Abstract
Objectives
The objective of this study was to determine if hypoglycemia is an effect of overnight fasting and gonadectomy in kittens, as well as to determine predictors of baseline and postoperative blood glucose.
Methods
This was a prospective observational study. Seventy-five kittens between the age of 8 and 16 weeks undergoing routine castration or ovariohysterectomy at an animal shelter were included. Two blood glucose measurements were analyzed per kitten after an overnight fast: a baseline reading prior to preoperative examination, and a reading immediately postoperatively. Predictors of the baseline and postoperative blood glucose levels were determined using multi-level mixed-effects linear regression.
Results
Kittens, when fasted overnight, were not hypoglycemic (<60 mg/dl). No kittens exhibited clinical signs consistent with hypoglycemia. No kittens had a blood glucose <70 mg/dl postoperatively. Postoperative hyperglycemia (>150 mg/dl) was observed in 44% of kittens. The only predictor of fasted blood glucose levels was body condition score. The only predictor of postoperative blood glucose levels was the fasting blood glucose value.
Conclusions and relevance
Overnight fasting prior to elective sterilization in 8- to 16-week-old kittens did not result in hypoglycemia. Concern regarding hypoglycemia after a prolonged fast in kittens may be unwarranted for short procedures in healthy animals.
Introduction
Fasting dogs and cats prior to anesthesia and surgery is a common practice in veterinary medicine. Although there are few objective data to support this practice, an overnight fast is commonly recommended prior to an anesthetic procedure in adult animals, with water remaining freely available. 1 The most common rationale for preoperative fasting is a reduction in the occurrence of vomiting or gastroesophageal reflux that may result in pulmonary aspiration; 2 however, there is evidence in dogs and humans that prolonged fasting may not result in a reduction in gastric contents or the likelihood of a reflux or aspiration event.3–5
Further complicating recommendations regarding fasting times are altered protocols in patients <6 months of age, in which concerns exist regarding the development of hypoglycemia after a prolonged fast.1,6 Hypoglycemia may develop in pediatric animals as a result of decreased hepatic glycogen stores, as well as immature renal and hepatic tissue, greater myocardial demand or a combination of these factors. 5 A current preoperative protocol for juvenile patients undergoing gonadectomy in a spay–neuter program is to be fasted for no more than 4 h prior to surgery. 6
Although shortened fasting periods and postoperative glucose supplementation are routinely recommended for pediatric patients, no study has established the risk of hypoglycemia in kittens undergoing brief elective gonadectomy after a typical overnight fast. Our aim was to document perioperative blood glucose concentrations in healthy 8- to 16-week-old kittens undergoing elective gonadectomy after an overnight fast, and to evaluate patient and procedural factors predictive of perioperative blood glucose levels. We hypothesized that healthy kittens would not be hypoglycemic after an overnight fast and brief elective gonadectomy.
Materials and methods
Kittens aged between 8 and 16 weeks presenting for routine gonadectomy through an animal shelter were enrolled in the study. Kittens were excluded from the study if documented to be outside the 8- to 16-week-old age range, or if not healthy enough to undergo anesthesia and gonadectomy. The age of the kittens was determined from shelter records and confirmed by weight and dentition by a veterinarian (REK). Kittens were housed with littermates if present or singly if not. As per the existing shelter protocol, kittens had food removed no later than midnight prior to surgery. Water remained freely available. The research protocol was approved by the Midwestern University Institutional Animal Care and Use Committee and was conducted in the shelter facility.
Preoperative physical examinations were performed the morning of surgery. Data recorded included sex, age, weight and body condition score (BCS) on a scale from 1–9. 7 BCS was assigned by a single person (REK) for all kittens. At the start of the examination, a small aliquot (0.3 μl) of blood was drawn using a lancing device provided by the manufacturer of the glucometer (AlphaTRAK 2; Abbott Laboratories), set to a depth of 4, from the right metacarpal pad. A new lancet was used for each kitten and each blood glucose measurement. All samples were analyzed immediately after lancing with a single glucometer calibrated as per the manufacturer’s instructions.
