamese cat. Wright-Giemsa stain
Low numbers of band neutrophils may be observed in normal cats. Band neutrophils have nuclei with parallel sides and chromatin that is less condensed than that of mature neutrophils (Figure 1b). No area of the nucleus has a diameter less than two-thirds the diameter of any other area of the nucleus. Band nuclei often appear U- or S-shaped in blood films. Unless toxic, their cytoplasm resembles that of mature neutrophils. 1

A Barr body (sex chromatin lobe or drumstick) is present in a low percentage of mature neutrophils from females and contains the inactivated χ chromosome (Figure 1c). Vesicular appendages (blebs) have occasionally been recognized protruding from the nuclei of cat neutrophils (Figure 1c). 2 The significance of nuclear blebs in cats is unknown, but they have been seen in blood from a guinea pig after irradiation and in blood from humans with vitamin B12 deficiency, hematopoietic neoplasms, and after chemotherapy and irradiation.2,3
Granules
Neutrophil granules either do not stain or appear light pink in color with Romanowsky-type blood stains. Toxic granulation refers to the presence of magenta staining cytoplasmic granules, which consist of primary granules that have retained the staining intensity normally observed in promyelocytes in the bone marrow. The presence of toxic granulation and cytoplasmic basophilia suggests severe toxemia. Toxic granulation is most often seen in horses and is rarely seen in cats. It must be differentiated from reddish granulation in the cytoplasm of healthy Birman cats and in some healthy Siamese and Himalayan cats (Figure 1d).1,4

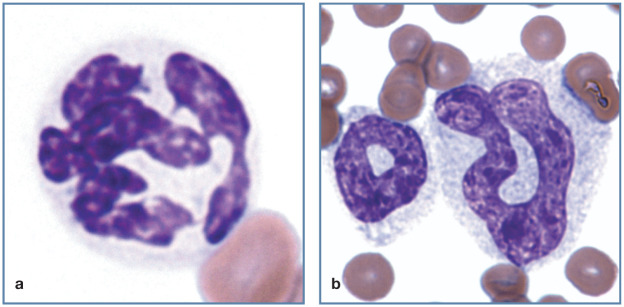
Similar blue-to-magenta staining granulation occurs in the cytoplasm of neutrophils from cats with certain inherited lysosomal storage disorders, including mucopolysaccharidosis types VI and VII, and GM2 gangliosidosis. 1 Exceptionally large granules are present in neutrophils from Persian cats with hereditary Chédiak-Higashi syndrome (Figure 2a). 5
Figure 2.
Morphology of feline neutrophilic cells. (a) Large granules in the cytoplasm of a Persian cat with Chédiak- Higashi syndrome. (b) Neutrophil with Döhle bodies as the only evidence of cytoplasmic toxicity. (c) Large toxic neutrophil with foamy basophilic cytoplasm containing multiple darker blue Döhle bodies. Reproduced from Harvey (2012), 1 with permission. (d) Band neutrophil with foamy basophilic cytoplasm containing Döhle bodies. Wright-Giemsa stain
Toxic cytoplasm
When the cytoplasm of a neutrophilic cell has increased basophilia, foamy vacuolation and/or contains Döhle bodies, it is said to be toxic (Figures 2b–d and 3). A semiquantitative method for recording toxic cytoplasm is given in Table 1 of Part 1. These morphologic abnormalities develop in neutrophilic cells within the bone marrow prior to their release into the circulation. 6 Toxic cytoplasm is primarily seen in association with strong inflammatory conditions. Nuclear abnormalities, including karyolysis, karyorrhexis, hyposegmentation, ring formation and binucleation, may also be present in neutrophils with toxic cytoplasm. 1 Toxic neutrophils are most often associated with bacterial infections (eg, pneumonia, peritonitis, septicemia); however, they may also be observed in viral infections (eg, parvovirus and upper respiratory viruses), and some severe metabolic disorders (eg, ketoacidotic diabetes and hepatic lipidosis). 7
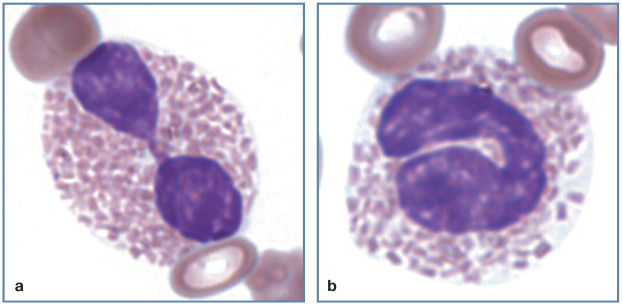
Figure 3.
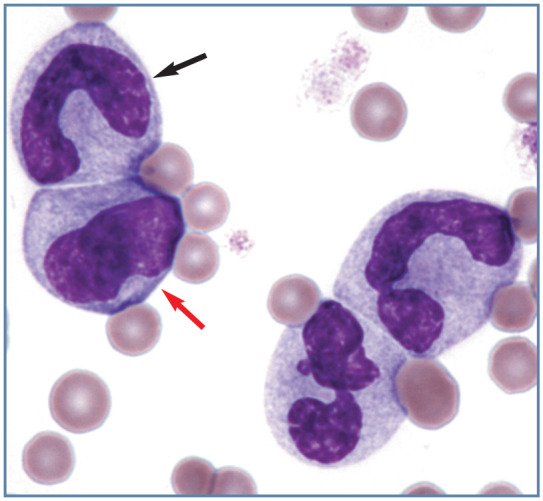
Toxic left shift in blood from a cat with pyothorax. A band neutrophil (black arrow), early neutrophilic metamyelocyte (red arrow) and two neutrophils exhibit markedly basophilic cytoplasm. Wright-Giemsa stain
A unique feature in cats is the presence of Döhle bodies in neutrophils from some apparently healthy animals (Figure 2b). Döhle bodies are bluish, angular cytoplasmic inclusions of neutrophils and their precursors. They are composed of retained aggregates of rough endoplasmic reticulum. By themselves, these inclusions are believed to represent evidence of mild toxicity. In other species they typically occur with more pronounced evidence of cytoplasmic toxicity (cytoplasmic basophilia and/or foamy cytoplasmic vacuolation). 1
Cytoplasmic toxicity is often present in cats with pronounced left shifts in blood, but can occur without a left shift. Neutrophil counts may be low, normal or high when toxic cells are present. 7
Left shift
In normal cats, mature neutrophils and, sometimes, low numbers of band neutrophils are released from bone marrow into the blood. The presence of increased numbers of non-segmented neutrophilic cells (see box) in the blood is referred to as a left shift. Increased numbers of band neutrophils are seen in blood (Figure 2d), with metamyelocytes and myelocytes present less often, and promyelocytes and myeloblasts rarely encountered (Figure 3). 1
Left shifts are usually associated with inflammatory conditions. These inflammatory conditions are often infectious, but they may be non-infectious. The term ‘leukemoid reaction’ is used when a marked neutrophilia with left shift back to at least myelocytes occurs in association with an inflammatory condition (Figure 4). 1 Leukemoid responses are generally associated with localized purulent inflammatory conditions. 8 Prominent left shifts are also present in animals with chronic myeloid leukemia and Pelger–Huët anomaly; however, Pelger–Huët anomaly is rare and chronic myeloid leukemia has not been clearly documented in cats.
