Abstract
Objectives
The aim of this study was to assess whether, in contrast to serum creatinine, which is higher in Birman cats than in other breeds, the serum concentration of symmetric dimethylarginine (SDMA) is comparable in clinically healthy Birmans and in the general feline population. This could allow, in this breed, to better evaluate chronic kidney disease (CKD).
Methods
Serum creatinine and SDMA were measured in clinically healthy Birmans (n = 50) and in cats of other breeds (n = 46), and the results were statistically compared. A breed-specific reference interval (RI) was established for Birmans and compared with the RI for the general feline population (0.0–14.0 µg/dl).
Results
Creatinine (1.58 ± 0.36 mg/dl) and SDMA (12.2 ± 2.8 µg/dl) were higher (P <0.001) in Birmans than in cats of other breeds (1.19 ± 0.17 mg/dl; 10.3 ± 2.5 µg/dl). In 20/50 Birman cats (40.0%) serum creatinine was higher than both the non-breed-specific RI of our laboratory and the threshold recommended to classify cats as IRIS stage 2 (1.6 mg/dl). The concentration of SDMA was higher than the pre-existing RI in 10/50 Birmans (20.0%) and in four cats of other breeds (8.7%). Among Birmans, the proportion of cats with SDMA >14 µg/dl was lower (P <0.017) than the proportion of cats with creatinine >1.60 mg/dl. However, the deviation from the upper limit of the RI was lower than the analytical variability of the method in 7/10 Birmans and in 4/4 cats of other breeds. The breed-specific RI (3.5–18.7 µg/dl) overlapped with the pre-existing one.
Conclusions and relevance
SDMA may be a better marker of CKD in Birman cats than creatinine when non-breed-specific RIs are utilised. The coupled analysis of creatinine and SDMA could help prevent errors in diagnosing and staging CKD in Birman cats.
Introduction
Birman cats, as well as cats of other breeds such as Siberian and, to a lesser extent, Siamese and Somali cats, have a physiologically high concentration of serum creatinine and urea compared with the general feline population.1,2 Although the mechanism responsible for this breed peculiarity is unknown, the high serum level of creatinine may hamper the interpretation of laboratory profiles since creatinine and urea are commonly included in laboratory panels for diagnosing and staging feline chronic kidney disease (CKD). 3 CKD is common in cats, and owners or breeders frequently require clinical or laboratory investigation to monitor renal functions in their cats, especially in the elderly, as recommended by the IRIS guidelines. 4
Moreover, Birman cats have been reported to be prone to developing feline infectious peritonitis, 5 a disease in which renal function is frequently altered, 6 and may be affected by hereditary polycystic kidney disease, as in other Persian-related cats. 7 Therefore, in this breed, clinical and laboratory investigation are frequently also performed in young cats. In all these clinical scenarios, CKD risks being overestimated as a result of the physiologically high serum creatinine concentration in Birman cats; the detection of high serum creatinine often then induces owners and vets to perform unnecessary and stressful clinical and laboratory work-ups to exclude the presence of CKD.
Recently, symmetric dimethylarginine (SDMA) has been proposed as a novel marker for an early diagnosis of CKD in cats.8–10 SDMA is the methylated form of the amino acid arginine, which is released into the circulation during the normal catabolism of body proteins.8,11 As for creatinine, SDMA is excreted by the kidneys, and the correlation between glomerular filtration rate (GFR) and creatinine and GFR and SDMA is almost identical. 9 Therefore, the serum concentration of SDMA increases when the GFR decreases, as it occurs during CKD. According to another study, the increase of SDMA above the reference interval (RI) occurs earlier than the increase of creatinine in cats with CKD. 10
Based on our knowledge of the metabolism of SDMA, 11 which is different from that of creatinine, 12 it is very likely that (despite the high creatinine concentration) in clinically healthy Birman cats SDMA has the same concentration as that recorded in the general population of clinically healthy cats.
Therefore, SDMA may be a good marker to monitor the health status in this feline breed, thus reducing the number of unnecessary samplings to exclude CKD.
