Abstract
Objectives
The aim of the study was to evaluate the cardiorespiratory effects, quality of sedation and recovery of intramuscular alfaxalone–dexmedetomidine–butorphanol (ADB) and ketamine–dexmedetomidine–butorphanol (KDB), in cats.
Methods
Nine adult, healthy cats (6.63 ± 1.42 kg) were enrolled in a blinded, randomized, crossover experimental design. Cats were sedated twice intramuscularly, once with ADB (alfaxalone 1 mg/kg, dexmedetomidine 0.005 mg/kg, butorphanol 0.2 mg/kg), and once with KDB (ketamine 5 mg/kg, dexmedetomidine 0.005 mg/kg, butorphanol 0.2 mg/kg), in random order. Data collected included heart rate (HR), arterial blood pressure and blood gas analysis, respiratory rate and sedation score. Analysis of variance with Bonferroni post-hoc correction was used for parametric data, and a Wilcoxon signed rank test was used for non-parametric data. Significance was set at P <0.05.
Results
Total sedation time was shorter for ADB (90.71 ± 15.12 mins vs 147.00 ± 47.75 mins). Peak sedation was observed within 15 mins in both groups. Quality of recovery was excellent in both groups. HR decreased over time in both groups. Diastolic and mean arterial pressure decreased over time for ADB, becoming significant after 30 mins. All cardiovascular variables were within the clinically acceptable range in both groups. Arterial partial pressure of oxygen was significantly decreased from baseline for KDB at all time points (73 ± 2.5 mmHg [9.7 ± 0.3 kPa] vs ADB 83 ± 2.6 mmHg [11 ± 0.3 kPa]). Hypoventilation was not observed.
Conclusions and relevance
Both protocols produced acceptable cardiovascular stability. Sedation and recovery quality were good, albeit sedation was shorter with ADB. Although oxygenation was better maintained in the ADB group, all sedated cats should receive oxygen supplementation.
Introduction
Cats are commonly sedated to perform diagnostic or minimally invasive procedures, minimize stress and facilitate handling. An ideal sedation protocol allows for quick and smooth decreased responsiveness while maintaining cardiopulmonary function and providing quiet recovery. Given that monitoring of physiologic variables is usually more limited during sedation 1 compared with general anesthesia, it is important that the drug combination provides good cardiorespiratory stability.
Alfaxalone was approved by the Food and Drug Administration in 2012 as an anesthetic induction agent in cats and dogs. It is a short-acting neurosteroid with rapid onset of action, producing dose-dependent changes in cardiovascular and respiratory variables with hypoventilation and apnea when given intravenously at supraclinical doses. 2 Alfaxalone is licensed for intramuscular administration in Australia, New Zealand and South Africa and can be given via this route, which makes it a desirable agent for sedation in cats.
The mortality rate of cats undergoing sedation is significantly higher than dogs (0.12% vs 0.07%). 1 The main causes of death in these patients are related to cardiorespiratory failure. As such, it is particularly important to establish safe sedation protocols that pose minimal alterations on the cardiorespiratory system. The objective of this study is to evaluate the effects of alfaxalone in combination with dexmedetomidine and butorphanol on the cardiorespiratory system and quality of sedation and recovery in cats. We hypothesized that sedation time will be shorter with alfaxalone than with ketamine, and that alfaxalone provides better cardiopulmonary stability than ketamine in combination with dexmedetomidine and butorphanol.
Material and methods
Animals
Nine adult domestic shorthair cats, seven neutered males and two spayed females, weighing 6.63 ± 1.42 kg (mean ± SD) (range 5.1–8.5 kg) and aged 6.1 ± 2.8 years were used. Cats were considered healthy based on physical examination, packed cell volume, total solids, and serum biochemistry prior to every treatment. Animals were group housed in approved facilities, fed with commercially available cat food (Laboratory Feline Diet 5003*) and access to water ad libitum. The study was approved by the local Institutional Animal Care and Use Committee (IACUC 15-116).
Experimental design
The study was conducted in a blinded, randomized, crossover experimental design. Randomization was undertaken within Excel (Microsoft). Each cat was treated twice with a 7 day washout between treatments. The following treatments were employed: alfaxalone (Alfaxan; Jurox) 1 mg/kg, dexmedetomidine (Dexdomitor; Zoetis) 0.005 mg/kg, butorphanol (Torbugesic; Fort Dodge Animal Health) 0.2 mg/kg (ADB) intramuscularly or ketamine (Ketaset; Zoetis) 5 mg/kg, dexmedetomidine (Dexdomitor; Zoetis) 0.005 mg/kg, butorphanol (Torbugesic; Fort Dodge Animal Health) 0.2 mg/kg (KDB) intramuscularly.