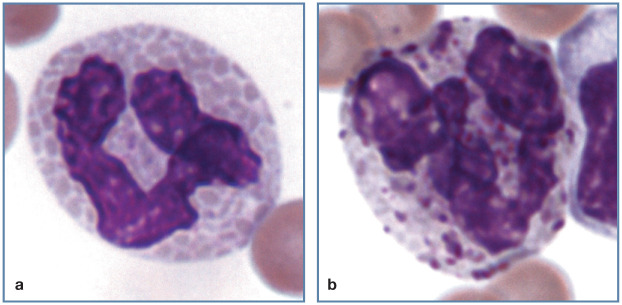
Figure 4.
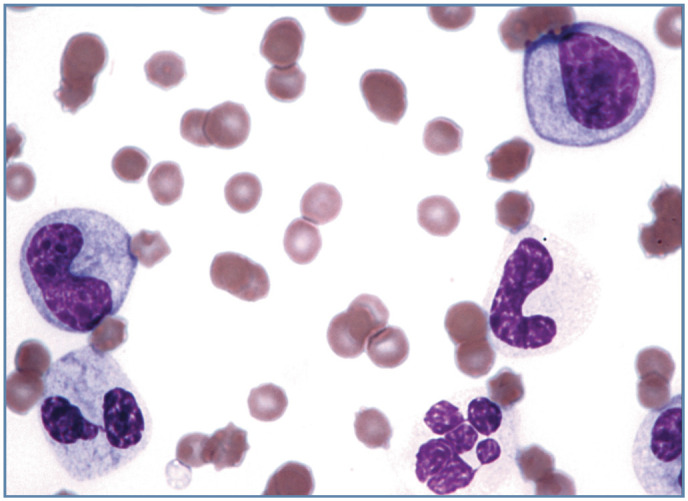
Toxic left shift in a cat with a leukemoid reaction (leukocyte count 101 × 106/l) resulting from a large draining abscess containing maggots. A toxic neutrophil (bottom left), toxic metamyelocyte (left), toxic myelocyte (top right), band neutrophil (right center) and neutrophil (bottom right) are present. Wright-Giemsa stain
Pelger–Huët anomaly
Cats (as well as dogs and horses) that are heterozygous for inherited Pelger-Huët anomaly exhibit hyposegmentation of neutrophilic cells. The cells have condensed nuclear chromatin with few or no nuclear constrictions (Figure 5). Nuclei may be round, oval, kidney, band, peanut or bilobed in shape. These heterozygous animals show no clinical signs associated with this disorder. Homozygous affected animals die in utero or shortly after birth.9,10 Pseudo-Pelger-Huët cells may occur transiently with infections (especially feline leukemia virus [FeLV]), in myeloid neoplasms or, occasionally, with chemotherapy.11,12
Figure 5.
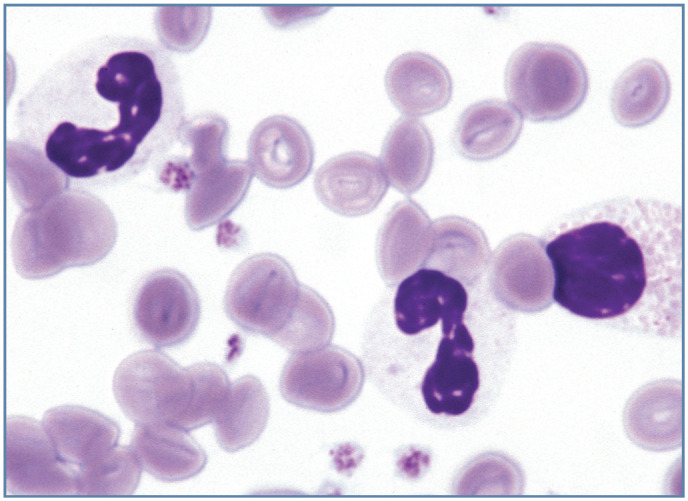
Band neutrophil (left), bilobed neutrophil (center) and eosinophilic myelocyte (right) in the blood of a cat with Pelger-Huët anomaly. Wright-Giemsa stain. Reproduced from Harvey (2012), 1 with permission
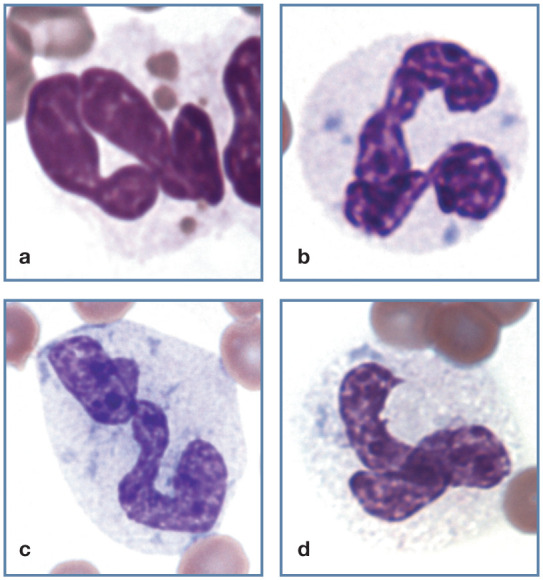
Hypersegmentation (right shift)
Hypersegmentation has generally been defined as the presence of five or more distinct nuclear lobes within a neutrophil (Figure 6a). Hypersegmentation occurs as a normal aging process and most often reflects prolonged transit time in blood, as can occur with resolving chronic inflammation, glucocorticoid administration or hyperadrenocorticism. 1 Hypersegmentation may also be present in myeloid neoplasms. 13 Neutrophilic hypersegmentation has been described in a cat with folate deficiency. 14
Figure 6.
Abnormal neutrophil morphology. (a) Hypersegmented neutrophil in blood from a cat with myelodysplastic syndrome. Reproduced from Harvey (2012), 13 with permission. (b) Toxic donut-shaped neutrophil and toxic giant band neutrophil in blood from a cat with pyothorax. Wright-Giemsa stain
Dysgranulopoiesis
Giant neutrophils with nuclear abnormalities are most often seen in cats with intense inflammatory diseases and/or dysgranulopoiesis (Figure 6b). They may exhibit normal nuclear morphology or appear hypersegmented. Dysgranulopoiesis is seen in acute myeloid leukemias, myelodysplastic syndromes, FeLV infections, and feline immunodeficiency virus (FIV) infections. Neutrophils with donut-shaped nuclei also appear to be more common in cats with intense inflammatory responses, as well as with myeloid neoplasms. 1
Acute myeloid leukemia
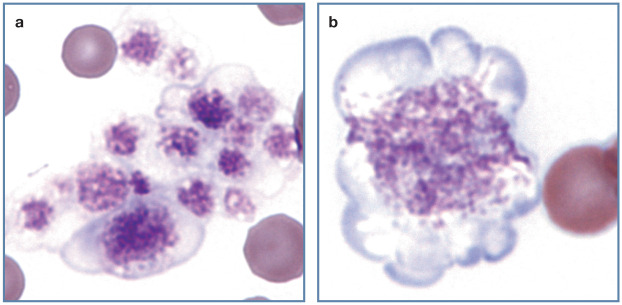
Myeloid neoplasms are characterized by the clonal proliferation of one or more of the nonlymphoid marrow cell lines (granulocytic, monocytic, erythrocytic or megakaryocytic) in bone marrow. In cats with acute myeloid leukemia, myeloblasts and other immature granulocytes may be present in blood, either alone (Figure 7), with monoblasts or with erythroblasts (Figure 8). 1
Figure 7.