Kittens that qualified for enrollment in the study were entered into a spreadsheet and assigned a random decimal number between 0 and 1 (Microsoft Excel for Mac 15). The kittens were sorted by this number in order to create a random surgical order. Anesthesia was induced using a single intramuscular administration of a combination of dexmedetomidine (0.02 mg/kg [0.04 mg/lb]), ketamine (2.75 mg/kg [6.06 mg/lb]) and butorphanol (0.275 mg/kg [0.606 mg/lb]). Additional rescue analgesia was available. Female kittens were intubated and maintained at a surgical plane of anesthesia throughout the surgery on isoflurane in oxygen. All sterilization procedures were performed by the same veterinarian (REK). The second blood glucose reading was taken from the left metacarpal pad immediately after completion of surgery. Kittens were monitored for signs of clinical hypoglycemia, particularly lethargy, tremors and seizures.
Hypoglycemia was defined as a blood glucose <60 mg/dl; hyperglycemia was defined as blood glucose >150 mg/dl. 8 Blood glucose values were tested for normality using the Shapiro–Wilk normality test, and were found to be non-normally distributed. A non-parametric Wilcoxon rank-sum test was used to compare median blood glucose values between kittens with a BCS of ⩽4 and kittens with a BCS of 5. Predictors of the baseline and postoperative blood glucose levels were determined using mixed-effects linear regression clustered on day and litter (Stata version 14). Significance was set at P <0.05.
Results
Data collection took place over 3 days. A total of 75 kittens were enrolled in the study; 40 male (53%) and 35 female (47%) with a mean age of 10.3 weeks (range 8–16 weeks). The mean weight was 1.1 kg (2.4 lbs) (range 0.9–1.6 kg [2–3.5 lbs]). One kitten had a BCS of 3, 60 kittens a BCS of 4 and 14 kittens a BCS of 5. Baseline blood glucose was collected in the morning between the hours of 8:26 and 10:46 a.m. Mean time of fast at baseline sample collection was 570 mins (range 506–646 mins).
The median baseline blood glucose value was 104 mg/dl (range 61–161 mg/dl) (Figure 1). Median baseline blood glucose was 99 mg/dl for kittens with a BCS of 3 or 4, while it was 123 mg/dl for kittens with a BCS of 5. The baseline blood glucose value was significantly different (P = 0.001) between kittens with a BCS of 3 or 4 and kittens with a BCS of 5. BCS was the only demographic variable that significantly predicted (P = 0.005) the baseline blood glucose reading. The baseline blood glucose concentration was predicted to be 16 mg/dl higher for a BCS of 5 vs a BCS of 4 after controlling for clustering by litter and day. Not significant were sex, age and weight.
Figure 1.
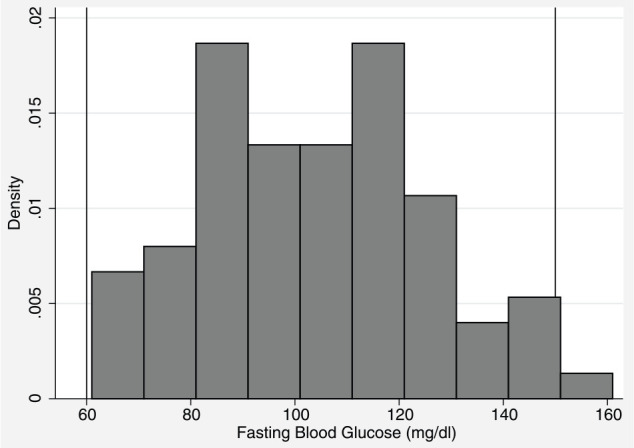
Baseline blood glucose values after overnight fasting. Lines at limit for hypoglycemia (60 mg/dl) and hyperglycemia (150 mg/dl)
The median postoperative blood glucose value was 146 mg/dl (range 74–283 mg/dl) (Figure 2). The postoperative blood glucose was 109 mg/dl for the kitten with a BCS of 3. The median postoperative blood glucose for kittens with a BCS of 4 was 146 mg/dl, and 132 mg/dl for kittens with a BCS of 5 (Figure 3). Postoperative blood glucose increased for 89% of kittens (Figure 4). The median change between the first and second blood glucose values was 42 mg/dl (range −59 to 163 mg/dl). The kitten with a BCS of 3 increased by 9 mg/dl, kittens with a BCS of 4 had a median increase of 44 mg/dl and kittens with a BCS of 5 had a median increase of 19 mg/dl. No demographic variables, including BCS, were predictive of the postoperative blood glucose value. The value of the initial baseline blood glucose measurement was the only variable that predicted the postoperative blood glucose reading. For every additional 1 mg/dl baseline blood glucose reading above 60 mg/dl, the postoperative reading was predicted to increase by 0.5 mg/dl (P = 0.013). The duration of surgery, duration of anesthesia (induction through end of surgery) and postoperative temperature were not predictive of the postoperative blood glucose values. No clinical signs of hypoglycemia were observed at any time point.
