Abstract
Objectives
The objective was to identify clinical or ultrasonographic results associated with ureteral obstruction or outcome in cats with azotaemia.
Methods
This was a retrospective cross-sectional study of cats with azotaemia (serum creatinine >180 μmol/l) that had ultrasonography of the urinary tract, ultrasound images available for review and received treatment for azotaemia. Cats with pre-renal azotaemia or urethral obstruction were excluded. Associations between clinical and ultrasonographic results and the dependent variables ‘tentative diagnosis of ureteral obstruction’, ‘pyelography positive for ureteral obstruction’ and ‘death in hospital’ were tested using binary logistic regression.
Results
In total, 238 cats satisfied the inclusion criteria. Median age was 7 years (range 2 weeks to 20 years), duration of clinical signs was 7 days (range 1 day to 6.3 years) and serum creatinine was 417 μmol/l (range 184–2100 μmol/l). Tentative diagnosis of ureteral obstruction in 92/238 (39%) cats was significantly associated with unilateral enlarged kidney on palpation, and dilated renal pelvis and calculi within the ureter on ultrasonography. Pyelography was performed in 49/92 (53%) cats (16 bilateral) with a tentative diagnosis of ureteral obstruction, and was positive for obstruction in 46/65 (71%) instances. No significant differences in ultrasonographic signs were found between cats with obstructed and non-obstructed ureters. Receiver–operating characteristic analysis of renal pelvic diameter as a diagnostic test for ureteral obstruction found an area under the curve not significantly different from 0.5. There was good agreement between results of radiography and ultrasonography for presence of urinary calculi (kappa 0.67). Treatment was medical in 171 (72%) cats and surgical (ureteral stent or by-pass device) in 67 (28%). Death in hospital was significantly associated with serum creatinine and presence of peritoneal fluid, but not with clinical diagnosis, ultrasonographic signs or treatment method.
Conclusions and relevance
Ultrasonography may be used to identify azotaemic cats at greatest risk of ureteral obstruction, but when using pyelography as the reference test ultrasonography appears to be inaccurate for diagnosis of ureteral obstruction.
Introduction
Cats may present with azotaemia as a result of an acute kidney injury (AKI) or the cumulative effects of chronic kidney disease (CKD).1,2 History and clinical signs associated with azotaemia in cats are predominantly non-specific, such as inappetence, lethargy, vomiting and weight loss; 3 hence, diagnosis relies on determination of serum creatinine. Ureteral obstruction by calcium oxalate calculi is the most frequent, and increasingly frequent, cause of AKI in cats.3–5 Like azotaemia, ureteral obstruction does not usually cause characteristic clinical signs. For example, nephromegaly or signs of pain on palpation of the affected kidney are observed infrequently. 6 For this reason, radiography or ultrasonography is indicated to look for ureteral calculi and signs of obstruction in cats presenting with azotaemia. 3 Given that unilateral ureteral obstruction will not result in azotaemia if the contralateral kidney is functioning well, it is essential to assess both kidneys for signs of obstruction and pre-existing renal damage.
Diagnosis of ureteral obstruction in cats can be challenging. Accuracy of survey radiography or ultrasonography for detection of ureteral calculi is only moderate, 3 with instances of false-negative and false-positive results having been recorded for both modalities. 3 Other ultrasonographic findings associated with ureteral obstruction include ipsilateral nephromegaly, pelvic and/or ureteral dilatation and perinephric fluid. 6 Renal size and the degree of pelvic dilatation are variable in cats with normal renal function, and normal values overlap with values observed in cats with renal disease or urinary obstruction.6,7 Two studies found that a renal pelvic diameter >13 mm was consistently associated with ureteral obstruction,7,8 but the majority of obstructed kidneys have less marked pelvic dilatation, so may appear similar to kidneys affected by CKD or pyelonephritis, which also frequently exhibit pelvic dilatation. 8 For these reasons, cats considered equivocal for ureteral obstruction at ultrasonography are candidates for antegrade pyelography.9,10 Antegrade pyelography is considered the most accurate test for ureteral obstruction. 10 Passage of contrast into the bladder proves ureteral patency, whereas lack of contrast passage indicates at least partial obstruction and usually identifies the site of an obstructive lesion. In addition to calculi and deposits of solidified blood that may cause ureteral obstruction, 11 ureteral strictures following previous bouts of ureteral calculi or surgery to treat ureteral obstruction may be demonstrated using pyelography. 12
Ability to distinguish cats with ureteral obstruction from other cats presenting with azotaemia enables more specific choice of treatment. Methods for treatment of ureteral obstruction in cats include medical management, 13 surgery, 13 ureteral stents14–17 and subcutaneous ureteral bypass (SUB) devices.18,19 A study of cats with ureteral obstruction treated using either a ureteral stent or SUB found no factors that were associated with survival to discharge, but relatively high blood urea nitrogen at presentation, high creatinine at discharge and overhydration of cats during hospitalisation were associated with decreased overall survival. 20 No studies to date have tested associations between imaging findings and outcome in cats presenting with azotaemia.
