Abstract
Objectives
The objective of this study was to identify the renal ultrasonographic (US) findings most strongly associated with azotaemia in cats.
Methods
US findings in 238 cats with (serum creatinine >180 μmol/l) and 270 cats without azotaemia were compared in a retrospective case-control study. Cats with pre-renal azotaemia or urethral obstruction were excluded. Data extracted from the medical records included age, body weight and body condition score (BCS). Quantitative and subjective US findings were extracted from archived ultrasound images and contemporaneous reports.
Results
In non-azotaemic cats, mean ± SD renal length was 40.1 ± 5.5 mm. Male cats had larger kidneys than female cats (mean difference 5.2 mm; P = 0.001) and, on average, the right kidney was slightly larger than the left (mean difference 1.6 mm; P = 0.01). Azotaemic cats had significantly lower mean body weight and BCS, and greater mean age and renal pelvic diameter. Renal pelvic diameter was negatively correlated with urine specific gravity (ρ –0.44, P <0.001). Compared with non-azotaemic cats, there was no difference in mean renal length of azotaemic cats because the numbers with enlarged kidneys and small kidneys were similar. Radiologists’ subjective assessments of renal size differed markedly between azotaemic and non-azotaemic cats, with azotaemic cats more likely to be recorded falsely as having abnormally small or enlarged kidneys. US findings significantly associated with azotaemia were perinephric fluid (odds ratio [OR] 26.4, 95% confidence interval [CI] 3.4–207.7), small kidneys (OR 8.4, 95% CI 4.0–17.4), hyperechoic renal cortex (OR 4.1, 95% CI 2.2–7.6), loss of corticomedullary differentiation (OR 4.1, 95% CI 1.8–9.6), renal calculi (OR 2.7, 95% CI 1.4–4.9), enlarged kidneys (OR 2.5, 95% CI 1.2–5.5) and dilated renal pelvis (OR 1.6, 95% CI 1.3–1.9).
Conclusions and relevance
Perinephric fluid was the US finding most strongly associated with azotaemia in this study and may merit more emphasis than it has received to date. Bias in radiologists’ subjective assessments of renal size suggests that other subjective findings will also be biased.
Introduction
Renal azotaemia can occur because of an acute kidney injury (AKI) or the cumulative effects of chronic kidney disease (CKD).1,2 In cats, causes of AKI include ureteral obstruction,3,4 ethylene glycol or lily toxicity 3 and pyelonephritis, 3 and causes of CKD include polycystic kidney disease,5–8 interstitial inflammation and fibrosis, 9 and nephrolithiasis. 10 AKI and CKD can be present together; for example, when ureteral obstruction occurs as a sequel to nephrolithiasis.4,11 In patients with azotaemia, ultrasonography is indicated to examine the kidneys in order to distinguish AKI from CKD, 12 particularly to look for signs of urinary obstruction, 13 which requires specific treatment. Given that unilateral renal abnormalities will not result in azotaemia if the contralateral kidney is functioning well, it is essential to examine both kidneys.
Ultrasonographic (US) abnormalities can be divided into subjective findings, including abnormal renal shape and echogenicity, and quantitative findings, including abnormal renal length and pelvic dilatation. 14 Both subjective and quantitative abnormal findings can occur in cats with or without azotaemia because of overlap between the normal and abnormal ranges, because of the occurrence of subclinical renal disease that does not cause azotaemia and because the kidneys may be affected secondarily in cats with various non-renal diseases, such as cardiomyopathy or acromegaly.15,16 It is unclear what subjective or quantitative US findings are most strongly associated with azotaemia and therefore are most likely to represent accurate signs of clinically significant renal disease. For example, renal length is routinely measured during abdominal US examinations and there have been multiple studies of normal renal size in cats;17–22 however, the diagnostic accuracy of renal length measurements has not been assessed.
The aim of the present study was to compare US findings in cats presenting with and without azotaemia in order to identify the US findings most strongly associated with azotaemia.