Procedures
Food but not water was withheld for at least 12 h prior to treatment.
On the day of the experiment each cat was weighed, a physical examination was performed and blood was sampled for biochemistry. For instrumentation, cats were anesthetized with isoflurane in oxygen delivered into a chamber. When righting reflex was lost, cats were taken out of the chamber and anesthesia was continued using isoflurane in 100% oxygen, delivered with a tight-fitting mask attached to a non-rebreathing system. An intravenous catheter was placed into the left cephalic vein, and an arterial catheter into the coccygeal artery, for invasive blood pressure measurement and arterial blood gas sampling. Cats were allowed to recover for a minimum of 1 h or until they reached a sedation score of 0. Sedation was scored using subjective scoring criteria described by Granholm et al 2006 (see Appendix 1 in the supplementary material). 3
After full recovery, baseline values for heart rate (HR), arterial blood pressure (BP), respiratory rate (RR), sedation score (SED), body temperature and arterial blood gas values were recorded. The arterial catheter was connected to an electronically zeroed fluid-filled disposable pressure transducer, placed at the level of the heart, and connected to a multiparameter monitor (VetTrends V; System Vet). The assigned treatment was administered as one injection into the epaxial muscle with a 22 G needle. Reaction to drug injection was scored as reaction or non-reaction.
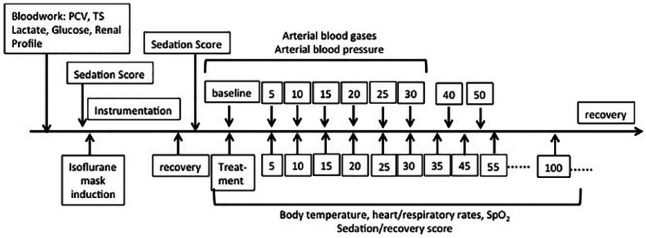
When cats lost their righting reflex, they were placed in right lateral recumbency on a heating blanket connected to a multiparameter monitor (VetTrends V; System Vet) recording electrocardiogram (ECG), systolic (SAP), diastolic (DAP) and mean (MAP) arterial pressures, and blood oxygen saturation (SpO2), RR and SED were recorded. Rectal temperature was measured with a commercially available digital thermometer. One milliliter of each arterial blood gas sample was collected and immediately analyzed with a commercially available analyzer (ISTAT; ABAXIS). All variables were recorded every 5 mins post-injection for 30 mins. After 30 mins, the arterial catheter was removed and ECG, SpO2, HR, RR and SED continued to be monitored every 5 mins for another 30 mins and then every 10 mins thereafter, until animals reached an SED of 0. The chronological order of the data collection is given in Figure 1.
Figure 1.
Chronological order of the data collection with time given in minutes. PCV = packed cell volume; TS = total solids; SpO2 = blood oxygen saturation
An overall recovery score was given by using a subjective 5-point scale, described by Pascoe et al 2006 (see Appendix 2 in the supplementary material). 4 Recovery was considered prolonged when the sedation score was ⩾5 at 120 mins or any time point after that.
Statistical analysis
Data analyses were performed using JMP (SAS Software; USA). A repeated measures analysis of variance (ANOVA) with a mixed effect model was used to analyze HR, BP, RR, body temperature, and the partial pressure of oxygen and carbon dioxide in arterial blood (PaO2 and PaCO2, respectively). Treatment and time points were entered as fixed effects and animals as random effect. When time point effects were detected, a Bonferroni post-hoc correction was performed to compare each time point against baseline with the treatment. When treatment effects were detected, treatments were compared by each time point with a paired t-test.
Total sedation time (time from treatment injection till reaching sedation score of 0), total general anesthesia time (time from starting delivery of inhalant to discontinuing inhalant anesthesia), time between the end of anesthesia and injection of treatment, and recovery score were assessed with a Wilcoxon signed rank test. Significance was set at P <0.05.
Results
All cats completed the study, with nine cats in each treatment group. In one cat of the KDB group, the arterial catheter was lost during recovery from general anesthesia. Only SED, HR, RR and body temperature were included from this cat for data analysis. In one cat of the ADB group, the arterial catheter was not working properly after sedation. Only blood gases but no blood pressure reading could be obtained.