Figure 2.
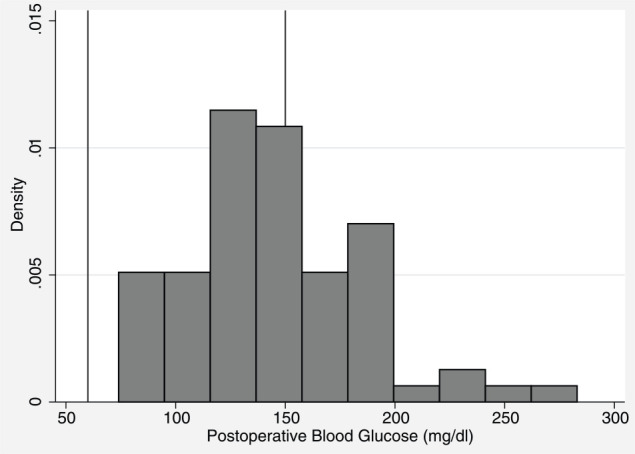
Postoperative blood glucose values. Lines at limit for hypoglycemia (60 mg/dl) and hyperglycemia (150 mg/dl)
Figure 3.
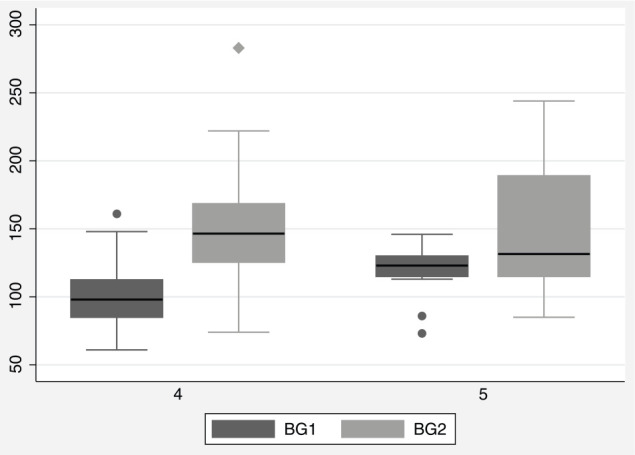
Box plot showing baseline (BG1) and postoperative (BG2) blood glucose values for kittens with BCS 4 and 5. Black line at median value
Figure 4.