Materials and methods
For this retrospective case-control study, electronic medical records of the Queen Mother Hospital for Animals (QMHA) between June 2009 and May 2015 were reviewed. The criteria for inclusion were cats presented for the first time during this period that had ultrasonography of the urinary tract, ultrasound report and images available for review, and had serum creatinine determination. Azotaemia was defined as serum creatinine at presentation >180 μmol/l as determined by the clinical pathology laboratory, or plasma creatinine >140 μmol/l as determined by bench-top biochemistry analyser (Bioprofile 300; Nova Biomedical). Cats with pre-renal azotaemia or urethral obstruction were excluded. Determination of pre-renal azotaemia was based on serum creatinine on admission above the reference interval (RI), a urine specific gravity (USG) >1.040 and no US evidence of urinary obstruction. 3
Cases were collected in two stages: all azotaemic cats that satisfied the inclusion criteria were collected first for use in a study of ureteral obstruction, 23 followed by collection of a similar number of non-azotaemic cats presented during the same time period, regardless of their clinical diagnosis. Data extracted from the medical records included signalment, body weight, body condition score (BCS), serum creatinine and USG. US findings were extracted from contemporaneous reports written by six different Board-certified ultrasonographers employed at the QMHA during the period covered by the study. Archived ultrasound images were reviewed in order to add renal measurements absent from the report. US findings included objective renal length in sagittal or dorsal images, objective transverse pelvic diameter, subjective renal size, subjective pelvic and ureteral dilatation, renal shape (irregular, asymmetrical, nodule or mass lesion), presence of renal scars (focal depressions in the cortical surface with adjacent hyperechoic cortical segment), echogenicity of the cortex and medulla, presence of renal cysts, calcification of the renal parenchyma, presence of perinephric fluid, and presence of renal, ureteral or bladder calculi (Table 1). Clinical and US data for each cat were derived from the first period of hospitalisation only.
Table 1.
Ultrasonographic criteria
| Criterion | Value recorded |
|---|---|
| Renal length | mm |
| Pelvic transverse diameter | mm |
| Subjective renal size | Normal; small; enlarged |
| Subjectively dilated pelvis | No; slight; marked |
| Subjectively dilated ureter | No; slight; marked |
| Renal shape | Normal; irregular; asymmetrical; rounded; nodule; mass |
| Cortical scars | None; slight; marked |
| Echogenicity of cortex | Normal; increased; heterogeneous |
| Echogenicity of medulla | Normal; increased; heterogeneous; medullary rim sign; halo signs; loss of corticomedullary differentiation |
| Calcification of renal parenchyma | None; present |
| Renal cyst | Single cyst; polycystic renal disease |
| Perinephric fluid | None; slight; marked; perinephric pseudocyst |
| Renal calculi | None; single; multiple |
| Calculi in ureter | None; single; multiple |
| Calculi in bladder | None; single; multiple |
Non-azotaemic cats
In non-azotaemic cats, renal length was tested for normality. The effect of sex, body weight and laterality (left or right) on renal length was tested using a linear mixed-effects model that accounted for repeated measures from each cat. Correlation between USG and renal pelvic diameter (neither of which were normally distributed) was tested using Spearman’s coefficient (ρ). Associations between the age of non-azotaemic cats and the likelihood of abnormal US findings affecting either kidney were tested using binary logistic regression.
Comparisons between azotaemic and non-azotaemic cats
Continuous variables were tested for normality and differences in continuous variables between azotaemic and non-azotaemic cats tested using an independent samples t-test. The differences in BCS and renal pelvic diameter between azotaemic and non-azotaemic cats were tested using Mann–Whitney tests. Subjective US findings for the left and right kidney in each cat were aggregated for statistical analysis. Pairwise testing of differences in prevalence of US findings between azotemic and non-azotemic cats were tested using χ 2 methodology. Associations between US findings and azotaemia were tested using binary logistic regression with step-wise removal of non-significant variables. Results were expressed as odds ratio (OR) and 95% confidence intervals (CI). Statistical tests were undertaken using a proprietary application (SPSS Statistics, version 22; IBM). Differences with a P value <0.05 were considered significant.
Results
Records were obtained from 238 azotaemic cats and 270 non-azotaemic cats. There were 286 males (275 neutered) and 222 females (210 neutered). Median age was 7 years (range 2 weeks–22 years). There were 286 (56%) domestic shorthair cats, 23 (5%) Persians, 30 (6%) domestic longhairs, 21 (4%) British Shorthairs, 18 (4%) Ragdolls, 16 (3%) Bengals, 16 (3%) Siamese, 15 (3%) mixed breed cats, 14 (3%) Burmese, 10 (2%) Maine Coons, eight (2%) Birmans, eight (2%) British Blues, six (1%) Tonkinese and 15 other feline breeds with fewer than five affected individuals. Median BCS was 4/9 (range 1–8) and mean body weight was 4.1 kg (range 0.9–8.5 kg).