The aim of this study was to assess whether clinically healthy Birman cats (as a representative breed with high creatinine concentration) have normal SDMA concentrations when compared with a general feline RI and to define the breed-specific RI for SDMA, if needed. This would provide a better evaluation of renal function in this breed.
Materials and methods
Caseload
This study was undertaken on blood samples collected from clinically healthy Birman cats, within a collaboration with the Italian association of Birman cats breeders. The cats included in this study originated from three different Italian regions: Lombardy (n = 62), Piedmont (n = 21) and Tuscany (n = 13). Cats were subjected to periodical veterinary visits that included routine bloodwork, urinalysis, abdominal ultrasound and genetic screening for hereditary disorders. The inclusion criteria for Birman cats were no clinical history of disease, including the absence of abnormalities in plasmatic or urinary biomarkers of renal disease (eg, serum creatinine and urea within breed-specific RIs, urine specific gravity <1.025 and absence of proteinuria), the absence of any abnormality detected by physical examination and diagnostic imaging, and the absence of laboratory changes compared with breed-specific RIs for all the analytes included in the laboratory bloodwork performed within this study.
As a control group, we used blood samples from cats belonging to breeds other than Birmans, collected at our institution during annual wellness visits, before elective surgery (eg, castration, dental scaling) or within clinical examination for diseases not associated with decreased glomerular filtration rate (mostly non-metastatic cutaneous tumours not associated with systemic clinical or laboratory abnormalities or cachexia). The inclusion criterion for these control cats was a serum concentration of creatinine <1.6 mg/dl (ie, the RI of our laboratory, which corresponds to the threshold considered as important for the IRIS staging of CKD 4 ) and the absence of other abnormalities in routine blood and urine analysis.
The study was approved by the Institutional Animal Care and Use Committee (approval number: 3/2016).
In both groups, samples from cats younger than 6 months or affected by diseases potentially influencing the GFR and samples presenting gross abnormalities (eg, haemolysis or lipaemia) were excluded from the study.
Samples from Birman cats were collected from November 2015 to June 2016 from the cephalic vein by the referring veterinarians upon informed consent from the owners. Blood was placed in tubes without anticoagulant, centrifuged (1800 g for 8 mins) within 1 h of sampling and frozen at –20°C to be periodically sent to our department, where samples were analysed within 1 month of sampling. Specifically, samples were thawed and used to perform a basic panel of biochemical tests to exclude the presence of laboratory abnormalities. This panel consisted of creatinine, urea, total protein, albumin, alanine aminotransferase, gamma-glutamyl transpeptidase and cholesterol, and was performed using an automated spectrophotometer (Cobas Mira) with reagents provided by Hagen Diagnostica.
The remaining aliquots of serum samples were sent to IDEXX Laboratories to perform SDMA analysis using enzyme multiplied immunoassay technology. SDMA was measured in duplicate or, when the difference between the two measurements exceeded 2.0 µg/dl, in triplicate. The mean values of the replicas recorded in each cat were included in this study and used for group comparison. Conversely, in order to fulfil the requirements of the American Society for Veterinary Clinical Pathology (ASVCP) guidelines for establishment of RIs, 13 only the results of the first reading were used to assess the validity of pre-existing RIs and to calculate the breed-specific RI as described below.
Urine samples were collected by cystocentesis only in a limited number of cats (18 Birmans and 23 cats of other breeds); therefore, results regarding urinalysis were not included in the statistical comparisons mentioned below.
Statistical analysis
Results regarding age, creatinine and SDMA recorded in Birman and in cats of other breeds were compared with a Mann–Whitney U-test, using statistical software (Analyse-it version 2.21). The same software was used to assess in both breed groups; using the Pearson’s χ2 test, the possible differences in the proportion of cats with increased SDMA compared with the RIs. Similarly, the Pearson’s χ2 test was used to compare, within the group of Birman cats, the proportion of cats with SDMA higher than the RI and the proportion of cats with creatinine >1.60 mg/dl, as well as the proportion of cats with increased SDMA and creatinine <1.60 mg/dl and the proportion of cats with increased SDMA and creatinine >1.60 mg/dl.
