Abstract
Objectives
Felis catus papillomavirus type 2 (FcaPV-2) commonly infects the skin of domestic cats, and mounting evidence suggests that the virus could be involved in a subset of feline skin cancers. The reason why some cats develop FcaPV-2-induced disease and others do not is currently unknown. However, it has been shown that kittens in different litters have markedly different FcaPV-2 DNA loads and the aim of this study was to determine whether these differences could be due to inherent differences in susceptibility to infection. Such differences could potentially explain why only a small proportion of cats develop FcaPV-2-associated skin disease.
Methods
Repeated skin swabs were taken to measure FcaPV-2 DNA loads in queens and kittens in a research colony. The kittens either stayed in their original litters or were moved between litters; eventually, all of the kittens were housed together. A subset of samples was also analysed for FcaPV-2 mRNA.
Results
While there were initially large differences in FcaPV-2 DNA loads between litters of kittens, these differences disappeared when the kittens were moved between litters or housed together. Importantly, the viral DNA loads changed too rapidly to be due to the acquisition or clearance of infection. In contrast, the differences in viral DNA loads between the different queens were sustained throughout the experiment. FcaPV-2 mRNA was also detected in samples from 1- to 8-day-old kittens.
Conclusions and relevance
The results suggest that the FcaPV-2 DNA load in a swab sample from an individual kitten largely reflects the overall level of FcaPV-2 shedding in the group of in-contact cats, rather than the infection status of the individual kitten. Therefore, there was no evidence for inherent differences in susceptibility to infection. However, the finding of FcaPV-2 mRNA suggests that at least some kittens do become infected with FcaPV-2 early in life.
Introduction
Papillomaviruses (PVs) are double-stranded DNA viruses that infect stratified squamous epithelia in a wide range of animal species. While many PV infections are asymptomatic, some PV types can cause hyperplastic warts and a small number of PV types can cause cancer, including the well-known example of cervical cancer in women caused, predominantly, by human PV types 16 and 18.
Domestic cats do not develop cutaneous warts and PV-induced disease has historically been considered to be rare in cats. However, more recent studies have shown that PV infection causes feline sarcoids, feline viral plaques and Bowenoid in situ carcinomas (BISCs).1–3 In addition, studies have consistently detected PV DNA more frequently in feline cutaneous squamous cell carcinomas (SCCs) than in other skin tumours, suggesting that PVs may also play a role in the development of these common and aggressive skin cancers.3,4 The mostly commonly detected PV type in feline viral plaques, BISCs and SCCs is Felis catus papillomavirus type 2 (FcaPV-2).2,4 The recent detection of FcaPV-2 gene expression in around one-third of feline cutaneous SCCs, and the transforming properties of these viral genes seen in cell culture, provides further evidence for a role of this virus in cancer development.5,6
At present, the epidemiology of FcaPV-2 infection in cats is poorly understood. In a previous study, FcaPV-2 DNA was found in skin swabs from 91% of 2-day-old kittens and, interestingly, some litters of kittens had very high viral DNA loads, whereas others had low viral DNA loads. 7 The cause of this variability in viral DNA load was unknown; however, it was hypothesised that some litters of kittens may be more or less susceptible to infection with FcaPV-2. In people, around a third of babies receive maternal antibodies against several different HPV types and these antibodies may protect against infection. 8 Alternatively, genetic differences in the major histocompatibility complex class I and class II molecules have been shown to influence HPV-induced disease in people;9,10 therefore, some kittens may be genetically less susceptible to infection. If some cats are less susceptible to FcaPV-2 infection than others, this may explain why infection is asymptomatic in most cats but a small proportion develop pre-neoplastic or neoplastic disease. However, another possible reason for the differing FcaPV-2 DNA loads in the different litters could be that the FcaPV-2 DNA load on the young kittens simply reflects the level of virus being shed by other cats in the household, rather than from the kittens themselves.