Median serum creatinine in azotaemic cats was 417 μmol/l (range 184–2100 μmol/l) as determined by the clinical pathology laboratory (n = 199) and median USG was 1.018 (range 1.006–1.050). Ureteral obstruction was the most frequent tentative diagnosis, recorded in 92/238 (39%) azotaemic cats, and of these 38 (41%) were proved to have ureteral obstruction by pyelography. 23
Non-azotaemic cats
Renal length had a normal distribution (mean ± SD 40.1 ± 5.5 mm. Five (2%) juvenile (<9 months old) non-azotaemic cats were excluded from this calculation. There were significant effects on renal length for sex (P <0.001) and laterality (P <0.01), but not body weight (P = 0.85). Males had larger kidneys than females (mean 42.1 mm vs 36.9 mm) and the right kidney was larger than the left (mean 40.9 mm vs 39.3mm).
In non-azotaemic cats, there was a significant association between likelihood of abnormal US findings and increasing age (P = 0.001). Specifically, the prevalence of dilated renal pelvis (OR 1.2, 95% CI 1.07–1.32), hyperechoic renal cortex (OR 1.1, 95% CI 1.02–1.21) and calcification of renal parenchyma (OR 1.3, 95% CI 1.02–1.58) increased with age.
Comparisons between azotaemic and non-azotaemic cats
Azotaemic cats had significantly lower mean body weight and BCS, and greater mean age and renal pelvic diameter compared with non-azotaemic cats (Table 2). No significant difference in median renal length was observed because the numbers of azotaemic cats with enlarged kidneys and small kidneys were similar (see below).
Table 2.
Comparison of continuous variables cats with and without azotaemia
| Variable | Azotaemic (n = 238) | Non-azotaemic (n = 270) | P value |
|---|---|---|---|
| Age (years) | 8.1 ± 4.7 | 6.0 ± 4.0 | <0.001 |
| Body weight (kg) | 3.9 ± 1.1 | 4.3 ± 1.3 | 0.02 |
| Body condition score (/9) | 4 (1–8) | 4 (2–8) | 0.02 |
| Urine specific gravity | 1.018 (1.006–1.050) | 1.041 (1.011–1.070) | <0.0001 |
| Renal length (mm) | 39.3 ± 8.5 | 40.1 ± 5.5 | 0.11 |
| Renal pelvic diameter (mm) | 2.0 (0–50) | 0 (0–12) | <0.001 |
Values are mean ± SD for age, body weight and renal length; values are median (range) for body condition score, urine specific gravity and renal pelvic diameter
Subjective renal length was reported to be abnormal in 182 (76%) azotaemic cats, with 101 (42%) having subjectively small kidneys and 81 (34%) having subjectively enlarged kidneys; however, based on using renal size in the non-azotaemic cats in this study as a RI, small kidneys (<29 mm) and enlarged kidneys (>51 mm) were present in only 16% and 6% cats with azotaemia, respectively. Plots of the probability that renal length was recorded as subjectively normal vs actual renal length demonstrate markedly different distributions in non-azotaemic and azotaemic cats (Figure 1). In non-azotaemic cats, the kidneys were more likely to be recorded as subjectively normal in size in the range 29–51 mm, which corresponds closely with the RI observed in these cats. In contrast, the kidneys of azotaemic cats were more likely to be recorded as subjectively normal in the narrower range of 35–45 mm.
Figure 1.