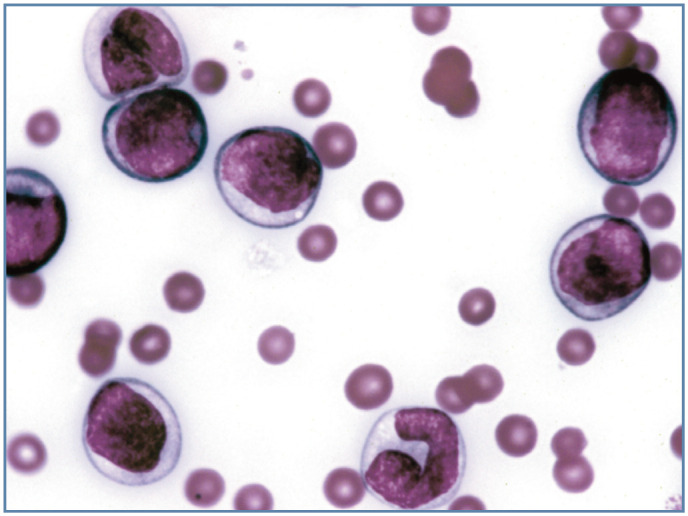
Acute myeloid leukemia in a cat. Most round cells (myeloblasts and myelocytes) were peroxidase positive. A band neutrophil (bottom center) is also present. Wright-Giemsa stain
Figure 8.
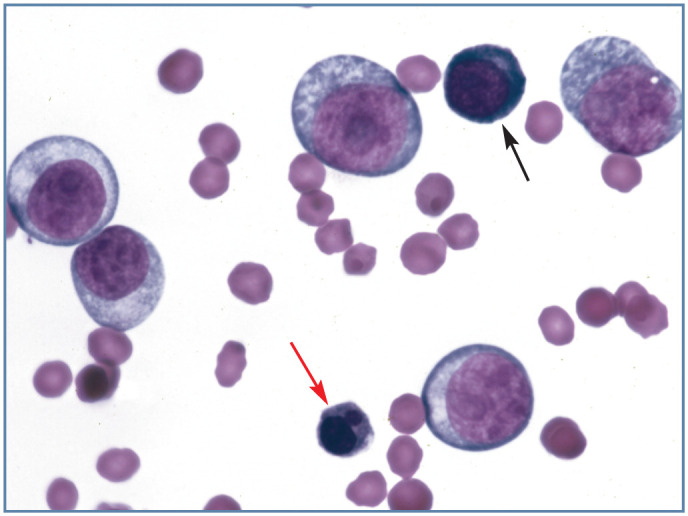
Blood from a cat with erythroleukemia (AML-M6). Large pale cells are predominantly myeloblasts. A basophilic rubriblast (black arrow) and a rubricyte with nuclear lobulation (red arrow) are also present. Wright-Giemsa stain
Infectious agents
Bacteria are rarely recognized in neutrophils present in feline blood films even though bacteremia is common in cats. Except for Mycobacterium species, bacterial rods and cocci stain blue with Romanowsky-type blood stains (Figure 9a). In contrast, Mycobacterium organisms appear as unstained rods within the cytoplasm (Figure 9b); with acid-fast stains these organisms stain red. 1 Hepatozoon felis gamonts may be seen in neutrophils and monocytes (Figure 9c). 15 This organism appears to cause subclinical disease in cats. 16 Leishmania organisms have also been reported in neutrophils from a cat (Figure 9d). 17
Figure 9.
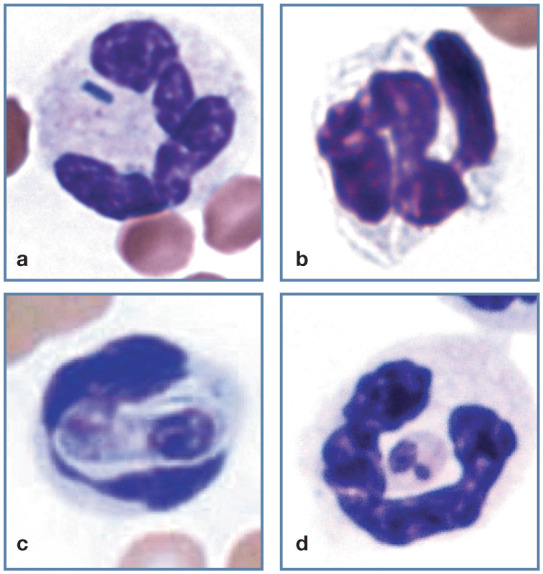
Feline neutrophils with phagocytized infectious organisms. (a) Bacterial rod in a neutrophil from a cat with a bacteremia. (b) Mycobacterium organisms in a neutrophil; these do not stain and appear as clear linear structures. (c) Hepatozoon felis gamont in a neutrophil. Reproduced from Lloret et al (2015), 15 with permission. Image courtesy of G Baneth. (d) Leishmania amastigote in a feline neutrophil. Reproduced from Harvey (2012), 1 with permission. Image courtesy of M Santos
Eosinophils
Eosinophils are so named because their granules have an affinity for eosin, the red dye in Romanowsky-type blood stains. In most animal species, eosinophils have round granules, but those from domestic cats have rod-shaped granules. The nucleus of eosinophils is similar to that of neutrophils, but tends to be less lobulated (often divided into only two lobes) (Figure 10a). Band eosinophils are common in some animals (Figure 10b); they are not usually separated from segmented eosinophils during differential counts because they are generally of little clinical significance. Like neutrophils, hyposegmentation of eosinophils is seen in cats with Pelger-Huët anomaly.
Figure 10.
Feline eosinophils containing rod-shaped granules. (a) Bilobed mature eosinophil. (b) Band eosinophil. Wright-Giemsa stain
Eosinophilia occurs in disorders that result in increased interleukin (IL)-5 production. It may accompany parasitic diseases, especially those caused by nematodes and flukes. Eosinophilia may also occur in association with eosinophilic inflammatory conditions of organs that normally contain numerous mast cells, such as skin, lung and intestine; and may be present in animals with IgE-mediated allergic hypersensitivity reactions, such as flea-bite allergies and feline asthma. Marked eosinophilia with extensive eosinophilic organ infiltrates in cats and humans has been classified as either chronic eosinophilic leukemia or hypereosinophilic syndrome. 1
Basophils
The cytoplasm of basophils is generally pale blue in color. The basophils of domestic cats are distinctive. Most of their granules are round or oval and stain light lavender (or mauve) in color (Figure 11a). Some basophils have purple granules in addition to the light lavender ones, as is seen in basophil precursors in the bone marrow (Figure 11b). The granules typically fill the cytoplasm, giving the nucleus of feline basophils a moth-eaten appearance. Band basophils are not usually separated from segmented basophils during differential counts because they are generally of little clinical significance, and they may be difficult to identify with certainty when granules obscure the nucleus.