The purpose of this study was three-fold. Firstly, to test the hypothesis that the differing FcaPV-2 DNA loads in kittens were due to some kittens being inherently more or less susceptible to FcaPV-2 infection. This was carried out by measuring the FcaPV-2 DNA loads on kittens that were cross-fostered early in life and in kittens that were mixed at weaning. If the variation in FcaPV-2 DNA loads between different litters of kittens was due to inherent differences in susceptibility to FcaPV-2 infection, then this variation would be expected to persist, regardless of the changes in environment. Second, it was considered possible that the FcaPV-2 DNA detected on the young kittens was the result of environmental contamination and the kittens themselves were not truly infected. To investigate this possibility, samples were collected and analysed for the presence of FcaPV-2 mRNA. While FcaPV-2 DNA could be present in sloughed skin cells in the environment, FcaPV-2 RNA is only present in the deeper layers of infected skin, making the detection of FcaPV-2 RNA a more reliable measure of infection. The third objective of the study was to measure the FcaPV-2 DNA loads of the queens over an extended period, including times when they were isolated from other cats. This would assess whether the queens were likely to be shedding FcaPV-2 DNA.
This study is the first to evaluate FcaPV-2 DNA loads in repeated skin swabs from kittens that have been moved between different environments. Additionally, to our knowledge, this study is the first time that PV RNA has been evaluated in skin swabs from any domestic animal species.
Materials and methods
Selection of cats
Skin swabs were taken from four pregnant queens at the Centre for Feline Nutrition at Massey University, Palmerston North, New Zealand. Two queens were identified with a high FcaPV-2 DNA load (queens 1 and 3) and two with a low FcaPV-2 DNA load (queens 2 and 4). The four queens that were selected had a total of 17 kittens, all of which were included in the study. This study was approved by the Massey University animal ethics committee.
Swab collection for DNA analysis
Cotton-tipped swabs (Protec Solutions) were first moistened in sterile saline then firmly drawn across a 5 × 3 cm area of haired skin five times, according to a set pattern (Figure 1). All swabs were taken by one author (NAT). Two swabs were collected from each cat, one from the dorsum between the shoulder blades and the other from the abdomen cranial to the umbilicus. The two swab heads were collected into 500 µl sterile saline and processed as previously described. 7
Figure 1.
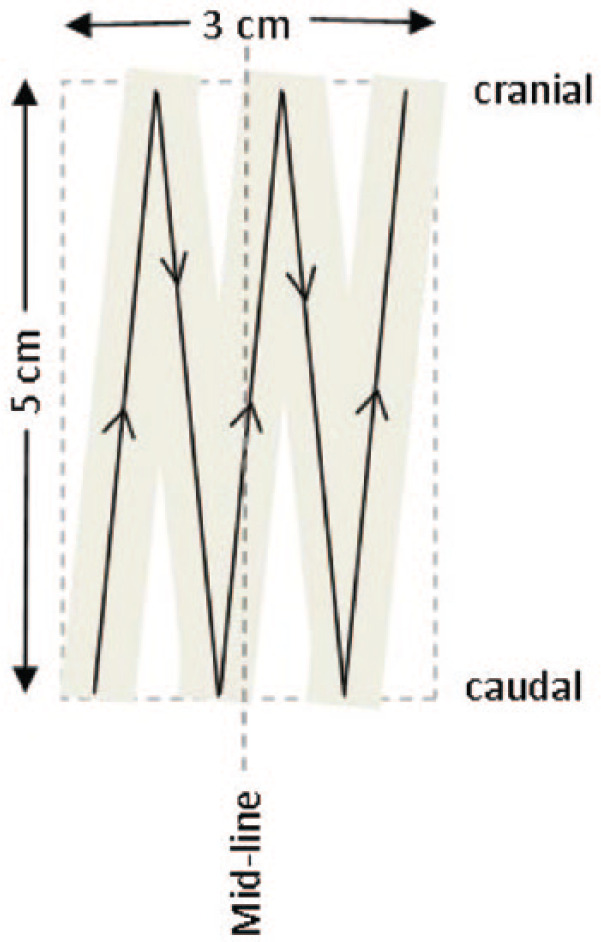
Swabbing technique. A saline-soaked cotton-tipped swab was drawn firmly across the skin in the pattern shown. The haircoat was not clipped and the cranial strokes were directed under the haircoat against the direction of hair growth to ensure good skin contact
Sampling time points
Swabs were collected from all queens 2 and 4 weeks prior to parturition. Following parturition, the queens and kittens were swabbed when the kittens were 1 day old, then before and after cross-fostering, and then weekly until the kittens were weaned and established in an outdoor run at 12–15 weeks of age (Figure 2). Once they were in the outdoor run, samples were taken every 4 weeks until the kittens were approximately 32 weeks of age.