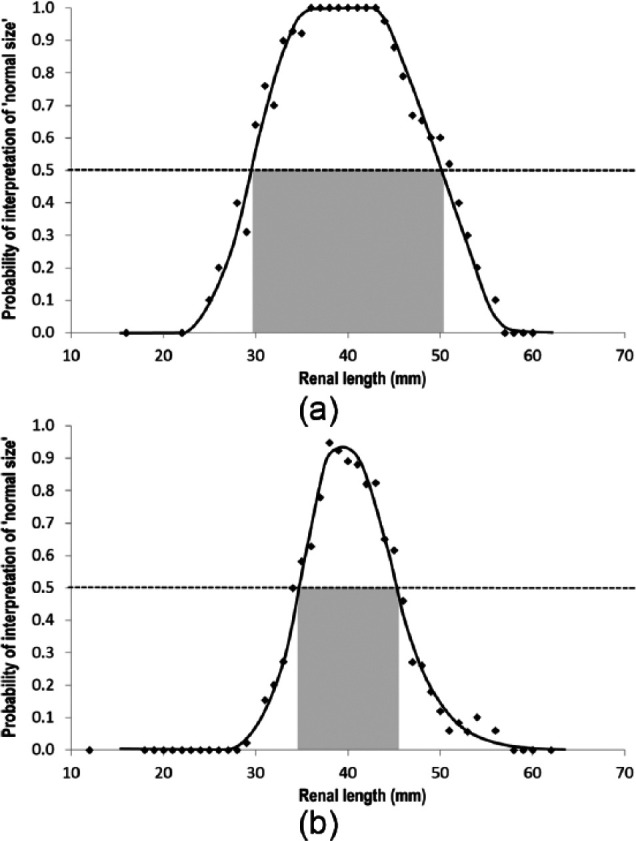
Plots of the probability that renal length was recorded as subjectively normal vs actual renal length in (a) non-azotaemic cats and (b) azotaemic cats. Probability of a kidney being reported as normal size exceeds 50% in the range of 29–51 mm (grey zone) for non-azotaemic cats and the narrower range of 35–45 mm for azotaemic cats. Trend lines were drawn manually for illustration purposes only
Renal pelvic diameter was negatively correlated with USG (ρ −0.44, P <0.001) (Figure 2). If the 38 cats with proven ureteral obstruction were excluded, the correlation between renal pelvic diameter and USG was not changed significantly.
Figure 2.
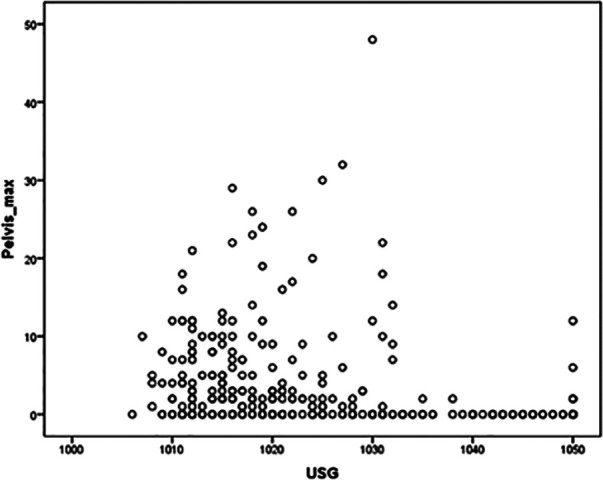
Scatter plot of renal pelvic diameter against urine specific gravity in 368 cats. There is a moderate negative correlation (ρ = −0.44, P <0.001)
Abnormalities affecting the kidneys, ureters and/or bladder were observed in 226 (95%) cats with azotaemia and 126 (47%) cats without azotaemia (Table 3). On the basis of pairwise testing, all recorded US findings were significantly more prevalent in azotaemic cats, except renal nodule or mass, polycystic disease, the medullary rim sign, halo sign and perinephric pseudocyst, which occurred with similar frequency in azotaemic and non-azotaemic cats. In the final regression model, US findings significantly associated with azotaemia were perinephric fluid (OR 26.4, 95% CI 3.4–207.7), small kidneys (OR 8.4, 95% CI 4.0–17.4), hyperechoic renal cortex (OR 4.1, 95% CI 2.2–7.6), loss of corticomedullary differentiation (OR 4.1, 95% CI 1.8–9.6), renal calculi (OR 2.7, 95% CI 1.4–4.9), enlarged kidneys (OR 2.5, 95% CI 1.2–5.5) and dilated renal pelvis (OR 1.6, 95% CI 1.3–1.9).
Table 3.