Within the ADB group, cartridge error did not allow PaO2 to be obtained for single cats at T10, T20, and T25, and PaCO2 for one cat at T20 and for two cats at T25.
One cat developed a sinus bradycardia with sinus pause followed by a junctional escape rhythm after 60 mins with KDB and 40 mins with ADB. Intramuscular atipamezole (Antisedan; Zoetis) was administered, which resolved the arrhythmias. Post antagonism, the data from this cat was excluded from further analysis.
In total, dexmedetomidine was antagonized in three cats with atipamezole due to prolonged recovery, for KDB at 140 and 150 mins and for ADB at 145 mins. At these times, cats still had an SED of 6/9 and 8/9 with KDB and 5/9 with ADB without improvement. Post-reversal, the data from these cats was excluded from further analysis.
General anesthesia time for instrumentation and time between the end of general anesthesia to injection of treatment was not significantly different for ADB and KDB (30.78 ± 4.92 vs 29.56 ± 6.15, and 63.78 ± 25.09 vs 72.56 ± 27.36 mins, respectively).
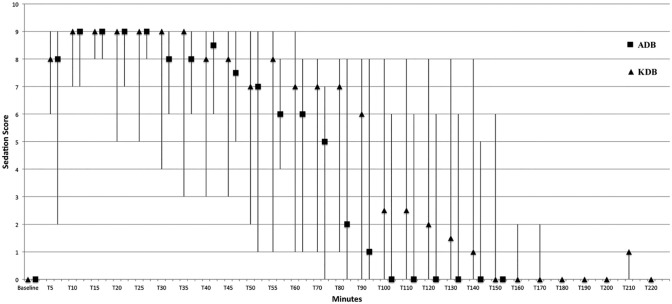
Total sedation time was significantly longer (P <0.04) for KDB (147.00 ± 47.75 mins) than for ADB (90.71 ± 15.12 mins). Baseline SED was 0 in all cats in both groups. Median SED were greater than baseline 5 mins after ADB or KDB administration. All cats were in lateral recumbency within 5 mins post-injection, and peak sedation was observed within 10 mins post-injection. Median SEDs were similar for both treatments, within the first 35 mins post-injection. A median SED of greater than 8 was observed for 25 mins after reaching peak sedation for both groups (Figure 2).
Figure 2.
Sedation score for nine cats receiving alfaxalone, dexmedetomidine, butorphanol (ADB) or ketamine, dexmedetomidine, butorphanol (KDB) intramuscularly. Data are reported as median with maximum and minimum values represented by vertical bars at each time point measured in mins
Recovery quality was not different between groups with a median score of 4.50 (range 5–4) for ADB and 5 (range 5–2) for KDB. All cats recovered quietly, without any adverse events.
Baseline cardiovascular values were not different between treatments. A significant decrease in HR from baseline (P <0.0002) was observed for both ADB and KDB over time. Mean HR was lower in ADB compared with KDB during the first 35 mins of sedation (ADB 144 ± 29 versus KDB 157 ± 33 bpm).
Due to non-compliance of some cats, baseline BP values could only be obtained from four cats in the ADB group and five cats in the KDB group, and no difference was found between groups. For the first 25 mins post-injection SAP, DAP and MAP did not change markedly from baseline with either treatment. At 30 mins, DAP and MAP for ADB were significantly lower compared with baseline (P <0.0095, and P <0.04). No significant differences in SAP, DAP and MAP were observed between treatments.
In both groups, the RR decreased significantly from baseline within 5 mins post-injection (P <0.0001) and did not return to baseline by the end of sedation (SED = 0). RR appeared to be higher in ADB during the first 35 mins of sedation (38 vs 32 breaths/min).
Overall baseline values for PaO2 were similar for both groups (ADB 97.2 ± 9.7 mmHg, KDB 99.3 ± 12.1 mmHg). Marked decreases from baseline were observed for KDB (P <0.0001) at any given time point of measurement, with mean PaO2 below 75 mmHg for over 25 mins post-injection. Five cats in the KDB group had a PaO2 below 80 mmHg at every single time point post-injection. At T10 and T15 seven cats and at T20 and T25 six cats had a PaO2 less than 80 mmHg.
For ADB, mean PaO2 did decrease significantly from baseline at 10, 20, 25 and 30 mins post-injection (P <0.02); however, mean values did not decrease below 80 mmHg at any time point. At T5, 10 three cats, at T20 and T30 two cats, and at T15 and T30 one cat in the ADB group had a PaO2 <80 mmHg. Comparing between treatments, the mean PaO2 was significantly higher for ADB when compared with KDB at 10 mins (P <0.03) and 15 mins (P <0.01). Overall, the mean PaO2 for ADB was consistently higher than KDB at any given time point (Figure 3).