Figure 11.
Feline basophils. (a) Basophil containing many light lavender granules in the cytoplasm. (b) Basophil with a mixture of light-lavender and purple granules. The pale granules overlying the nucleus give it a moth-eaten appearance. Wright-Giemsa stain
Basophils can be difficult to recognize in blood films prepared with aqueous stains, such as Diff-Quik, because granules do not stain as well with these stains. Basophilia is generally associated with immunoglobulin (Ig)E-mediated disorders. When present, basophilia usually accompanies eosinophilia. Basophilia may occur in some cats with mast cell tumors, primarily non-cutaneous types. 1
Lymphocytes
Most lymphocytes reside within lymphoid organs (lymph nodes, thymus, spleen and bone marrow), with only a small percentage circulating in the blood. T lymphocytes are most numerous, followed by B lymphocytes and natural killer (NK) cells.
Most lymphocytes are small to medium in size, but some large lymphocytes may be present (Figure 12a–c). They have high (>1.0) nuclear-cytoplasmic (N:C) ratios, with the highest ratios in small lymphocytes and the lowest ratios in large NK cells. The cytoplasm of unstimulated blood lymphocytes is usually pale blue in color. Granules are generally absent or low in number, unless the cell is a granular lymphocyte. Lymphocyte nuclei are usually round but may be oval or slightly indented. Nuclear chromatin varies from condensed and densely staining, to a pattern of light and dark staining areas, to lighter-staining nuclei with smooth chromatin. 1
Figure 12.
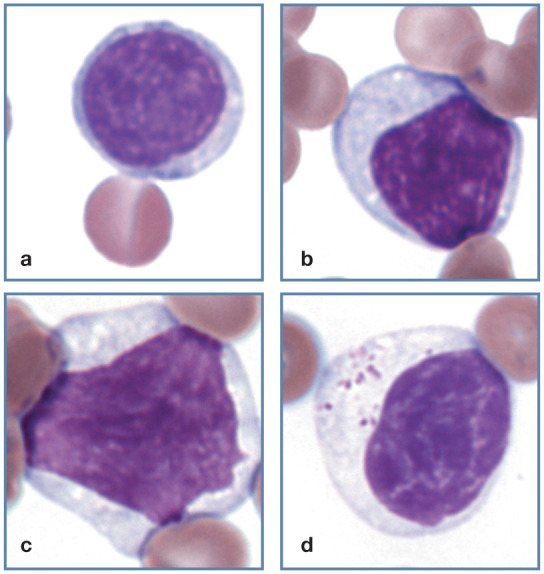
Normal lymphocyte morphology. (a) Small lymphocyte. (b) Medium-sized lymphocyte. (c) Large lymphocyte. (d) Granular lymphocyte. Wright-Giemsa stain
Granular lymphocytes
Like other domestic mammal species, a low percentage of cat lymphocytes in blood have focal accumulations of red or purple staining granules within the cytoplasm (Figure 12d). These granular lymphocytes may be cytotoxic T lymphocytes or NK cells. 1 Increased numbers of granular lymphocytes may occur during acute FIV infections. 18 Lymphocytes with exceptionally large magenta staining granules may be seen in the blood and bone marrow of cats with metastatic large granular lymphomas (Figure 13). Most of these large granular lymphomas appear to originate as intestinal tumors composed of cytotoxic T lymphocytes. This neoplasm is not associated with FeLV infection. 19 Basophilic granules may be seen in the lymphocytes of cats with inherited mucopolysaccharidosis types VI and VII. 1
Figure 13.
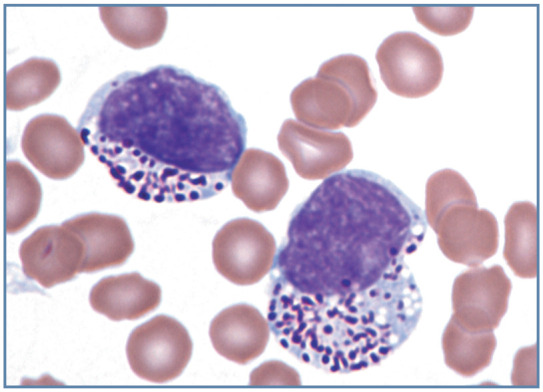
Two neoplastic large granular lymphocytes in blood from a cat with metastatic large granular lymphoma. Granules are larger than those seen in non-neoplastic granular lymphocytes. Wright-Giemsa stain
Vacuolated lymphocytes
Cytoplasmic vacuoles may be seen in lymphocytes associated with a variety of neoplastic and non-neoplastic disorders. Discrete vacuoles may occur in the cytoplasm of lymphocytes from cats with inherited lysosomal storage diseases, including mucopolysaccharidosis type VII, GM1 and GM2 gangliosidosis (Figure 14a), α-mannosidosis and Niemann-Pick type diseases. Basophilic granules and vacuoles may not become apparent in some lysosomal disorders until the affected animal reaches adulthood. 1
Figure 14.
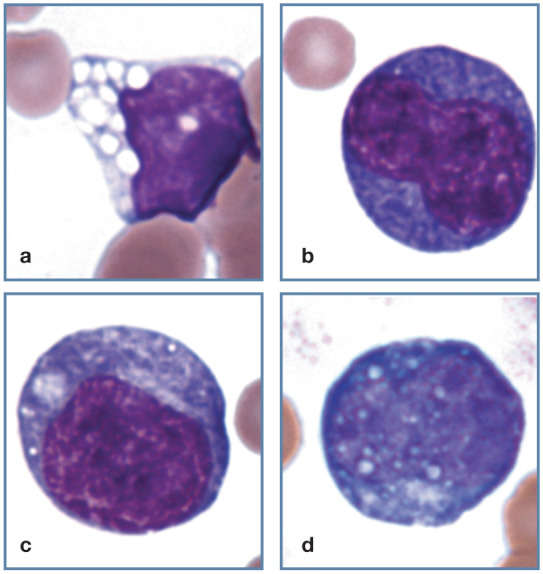
Abnormal lymphocyte morphology. (a) Vacuolated lymphocyte from a Korat cat with inherited GM2 gangliosidosis. (b,c) Reactive lymphocytes with increased cytoplasmic basophilia. (d) Reactive lymphocyte with cytoplasmic Russell bodies containing immunoglobulin. Wright-Giemsa stain
Reactive lymphocytes
Lymphocytes proliferate in response to antigenic stimulation. They increase in size and exhibit increased cytoplasmic basophilia (Figure 14b,c). These antigenically stimulated cells mostly remain in peripheral lymphoid tissues, but may enter the circulation, although usually in low numbers. Some reactive lymphocytes are large with convoluted nuclei similar to monocytes, except that their cytoplasm is more basophilic (navy blue color) than cytoplasm seen in monocytes. These cells can also be difficult to differentiate from some neoplastic lymphocytes. Some reactive lymphocytes are plasmacytoid (plasma cell-like) in appearance. Occasional lymphocytes contain pinkish or bluish globules (Russell bodies) within the cytoplasm (Figure 14d). 1
Neoplastic lymphocytes
Lymphocytes that are present in high numbers in the blood of cats with chronic lymphocytic leukemia have the morphology of normal lymphocytes. Lymphoblasts and prolymphocytes are present in the blood of some cats with lymphoma and acute lymphoblastic leukemia (Figure 15). These neoplastic lymphocytes are large and, compared with normal blood lymphocytes, exhibit increased cytoplasmic basophilia and less condensed nuclear chromatin. Nucleoli may or may not be clearly visible in the nuclei of these neoplastic cells. 1
Figure 15.