Comparison of ultrasonographic signs in cats with and without azotaemia
| Ultrasonographic signs | Azotaemic (n = 238) | Non-azotaemic (n = 270) | P value |
|---|---|---|---|
| Subjectively small kidney | 101 (42) | 15 (6) | <0.001 |
| Subjectively enlarged kidney | 81 (34) | 21 (8) | <0.001 |
| Subjectively dilated pelvis | 152 (64) | 17 (6) | <0.001 |
| Subjectively dilated ureter | 86 (36) | 3 (1) | <0.001 |
| Irregular renal shape | 69 (29) | 14 (5) | <0.001 |
| Rounded kidney | 9 (4) | 0 | 0.001 |
| Renal nodule or mass | 1 (0.4) | 1 (0.4) | 1 |
| Cortical scars | 41 (17) | 18 (7) | <0.001 |
| Hyperechoic renal cortex | 105 (44) | 31 (11) | <0.001 |
| Hyperechoic renal medulla | 74 (31) | 20 (7) | <0.001 |
| Medullary rim sign | 22 (9) | 27 (10) | 0.88 |
| Halo sign | 0 | 0 | 1 |
| Loss of corticomedullary differentiation | 61 (26) | 14 (5) | <0.001 |
| Calcification of renal parenchyma | 12 (5) | 3 (1) | 0.01 |
| Polycystic disease | 12 (5) | 16 (6) | 0.70 |
| Perinephric fluid | 54 (23) | 1 (0.4) | <0.001 |
| Perinephric pseudocyst | 1 (0.4) | 0 | 0.47 |
| Renal calculi | 92 (39) | 13 (5) | <0.001 |
| Calculi in ureter | 77 (32) | 1 (0.4) | <0.001 |
| Calculi in bladder | 49 (21) | 12 (4) | <0.001 |
Data are n (%)
Discussion
The cats in this series represent heterogeneous samples of azotaemic and non-azotaemic cats. In the majority of azotaemic cats, a specific final diagnosis was not determined, 23 and diagnosis in non-azotaemic cats was not recorded for the purposes of the present study. Under these circumstances, only relatively general conclusions are possible about the meaning of the US findings. For example, perinephric fluid was the US finding most strongly associated with azotaemia; the majority of possible US signs of renal disease had low sensitivity for azotaemia and low specificity, with abnormalities affecting the kidneys reported frequently in cats without azotaemia. Although ultrasonography is recommended for investigation of individual cats with CKD, 24 it is not recommended as a screening test for CKD in cats because of the poor correlation between US findings and renal function and the prevalence of US abnormalities in non-azotaemic cats, 21 the significance of which is difficult to assess.
Abnormalities affecting the kidneys were reported in 47% of non-azotaemic cats in the present study. The increasing prevalence with age of dilated renal pelvis, hyperechoic renal cortex and calcification of the renal parenchyma may reflect subclinical renal disease that gradually becomes more marked over time.1,9 Other studies have also found a high prevalence of US abnormalities in cats without azotaemia. For example, US changes, including segmental cortical lesions and abnormal renal capsule, were detected in 66/133 (50%) healthy Ragdoll cats and 25/62 (40%) healthy cats of other breeds. 25 The occurrence of abnormalities affecting the kidneys in cats without azotaemia is not surprising because azotaemia is an insensitive indicator of renal function, 26 which becomes elevated only when a large proportion of functional nephrons have been lost and may be low despite clinically significant renal disease in cats with low body weight.27,28 Insensitivity of serum creatinine means that the group of non-azotaemic cats in the present study will include individuals with renal disease. More accurate quantitative assessment of renal function requires measurement of glomerular filtration rate; 28 however, serum creatinine remains the test most widely used for assessment of renal function in cats (and dogs) because it is widely available and inexpensive compared with measurement of glomerular filtration rate.
Polycystic renal disease and medullary rim sign occurred with similar frequency in azotaemic and non-azotaemic cats. Polycystic renal disease encompasses a wide spectrum of severity, including subclinical.29,30 That polycystic renal disease was found just as frequently in cats without signs of renal dysfunction as in cats with azotaemia emphasises that its significance in an individual feline patient must be interpreted in combination with other clinical findings. As previously reported in dogs, 31 there appears to be no association between the medullary rim sign and clinical renal disease in cats.
Occurrence of perinephric fluid has been reported previously in cats with azotaemia,23,32 and has been associated with hyperkalaemia in cats with urinary obstruction. 33 Although perinephric fluid was not significantly associated with ureteral obstruction or severity of renal dysfunction in some previous studies,23,32 it was the US finding most strongly associated with azotaemia in the present study and therefore may merit more emphasis than it has received to date. Perinephric fluid may be distinguished from subcapsular collections or perinephric pseudocyst when it has a pointed shape on its non-renal border and/or is confluent with hypoechoic or anechoic fluid dissecting between fat in the retroperitoneum. By analogy to cardiac failure, in which pulmonary oedema is a more accurate indicator of cardiac dysfunction than the radiographic appearance of the cardiac silhouette, 34 perinephric fluid appears to be a more accurate sign of renal dysfunction than the US appearance of the kidneys. Perinephric fluid is liable to accumulate in cats with renal disease when renal ultrafiltrate leaks into the renal interstitium in a sufficient quantity to overwhelm the drainage capacity of capsular lymphatics. 32 Perinephric fluid in cats with renal dysfunction is more likely to be observed after administration of intravenous fluids than at presentation, when cats are liable to have hypovolaemia.