Figure 2.
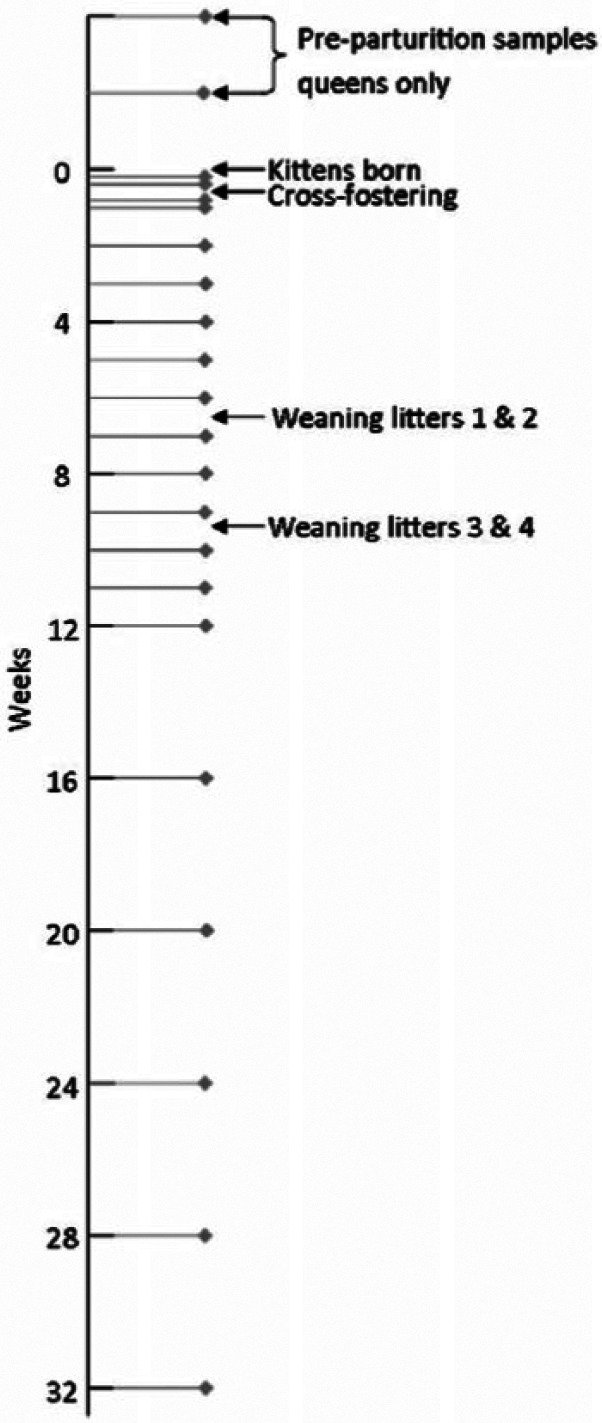
Sampling time points. In this general schedule of sampling time points, time 0 is when the kittens were born. The exact timing of the samples differed by 1–2 days from the general schedule as each set of two litters was born around 4 days apart. The kittens and queens were sampled at the same time points, although two extra swabs were taken from the queens in the 4 weeks prior to parturition
The timing of the cross-fostering occurred as follows: queen 1 gave birth to four live kittens although two died several days later as a result of complications at birth. Four days later queen 2 gave birth to five kittens and the next day, one kitten was cross-fostered from queen 2 (low viral DNA load) onto queen 1 (high viral DNA load). The kitten cross-fostered onto queen 1 died as a result of misadventure at around 2 weeks of age. Queen 3 gave birth to five kittens. Four days later, queen 4 gave birth to three kittens. The next day one kitten was removed from queen 3 (high viral DNA load) and cross-fostered onto queen 4 (low viral DNA load).