In order to assess whether the concentration of SDMA in Birman cats falls within the RIs for the general population of clinically healthy cats determined by the producer of the assay, we applied the method of transference of RIs, which, with some intrinsic limitation, is widely used and recommended by the current guidelines. 13 This method validates pre-existing RIs if <10% of 20 randomly selected results fall outside the claimed RI. A breed-specific RI was then established for Birman cats using an Excel spreadsheet with the Reference Value Advisor (version 2.0) set of macroinstructions, 14 which, according the recommendations of the International Federation of Clinical Chemistry and Laboratory Medicine Clinical and Laboratory Standards Institute, 15 performs descriptive statistics, tests of normality (Anderson–Darling with histograms and Q–Q plots and Box–Cox transformation) and outlier analysis. Both Dixon–Reed and Tukey tests were used to detect outliers, which were retained unless clear reasons to classify them as aberrant observations were found, as recommended by the ASVCP guidelines. 13 RIs were calculated using a non-parametric bootstrap method, that calculated also the 90% confidence interval of RIs. Linear regression was used to assess the possible influence of age on RIs of SDMA in Birman cats. 16
Results
Group composition
The study population was composed of 96 cats that fulfilled the inclusion criteria.
Of these, 50 were Birman cats (21 female, six spayed female, 17 males, six neutered males; age range 6 months to 13 years [median 4 years]). The group of Birman cats included only two cats younger than 1 year, which, according to the information reported by IDEXX, may have slightly higher SDMA concentrations.
The remaining 46 cats belonged to eight different breeds (35 domestic shorthairs, two Persians, two Exotic Shorthairs, two Maine Coons, two British Shorthairs, one Devon Rex, one Scottish Fold, one Siberian cat). This group was composed of nine females, 18 spayed females, four males and 15 neutered male cats (age range 6 months–17 years, median 9 years). Only one cat in this group was younger than 1 year.
The age of the Birman cats was significantly lower than the age of cats of other breeds (P <0.001).
As specified in the inclusion criteria, all the Birman cats were clinically healthy and cats of other breeds did not have clinical signs consistent with kidney disease. Results of urinalysis, when available, were unremarkable. In particular, urine specific gravity was not consistent with a renal disease neither in Birman cats (range 1.025–1.080; median 1.040) nor in cats of other breeds (range 1.030–1.086; median 1.060).
Creatinine and SDMA concentration
Both creatinine (mean ± SD 1.58 ± 0.36 mg/dl; median 1.47 mg/dl) and SDMA (mean ± SD 12.2 ± 2.8 µg/dl; median 12.0 µg/dl) were significantly higher (P <0.001 for both) in Birman cats than in cats of other breeds (mean ± SD 1.19 ± 0.17 mg/dl [median 1.17 mg/dl] for creatinine; mean ± SD 10.3 ± 2.5 µg/dl [median 9.7 µg/dl] for SDMA). The comparison of results regarding creatinine and SDMA revealed that in the Birman group, the proportion of cats with SDMA >14 µg/dl (n = 10/50) was significantly lower (P <0.017) than the proportion of cats with creatinine levels >1.60 mg/dl (n = 20/50). However, the distribution of data (Figure 1) revealed that despite the relatively wide difference in creatinine between groups, a higher degree of overlap was found for SDMA. No significant sex-related differences were found for SDMA among Birman cats (P = 0.963) or among cats of other breeds (P = 0.615). Similarly, the linear regression tests did not show significant differences by age between the two groups (P = 0.684 and P = 0.585, respectively).
Figure 1.