Figure 3.
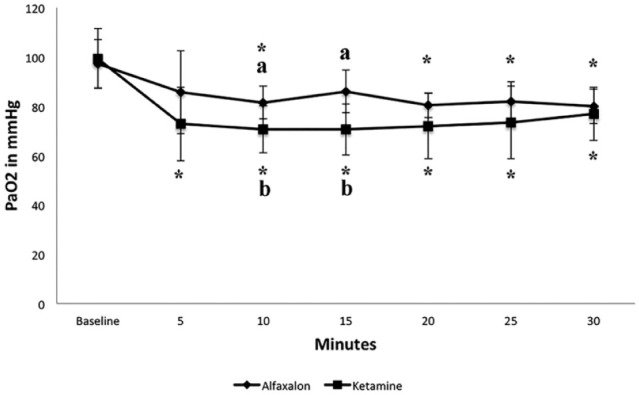
Partial pressure of oxygen in arterial blood (PaO2) in mmHg reported as mean value for nine cats receiving alfaxalone, dexmedetomidine, butorphanol (ADB) and 8/9 cats receiving ketamine, dexmedetomidine, butorphanol (KDB) intramuscularly. *Within a group, values differ significantly (P ⩽0.05) from the value at time 0 (= baseline). a,b Between groups, value differs significantly (P ⩽0.05) at measured time point
PaCO2 did increase for both treatments from baseline over time. For KDB, this increase was significant 5 mins post-injection (P <0.008) and for any other time point thereafter (P <0.001). For ADB a significant increase from baseline was observed at 20 mins (P <0.04) and at 30 mins (P <0.015). When comparing treatments against each other, the PaCO2 was lower for ADB than KDB at any time, reaching significance at 5 and 10 mins post-injection (P <0.012 and P <0.005). For both treatments, the mean PaCO2 maintained within a normal physiologic range after sedation.
SpO2 was below 95% within 5 mins post-injection for both treatments, and remained low. SpO2 readings were lower for ADB than KDB at T5, T25 and T30, inconsistent with what was observed for PaO2 (ADB had consistently higher PaO2 values). Cardiorespiratory data is summarized in Table 1 for the first 30 mins of sedation.
Table 1.
Results of blood gas analysis, cardiorespiratory variables and body temperature for nine cats after administration of alfaxalone (1 mg/kg), butorphanol (0.2 mg/kg), dexmedetomidine (5 μg/kg) (ADB) and 8/9 cats after ketamine (5 mg/kg), butorphanol (0.2 mg/kg), dexmedetomidine (5 μg/kg) (KDB)
| Variable | Treatment | Baseline | Time after drug administration (mins) |
|||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 30 | |||
| PaO2 (mmHg) | ADB KDB |
97.2 ± 9.7 99.3 ± 12.1 |
85.7 ± 16.8 72.8 ± 15 * |
81.5 ± 6.7
*
a
70.6 ± 9.5 * b |
86 ± 8.7a
70.6 ± 10.4 * b |
80.4 ± 5.1
*
71.9 ± 13.2 * |
82 ± 7.9
*
73.5 ± 14.8 * |
80 ± 7.0
*
76.9 ± 10.9 * |
| PaCO2 (mmHg) | ADB KDB |
29.7 ± 6.2 29 ± 4.8 |
30.7 ± 6.2a
36.8 ± 1.8 * b |
31.7 ± 3a
39.2 ± 5.3 * b |
32.7 ± 4.6 38.4 ± 7.4 * |
33.7 ± 3.1
*
40.2 ± 6.2 * |
34 ± 4.5 40 ± 6.8 * |
35.3 ± 5.6
*
38.2 ± 4.9 * |
| SpO2 | ADB KDB |
91.4 ± 4.8 92.7 ± 6.1 |
93.3 ± 3.5 90.3 ± 4.3 |
90.6 ± 4.6 87.8 ± 2.6 |
92.1 ± 5.2 90.1 ± 2.5 |
88.7 ± 8.1 90.1 ± 4.5 |
87.2 ± 8.9 90.7 ± 4.1 |
|
| MAP (mmHg) | ADB KDB |
120 ± 7.3 120.8 ± 10.6 |
124.3 ± 6.6 112.5 ± 5.6 |