Swabs for RNA analysis
In addition to the swab samples collected for DNA analysis, swabs were also collected for RNA analysis. The swabs for RNA analysis were taken in a similar manner to the DNA swabs. Initial efforts to detect RNA in the skin swabs were unsuccessful; however, part-way through the kittening season it was discovered that collecting the swabs into Ambion RNAlater solution (Life Technologies), rather than saline, yielded small quantities of extractible RNA. This method was used to collect swabs from two queens and their kittens when the kittens were 1, 3, 8 and 15 days of age. A total of 35 swabs were collected for RNA analysis. Skin biopsies were also collected at post-mortem examination from the three kittens that died. These were taken from the dorsal area of skin that had been previously swabbed and were kept at −70°C prior to RNA extraction.
DNA and RNA extraction
DNA was extracted from the samples using the High Pure PCR Template Preparation kit (Roche Applied Science) as previously reported. 7 RNA was extracted from the swab and tissue samples using the ReliaPrep RNA Tissue Miniprep system (Promega) according to the manufacturer’s recommendations. The tissue samples required an additional step of homogenising 40 mg of each sample with a Mini-Beadbeater-16 (BioSpec) prior to DNA or RNA extraction. RNA was reverse transcribed into cDNA with the Transcriptor first strand cDNA synthesis kit (Roche Applied Science) using 0.6 µg total RNA, and both random hexamer and oligo-dT primers according to the manufacturer’s recommendations.
Real-time PCR
A previously developed real-time PCR assay was used to quantify the FcaPV-2 DNA in the swab samples. 7 Quantities were normalised to copies per swab as normalising to feline genomic DNA was meaningless given the virus-containing mature skin cells did not have nuclei. To detect FcaPV-2 E6/E7 mRNA, the same real-time PCR assay was used, but with a cDNA template, as previously described. 5 As the same sequence was present in viral DNA and cDNA, a negative RT control was included for every sample to exclude the presence of genomic DNA in the cDNA samples. Reverse transcriptase real-time PCR was also used to detect mRNA coding for the FcaPV-2 capsid (L1) protein, and two F catus RNA reference genes (beta-actin and Abelson proto-oncogene 2 non-receptor tyrosine kinase), as previously described. 5
Statistical analysis
Data were analysed using Minitab 17.2.1. Log base 10 transformed data were used for statistical analyses. Samples that had no detectable virus were set at half the minimum detectable level of the assay prior to log transformation. Results were back transformed and reported as geometric means and 95% confidence intervals (CIs).
Results
Identification of litters with high and low FcaPV-2 DNA loads
Queens 1 and 3 had consistently high FcaPV-2 DNA loads over the course of the study, with mean FcaPV-2 DNA loads of 15,849 (95% CI 5623–44,668) and 10,234 (95% CI 3890–26,915) copies of viral DNA per swab (Figure 3). High viral DNA loads were also found on the kittens from queens 1 and 3, from as early as 1 day of age, with mean FcaPV-2 copy numbers of 1995 (95% CI 417–9550) and 11,749 (95% CI 3548–39,811) copies per swab. These queens and their kittens were designated the high-viral-load litters. In comparison, the viral DNA load was significantly lower in Queens 2 and 4, with mean viral loads of 282 (95% CI 100–832) and 457 (95% CI 170–1259) copies, respectively (P <0.001). The kittens from queens 2 and 4 also had significantly lower viral loads, with means of 69 (95% CI 20–229) and 83 (95% CI 22–324) copies of FcaPV-2 DNA per swab, at 1 day of age (P <0.001). These queens and kittens were designated the low-viral-load litters. The quantity of FcaPV-2 DNA found on the kittens prior to weaning was strongly correlated to the quantity on their queen (r = 0.833; P <0.05).