The aim of the present study was to review the records of a series of cats presenting with azotaemia in order to identify clinical or ultrasonographic findings associated with ureteral obstruction or outcome.
Methods
For this retrospective cross-sectional study, electronic medical records of the Queen Mother Hospital for Animals between June 2009 and May 2015 were reviewed. The criteria for inclusion were cats presented for the first time during this period, that had azotaemia, ultrasonography of the urinary tract, ultrasound report and images available for review, and received treatment for azotaemia. Azotaemia was defined as serum creatinine at presentation >180 μmol/l, as determined by the clinical pathology laboratory, or plasma creatinine >140 μmol/l as determined by a bench-top biochemistry analyser (Bioprofile 300; Nova Biomedical). Cats with pre-renal azotaemia or urethral obstruction were excluded. Determination of pre-renal azotaemia was based on serum creatinine on admission above the reference interval, a urine specific gravity (USG) >1.040 and no ultrasonographic evidence of urinary obstruction. 5
Data extracted from the medical records included signalment, history, clinical signs, results of haematology, serum chemistry and urinalysis, ultrasound findings, results of survey (non-contrast) radiography, results of pyelography, diagnosis, treatment and outcome (death in hospital or discharged alive). Categories of diagnosis included specific renal disease (when determined), tentative diagnosis of obstruction (including cats subsequently proven to be obstructed) and undiagnosed.
Ultrasound findings were extracted from archived ultrasound images and contemporaneous reports written by six different board-certified radiologists employed at the Queen Mother Hospital for Animals during the period covered by the study. Objective renal length in sagittal or dorsal images, objective transverse pelvic diameter, subjective renal size, subjective pelvic and ureteral dilatation, renal shape, presence of renal scars (focal depressions in the cortical surface with adjacent hyperechoic cortical segment), echogenicity of the cortex and medulla, presence of calculi, presence of perinephric or peritoneal fluid and results of radiography were recorded (Table 1). Radiographs were reviewed for presence of urinary calculi. Results of pyelography were recorded as normal, abnormal/non-obstructed or obstructed.
Table 1.
Ultrasonographic and radiographic criteria
| Criterion | Value recorded |
|---|---|
| Renal length | mm |
| Pelvic diameter | mm |
| Subjective renal size | Normal; small; enlarged |
| Subjectively dilated pelvis | No; slight; marked |
| Subjectively dilated ureter | No; slight; marked |
| Renal shape | Normal; irregular; asymmetric; rounded; nodular; renal mass |
| Cortical scars | None; slight; marked |
| Echogenicity cortex | Normal; increased; heterogeneous |
| Echogenicity medulla | Normal; increased; heterogeneous; medullary rim sign; loss of corticomedullary differentiation |
| Perinephric fluid | None; slight; marked |
| Peritoneal fluid | None; slight; marked |
| Calculi in kidney | None; single; multiple |
| Calculi in ureter | None; single; multiple |
| Calculi in bladder | None; single; multiple |
| Calculi on radiographs | None; renal; ureteral; bladder |
| Pyelogram | Normal; abnormal, non-obstructed; obstructed |
| Other findings | Single renal cyst; polycystic renal disease |
Clinical and ultrasonographic data for analysis were derived from the first period of hospitalisation only. Continuous data were summarised using median and range. Serum creatinine values as determined by the bench-top biochemistry analyser were not included in summary data nor used in analysis because the reference interval and operating range were different to those used by the clinical pathology laboratory, which took precedence. Associations between clinical and ultrasound results and the dependent variables ‘tentative diagnosis of ureteral obstruction’, ‘pyelography positive for ureteral obstruction’ and ‘death in hospital’ were tested using binary logistic regression with step-wise removal of non-significant variables. Results of the final regression models were expressed as odds ratio (OR) and 95% confidence interval (CI). The association between degree of renal pelvic dilatation on ultrasonography and pyelographic diagnosis of obstruction was tested using receiver–operating characteristic (ROC) curve analysis. For each cat that had ultrasonography and survey radiography, agreement between studies with respect to presence of calculi was tested using the kappa statistic. Statistical tests were undertaken using a proprietary application (SPSS Statistics, version 22; IBM). Differences with P <0.05 were considered significant.