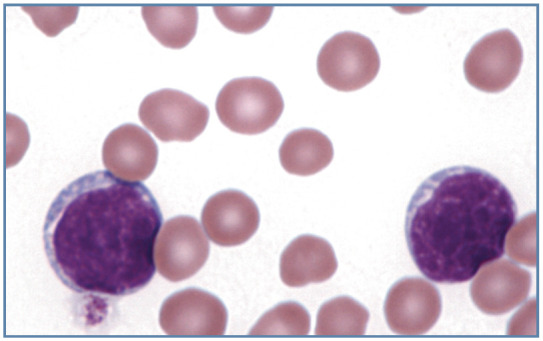
Blood film from a cat with acute lymphoblastic leukemia, showing two large lymphoblasts. Wright-Giemsa stain
Plasma cells
Plasma cells have lower N:C ratios and increased cytoplasmic basophilia compared with resting lymphocytes. The presence of prominent Golgi may create a pale perinuclear area in the cytoplasm. Plasma cells typically have eccentrically located nuclei with coarse chromatin clumping in a mosaic pattern. They are present in lymphoid organs (except the thymus), and are rarely observed in blood even when plasma cell neoplasia (myeloma-related disorders) is present (Figure 16).20,21
Figure 16.
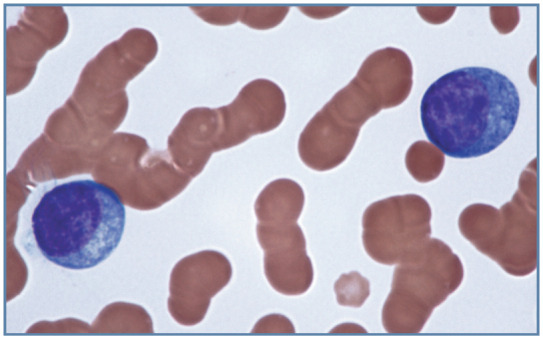
Neoplastic plasma cells in the blood of a cat with a metastatic plasma cell neoplasm involving bone marrow. There is increased basophilia between cells and prominent rouleaux formation because a monoclonal hyperglobulinemia was present. Wright-Giemsa stain
Monocytes
Monocytes are usually larger than lymphocytes, have nuclei that are more variable in shape and N:C ratios of ⩽1.0. The monocyte nucleus may be round, kidney-shaped or convoluted, with chromatin that is diffuse or mildly clumped (Figure 17a). The cytoplasm is typically gray–blue and often contains variably sized vacuoles (Figure 17b); less often, dust-like pinkish or reddish-purple granules may be visible. Monocytes develop into macrophages after they leave the blood and enter tissue. In some disorders, mononuclear phagocytes in blood become activated and enlarged, and resemble macrophages (Figure 17c). 1
Figure 17.
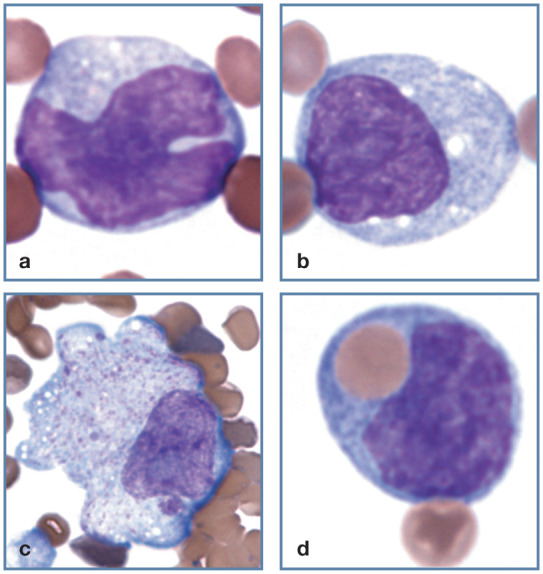
Mononuclear phagocyte morphology. (a,b) Normal monocyte morphology. (c) Macrophage at the feathered end of a blood film from a cat with Mycoplasma haemofelis infection. The magnification of this image is less than others in this group. (d) Monocyte with a phagocytized erythrocyte in blood from a cat with M haemofelis infection. Wright-Giemsa stain
Erythrophagocytosis by monocytes may be observed in primary or secondary immune-mediated hemolytic anemia (Figure 17d). Remarkably large macrophages containing schizonts of Cytauxzoon felis may be seen in cats with cytauxzoonosis, especially at the feathered end of the blood film (Figure 18).
Figure 18.
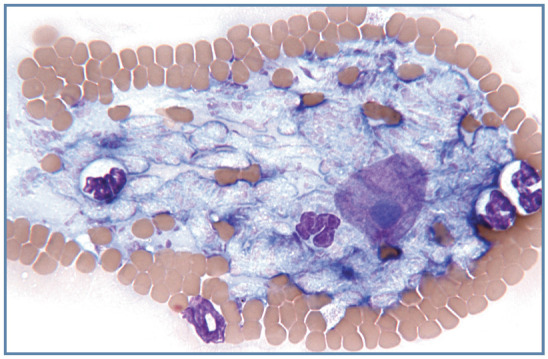
Disrupted intermediate Cytauxzoon felis schizont at the feathered end of a blood film. The large round magenta nucleus containing a blue nucleolus is from the host macrophage. Wright–Giemsa stain
Mast cells
Mast cells have biochemical characteristics similar to basophils and share a common progenitor cell in the bone marrow. However, they are not normally found in blood because they develop in tissues from morphologically unidentifiable precursor cells released into blood from bone marrow. Mast cells have round nuclei, rather than the segmented nuclei of basophils. They contain large numbers of purple to dark blue granules. Granules in cat basophils are generally larger than those in mast cells and most appear light lavender with methanolic, Romanowsky-type blood stains (Figure 19). In contrast to dogs, where mast cells may be present in low numbers in various disorders, mast cells are rarely seen in the blood of cats in the absence of mast cell neoplasms. 22
Figure 19.