Figure 3.
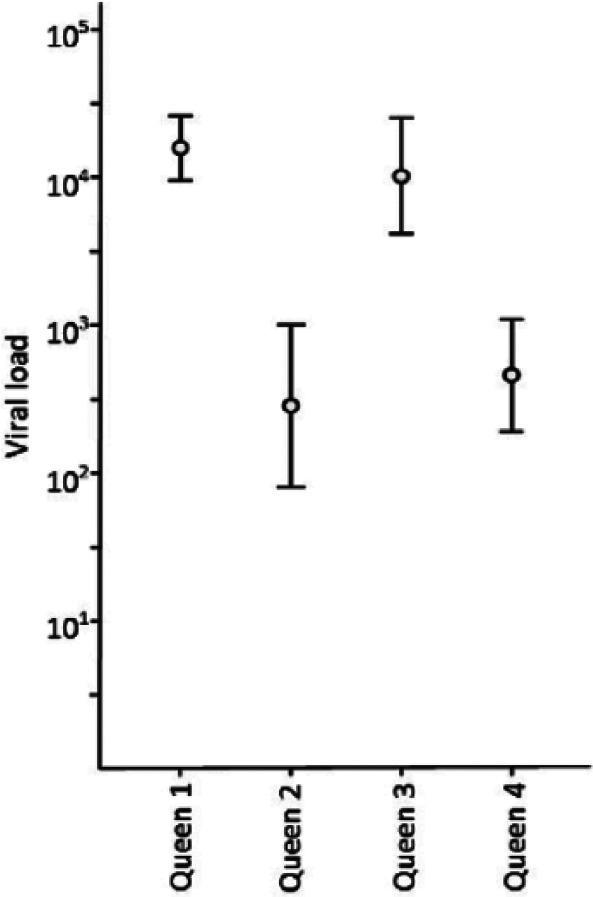
Mean viral DNA load of the queens. The mean viral load (copies of Felis catus papillomavirus type 2 DNA per swab) with 95% confidence interval, for the four queens over the duration of the study. Between 17 and 20 samples were taken from each queen over a 10–12 month period. During this time the queens were housed individually, with their kittens, and in the outdoor run with other adult cats, yet the viral load on each queen remained relatively constant
FcaPV-2 DNA load after cross-fostering
Cross-fostering a kitten from a low-viral-load litter to a high-viral-load litter, resulted in a marked increase in the quantity of FcaPV-2 DNA detected: from 54 copies to 6064 copies after 2 days and 158,966 copies after 7 days (Figure 4). In reverse, cross-fostering a kitten from a litter with a high viral load to a litter with a low viral load resulted in a marked decrease in the quantity of FcaPV-2 DNA in swabs from that kitten: from 16,103 copies per swab to 14 copies per swab over 3 days (Figure 5). Interestingly, although queen 4 and her kittens generally maintained a low viral DNA load, a transient increase in the quantity of FcaPV-2 DNA detected on these cats occurred 5 days after the addition of a cross-fostered kitten from a litter with a high viral load.
Figure 4.