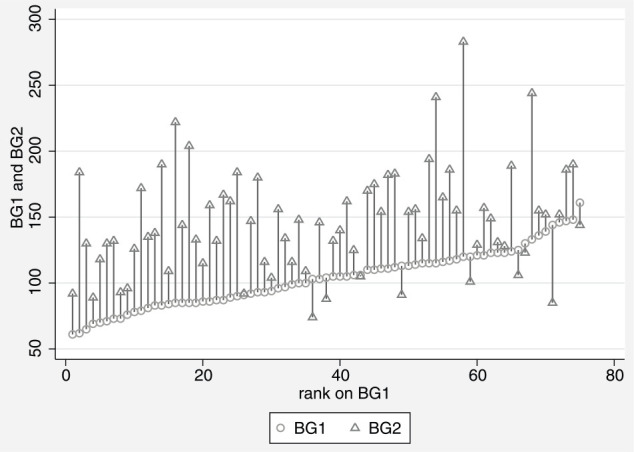
Paired plot showing postoperative blood glucose value (triangle BG2) ordered by baseline blood glucose value (circle BG1)
No surgical complications occurred. Median surgical time was 5 mins (range 4–19 mins) for ovariohysterectomy and 1 min for castration (range 1–15 mins), with all surgical times rounded to the nearest minute. A single male cat was bilaterally cryptorchid with intra-abdominal testicles; this cat had a 15 min surgery. Excluding the cryptorchid cat, the range for castration time was 1–2 mins. Surgeries occurred over a several-hour period on each day of data collection (day 1: 4.5 h; day 2: 4.0 h; day 3: 2.8 h), with the length of individual fast prior to the postoperative blood glucose measurement varying depending on where each kitten fell within the random surgical order (mean 758 mins, range 638–946 mins). The time of day that surgery was performed was not significant for any blood glucose sampling time point.
Discussion
No kitten was hypoglycemic pre- or postoperatively. This contrasts with conventional wisdom that kittens would become hypoglycemic after a prolonged fast. 6 In the kittens of our study, this may be owing to hepatic metabolism and glomerular filtration rate reaching maturity by 8–12 weeks of age. 9 Other factors that may cause hypoglycemia in pediatric animals are decreased hepatic glycogen stores, as well as immature renal and hepatic tissue. 5 Perioperative handling could also play a role in the development of increased blood glucose; cats are known to develop profound increases in glucose in response to a stressful event, including restraint and handling. A prior study identified a rapid and significant increase in glucose in all cats subjected to a short bath, with a relationship seen between struggling and the concentration of glucose. 10
Postoperative glucose levels changed by a median of 42 mg/dl (range −59 to 163 mg/dl). This may have been due to a stress response from surgery that triggers the release of glucagon and diminishes insulin secretions. 11 In children, hyperglycemia associated with surgery is more common than hypoglycemia due to a hypermetabolic stress response, which increases glucose production and causes insulin resistance. 12 Intraoperative stimulation is an important component of this stress response. 11 Perioperative hyperglycemia in humans, including pediatric patients, has been associated with a variety of deleterious effects, including postoperative mortality, and cardiovascular, respiratory, neurologic and infectious morbidity.13–15 Hyperglycemia may not be of significant consequence to healthy kittens when it occurs in a transient fashion; however, simple interventions to decrease the severity of hyperglycemia, such as minimization of preoperative stress (restraint, handling, auditory input) and reduction, where possible, of intraoperative stimulation may be warranted.
Kittens that had lower blood glucose values preoperatively tended to have a lower BCS. Body weight was not significant, likely owing to a cohort of young animals where weight varies based on age. Weight does not assess the lean-to-fat ratio or the amount of body fat covering an animal, whereas BCS is a means of subjectively capturing this data. 7 The association between blood glucose and BCS could be due to a greater amount of fat stores that can be more readily mobilized for energy.
Postoperative glucose was predicted only by each patient’s baseline glucose level. This suggests that a proportional increase in blood glucose occurred for most patients from the fasted baseline. The relatively uniform anesthetic and surgical duration may have masked a true effect of these interventions on blood glucose, however, and further studies are warranted to evaluate the effect of longer anesthetic and surgical procedures.
In addition to short anesthetic and surgical procedures, other limitations of this study include the absence of a blood glucose measurement late in the recovery period, which may have been useful in identifying occult hypoglycemia. As the response to the physiologic stress of examination, anesthesia and surgery resolves, blood glucose levels may decrease, which would not have been captured by this study.