Results
Records were found of 238 cats that satisfied the inclusion criteria (Figure 1). There were 121 males (115 neutered) and 127 females (111 neutered). Their median age was 7 years (range 2 weeks–20 years). There were 134 (56%) domestic shorthair cats, 13 (6%) British Shorthair cats, 12 (5%) domestic longhairs, 11 (5%) Ragdolls, nine (4%) Burmese, nine (4%) Persians, eight (3%) Bengals, seven (3%) Siamese, six (3%) mixed breed cats, four (2%) Birmans, four (2%) British Blues, four (2%) Maine Coons and 11 other feline breeds with fewer than four affected individuals.
Figure 1.
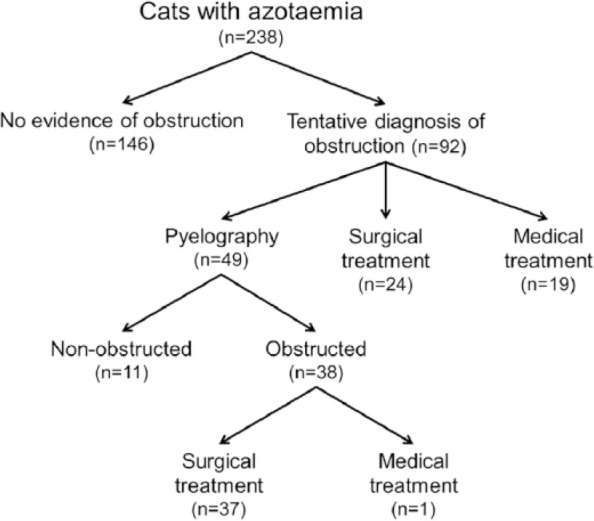
Summary of diagnostic categories and management of 238 cats with azotaemia
Urinary calculi had been identified prior to referral in 12 (5%) cats. Clinical signs included lethargy in 152 (64%) cats, inappetence in 142 (60%), vomiting in 90 (38%), polydipsia/polyuria in 65 (27%), weight loss in 60 (25%), straining to urinate in 23 (10%), signs of abdominal pain in 13 (5%) and diarrhoea in eight (3%). The median duration of clinical signs was 7 days (range 1 day–6.3 years). Median body condition score was 4/9 (range 1–8), body weight was 3.7 kg (range 0.9–7.1kg), heart rate was 178 beats/min (range 100–250 beats/min) and respiratory rate was 32 breaths/min (range 10–136 breaths/min). Rectal temperature values were available for 192 cats. Median rectal temperature was 37.9ºC (range 32.8–40.1ºC), 111 (58%) cats had subnormal rectal temperature (<38.1ºC) and 11 (6%) were pyrexic (>39.2ºC). During hospitalisation, 12 (5%) cats developed signs of overhydration, including pleural and peritoneal effusion.
All cats had azotaemia on presentation, as per the inclusion criteria. Median serum creatinine was 417 μmol/l (range 184–2100 μmol/l) as determined by the clinical pathology laboratory (n = 199). Additional results of haematology and serum chemistry determinations for 199 cats were available for review. Median haematocrit was 0.29 (range 0.09–0.49); 88 (44%) cats were anaemic (haematocrit <0.29). Total serum protein was 72 g/l (range 19–112 g/l); 12 (6%) cats had hypoproteinaemia (total protein <60 g/l). Eighty-two (41%) cats had hyperkalaemia (serum potassium >4.6 mmol/l).