Cross-fostering from a low- to high-viral-load litter. The mean viral load (copies of Felis catus papillomavirus type 2 DNA per swab), with 95% confidence interval band, is shown for both the high-viral-load litter (darker shading) and low-viral-load litter (lighter shading). During the first week of life, one kitten was cross-fostered from the low-viral-load litter to the high-viral-load litter as indicated by the points. One skin swab was collected from this kitten prior to cross-fostering (white point) and two swabs were collected after the cross-fostering (black points). The cross-fostered kitten died at 2 weeks of age. Note that the viral load of this kitten increased markedly when moved to the high-viral-load litter. At around 6 weeks of age the kittens were weaned and moved to an outdoor run as indicated by the vertical line. This resulted in a loss of the difference in viral load between the two litters
Figure 5.
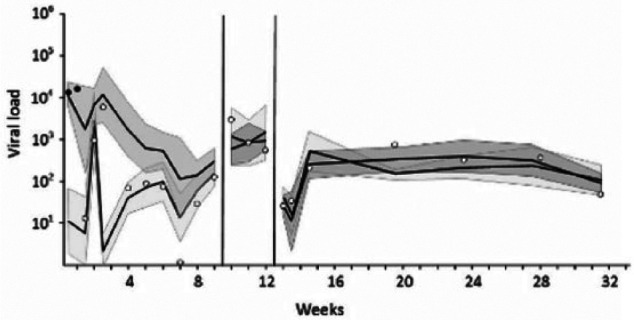
Cross-fostering from a high- to low-viral-load litter. The mean viral load (copies of Felis catus papillomavirus type 2 DNA per swab), with 95% confidence interval band, is shown for the kittens in both the high-viral-load litter (darker shading) and low-viral-load litter (lighter shading). During the first week of life, one kitten was cross-fostered from the high-viral-load litter to the low-viral-load litter as indicated by the points. Black points indicate the viral load of this kitten prior to cross-fostering, while white points indicate the viral load after cross-fostering. Note that the viral load of this kitten changed rapidly to reflect that of its new litter. For this group of kittens, weaning and then moving to the outdoor run happened as two separate events, the timing of which is indicated by the vertical lines. Note how the differences in viral load seen prior to weaning were no longer present after weaning
FcaPV-2 DNA load after weaning
When the kittens were separated from their queens at weaning, the FcaPV-2 DNA load on the kittens from the high-viral-load litters dropped and the FcaPV-2 DNA load on the kittens from the low-viral-load litters increased, so that all of the kittens had a similar viral load that was relatively stable for the remainder of the study, at around 93 copies of FcaPV-2 DNA per swab (95% CI 69−123). Overall, there was a statistically significant difference in viral load between the high and low groups before weaning (P <0.001) but not after weaning (P <0.758).
Detection of FcaPV-2 RNA
FcaPV-2 mRNA was found in three swab samples and one tissue biopsy, which came from 3/9 (33%) kittens. FcaPV-2 late viral (L1/L2) gene expression was detected in two swabs from one kitten from a low-viral-load litter (days 1 and 8), and in one swab from a kitten from a high-viral-load litter (day 3). Additionally, FcaPV-2 early gene (E6/E7) mRNA was detected in a skin biopsy from one kitten, from a high-viral-load litter, that died 4 days after birth. There was no significant association between the presence of FcaPV-2 mRNA and the FcaPV-2 DNA load of the kittens.
Discussion
The aim of this study was to investigate whether the previously observed differences in FcaPV-2 DNA load between different litters of kittens could relate to differing susceptibilities to FcaPV-2 infection, as this may explain why some cats develop disease due to FcaPV-2 infection and others do not. However, the results of the cross-fostering in this study revealed that the FcaPV-2 DNA load on kittens changed markedly when they were moved to different litters. These changes occurred over 2–7 days; much faster than the complete PV lifecycle, which is tightly linked to the differentiation process of the epidermis and generally takes weeks to months. 11 Therefore, the FcaPV-2 DNA on these kittens did not appear to be due to viral replication in the kitten’s skin, rather it was consistent with virus shed from the queens that was present on the skin surface. This was further supported by the results obtained later in the study when the kittens were mixed together in a new environment, which resulted in the loss of the observed differences in viral load between the different litters. Therefore, the results of this study suggest that the FcaPV-2 DNA load detected on kittens relates to the level of environmental contamination rather than inherent differences in their susceptibility to FcaPV-2 infection.