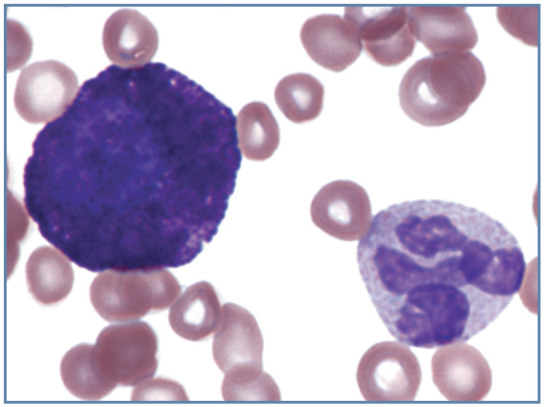
Mast cell (left) and basophil (right) in blood from a cat with splenic mast cell neoplasia. The round nucleus of the mast cell is nearly obscured by the large numbers of cytoplasmic granules present. Wright-Giemsa stain
Platelet morphology – normal and abnormal
Blood platelets (thrombocytes) are small, round-to-oval, anucleated cell fragments (thin discs when unstimulated) that are produced by megakaryocytes in the bone marrow. Platelet cytoplasm appears light blue with many small reddish-purple granules when visualized using Romanowsky-type blood stains. Cat platelets are larger and more variable in size than those of other domestic mammals (Figure 20a). The unique structure of the M loop region of the α1-tubulin gene may contribute to the variability in platelet size. 23
Figure 20.
Morphology of platelets. (a) Aggregate demonstrating the large size and variable size of feline platelets. The platelet at the bottom is larger than seen in most cats. (b) Macroplatelet in blood from a cat with erythroleukemia. Wright-Giemsa stain
Cat platelets are especially prone to be activated and aggregate during blood collection and handling. Partially activated platelets are no longer discs but have thin cytoplasmic processes extending from a spherical cell body. When platelets are more fully activated, their granules are crushed together by a surrounding web of microtubules and microfilaments, and this central aggregate of platelet granules may be mistaken for a nucleus (Figure 20b). Following degranulation, platelet aggregates appear as light blue material which may potentially be overlooked by an inexperienced observer (Figure 21).
Figure 21.
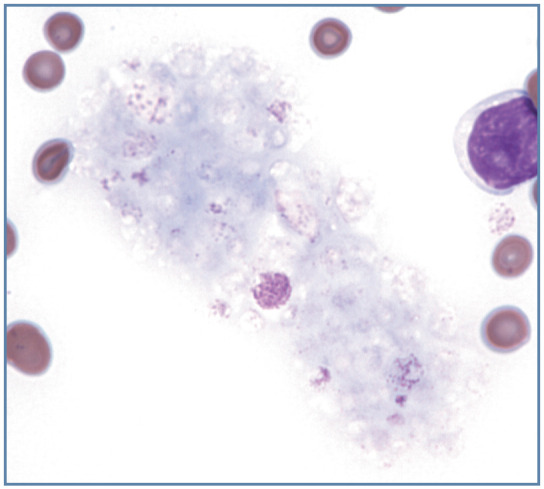
Aggregate of largely degranulated platelets. Wright-Giemsa stain
The presence of platelet aggregates results in erroneously low platelet counts. Even in the absence of platelet aggregation, many electronic cell counters cannot accurately count cat platelets in whole blood, because their large size makes them difficult to separate from erythrocytes based on cell volume. Consequently, it is essential that a stained blood film is examined for platelet numbers before a thrombocytopenia is recorded in a hematology report. 1
As in other mammalian species, the presence of frequent macrothrombocytes in a thrombocytopenic cat suggests that enhanced thrombopoiesis is present; macrothrombocytes may also be present in thrombocytopenic cats with FeLV infection and/or myeloid neoplasia (Figure 20b). 13 Large enlongated platelets were observed in blood from a Ragdoll cat that was homozygous for the F385Y change in the gene encoding for β1-tubulin (Figure 22). 23
Figure 22.
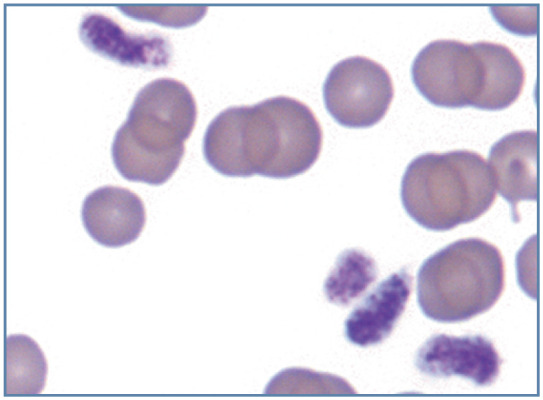
Large elongated platelets from a Ragdoll cat that was homozygous for the F385Y change in the gene encoding for β1-tubulin. Wright-Giemsa stain. Courtesy of EA Spangler
Anaplasma platys (formerly Ehrlichia platys) is a rickettsial parasite that specifically infects platelets and causes infectious cyclic thrombocytopenia in dogs. Similar-appearing inclusions have been seen in platelets from a cat, and this organism has been identified in cats using PCR. 24
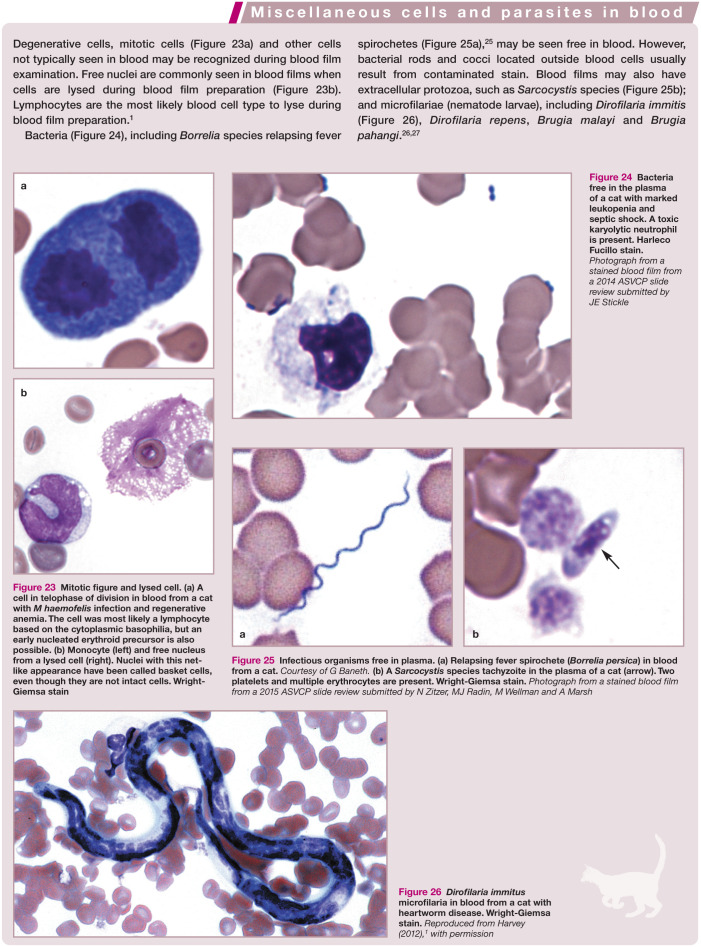
Figure 23.