Results of urinalysis for 196 cats were available for review. Median USG was 1.017 (range 1.006–1.050), pH was 6.0 (range 5.0–9.0), semi-quantitative urine protein was increased in 110 (56%) cats (1+ [n = 80], 2+ [n = 19], 3+ [n = 11]), urine blood was increased in 65 (33%) cats (1+ [n = 18], 2+ [n = 23], 3+ [n = 24]) and urine glucose was increased in 34 (17%) cats (1+ [n = 18], 2+ [n = 6], 3+ [n = 10]). One hundred and twenty-five (64%) cats had isosthenuria (USG <1.020). Culture of urine grew bacteria in 26 (13%) instances, including Escherichia coli in 15 cats, mixed populations of bacteria in three, Staphylococcus species in one, Enterococcus species in one and Micrococcus species in one.
Ureteral obstruction was recorded as the tentative diagnosis in 92/238 (39%) cats. In the final regression model, tentative diagnosis of ureteral obstruction was significantly associated with unilateral enlarged kidney on palpation (OR 17.2, 95% CI 3.9–75.5), dilated renal pelvis (OR 27.6, 95% CI 6.7–112.7) and calculi within the ureter (OR 62.8, 95% CI 11.4–347.4) on ultrasound. These results indicate the clinical and ultrasound findings used most frequently by attending clinicians as the basis for tentative diagnosis prior to pyelography or other method of definitive diagnosis. Although dilated ureter was significantly associated with ureteral obstruction on the basis of pairwise testing, dilated ureter was not included in the final regression model because it was highly correlated with dilated renal pelvis. No other clinical signs, blood test results or ultrasound findings were associated with tentative diagnosis of ureteral obstruction.
Of the 92 cats with a tentative diagnosis of ureteral obstruction, 49 (53%) had pyelography, including 16 cats that had bilateral pyelograms. Of 65 kidneys subjected to pyelography, 46 (71%) were proved to have ureteral obstruction, nine (14%) had an abnormal but non-obstructed ureter (Figure 2) and 10 (15%) had no ureteral abnormality identified. No significant differences in ultrasound signs were found between cats with obstructed and non-obstructed ureters at pyelography (Table 2). ROC analysis of renal pelvic diameter as a diagnostic test for ureteral obstruction found an area under the curve 0.60 (95% CI 0.44–0.75) (P = 0.08). An identical result was obtained using the pelvic diameter as a percentage of renal length as a diagnostic test for ureteral obstruction (Figure 3). Of the 46 kidneys with proven ureteral obstruction, 18 (39%) had ultrasonographic signs compatible with pre-existing chronic nephropathy, including irregular kidney shape in 13 (28%), cortical scars in nine (20%), reduced kidney size in six (13%) and parenchymal calcification in one (2%).
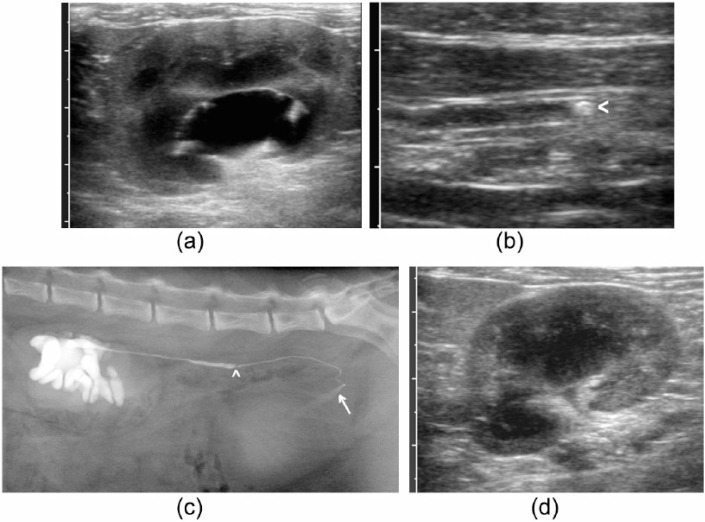
Figure 2.
Example of a cat with pelvic dilatation and ureteral calculus compatible with ureteral obstruction but patent ureter on pyelography. (a) Dorsal plane ultrasound image of the left kidney at presentation. Pelvic diameter was recorded as 10 mm; (b) dilated left ureter containing a calculus (arrowhead); (c) lateral radiograph after pyelography showing localised dilatation of the left ureter cranial to an intraluminal filling defect compatible with a calculus (arrowhead) and passage of contrast into the bladder (arrow); (d) dorsal plane ultrasound image of the left kidney at follow-up examination after medical management for 1 month, in which pelvic dilatation has resolved
Table 2.