To investigate whether all the FcaPV-2 DNA detected on the kittens was due to environmental contamination, swabs and skin biopsy samples were evaluated for the presence of FcaPV-2 mRNA. The detection of FcaPV-2 late gene (L1/L2) mRNA in three swabs from two kittens at 1–8 days of age, and the detection of FcaPV-2 early gene (E6/E7) mRNA in a skin biopsy from one kitten that died 4 days after birth, supported the presence of true infection in these kittens and suggests that at least some of the detected FcaPV-2 DNA is the result of true infection. The possibility that the mRNA could have been derived from an infection of another cat was considered. However, PV gene expression is tightly linked to the differentiation process of skin and E6/E7 mRNA is only present in deep epidermal layers. A change in viral promoter activity in the superficial skin layers means these genes are no longer transcribed and so PV E6/E7 mRNA would not be present in contaminating skin cells shed from the skin surface of another cat. 12 Similarly, it is unlikely that L1/L2 mRNA would be present in mature skin cells shed from another cat but present on the kitten’s skin, as the cornification process that these cells undergo prior to sloughing would degrade any residual viral mRNA, along with the cell nucleus (whereas the PV DNA is protected inside the PV capsid). 13
Surprisingly, the kitten that had FcaPV-2 mRNA in two swab samples was from a low-viral-load litter, and the FcaPV-2 DNA load on this kitten remained low for the duration of the study. Therefore, it appears that cats can be infected by FcaPV-2 despite having consistently low levels of FcaPV-2 DNA in swab samples.
FcaPV-2 mRNA was found in samples from 3/9 (33%) kittens in this study. However, this probably underestimates the proportion of kittens that became infected with FcaPV-2. The quantity of mRNA in the swab samples was low owing to the small number of nucleated cells collected by this method. Therefore, some true FcaPV-2 infections may not have been detected because too few infected cells were collected. By comparison, the biopsies collected at post-mortem examination contained numerous nucleated cells and large quantities of mRNA. However, skin biopsies are also an insensitive measure of true FcaPV-2 infection because they represent only a small area of skin and may miss focal FcaPV-2 infections. While FcaPV-2 DNA is shed from the skin surface and distributed widely in mature skin cells, FcaPV-2 mRNA is restricted to the focal area of epidermis overlying the infected basal cells. Therefore, the chance of detecting FcaPV-2 mRNA in a skin biopsy from an infected cat is likely to be low. Owing to the lack of a sensitive measure of true FcaPV-2 infection it was not possible to determine the prevalence of FcaPV-2 infection in this study, but the presence of FcaPV-2 gene expression in a tissue biopsy and several skin swabs suggests that some of the kittens were infected with FcaPV-2.
In contrast to the kittens, significant differences in viral DNA load were sustained between the queens for the duration of the study, regardless of their environment. In people, cutaneous PVs are thought to be acquired during early childhood.14,15 After an initial period of viral replication, the development of a cell-mediated immune response suppresses viral replication so that PV DNA is only detectable in low levels in the basal cells. 16 The shedding of significantly different amounts of virus from the adult cats may indicate that some cats fail to clear or suppress the FcaPV-2 infections acquired as kittens or that adult cats frequently become re-infected with the virus. This suggests that cats may have inherent differences in their abilities to suppress FcaPV-2-replication, but this does not manifest until later in life. Such differences may influence which cats subsequently develop FcaPV-2-induced skin disease.