118.9 ± 6.8 113.3 ± 14.7 |
116.9 ± 8.8 111 ± 14.8 |
115.9 ± 9 112.1 ± 12 |
116.6 ± 10.7 113.9 ± 9.5 |
112 ± 10.8
*
111.5 ± 10 |
| SAP (mmHg) | ADB KDB |
134.5 ± 6.6 137 ± 19 |
132 ± 5.4 123 ± 18.7 |
128.1 ± 7.5 128 ± 20.9 |
127 ± 9.7 125 ± 15 |
124 ± 10 127 ± 10.5 |
125.3 ± 10.9 128.9 ± 8.4 |
123.4 ± 12.9 129 ± 6.2 |
| DAP (mmHg) | ADB KDB |
110 ± 8.2 108 ± 6.4 |
118 ± 7.8 105.1 ± 16.4 |
111 ± 7.2 105.3 ± 16.5 |
108.5 ± 8.6 101.8 ± 16.7 |
108.9 ± 10.5 102.3 ± 14.5 |
108.9 ± 12.9 102.7 ± 11.3 |
103 ± 8.7
*
99.6 ± 12.1 |
| HR (beats/min) | ADB KDB |
201 ± 31 205 ± 39 |
161 ± 45
*
170 ± 33 * |
147 ± 32
*
157 ± 28 * |
142 ± 25
*
157 ± 37 * |
139 ± 24
*
154 ± 32 * |
138 ± 25
*
154 ± 31 * |
143 ± 24
*
154 ± 38 * |
| RR (breaths/min) | ADB KDB |
64 ± 26 53 ± 16 |
37 ± 12*
36 ± 13* |
37 ± 5*
30 ± 10* |
41 ± 5 34 ± 12 |
37 ± 7 30 ± 7 |
38 ± 9*
31 ± 6* |
39 ± 9*
33 ± 7* |
| Temperature (ºC) | ADB KDB |
38.1 ± 0.4 37.8 ± 0.6 |
37.9 ± 0.4 38 ± 0.7 |
38.2 ± 0.4 38.1 ± 0.7 |
37.9 ± 0.6 38.3 ± 0.6 |
37.9 ± 0.6 38.2 ± 0.5 |
37.9 ± 0.5 38.1 ± 0.6 |
37.8 ± 0.5 37.9 ± 0.6 |
Data are given as mean ± SD
Significantly (P ⩽0.05) different from baseline value
In each column, values with different superscript letters are significantly (P <0.05) different
PaO2 = partial pressure of oxygen in arterial blood; PaCO2 = partial pressure of carbon dioxide in arterial blood; SpO2 = blood oxygen saturation; MAP = mean arterial pressure; SAP = systolic arterial pressure: DAP = diastolic arterial pressure; HR = heart rate; RR = respiratory rate
Body temperature steadly declined over time in both groups, reaching significance at 45 mins (ADB) and 90 mins (KDB). Temperature did not return to baseline by the end of the observations. One cat in the ADB and three cats in the KDB became moderately hypothermic (temperature ⩽36.5ºC).
Discussion
This study showed that ADB and KDB combinations induced lateral recumbency within 5 mins following a single intramuscular injection in cats. Furthermore, total sedation time with the alfaxalone combination was significantly shorter than with the ketamine combination. We were unable to detect a difference in quality of sedation, recovery and cardiovascular effects between both treatments. In addition, altough pulmonary effects were different between the treatments, with KDB causing hypoxemia in a greater number of animals, some individual cats also developed hypoxemia with the alfaxalone combination.