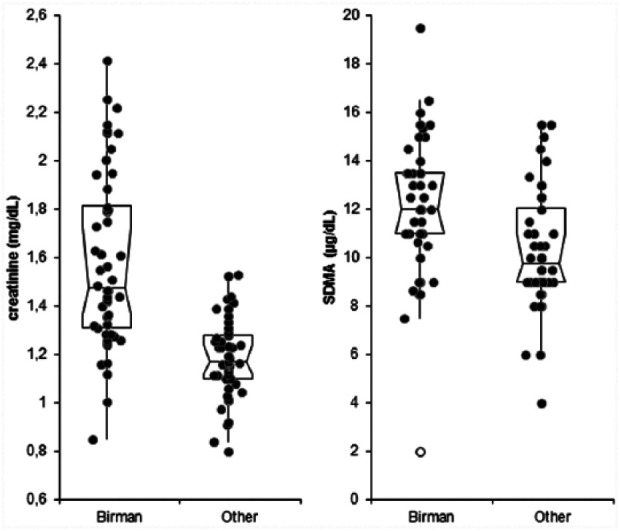
Distribution of results of creatinine and symmetric dimethylarginine (SDMA) recorded in Birman cats and in cats of other breeds. Boxes indicate the interquartile interval (25th–75th percentile); the horizontal line corresponds to the median value; the vertical lines are the limits of suspected outlier distribution according to the Tukey rule. Each black dot corresponds to an individual value. Far outliers are indicated with the open circles
The analysis of individual data revealed that in 20/50 Birman cats (40%) the concentration of serum creatinine was higher than 1.60 mg/dl (Table 1), which corresponds to the upper RI of the laboratory, as calculated in a population of 94 cats following the ASVCP guidelines for determination of de novo RIs. 13 This upper reference limit is lower than that used in other commercial laboratories, whose upper reference limit may be as high as 2.3 mg/dl. In our case the upper reference limit corresponds to the threshold recommended for staging patients according to IRIS stage 2 classification. 4 In 5/20 cats the deviation from the IRIS threshold was <10%, which may be consistent as an acceptable analytical error for creatinine, 17 whereas in the remaining 15 cases the deviation from the threshold largely exceeded 10%. However, all the values were below the upper limit of the breed-specific RI established for Birman cats in a previous study (2.5 mg/dl). 2
Table 1.
Individual data recorded in Birman cats and in cats of other breeds
| Birman cats |
Cats of other breeds |
||||||
|---|---|---|---|---|---|---|---|
| Cat number |
Age (years) | Creatinine (mg/dl) | SDMA (µg/dl) | Cat number |
Age (years) | Creatinine (mg/dl) | SDMA (µg/dl) |
| 1 | 12 | 1.89 | 13.5 | 1 | 1 | 1.05 | 11.0 |
| 2 | 4 | 1.32 | 10.0 | 2 | 0.5 | 1.26 | 14.0 |
| 3 | 8 | 1.62 | 13.5 | 3 | 0.5 | 0.92 | 12.5 |
| 4 | 1 | 1.29 | 14.0 | 4 | 12 | 0.84 | 6.0 |
| 5 | 2 | 1.28 | 11.0 | 5 | 2 | 0.98 | 9.0 |
| 6 | 1 | 1.12 | 2.0 | 6 | 1 | 1.01 | 15.5 |
| 7 | 5 | 1.95 | 15.5 | 7 | 1 | 1.02 | 9.5 |
| 8 | 1 | 1.24 | 12.5 | 8 | 12 | 1.06 | 8.0 |
| 9 | 4 | 1.37 | 10.7 | 9 | 9 | 1.10 | 15.5 |
| 10 | 2 | 1.49 | 13.5 | 10 | 16 | 1.10 | 9.5 |
| 11 | 4 | 1.40 | 11.0 | 11 | 1.5 | 1.12 | 10.0 |
| 12 | 1 | 1.01 | 7.5 | 12 | 5 | 1.12 | 8.5 |
| 13 | 2 | 1.80 | 15.3 | 13 | 2 | 1.12 | 4.0 |
| 14 | 2 | 1.26 | 11.0 | 14 | 2 | 1.14 | 9.0 |
| 15 | 3 | 1.42 | 13.5 | 15 | 3 | 1.15 | 10.0 |
| 16 | 2 | 1.47 | 14.5 | 16 | 7 | 1.16 | 8.0 |
| 17 | 8 | 1.36 | 9.0 | 17 | 4 | 1.16 | 10.0 |
| 18 | 9 | 2.11 | 11.0 | 18 | 9 | 1.16 | 10.5 |
| 19 | 4 | 1.57 | 8.7 | 19 | 5 | 1.17 | 9.0 |