Ultrasonographic signs in kidneys with and without ureteral obstruction
| Ultrasonographic signs | Pyelographic diagnosis |
|
|---|---|---|
| Obstructed |
Non-obstructed |
|
| (n = 46) | (n = 19) | |
| Median (range) renal length (mm) | 44 (21–56) | 41 (32–51) |
| Median (range) pelvic diameter (mm) | 9 (3–30 mm) | 7 (2–24) |
| Median (range) pelvic diameter as a proportion of renal length (%) | 23 (7–81) | 19 (6–56) |
| Subjectively dilated pelvis | 46 (100) | 19 (100) |
| Subjectively dilated ureter | 39 (85) | 14 (74) |
| Abnormal renal shape | 12 (26) | 8 (42) |
| Cortical scars | 9 (20) | 3 (16) |
| Abnormal cortical echogenicity | 12 (26) | 7 (37) |
| Abnormal medullary echogenicity | 10 (22) | 7 (37) |
| Perinephric or peritoneal effusion | 14 (30) | 7 (37) |
| Calculi in kidney | 18 (39) | 10 (53) |
| Calculi in ureter | 27 (59) | 14 (74) |
| Calculi in bladder | 8 (17) | 2 (11) |
Data are n (%) unless otherwise indicated. There are no significant differences between kidneys with and without ureteral obstruction
Figure 3.
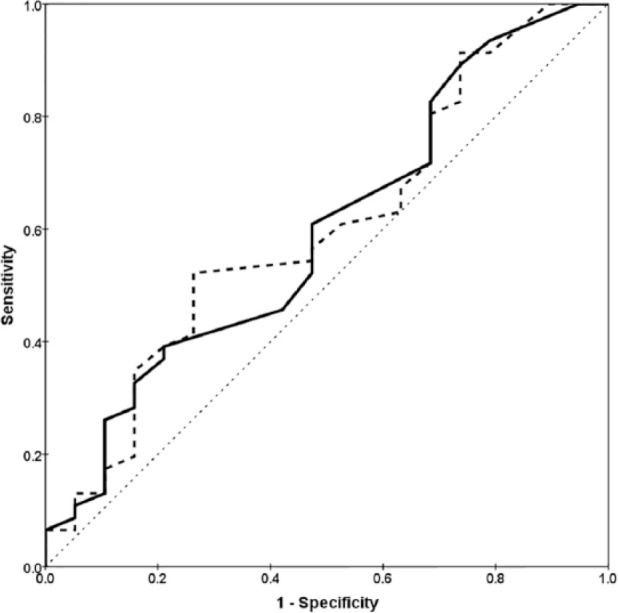
Receiver–operating characteristic plots for renal pelvic diameter (solid line) and pelvic diameter as a percentage of renal length (dashed line) as diagnostic tests for ureteral obstruction. Neither curve encompasses an area significantly different from 0.5 (diagonal dotted line)
Abdominal survey radiographs were obtained in 70/238 (29%) cats. Results for radiography and ultrasonography with respect to calculi affecting the kidneys, ureters or bladder agreed in 61 cats and disagreed in nine cats (Table 3). Overall there was good agreement between results for radiography and ultrasonography for presence of urinary calculi, kappa 0.67 (95% CI 0.47–0.87).
Table 3.
Results of radiography and ultrasonography for presence of urinary calculi
| Radiography |
|||
|---|---|---|---|
| + | – | ||
| Ultrasonography | + | 47 | 7 |
| – | 2 | 14 | |
| Kappa = 0.67 (95% CI 0.47–0.87) | |||
+ = calculi identified; – = no calculi identified; CI = confidence interval
Treatment for azotaemia was medical in 171/238 (72%) cats and surgical (ureteral stent or bypass device) in 67/238 (28%). The 67 cats treated surgically included six with abnormal but non-obstructed ureters on pyelography. Median period of hospitalisation was 5 days (range 1–22 days), after which 184 (77%) cats were discharged and 54 (23%) cats died or were euthanased. In the final regression model, death in hospital was associated with serum creatinine (OR 1.002, 95% CI 1.001–1.004) and presence of peritoneal fluid (OR 1.6, 95% CI 1.0–2.7) but not with clinical diagnosis, results of other blood or urine tests, ultrasound findings or treatment method. Plot of probability of death vs serum creatinine corresponded closely to a linear function (Figure 4). Only serum creatinine results as determined by the clinical pathology laboratory were used for the analysis and plot.