In the past, several sedation protocols including alfaxalone have been investigated, with controversial results with regards to sedation and recovery quality. One study 5 reported that cats receiving alfaxalone 5 mg/kg, dexmedetomidine 0.01 mg/kg, with or without hydromorphone 0.1 mg/kg intramuscularly, were moderately to profoundly sedated but still responsive to sounds. In our cats, the sedation was very predictable for either treatment, with every cat being in lateral recumbency within 5 mins and achieving peak sedation (SED >8) within 10 mins. Peak sedation lasted for 25 mins, with signs of recovery slowly appearing after that. A possible explanation for the more consistent sedation may be the difference in drug combinations and the dosage of alfaxalone. The combination of a higher dose of alfaxalone with a pure mu agonist may have exaggerated negative side effects in cats, which can be seen when alfaxalone is given alone at dosages above 5 mg/kg. 6
In a study evaluating different dosages of alfaxalone given to cats as sole agent, 6 the following side effects were observed during recovery: ataxia, muscle tremors, opisthotonus-like posture, paddling of the forelimbs and vomiting. Similar observation was made by Grubb et al 2013, 5 where recovery was reported as unpredictable, marked by an extreme responsiveness to acoustic stimuli, when alfaxalone and dexmedetomidine with or without hydromorphone were given intramuscularly. When alfaxalone was compared with propofol as an intravenous induction agent for short procedures in cats premedicated with buprenorphine and acepromazine, more cats in the alfaxalone group were reported to paddle and tremble during the recovery. 7 With our protocol no such observation was made, and the recovery score between groups was not significantly different between treatments. Adami et al 2016 8 found similar sedation quality in cats as we did; however, higher dosages were recommended based on their results. This may be due to the fact that in the aforementioned study cats were undergoing minimally invasive procedures, whereas in our study no painful procedures were performed. Hence, our dosages may be more relevant when diagnostics such as physical exam, radiographs or ultrasound are performed.
Within both treatments the heart rate decreased significantly from baseline within 5 mins post-injection, but was well maintained in all cats throughout the study. This effect has been reported with other studies using alpha (α)2-agonists in cats with or without the co-administration of an opioid and ketamine or alfaxalone.9–13 Dexmedetomidine promotes a biphasic decrease in HR by initially activating peripheral α2-adrenoreceptors, increasing systemic vascular resistance, followed by a longer lasting centrally mediated increase in vagal activity. 14 Butorphanol given to isoflurane anesthetized cats intravenously at two different dosages did not change HR significantly. 15 Ketamine is reported to have positive chronotropic effects by its central action inhibiting efferent cardiac vagal stimulation and therefore increasing HR. 16 As other studies reported, we were not able to see a significant increase in HR 10 from baseline, which might be a result of a blunting effect from butorphanol and dexmedetomidine. The HR appeared higher for KDB within the first 35 mins, which may reflect ketamine’s positive chronotrope effect. We therefore conclude that dexmedetomidine was the major contributor to the decrease in HR.
The baseline blood pressure measurement occurred with the cats standing and being distracted. Although the animals were used to handling, this interaction may still have caused stress in our cats, reflected by the mean HR of 200 beats/min (ADB) to 205 beats/min (KDB) and a MAP of 120 mmHg (white-coat effect). 17 Although the blood pressure slowly declined over time, reaching significance after 30 mins in the ADB group (MAP and DAP), the values approached normality with no hypotension or hypertension being observed after sedation in either group.
Hypoxemia is defined as a PaO2 of less than 80 mmHg, with values below 60 mmHg considered severe hypoxemia. In both treatments, we observed a decrease in mean PaO2 over time. For KDB, mean PaO2 was consistently less than 80 mmHg at all measurements, in contrast to ADB where the mean PaO2 never decreased below 80 mmHg. However, individual cats in the ADB group also developed hypoxemia at different timepoints, but the occurrence was not as frequent as in the KDB group where five cats were hypoxemic at any given time point.
Since all cats were healthy, breathing room air, and no hypoventilation occurred, formation of pulmonary atelectasis (due to positioning), leading to V/Q mismatch, may have been the underlying mechanisms for the decrease in oxygenation in both groups. In conscious dogs, lateral recumbency is associated with lower PaO2 than sternal recumbency. 18 All cats were positioned in right lateral recumbency throughout the study. We cannot exclude that administering 100% oxygen during general anesthesia (instrumentation phase) caused atelectasis; 19 however, all animals were allowed to recover for at least 60 mins before sedation and baseline values for PaO2 and PaCO2 were not different between treatments and were within the normal physiologic range. Sedation has depressant effects on tidal volume, alveolar ventilation and chest wall mechanics, 20 possibly promoting atelectasis during sedation in lateral recumbency, contributing to ventilation-perfusion (V/Q) mismatch. Zeiler et al 2014 21 reported a decreased PaO2 in cats receiving ketamine and medetomidine with or without an opioid, which is similar to our KDB group. Interestingly, ketamine anesthesia has been described as having no effects in gas exchange in spontaneously ventilating human patients. 20 In contrast, when thiopental/halothane are used, atelectasis develops within 5 mins. 22
Because cats in the KDB group were consistently hypoxemic at any time point, and all cats were handled the same way, it seems reasonable to assume that the ketamine combination caused more severe V/Q mismatching. Despite the well known bronchodilatory effects of ketamine 23 and little effect of minute volume, 24 it appears that the association with dexmedetomidine and butorphanol resulted in greater depression and lower oxygenation than the alfaxalone protocol.