| 20 | 6 | 1.55 | 9.0 | 20 | 2 | 1.17 | 9.0 |
| 21 | 7 | 1.26 | 10.5 | 21 | 14 | 1.17 | 9.0 |
| 22 | 11 | 0.85 | 8.5 | 22 | 10 | 1.19 | 9.5 |
| 23 | 11 | 1.29 | 8.5 | 23 | 9 | 1.20 | 8.5 |
| 24 | 11 | 1.43 | 9.0 | 24 | 10 | 1.23 | 13.3 |
| 25 | 10 | 1.17 | 16.5 | 25 | 8 | 1.23 | 11.0 |
| 26 | 3 | 2.26 | 15.0 | 26 | 6 | 1.23 | 10.5 |
| 27 | 1 | 2.12 | 13.0 | 27 | 12 | 1.24 | 11.5 |
| 28 | 5 | 1.51 | 13.5 | 28 | 11 | 1.24 | 9.0 |
| 29 | 2 | 1.47 | 12.0 | 29 | 2 | 1.26 | 14.5 |
| 30 | 2 | 1.73 | 13.0 | 30 | 10 | 1.27 | 9.0 |
| 31 | 1 | 1.44 | 11.5 | 31 | 17 | 1.31 | 12.5 |
| 32 | 13 | 1.44 | 13.0 | 32 | 13 | 1.34 | 10.5 |
| 33 | 5 | 2.22 | 15.5 | 33 | 10 | 1.42 | 12.5 |
| 34 | 1 | 1.25 | 11.0 | 34 | 4 | 1.43 | 9.0 |
| 35 | 2 | 2.13 | 19.5 | 35 | 15 | 1.53 | 11.0 |
| 36 | 1 | 1.75 | 12.0 | 36 | 11 | 1.29 | 13.0 |
| 37 | 1 | 1.33 | 15.5 | 37 | 11 | 1.36 | 9.0 |
| 38 | 1.5 | 1.16 | 12.5 | 38 | 14 | 1.03 | 9.0 |
| 39 | 1.5 | 1.95 | 12.0 | 39 | 9 | 1.28 | 6.0 |
| 40 | 4 | 2.01 | 13.5 | 40 | 11 | 1.39 | 15.0 |
| 41 | 8 | 1.81 | 15.0 | 41 | 7 | 0.80 | 12.0 |
| 42 | 6 | 2.05 | 13.5 | 42 | 6 | 1.44 | 10.0 |
| 43 | 1.5 | 1.36 | 12.0 | 43 | 14 | 1.08 | 9.0 |
| 44 | 6 | 2.42 | 16.0 | 44 | 10 | 1.39 | 8.0 |
| 45 | 6 | 1.61 | 11.0 | 45 | 8 | 0.91 | 9.0 |
| 46 | 7 | 1.80 | 11.5 | 46 | 14 | 1.53 | 13.0 |
| 47 | 4 | 2.15 | 13.5 | ||||
| 48 | 5 | 1.31 | 12.0 | ||||
| 49 | 5 | 1.63 | 12.0 | ||||
| 50 | 5 | 1.79 | 9.0 | ||||
In bold are creatinine values higher than the threshold recommended by IRIS to classify cats in stage 2 of renal disease, and symmetric dimethylarginine (SDMA) values higher than the upper reference limit recommended by the producer of the test and included in the new IRIS staging system 4
Within the group of Birman cats, no significant differences (P = 0.213) in the concentration of SDMA were found between cats with creatinine <1.60 mg/dl (mean ± SD 11.6 ± 2.8 µg/dl, median 12.0 µg/dl) and cats with creatinine >1.60 mg/dl (mean ± SD 12.9 ± 2.8 µg/dl, median 13.0 µg/dl). The proportion of cats with SDMA >14.0 µg/dl was significantly higher (P = 0.045) in cats with creatinine higher >1.60 mg/dl (n = 7/20) than in cats with creatinine <1.60 mg/dl (n = 3/30). However, the proportion of cats with a substantial elevation in SDMA concentration (ie, >15.5 µg/dl) was not significantly different (P = 0.372) in cats with creatinine >1.60 mg/dl (n = 2/20) than in cats with creatinine <1.60 mg/dl (n = 1/30).
Finally, it is worth noting that in the only two Birman cats younger than 1 year of age the SDMA concentration was within the pre-existing RI (7.5 and 14 µg/dl, respectively).