Figure 4.
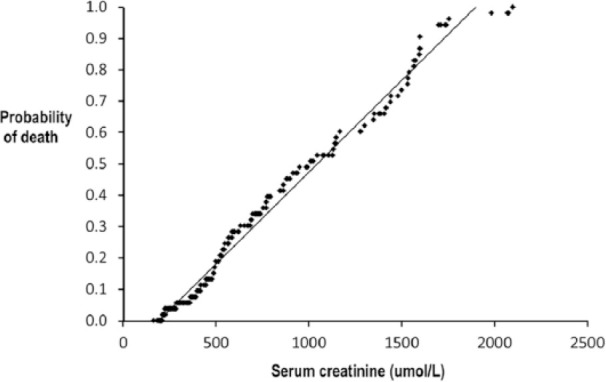
Plot of probability of death in hospital vs serum creatinine at presentation in 199 cats with azotaemia. Line is a fitted linear function
Discussion
The cats in this series likely represent a range of aetiologies for azotaemia, but in the majority a specific diagnosis was not determined. The focus of this study was cats with ureteral obstruction, but definitive evidence of obstruction was not obtained in all those with tentative diagnosis of ureteral obstruction. It is evident from the results that clinicians managing azotaemic cats in our hospital placed most emphasis on unilateral enlarged kidney on palpation, dilated renal pelvis and calculi within the ureter as signs of ureteral obstruction, and that many cats were treated surgically for ureteral obstruction on the basis of these signs without employing pyelography. Although pyelography is recognised as the definitive method for determination of ureteral obstruction, this does not mean it is necessary in every cat suspected of having an obstruction. Cats with azotaemia and evidence of ureteral calculi are clearly at risk of obstruction (some may have had previous obstruction that resolved); hence, there is an indication to treat them without proof that they are obstructed when presented. Presence of azotaemia proves bilateral renal insufficiency and, under this circumstance, the patient cannot tolerate ureteral obstruction; therefore, if risk factors for obstruction are identified ultrasonographically, treatment for obstruction is indicated because this is likely to be, or to become, a contributor to azotaemia.
The clinical scenario dictates the appropriateness of imaging in patients with azotaemia. In humans with newly detected renal dysfunction, ultrasonography is indicated to look for reversible causes, assess renal size and echogenicity, and thereby establish the chronicity of disease, but imaging is most useful in high-risk groups or in patients in whom there is a strong clinical suspicion of obstruction. 21 Similarly, ultrasonography is used routinely to examine the kidneys of cats with azotaemia in an attempt to distinguish causes of AKI, such as ureteral obstruction or ethylene glycol toxicity, from conditions associated with CKD, such as polycystic kidney disease or nephritis; however, there is notable overlap in the pathophysiology of these conditions, as the majority of cats presenting acutely with ureteral obstruction have pre-existing CKD. 22 It seems likely that in many instances, signs of pre-existing CKD in a cat presenting acutely with ureteral obstruction will reflect a prior undetected episode of obstruction.
Ultrasonography has utility as a means of selecting those azotaemic cats at greatest risk of ureteral obstruction, in which further assessment of obstruction is indicated. Only kidneys with pelvic dilatation on ultrasonography are candidates for pyelography because a degree of pelvic dilatation is necessary to enable surgical placement of a SUB device. However, in this study, when using the results of pyelography as the gold standard, ultrasonography was inaccurate for ureteral obstruction; therefore, management decisions based on ultrasonography alone may be flawed. The degree of dilatation of the renal pelvis and proximal ureter at any point in time will reflect the balance of urine output and the degree of any obstruction. Dilatation of the renal pelvis may be observed with high rates of urine output in non-obstructed animals; 23 hence, it must also be interpreted with caution. Conversely, it is plausible that urinary tract dilatation could be absent in an obstructed but volume-depleted patient. Pyelography should be considered whenever there is a need to prove ureteral obstruction.