Renal length in healthy cats has been studied in some detail. Using US measurements, the normal RI has been reported as 30.4–42.9 mm in 10 cats, 17 and 29.8–50.9 mm in 56 Ragdoll cats. 35 Minor, non-significant inter-breed differences were observed in renal length of Sphynx cats, British Shorthair and Ragdoll cats. 36 Multiple studies have found that the right kidney in cats is slightly longer than the left.20,22,36 Renal length in cats has also been positively correlated with body weight,20,35 fat accumulation in the kidney, 37 age 35 and male sex. 35 In a radiographic study, neutered cats had smaller kidneys than sexually intact cats. 18 The present study provides additional evidence that the RI for feline kidneys should be approximately 29–51 mm, that male cats have larger kidneys than female cats (mean difference 5.2 mm) and, on average, the right kidney is normally slightly larger than the left (mean difference 1.6 mm).
The diagnostic accuracy of renal length measurements is low because relatively few cats with azotaemia have either small or large kidneys. In the present study, small kidneys (<29 mm) and enlarged kidneys (>51 mm) were present in only 16% and 6% cats with azotaemia, respectively; hence the sensitivity of this measurement for azotaemia is low. However, the kidneys of azotaemic cats in the present study were more likely to be recorded as subjectively abnormal than kidneys of the same size in non-azotaemic cats. This finding indicates that ultrasonographers’ judgements about renal size were biased by the knowledge that patients had azotaemia. This observation raises questions about the validity of subjective renal size assessment in azotaemic cats specifically, and questions about the validity of subjective organ size assessment in clinical patients generally. Interpretation of diagnostic images (and other test results) tends to be more accurate if the clinical history is known; 38 however, one argument against providing clinical information is that readers may perceive abnormalities that are not present (false-positives). It appears that the ultrasonographers whose data were used for the present study employed different thresholds for abnormally small and abnormally large depending on the clinical history of azotaemia and therefore were recording abnormalities that may not have been present. It is important not to over-emphasise organ size as a diagnostic criterion because the normal size ranges for many anatomical structures are very wide (particularly in dogs); hence, there is marked overlap between normal and pathological ranges, which limits the sensitivity and specificity of tests based on measurements. 39
If subjective assessment of renal size is prone to bias, the same may be true of other subjective US findings significantly associated with azotaemia in the present study, such as hyperechoic renal cortex or loss of corticomedullary differentiation. Hyperechoic renal cortex is a finding based on subjective comparison of renal echogenicity with adjacent structures, such as the liver. The renal cortex in cats normally has similar echogenicity as the adjacent liver, 19 although this relationship may be affected by normal variations in fat deposition in the kidney and/or liver,37,40,41 or the type of ultrasound transducer used. 42 In cats, interstitial nephritis, interstitial necrosis and fibrosis are associated with increased renal cortical echogenicity. 43 Particularly in a cat with CKD, in which these conditions are suspected, renal echogenicity is liable to be over-interpreted. The low specificity of this US finding is also emphasised by the finding that it was the abnormality reported most frequently in cats without azotaemia in the present study.
Median renal pelvic diameter was significantly greater in azotaemic cats compared with non-azotaemic cats. This difference likely reflects both the occurrence of urinary obstruction in the group of azotaemic cats, which was proven in 38/238 (16%) instances, and their lower median USG, which will frequently be associated with polyuria. Based on all cats for which USG data were available, renal pelvic diameter was negatively correlated with USG. The tendency for pyelectasia in animals producing dilute urine represents a physiological variation in pelvic diameter occurring in response to different rates of urine output. This effect has been demonstrated experimentally in dogs. 44
Conclusions
On the basis of the present study, it appears that perinephric fluid is strongly associated with azotaemia and may be less prone to bias than subjective assessments of renal size or echogenicity. Therefore, it may prove to be one of the most accurate US signs of renal dysfunction.
Acknowledgments
We thank Yu-Mei Chang for helping with the statistical analysis.