Duplicate measurement of SDMA provided the same results in 32 cases (33.3%), showed a difference of 1 µg/dl in 42 cases (43.8%), a deviation of 2 µg/dl in 20 cases (20.8%) and a difference of 3 µg/dl in two cases (2.1%). The coefficient of variation of the duplicate measurements ranged from 0.0–20.2%, with a median value of 5.2%.
The concentration of SDMA was higher than the upper limit of the pre-existing RI (14 µg/dl) in 10/50 Birman cats (20.0%) and in four cats belonging to other breeds (8.7%). The proportion of individuals with increased SDMA compared with the RIs was not significantly different between groups (P = 0.094).
In 7/10 Birman cats with increased SDMA (14.0% of the total number of Birman cats included in this study), and in all the four cats of other breeds with increased SDMA, the deviation from the RIs did not exceeded 1.5 µg/dl. This value corresponds to the approximately 10% deviation that is consistent with the analytical variability of the method determined by the coefficient of variation recorded in most of the duplicate or triplicate measurements in this study, and is consistent with the coefficient of variation reported in the scientific literature (7% at values close to the upper limit of the RI). 18 Also in this case the proportion of cats with a deviation <10% compared with the RIs was not significantly different in Birman cats compared with cats of other breeds (P = 0.325).
However, within the Birman group, the proportion of cats with a deviation of more than 10% on the upper reference limit of SDMA (n = 3/50) was significantly lower (P <0.001) than the proportion of cats with a deviation of >10% on the upper reference limit of creatinine (n = 15/50). In fact, a deviation >1.5 µg/dl from the upper reference limit was found only in three Birman cats (6.0% of the total number of Birman cats included in the study), which had SDMA concentrations of 16.0, 16.5 and 19.5 µg/dl. Two of these three cats also had creatinine values >1.6 mg/dl. In the cats of other breeds the deviation from the upper reference limit was <1.5 µg/dl. Moreover, the proportion of cats with a deviation higher than 1.5 µg/dl from the upper reference limit was not significantly different between Birman cats and cats of other breeds (P = 0.076), likely owing to the low number of cats of other breeds.
However, the transference method did not validate, in Birman cats, the RI generated in the general feline population. Therefore, a breed-specific RI was generated for SDMA using the approach recommended by the ASVCP guidelines. This approach identified a single outlier that was in the lower and non-clinically significant part of the dataset (Figure 2), and defined a RI ranging from 3.4 µg/dl (90% confidence interval [CI] 2.0–8.3 µg/dl) to 19.2 µg/dl (90% CI 16.0–20.0 µg/dl).
Figure 2.
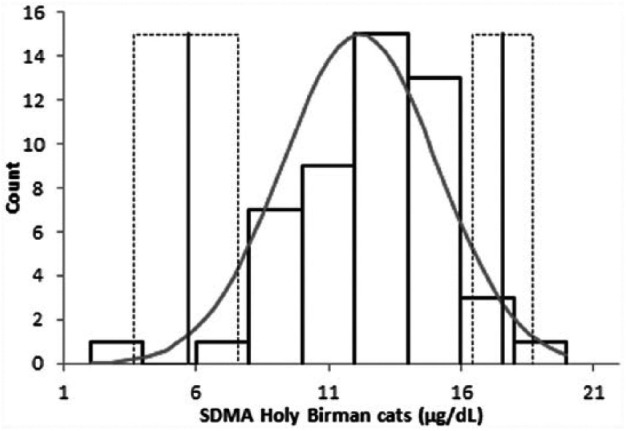
Distribution of data regarding symmetric dimethylarginine (SDMA) concentration in Birman cats. The bars indicate the frequency of each point value. Vertical solid lines indicate the limits of the reference intervals (dotted lines represent the 90% confidence intervals of each limit). The grey line indicates the fitted distribution, which depicts a Gaussian curve
Discussion
The results of this study confirmed the physiologically high serum concentration of creatinine in Birman cats, already reported in previous studies,1,2 either in terms of cats with creatinine values >1.6 mg/dl or in terms of magnitude of the increase compared with this threshold. In fact, the concentration of serum creatinine was significantly higher in Birman cats than in cats of other breeds, although this result is likely affected by a selection bias – normal creatinine concentration being an inclusion criterion for cats of other breeds. The threshold mentioned above is the upper reference limit of the laboratory and, more importantly, corresponds to the threshold for stage 2 IRIS classification, which is widely used independently of the RIs of each laboratory. 4 Therefore, over-diagnosis of CKD or errors in CKD staging of Birman cats may also occur for laboratories using higher RIs.