In general, SpO2 should reflect the animal’s SaO2, which, in turn, is closely related to the PaO2. As a result, higher PaO2 should translate into higher SpO2, and vice versa. Interestingly, although PaO2 for ADB was higher than for KDB, no difference was detected for the SpO2 values between groups. SpO2 depends on pulse pressure and tissue perfusion to obtain a signal. Erroneous readings may occur when a vasoconstrictor drug such as dexmedetomidine is administered, causing significant increase in systemic vascular resistance and a decrease in pulse pressure. This may lead to inaccurate values of SpO2 that do not reflect the actual SaO2. 25
Normal temperature in awake cats is 37.7–39.2°C. Impaired thermoregulation due to drug administration causing muscle relaxation, prevention of shivering and a cool ambient temperature can make sedated and anesthetized patients hypothermic. In our study, cats’ body temperature decreased over time, reflecting mild hypothermia in the ADB group. Cats receiving KDB maintained their temperature longer when compared with baseline; however, no significant difference between treatments was detected. In anesthetized cats, the prevalence of mild hypothermia (⩾36.5°C) was reported as 26.5% and moderate hypothermia (36.5–34°C) as 60.4%. 26 Overall, one cat in the ADB and three cats in the KDB became moderately hypothermic (temperature ⩽36.5ºC), which is less than reported in the study above. In contrast to what was observed here, the administration of butorphanol and ketamine has been associated with an increase in temperature above the physiologic value of 39.2°C. 27 There is no evidence in the literature, however, that suggests that alfaxalone has a direct effect on thermoregulation in cats. The results of this study emphasize that, regardless of the protocol used, it is important to monitor body temperature in sedated animals, and provide support when needed to avoid hypothermia or hyperthermia.
Conclusions
Both protocols provided good quality of sedation and recovery, and clinically acceptable cardiorespiratory changes. Although oxygenation was better maintained in cats given ADB, all sedated cats should receive oxygen supplementation. For shorter diagnostic procedures, ADB may be the better choice.
Supplemental Material
Adapted subjective scoring criteria used to evaluate sedation
Adapted subjective scoring criteria to evaluate the overall quality of recovery
Acknowledgments
We would like to thank Chin-Chi Liu for the statistical analysis and Roxanne Coffey, LVT, for her cat-whispering skills.
Footnotes
Supplementary material: The following files are available:
Appendix 1: Adapted subjective scoring criteria used to evaluate sedation.
Appendix 2: Adapted subjective scoring criteria to evaluate the overall quality of recovery.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 16 October 2017
References
- 1. Brodbelt DC, Blissitt KJ, Hammond RA, et al. The risk of death: the confidential enquiry into perioperative small animal fatalities. Vet Anaesth Analg 2008; 35: 365–373. [DOI] [PubMed] [Google Scholar]
- 2. Muir W, Lerche P, Wiese A, et al. The cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in cats. Vet Anaesth Analg 2009; 36: 42–54. [DOI] [PubMed] [Google Scholar]
- 3. Granholm M, McKusick BC, Westerholm FC, et al. Evaluation of the clinical efficacy and safety of dexmedetomidine or medetomidine in cats and their reversal with atipamezole. Vet Anaesth Analg 2006; 33: 214–223. [DOI] [PubMed] [Google Scholar]
- 4. Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg 2006; 33: 2–7. [DOI] [PubMed] [Google Scholar]
- 5. Grubb TL, Greene SA, Perez TE. Cardiovascular and respiratory effects, and quality of anesthesia produced by alfaxalone administered intramuscularly to cats sedated with dexmedetomidine and hydromorphone. J Feline Med Surg 2013; 15: 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamura J, Ishizuka T, Fukui S, et al. Sedative effects of intramuscular alfaxalone administered to cats. J Vet Med Sci 2015; 77: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathis A, Pinelas R, Brodbelt DC, et al. Comparison of quality of recovery from anaesthesia in cats induced with propofol or alfaxalone. Vet Anaesth Analg 2012; 39: 282–290 [DOI] [PubMed] [Google Scholar]