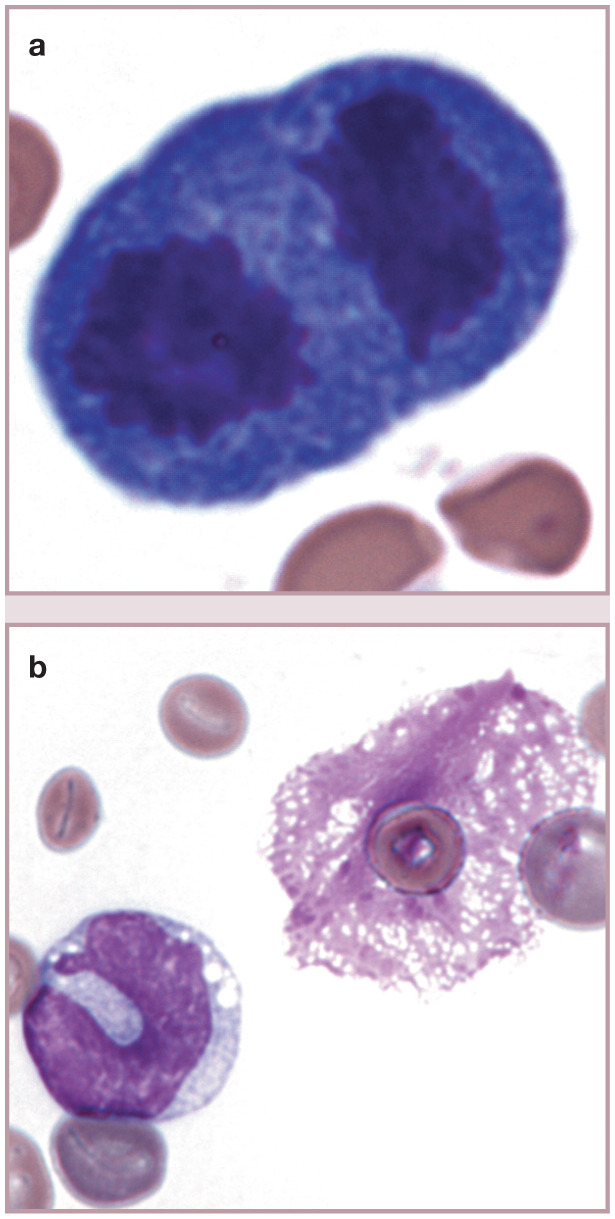
Mitotic figure and lysed cell. (a) A cell in telophase of division in blood from a cat with M haemofelis infection and regenerative anemia. The cell was most likely a lymphocyte based on the cytoplasmic basophilia, but an early nucleated erythroid precursor is also possible. (b) Monocyte (left) and free nucleus from a lysed cell (right). Nuclei with this net-like appearance have been called basket cells, even though they are not intact cells. Wright-Giemsa stain
Figure 24.
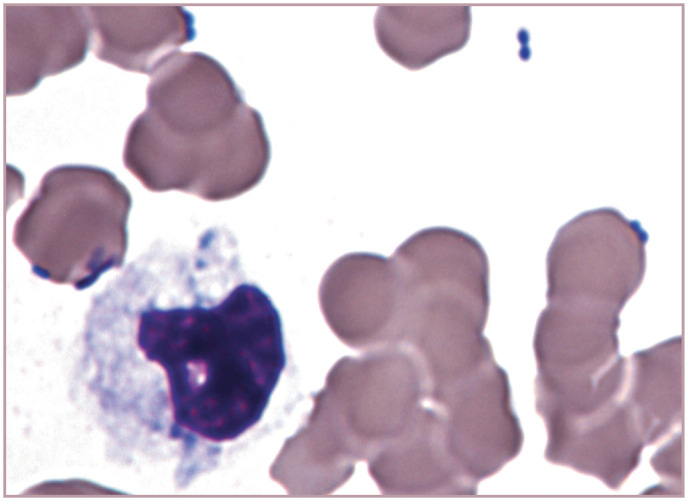
Bacteria free in the plasma of a cat with marked leukopenia and septic shock. A toxic karyolytic neutrophil is present. Harleco Fucillo stain. Photograph from a stained blood film from a 2014 ASVCP slide review submitted by JE Stickle
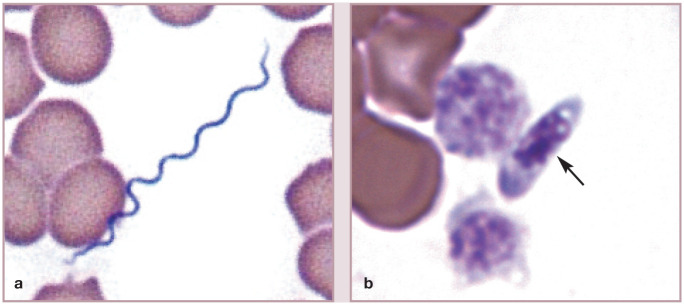
Figure 25.
Infectious organisms free in plasma. (a) Relapsing fever spirochete (Borrelia persica) in blood from a cat. Courtesy of G Baneth. (b) A Sarcocystis species tachyzoite in the plasma of a cat (arrow). Two platelets and multiple erythrocytes are present. Wright-Giemsa stain. Photograph from a stained blood film from a 2015 ASVCP slide review submitted by N Zitzer, MJ Radin, M Wellman and A Marsh
Figure 26.
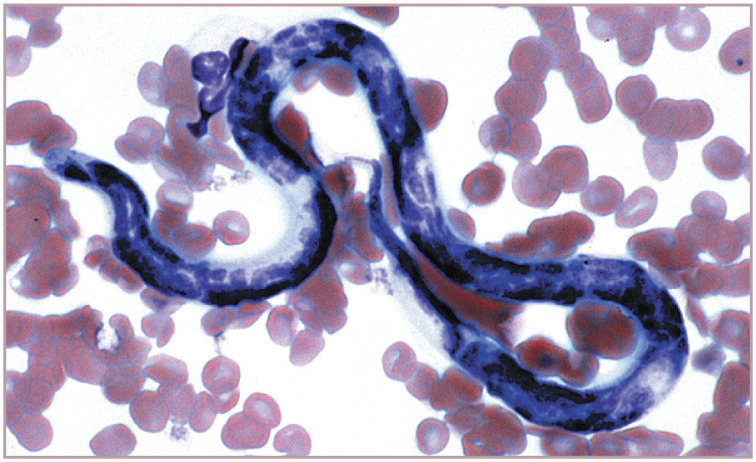
Dirofilaria immitus microfilaria in blood from a cat with heartworm disease. Wright-Giemsa stain. Reproduced from Harvey (2012), 1 with permission
Key Points
Cat neutrophils appear similar to those of other domestic mammals. Examination of neutrophils in the feline blood film may reveal cytoplasmic granules, toxic cytoplasm, left shift, Pelger-Huët anomaly, hypersegmentation, dysgranulopoiesis, acute myeloid leukemia or infectious agents.