Footnotes
Accepted: 19 September 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Khan TM, Khan KNM. Acute kidney injury and chronic kidney disease. Vet Pathol 2015; 52: 441–444. [DOI] [PubMed] [Google Scholar]
- 2. Balakrishnan A, Drobatz KJ. Management of urinary tract emergencies in small animals. Vet Clin North Am Small Anim Pract 2013; 43: 843–867. [DOI] [PubMed] [Google Scholar]
- 3. Segev G, Nivy R, Kass PH, et al. A retrospective study of acute kidney injury in cats and development of a novel clinical scoring system for predicting outcome for cats managed by hemodialysis. J Vet Intern Med 2013; 27: 830–839. [DOI] [PubMed] [Google Scholar]
- 4. Kyles AE, Hardie EM, Wooden BG, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984–2002). J Am Vet Med Assoc 2005; 226: 932–936. [DOI] [PubMed] [Google Scholar]
- 5. Domanjko-Petric A, Cernec D, Cotman M. Polycystic kidney disease: a review and occurrence in Slovenia with comparison between ultrasound and genetic testing. J Feline Med Surg 2008; 10: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barthez PY, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg 2003; 5: 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck C, Lavelle RB. Feline polycystic kidney disease in Persian and other cats: a prospective study using ultrasonography. Aust Vet J 2001; 79: 181–184. [DOI] [PubMed] [Google Scholar]
- 8. Cannon MJ, MacKay AD, Barr FJ, et al. Prevalence of polycystic kidney disease in Persian cats in the United Kingdom. Vet Rec 2001; 149: 409–411. [DOI] [PubMed] [Google Scholar]
- 9. Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet Pathol 2016; 53: 309–326. [DOI] [PubMed] [Google Scholar]
- 10. Pimenta MM, Reche A, Freitas MF, et al. Study of nephrolithiasis and ureterolithiasis in cats with chronic kidney disease. Pesqui Vet Brasil 2014; 34: 555–561. [Google Scholar]
- 11. Roberts SF, Aronson LR, Brown DC. Postoperative mortality in cats after ureterolithotomy. Vet Surg 2011; 40: 438–443. [DOI] [PubMed] [Google Scholar]
- 12. Ozmen CA, Akin D, Bilek SU, et al. Ultrasound as a diagnostic tool to differentiate acute from chronic renal failure. Clin Nephrol 2010; 74: 46–52. [PubMed] [Google Scholar]
- 13. Remer EM, Papanicolaou N, Casalino DD, et al. ACR appropriateness criteria on renal failure. Am J Med 2014; 127: 1041–8.e1. [DOI] [PubMed] [Google Scholar]
- 14. Debruyn K, Haers H, Combes A, et al. Ultrasonography of the feline kidney. J Feline Med Surg 2012; 14: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hickey MC, Jandrey K, Farrell KS, et al. Concurrent diseases and conditions in cats with renal infarcts. J Vet Intern Med 2014; 28: 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lourenco BN, Randall E, Seiler G, et al. Abdominal ultrasonographic findings in acromegalic cats. J Feline Med Surg 2015; 17: 698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walter PA, Feeney DA, Johnston GR, et al. Feline renal ultrasonography – quantitative-analyses of imaged anatomy. Am J Vet Res 1987; 48: 596–599. [PubMed] [Google Scholar]
- 18. Shiroma JT, Gabriel JK, Carter RL, et al. Effect of reproductive status on feline renal size. Vet Radiol Ultrasound 1999; 40: 242–245. [DOI] [PubMed] [Google Scholar]
- 19. Drost WT, Henry GA, Meinkoth JH, et al. Quantification of hepatic and renal cortical echogenicity in clinically normal cats. Am J Vet Res 2000; 61: 1016–1020. [DOI] [PubMed] [Google Scholar]
- 20. Park IC, Lee HS, Kim JT, et al. Ultrasonographic evaluation of renal dimension and resistive index in clinically healthy Korean domestic short-hair cats. J Vet Sci 2008; 9: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paepe D, Daminet S. Feline CKD diagnosis, staging and screening – what is recommended? J Feline Med Surg 2013; 15: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stocco AV, Sousa CAS, Gomes MS, et al. Is there a difference between the right and left kidney? A macroscopic approach in Brazilian Shorthair Cat. Arq Bras Med Vet Zootec 2016; 68: 1137–1144. [Google Scholar]