Classification of results of pyelography as obstructed or non-obstructed is a limitation of the present study. Partial obstruction is likely to have been present in at least some of the nine abnormal, but patent, ureters in the present study (Figure 3), but without any practical method of determining the degree of partial obstruction, a binary classification was considered necessary. In a cat with marked pelvic dilatation and a calculus in the ureter, there could be a clinically significant degree of obstruction even if some contrast passes on pyelography. Under these circumstances, reduction in azotaemia immediately following ureteral stenting or SUB placement could be a useful retrospective sign of ureteral obstruction.
There was good agreement between radiographic and ultrasonographic findings with respect to presence of urinary calculi. Discrepancies likely reflect instances in which calculi were present but not observed using one modality. Calculi may be missed ultrasonographically if the ureter cannot be adequately examined. Calculi may be missed radiographically if they are superimposed by other structures, such as parts of the gastrointestinal tract, or are not sufficiently opaque, which will include deposits of solidified blood. 11
Clinical signs in azotaemic cats in this series were similar to those previously described, with lethargy, inappetence, vomiting, polydipsia/polyuria and weight loss observed most frequently. Subnormal rectal temperature, anaemia and hyperkalaemia were also observed frequently. We excluded cats with pre-renal azotaemia because they represent a heterogeneous group of non-renal conditions, their azotaemia was relatively easily corrected in many cases and ultrasonography of the kidneys would not be expected to contribute to management of pre-renal azotaemia, except by documenting lack of signs of renal or post-renal conditions.
In this series, death in hospital was strongly associated with serum creatinine on presentation, but not with results of other blood or urine tests, ultrasound findings, clinical diagnosis of obstruction or treatment method. This result must be interpreted with caution. Serum creatinine concentration may be used as a prognostic indicator because creatinine is a marker of glomerular filtration rate and indicates the degree of functional renal damage. In CKD the creatinine concentration may be correlated with the true residual renal function and can help in predicting a prognosis (eg, the IRIS staging for CKD);24,25 however, in the acute phases of kidney injury or ureteral obstruction the reduction in the renal function may be transient and potentially reversible, therefore serum creatinine indicates only the degree of the current injury to the kidney but cannot be used as a reliable prognostic factor. 26 Serum creatinine results need to be considered carefully when applied to the management of an individual patient; although other studies have also found a strong correlation between degree of azotaemia and prognosis,24,25 high serum creatinine alone does not always indicate irreversible kidney damage and failure. The association found in the present study between peritoneal fluid, which can be a sign of overhydration in patients receiving intravenous fluids, and death in hospital corresponds with a previous study that found overhydration of cats during hospitalisation to be associated with decreased overall survival. 20
Conclusions
Ultrasonography may be used to identify azotaemic cats at greatest risk of ureteral obstruction, but when using pyelography as the reference test ultrasonography appears to be inaccurate for diagnosis of ureteral obstruction.
Footnotes
Accepted: 18 January 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Khan TM, Khan KNM. Acute kidney injury and chronic kidney disease. Vet Pathol 2015; 52: 441–444. [DOI] [PubMed] [Google Scholar]
- 2. Balakrishnan A, Drobatz KJ. Management of urinary tract emergencies in small animals. Vet Clin North Am Small Anim Pract 2013; 43: 843–867. [DOI] [PubMed] [Google Scholar]
- 3. Kyles AE, Hardie EM, Wooden BG, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984–2002). J Am Vet Med Assoc 2005; 226: 932–936. [DOI] [PubMed] [Google Scholar]
- 4. Lekcharoensuk C, Osborne CA, Lulich JP, et al. Trends in the frequency of calcium oxalate uroliths in the upper urinary tract of cats. J Am Anim Hosp Assoc 2005; 41: 39–46. [DOI] [PubMed] [Google Scholar]