The concentration of SDMA was increased in some Birman cats. Cats with increased SDMA should be classified, according to the most recent version of the IRIS guidelines, 4 as stage 1 when creatinine is <1.6 mg/dl or in higher stages when creatinine is >1.6 mg/dl. However, in most cases, the increase recorded in Birman cats was quantitatively minimal, and a small increase in SDMA above the RI could be secondary to analytical variability (based on our study) or represent expected outliers, as RIs are calculated to include only 95% of the general population. 19 An increase of similar magnitude was also found in a similar proportion in the cats of other breeds, as well as in a previous study in dogs, 20 in which SDMA in healthy puppies was as high as 16 µg/dl (upper reference limit 14 µg/dl). This confirms that such an increase in magnitude might not be relevant from a biological/diagnostic point of view.
In order to exclude that these cats have ongoing CKD, not diagnosed by serum creatinine owing to its lower sensitivity compared with SDMA, 10 repeated measurements over time should be performed as only persistent increases of SDMA should be considered consistent with ongoing CKD. 4 However, as further support to the hypothesis that the slight increases recorded in some cats were likely dependent on the analytical variability of the method, in both breed groups these changes were found in cats older than 8 years, which are potentially at risk of CKD 4 and in which SDMA may be an earlier marker of CKD than creatinine,10,21 as well as in cats younger than 8 years.
Despite the absence of statistical differences in the proportion of cats with increased SDMA, in three cats (6% of Birman cats) the increase in SDMA concentration was higher than due increase expected due to the analytical variability of the assay. All three cats, as well as the other cats included in the study, were clinically healthy either at the time of sampling or during a follow-up ranging from 12–18 months (including repeated measurement of creatinine and urine specific gravity), and did not have other laboratory abnormalities.
There were, however, a couple of exceptions. Two of the clinically healthy study cats had a concentration of serum creatinine higher than the non-breed-specific RI and the IRIS threshold for stage 2CKD. This confirms the risk of overestimation of CKD in Birman cats; although the lack of clinical signs over time suggests that these cats are likely healthy, based on laboratory results alone it is impossible to determine whether these cats are affected by an early phase of CKD or are really healthy but have, in addition to the increased creatinine concentration typical of this breed, a physiologically high concentration of SDMA or any other condition that induces a transient decrease of GFR. Moreover, urinalysis and measurement of the urine specific gravity, as well as other biochemical tests in blood (eg, electrolytes, acid–base status) usually included in panels for the evaluation of renal function, were not included in the present study. Therefore, it is not possible to definitely exclude that the Birman cats included in this study were affected by early phases of CKD. However, this hypothesis seems unlikely, as repeated visits, bloodwork and urinalysis were performed in all these cats, either before the inclusion in this study or over a follow-up of 12–18 months, without revealing any abnormality consistent with CKD. Also, the results of the urine specific gravity of the few cats on which urinalysis was performed within this study were not consistent with CKD.