The hypoglycemic threshold of 60 mg/dl was selected for this study based on reference intervals for adult cats, in which hypoglycemia is commonly defined as ⩽60 mg/dl; 8 however, specific reference intervals for fasted kittens are not established. Although the value of 60 mg/dl was selected based on available reference intervals for adult cats and no kittens displayed signs of hypoglycemia, it may be that clinically significant physiologic alterations take place at a blood glucose level above the threshold of 60 mg/dl selected for this study.
Conclusions
This study showed that overnight fasting of healthy kittens between 8 and 16 weeks of age prior to elective gonadectomy was well tolerated and did not result in hypoglycemia, suggesting that concerns over development of hypoglycemia due to an overnight fast and brief gonadectomy in healthy kittens may be unfounded. This study did not identify an ideal preoperative fasting period for kittens, and further study is warranted to find fasting times in this population that maximize patient comfort and minimize risk of aspiration, as well as to characterize glucose trends during longer surgical procedures.
Footnotes
Accepted: 27 April 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bednarski R, Grimm K, Harvey R, et al. AAHA anesthesia guidelines for dogs and cats. J Am Anim Hosp Assoc 2011; 47: 377–385. [DOI] [PubMed] [Google Scholar]
- 2. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology 1993; 78: 56–62. [DOI] [PubMed] [Google Scholar]
- 3. Hardy JF, Lepage Y, Bonneville-Chouinard N. Occurrence of gastroesophageal reflux on induction of anaesthesia does not correlate with the volume of gastric contents. Can J Anaesth 2008; 37: 502–508. [DOI] [PubMed] [Google Scholar]
- 4. Savvas I, Raptopoulos D, Rallis T. A ‘light meal’ three hours preoperatively decreases the incidence of gastro-esophageal reflux in dogs. J Am Anim Hosp Assoc 2016; 52: 1–7. [DOI] [PubMed] [Google Scholar]
- 5. Cook-Sather SD, Litman RS. Modern fasting guidelines in children. Best Pract Res Clin Anaesthesiol 2006; 20: 471–481. [DOI] [PubMed] [Google Scholar]
- 6. Griffin B, Bushby PA, McCobb E, et al. The Association of Shelter Veterinarians’ 2016 veterinary medical care guidelines for spay–neuter programs. J Am Vet Med Assoc 2016; 249: 165–188. [DOI] [PubMed] [Google Scholar]
- 7. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–17. [Google Scholar]
- 8. Koenig A. Hypoglycemia. In: Silverstein D, Hopper K. (eds). Small animal critical care medicine. St Louis, MO: Elsevier, 2015, pp 352–357. [Google Scholar]
- 9. Pettifer G, Grubb T. Neonatal and geriatric patients. In: Tranquilli W, Thurmon J, Grimm K. (eds). Lumb & Jones’ veterinary anesthesia and analgesia. Ames, IA: Blackwell Publishing, 2007, pp 985–981. [Google Scholar]
- 10. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002; 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 11. Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000; 85: 109–117. [DOI] [PubMed] [Google Scholar]
- 12. Sharma V, Sharma R, Singh G, et al. Preoperative fasting duration and incidence of hypoglycemia and hemodynamic response in children. J Chem Pharm Res 2011; 3: 382–391. [Google Scholar]
- 13. Filho NO, Alves RL, Fernandes AT, et al. Association of increased morbidity with the occurrence of hyperglycemia in the immediate postoperative period after elective pediatric neurosurgery. J Neurosurg Pediatr 2016; 17: 1–5. [DOI] [PubMed] [Google Scholar]
- 14. Wintergerst KA, Buckingham B, Gandrud L, et al. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics 2006; 18: 173. [DOI] [PubMed] [Google Scholar]
- 15. Palermo NE, Gianchandani RY, McDonnell ME, et al. Stress hyperglycemia during surgery and anesthesia: pathogenesis and clinical implications. Curr Diab Rep 2016; 16: 33. [DOI] [PubMed] [Google Scholar]