- 5. Segev G, Nivy R, Kass PH, et al. A retrospective study of acute kidney injury in cats and development of a novel clinical scoring system for predicting outcome for cats managed by hemodialysis. J Vet Intern Med 2013; 27: 830–839. [DOI] [PubMed] [Google Scholar]
- 6. Bua AS, Dunn ME, Pey P. Respective associations between ureteral obstruction and renomegaly, urine specific gravity, and serum creatinine concentration in cats: 29 cases (2006–2013). J Am Vet Med Assoc 2015; 247: 518–524. [DOI] [PubMed] [Google Scholar]
- 7. d'Anjou M-A, Bedard A, Dunn ME. Clinical significance of renal pelvic dilatation on ultrasound in dogs and cats. Vet Radiol Ultrasound 2011; 52: 88–94. [PubMed] [Google Scholar]
- 8. Quimby JM, Dowers K, Herndon AK, et al. Renal pelvic and ureteral ultrasonographic characteristics of cats with chronic kidney disease in comparison with normal cats, and cats with pyelonephritis or ureteral obstruction. J Feline Med Surg 2016; 18: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rivers BJ, Walter PA, Polzin DJ. Ultrasonographic-guided, percutaneous antegrade pyelography: technique and clinical application in the dog and cat. J Am Anim Hosp Assoc 1997; 33: 61–68. [DOI] [PubMed] [Google Scholar]
- 10. Adin CA, Herrgesell EJ, Nyland TG, et al. Antegrade pyelography for suspected ureteral obstruction in cats: 11 cases (1995–2001). J Am Vet Med Assoc 2003; 222: 1576–1581. [DOI] [PubMed] [Google Scholar]
- 11. Westropp JL, Ruby AL, Bailiff NL, et al. Dried solidified blood calculi in the urinary tract of cats. J Vet Intern Med 2006; 20: 828–834. [DOI] [PubMed] [Google Scholar]
- 12. Zaid MS, Berent AC, Weisse C, et al. Feline ureteral strictures: 10 cases (2007–2009). J Vet Intern Med 2011; 25: 222–229. [DOI] [PubMed] [Google Scholar]
- 13. Kyles AE, Hardie EM, Wooden BG, et al. Management and outcome of cats with ureteral calculi: 153 cases (1984–2002). J Am Vet Med Assoc 2005; 226: 937–944. [DOI] [PubMed] [Google Scholar]
- 14. Kulendra NJ, Syme H, Benigni L, et al. Feline double pigtail ureteric stents for management of ureteric obstruction: short- and long-term follow-up of 26 cats. J Feline Med Surg 2014; 16: 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manassero M, Decambron A, Viateau V, et al. Indwelling double pigtail ureteral stent combined or not with surgery for feline ureterolithiasis: complications and outcome in 15 cases. J Feline Med Surg 2014; 16: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berent AC, Weisse CW, Todd K, et al. Technical and clinical outcomes of ureteral stenting in cats with benign ureteral obstruction: 69 cases (2006–2010). J Am Vet Med Assoc 2014; 244: 559–576. [DOI] [PubMed] [Google Scholar]
- 17. Nicoli S, Morello E, Martano M, et al. Double-J ureteral stenting in nine cats with ureteral obstruction. Vet J 2012; 194: 60–65. [DOI] [PubMed] [Google Scholar]
- 18. Berent A, Weisse C, Wright M, et al. The use of a subcutaneous ureteral bypass (SUB) device for ureteral obstructions in cats. J Vet Intern Med 2011; 25: 754–755. [Google Scholar]
- 19. Berent AC. Ureteral obstructions in dogs and cats: a review of traditional and new interventional diagnostic and therapeutic options. J Vet Emerg Critical Care 2011; 21: 86–103. [DOI] [PubMed] [Google Scholar]
- 20. Horowitz C, Berent A, Weisse C, et al. Predictors of outcome for cats with ureteral obstructions after interventional management using ureteral stents or a subcutaneous ureteral bypass device. J Feline Med Surg 2013; 15: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Remer EM, Papanicolaou N, Casalino DD, et al. ACR appropriateness criteria on renal failure. Am J Med 2014; 127: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 22. Roberts SF, Aronson LR, Brown DC. Postoperative mortality in cats after ureterolithotomy. Vet Surg 2011; 40: 438–443. [DOI] [PubMed] [Google Scholar]
- 23. Jakovljevic S, Rivers WJ, Chun R, et al. Results of renal ultrasonography performed before and during administration of saline (0.9% NaCl) solution to induce diuresis in dogs without evidence of renal disease. Am J Vet Res 1999; 60: 405–409. [PubMed] [Google Scholar]
- 24. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 25. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 26. Lee YJ, Chan JP, Hsu WL, et al. Prognostic factors and a prognostic index in cats with acute kidney injury. J Vet Intern Med 2012; 26: 500–505. [DOI] [PubMed] [Google Scholar]