- 8. Adami C, Imboden T, Giovannini AE, et al. Combinations of dexmedetomidine and alfaxalone with butorphanol in cats: application of an innovative stepwise optimisation method to identify optimal clinical doses for intramuscular anaesthesia. J Feline Med Surg 2016; 18: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamont LA, Bulmer BJ, Grimm KA, et al. Cardiopulmonary evaluation of the use of medetomidine hydrochloride in cats. Am J Vet Res 2001; 62: 1745–1749. [DOI] [PubMed] [Google Scholar]
- 10. Selmi AL, Mendes GM, Lins BT. Evaluation of the sedative and cardiorespiratory effects of dexmedetomidine, dexmedetomidine-butorphanol, and dexmedetomidine-ketamine in cats. J Am Vet Med Assoc 2003; 222: 37–41. [DOI] [PubMed] [Google Scholar]
- 11. Khenissi L, Nikolayenkova-Topie O, Broussaud S, et al. Comparison of intramuscular alfaxalone and ketamine combined with dexmedetomidine and butorphanol for castration in cats. J Feline Med Surg 2017; 19: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McSweeney PM, Martin DD, Ramsey DS, et al. Clinical efficacy and safety of dexmedetomidine used as a preanesthetic prior to general anesthesia in cats. J Am Vet Med Assoc 2012; 240: 404–412. [DOI] [PubMed] [Google Scholar]
- 13. Santos L, Ludders JW, Erb HN, et al. Sedative and cardiorespiratory effects of dexmedetomidine and buprenorphine administered to cats via oral transmucosal or intramuscular routes. Vet Anaesth Analg 2010; 37: 417–424. [DOI] [PubMed] [Google Scholar]
- 14. Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999; 54: 146–165. [DOI] [PubMed] [Google Scholar]
- 15. Ilkiw JE, Pascoe PJ, Tripp LD. Effects of morphine, butorphanol, buprenorphine, and U50488H on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2002; 63: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 16. Inoue K, Arndt JO. Efferent vagal discharge and heart rate in response to methohexitone, althesin, ketamine and etomidate in cats. Brit J Anaesth 1982; 54: 1105–1116. [DOI] [PubMed] [Google Scholar]
- 17. Belew AM, Barlett T, Brown SA. Evaluation of the white-coat effect in cats. J Vet Int Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 18. McMillan MW, Whitaker KE, Hughes D, et al. Effect of body position on the arterial partial pressures of oxygen and carbon dioxide in spontaneously breathing, conscious dogs in an intensive care unit. J Vet Emerg Crit Care 2009; 19: 564–570. [DOI] [PubMed] [Google Scholar]
- 19. Staffieri F, De Monte V, De Marzo C, et al. Effects of two fractions of inspired oxygen on lung aeration and gas exchange in cats under inhalant anaesthesia. Vet Anaesth Analg 2010; 37: 483–490. [DOI] [PubMed] [Google Scholar]
- 20. Tokics L, Strandberg A, Brismar B, et al. Computerized tomography of the chest and gas exchange measurements during ketamine anaesthesia. Acta Anaesth Scan 1987; 31: 684–692. [DOI] [PubMed] [Google Scholar]
- 21. Zeiler GE, Brighton TD, Fosgate GT, et al. Anaesthetic, analgesic and cardiorespiratory effects of intramuscular medetomidine-ketamine combination alone or with morphine or tramadol for orchiectomy in cats. Vet Anaesth Analg 2014; 41: 411–420. [DOI] [PubMed] [Google Scholar]
- 22. Brismar B, Hedenstierna G, Lundquist H, et al. Pulmonary densities during anesthesia with muscular relaxation – a proposal of atelectasis. Anesthesiology 1985; 62: 422–428. [DOI] [PubMed] [Google Scholar]
- 23. Gateau O, Bourgain JL, Gaudy JH, et al. Effect of ketamine on isolated human bronchial preparations. Br J Anaesth 1989; 63: 692–695. [DOI] [PubMed] [Google Scholar]
- 24. Mankikian B, Cantineau JP, Sartene R, et al. Ventilatory pattern and chest wall mechanics during ketamine anesthesia in humans. Anesthesiology 1986; 65: 492–499. [DOI] [PubMed] [Google Scholar]
- 25. Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Resp Med 2013; 107: 789–799. [DOI] [PubMed] [Google Scholar]
- 26. Redondo JI, Suesta P, Gil L, et al. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 2012; 170: 206–209. [DOI] [PubMed] [Google Scholar]
- 27. Posner LP, Pavuk AA, Rokshar JL, et al. Effects of opioids and anesthetic drugs on body temperature in cats. Vet Anaesth Analg 2010; 37: 35–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adapted subjective scoring criteria used to evaluate sedation
Adapted subjective scoring criteria to evaluate the overall quality of recovery