- 23. Lamb CR, Cortellini S, Halfacree Z. Ultrasonography in the diagnosis and management of ureteral obstruction in cats. J Feline Med Surg 2018; 20: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sparkes AH, Caney S, Chalhoub S, et al. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J Feline Med Surg 2016; 18: 219–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paepe D, Bavegems V, Combes A, et al. Prospective evaluation of healthy Ragdoll cats for chronic kidney disease by routine laboratory parameters and ultrasonography. J Feline Med Surg 2013; 15: 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartmann H, Schmitz R, Reder S, et al. Relationship between endogenous serum creatinine and glomerular filtration rate in healthy dogs and cats and animals with renal disease. Tieraerztl Praxis 2008; 36: 111–118. [Google Scholar]
- 27. Braun JP, Lefebvre HP, Watson ADJ. Creatinine in the dog: a review. Vet Clin Pathol 2003; 32: 162–179. [DOI] [PubMed] [Google Scholar]
- 28. Finch N. Measurement of glomerular filtration rate in cats – methods and advantages over routine markers of renal function. J Feline Med Surg 2014; 16: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonazzi M, Volta A, Gnudi G, et al. Prevalence of the polycystic kidney disease and renal and urinary bladder ultrasonographic abnormalities in Persian and Exotic Shorthair cats in Italy. J Feline Med Surg 2007; 9: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paepe D, Saunders J, Bavegems V, et al. Screening of ragdoll cats for kidney disease: a retrospective evaluation. J Small Anim Pract 2012; 53: 572–577. [DOI] [PubMed] [Google Scholar]
- 31. Mantis P, Lamb CR. Most dogs with medullary rim sign on ultrasonography have no demonstrable renal dysfunction. Vet Radiol Ultrasound 2000; 41: 164–166. [DOI] [PubMed] [Google Scholar]
- 32. Holloway A, O’Brien R. Perirenal effusion in dogs and cats with acute renal failure. Vet Radiol Ultrasound 2007; 48: 574–579. [DOI] [PubMed] [Google Scholar]
- 33. Nevins JR, Mai W, Thomas E. Associations between ultrasound and clinical findings in 87 cats with urethral obstruction. Vet Radiol Ultrasound 2015; 56: 439–447. [DOI] [PubMed] [Google Scholar]
- 34. Lamb CR, Boswood A. Role of survey radiography in diagnosing canine cardiac disease. Compend Contin Educ Pract Vet 2002; 24: 316–326. [Google Scholar]
- 35. Debruyn K, Paepe D, Daminet S, et al. Renal dimensions at ultrasonography in healthy Ragdoll cats with normal kidney morphology: correlation with age, gender and bodyweight. J Feline Med Surg 2013; 15: 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Debruyn K, Paepe D, Daminet S, et al. Comparison of renal ultrasonographic measurements between healthy cats of three cat breeds: Ragdoll, British Shorthair and Sphynx. J Feline Med Surg 2013; 15: 478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeager AE, Anderson WI. Study of association between histologic features and echogenicity of architecturally normal cat kidneys. Am J Vet Res 1989; 50: 860–863. [PubMed] [Google Scholar]
- 38. Loy CT, Irwig L. Accuracy of diagnostic tests read with and without clinical information: a systematic review. JAMA 2004; 292: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 39. Lamb CR, Nelson JR. Diagnostic accuracy of tests based on radiologic measurements of dogs and cats: a systematic review. Vet Radiol Ultrasound 2015; 56: 231–244. [DOI] [PubMed] [Google Scholar]
- 40. Nicoll RG, O’Brien RT, Jackson MW. Qualitative ultrasonography of the liver in obese cats. Vet Radiol Ultrasound 1998; 39: 47–50. [DOI] [PubMed] [Google Scholar]
- 41. Yeager AE, Mohammed H. Accuracy of ultrasonography in the detection of severe hepatic lipidosis in cats. Am J Vet Res 1992; 53: 597–599. [PubMed] [Google Scholar]
- 42. Yabuki A, Endo Y, Sakamoto H, et al. Quantitative assessment of renal cortical echogenicity in clinically normal cats. Anat Histol Embryol 2008; 37: 383–386. [DOI] [PubMed] [Google Scholar]
- 43. Zotti A, Banzato T, Gelain ME, et al. Correlation of renal histopathology with renal echogenicity in dogs and cats: an ex-vivo quantitative study. BMC Vet Res 2015; 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jakovljevic S, Rivers WJ, Chun R, et al. Results of renal ultrasonography performed before and during administration of saline (0.9% NaCl) solution to induce diuresis in dogs without evidence of renal disease. Am J Vet Res 1999; 60: 405–409. [PubMed] [Google Scholar]