In contrast to other domestic mammals, which have round eosinophil granules, eosinophils from domestic cats have rod-shaped granules. The eosinophil nucleus is similar to that of neutrophils, but tends to be less lobulated.
The basophils of cats are distinctive, with light lavender granules typically filling the cytoplasm, giving the nucleus a moth-eaten appearance.
Examination of lymphocytes in the feline blood film may reveal those that are granular, vacuolated, reactive or neoplastic, and may also show plasma cells.
Monocytes are usually larger than lymphocytes, have lower nucleus to cytoplasmic ratios and have nuclei that are more variable in shape.
Mast cells are not normally found in blood. They have round nuclei and contain large numbers of purple to dark blue granules, and generally indicate the presence of mast cell neoplasia.
Feline blood platelets are round-to-oval anucleated cell fragments. They are larger and more variable in size than those of other domestic mammals.
Degenerative cells, mitotic cells and other cells not typically seen in blood may be recognized during blood film examination. Extracellular protozoa, microfilariae and bacteria may also be seen in blood; however, bacterial rods and cocci located outside of blood cells are usually the result of contaminated stain.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Harvey JW. Veterinary hematology. A diagnostic guide and color atlas. St Louis, MO: Elsevier Saunders, 2012. [Google Scholar]
- 2. Bessis M. Living blood cells and their ultrastructure. New York: Springer-Verlag, 1973, pp 337–338. [Google Scholar]
- 3. Bunting RW, Selig MK. Localization of DNA in ultrascopic nuclear appendages of polymorphonuclear white blood cells from patients with low serum B(12). J Histochem Cytochem 2002; 50: 1381–1388. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch VM, Cunningham TA. Hereditary anomaly of neutrophil granulation in Birman cats. Am J Vet Res 1984; 45: 2170–2174. [PubMed] [Google Scholar]
- 5. Kramer JW, Davis WC, Prieur DJ. The Chédiak-Higashi syndrome of cats. Lab Invest 1977; 36: 554–562. [PubMed] [Google Scholar]
- 6. Gossett KA, MacWilliams PS, Cleghorn B. Sequential morphological and quantitative changes in blood and bone marrow neutrophils in dogs with acute inflammation. Can J Comp Med 1985; 49: 291–297. [PMC free article] [PubMed] [Google Scholar]
- 7. Segev G, Klement E, Aroch I. Toxic neutrophils in cats: clinical and clinicopathologic features, and disease prevalence and outcome – a retrospective case control study. J Vet Intern Med 2006; 20: 20–31. [DOI] [PubMed] [Google Scholar]
- 8. Jain NC. Essentials of veterinary hematology. Philadelphia: Lea & Febiger, 1993. [Google Scholar]
- 9. Latimer KS, Rakich PM, Thompson DF. Pelger-Huët anomaly in cats. Vet Pathol 1985; 22: 370–374. [DOI] [PubMed] [Google Scholar]
- 10. Deshuillers P, Raskin R, Messick J. Pelger-Huët anomaly in a cat. Vet Clin Pathol 2014; 43: 337–341. [DOI] [PubMed] [Google Scholar]
- 11. Toth SR, Onions DE, Jarrett O. Histopathological and hematological findings in myeloid leukemia induced by a new feline leukemia virus isolate. Vet Pathol 1986; 23: 462–470. [DOI] [PubMed] [Google Scholar]
- 12. Wang E, Boswell E, Siddiqi I, et al. Pseudo-Pelger-Huet anomaly induced by medications: a clinicopathologic study in comparison with myelodysplastic syndrome-related pseudo-Pelger-Huet anomaly. Am J Clin Pathol 2011; 135: 291–303. [DOI] [PubMed] [Google Scholar]
- 13. Harvey JW. Anemia in a cat – myelodysplastic syndrome. NAVC Clinician’s Brief 2012; February: 60–64. http://www.cliniciansbrief.com/sites/default/files/Anemia%20in%20Cat.pdf (2012, accessed February 13, 2017).
- 14. Myers S, Wiks K, Giger U. Macrocytic anemia caused by naturally occurring folate-deficiency in the cat [abstract]. Vet Clin Pathol 1996; 25: 30. [Google Scholar]
- 15. Lloret A, Addie DD, Boucraut-Baralon C, et al. Hepatozoonosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2015; 17: 642–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baneth G, Sheiner A, Eyal O, et al. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasit Vectors 2013; 6: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcos R, Santos M, Malhao F, et al. Pancytopenia in a cat with visceral leishmaniasis. Vet Clin Pathol 2009; 38: 201–205. [DOI] [PubMed] [Google Scholar]
- 18. Sprague WS, TerWee JA, VandeWoude S. Temporal association of large granular lymphocytosis, neutropenia, proviral load, and FasL mRNA in cats with acute feline immunodeficiency virus infection. Vet Immunol Immunopathol 2010; 134: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roccabianca P, Vernau W, Caniatti M, et al. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8αα phenotype. Vet Pathol 2006; 43: 15–28. [DOI] [PubMed] [Google Scholar]
- 20. Mellor PJ, Haugland S, Smith KC, et al. Histopathologic, immunohistochemical, and cytologic analysis of feline myeloma-related disorders: further evidence for primary extramedullary development in the cat. Vet Pathol 2008; 45: 159–173. [DOI] [PubMed] [Google Scholar]
- 21. Takeuchi Y, Iizuka H, Kanemitsu H, et al. Myeloma-related disorder with leukaemic progression in a cat. J Feline Med Surg 2010; 12: 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garrett LD, Craig CL, Szladovits B, et al. Evaluation of buffy coat smears for circulating mast cells in healthy cats and ill cats without mast cell tumor-related disease. J Am Vet Med Assoc 2007; 231: 1685–1687. [DOI] [PubMed] [Google Scholar]
- 23. Boudreaux MK, Osborne CD, Herre AC, et al. Unique structure of the M loop region of beta1-tubulin may contribute to size variability of platelets in the family felidae. Vet Clin Pathol 2010; 39: 417–423. [DOI] [PubMed] [Google Scholar]
- 24. Lima ML, Soares PT, Ramos CA, et al. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz J Microbiol 2010; 41: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baneth G, Nachum-Biala Y, Halperin T, et al. Borrelia persica infection in dogs and cats: clinical manifestations, clinicopathological findings and genetic characterization. Parasit Vectors 2016; 9: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasomsitti P, Mak JW, Sucharit P, et al. Detection of antibodies in cats infected with filarial parasites by the indirect immunofluorescence technique. Southeast Asian J Trop Med Public Health 1983; 14: 353–356. [PubMed] [Google Scholar]
- 27. Nuchprayoon S, Junpee A, Nithiuthai S, et al. Detection of filarial parasites in domestic cats by PCR-RFLP of ITS1. Vet Parasitol 2006; 140: 366–372. [DOI] [PubMed] [Google Scholar]