Conclusions
The results of this study suggest that the FcaPV-2 DNA load in a swab sample from an individual kitten largely reflects the overall level of FcaPV-2 shedding in the group of in-contact cats, rather than the infection status of the individual kitten. Therefore, there was no evidence to support the hypothesis that some litters of kittens are more susceptible to infection than others, owing to genetic differences or the presence of maternal antibodies. However, the finding of FcaPV-2 mRNA in samples from 1- to 8-day-old kittens suggests that at least some kittens do become infected with FcaPV-2 early in life. The detection of sustained differences in FcaPV-2 DNA viral loads in the adult cats suggests that the long-term response to the virus may be more important than the early stages of FcaPV-2 infection in explaining why some cats develop FcaPV-2 disease and others do not.
Acknowledgments
The authors wish to acknowledge the support of the staff at the Massey University Centre for Feline Nutrition.
Footnotes
Accepted: 25 April 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by a Massey University Research Fund grant.
References
- 1. Munday JS, Knight CG, Howe L. The same papillomavirus is present in feline sarcoids from North America and New Zealand but not in any non-sarcoid feline samples. J Vet Diagn Invest 2010; 22: 97–100. [DOI] [PubMed] [Google Scholar]
- 2. Munday JS, Peters-Kennedy J. Consistent detection of Felis domesticus papillomavirus 2 DNA sequences within feline viral plaques. J Vet Diagn Invest 2010; 22: 946–949. [DOI] [PubMed] [Google Scholar]
- 3. Munday JS, Kiupel M, French AF, et al. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet Dermatol 2008; 19: 259–263. [DOI] [PubMed] [Google Scholar]
- 4. Munday JS, French AF, Peters-Kennedy J, et al. Increased p16CDKN2A protein within feline cutaneous viral plaques, bowenoid in situ carcinomas, and a subset of invasive squamous cell carcinomas. Vet Pathol 2011; 48: 460–465. [DOI] [PubMed] [Google Scholar]
- 5. Thomson NA, Munday JS, Dittmer KE. Frequent detection of transcriptionally active Felis catus papillomavirus 2 in feline cutaneous squamous cell carcinomas. J Gen Virol 2016; 97: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 6. Altamura G, Corteggio A, Pacini L, et al. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology 2016; 496: 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Thomson NA, Dunowska M, Munday JS. The use of quantitative PCR to detect Felis catus papillomavirus type 2 DNA from a high proportion of queens and their kittens. Vet Microbiol 2015; 175: 211–217. [DOI] [PubMed] [Google Scholar]
- 8. Heim K, Hudelist G, Geier A, et al. Type-specific antiviral antibodies to genital human papillomavirus types in mothers and newborns. Reprod Sci 2007; 14: 806–814. [DOI] [PubMed] [Google Scholar]
- 9. Beskow AH, Josefsson AM, Gyllensten UB. HLA class II alleles associated with infection by HPV16 in cervical cancer in situ. Int J Cancer 2001; 93: 817–822. [DOI] [PubMed] [Google Scholar]
- 10. Chen D, Juko-Pecirep I, Hammer J, et al. Genome-wide association study of susceptibility loci for cervical cancer. J Natl Cancer Inst 2013; 105: 624–633. [DOI] [PubMed] [Google Scholar]
- 11. Hooper CE. Cell turnover in epithelial populations. J Histochem Cytochem 1956; 4: 531–540. [DOI] [PubMed] [Google Scholar]
- 12. Johansson C, Schwartz S. Regulation of human papillomavirus gene expression by splicing and polyadenylation. Nat Rev Microbiol 2013; 11: 239–251. [DOI] [PubMed] [Google Scholar]
- 13. Eckhart L, Lippens S, Tschachler E, et al. Cell death by cornification. Biochim Biophys Acta 2013; 1833: 3471–3480. [DOI] [PubMed] [Google Scholar]
- 14. Antonsson A, Karanfilovska S, Lindqvist PG, et al. General acquisition of human papillomavirus infections of skin occurs in early infancy. J Clin Microbiol 2003; 41: 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol 2013; 133: 2788–2790. [DOI] [PubMed] [Google Scholar]
- 16. Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 2011; 414: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]