In any case, a significantly higher concentration of SDMA was found in Birmans cats compared with cats of other breeds. However, the overlap between the data of the two breed groups was less pronounced for creatinine than for SDMA, although the overlap of creatinine may be underestimated owing to the exclusion of cats with creatinine >1.6 mg/dl in the group of cats of other breeds. Moreover, the transference method did not allow validation of the pre-existing SDMA RI. Therefore, a novel and breed-specific RI was generated using the results of the first SDMA measurement (ie, excluding results of duplicate or triplicate measurements). In the generation of the new RI a single outlier was found, but it was retained in the dataset as low SDMA values do not have a clinical significance. The normality test revealed that the data had a Gaussian distribution (P = 0.094), but the RI was generated using the non-parametric method as recommended by the ASVCP guidelines when the sample size is small. 13
The breed-specific RI generated in this study for Birman cats (3.4–19.2 µg/dl) largely overlapped the pre-existing one (0.0–14.0 µg/dl), and both the lower and the upper reference limits had a wide CI, likely due to the low number of observations. Therefore, the pre-existing RI, referred to the general feline population, may be used as a clinical decision limit, independently of the newly established interval. 15 In fact, in routine practice values <3.4 µg/dl are likely not relevant, and – based on our knowledge of SDMA metabolism8,11 – do not have a clinical significance. The huge CI of the upper reference limit, depending on a single cat with very high SDMA value, suggests that the former limit of 14.0 µg/dl may still be used in routine practice. However, occasionally, values higher than the RI could be detected. Moreover, it should be noted that the lower CI for the upper reference limit is still >14.0 µg/dl and therefore this upper limit may be modified if further studies confirm that healthy Birman cats consistently have values higher than the variation due to the analytical variability of the methods.
All these results, although biased by the lack of confirmatory tests for CKD, such as evaluation of urine specific gravity or clearance tests to estimate the GFR, suggest that, independently of the breed, SDMA, which is considered a sensitive marker of CKD, does not have an absolute specificity, as reported in previous studies, 10 and that SDMA might have lower specificity for CKD in certain breeds since increased SDMA may be occasionally found also in Birman cats. However, the comparison of the frequency and magnitude of increased SDMA and increased creatinine in Birman cats, as well as the statistical analysis regarding the comparison of the proportion of cats with increased SDMA or creatinine within the Birman group, confirms the hypothesis of this study – that SDMA is less frequently and less severely increased than creatinine in Birman cats. However, the frequency of changes in the concentration of creatinine may be lower for laboratories that use higher non-breed-specific upper limits of the RIs for creatinine.
The design of this study was not adequate to investigate the mechanisms responsible for this difference. However, the different behaviour of the two analytes suggests that the high creatinine concentration of Birman cats does not depend on a decreased GFR, which would more likely induce increases of both the analytes.10–12 It has been demonstrated that SDMA is less affected by body mass than creatinine,21,22 and that, in other species, physiologically high creatinine concentrations are found in breeds with increased muscle mass. 23 However, although to our knowledge no studies have compared the muscle mass of feline breeds, the muscle mass of Birman cats seems to be not particularly different from those of other cats. Therefore, it is unlikely that the different behaviour of the two analytes depends on the muscle mass. Unfortunately, information about the body weight or the body condition score of the cats included in this study were incomplete and, therefore, it was not possible to compare the results of creatinine or SDMA with data regarding the muscle mass. However, although the relationship between these variables was outwith the scope of this study, it would be interesting to further explore this aspect in future studies.
Similarly, the differences between Birman cats and cats of other breeds cannot be explained by the significant difference recorded for age, which, if present, would have more likely induced the opposite effect (frequent increases of SDMA in the group of older cats, in which the likelihood of a reduced GFR increases) leading to higher values in cats of other breeds (which were older than Birman cats) than in Birman cats. This hypothesis is confirmed by the results of the Birman cats younger than 1 year, which had a concentration of SDMA within the pre-existing RI.
Conclusions
This study supports the idea that SDMA may be less frequently elevated than creatinine in apparently healthy Birman cats when non-breed-specific laboratory RIs are used. On one hand, this study confirms the importance of using a breed-specific RI for investigating renal function in Birman cats; on the other hand, SDMA could be recommended as a marker of choice for routine investigation of renal function in this breed (and possibly in other breeds with physiologically high creatinine levels) and it should be included in any general health screening profile of these cats. The analysis of both creatinine and SDMA would help to prevent the over-diagnosis of CKD or errors in staging renal disease in Birman cats. It would also help to limit further clinical and laboratory investigations in Birman cats to only those that have creatinine concentrations outside of the breed-specific RIs or SDMA concentrations that are largely outside of the RI of the general feline population.
Acknowledgments
The authors are grateful to Dr Sabine Lower (IDEXX Laboratories Italia) for their technical support, and to the Italian association of breeders of Birman cats (Agabi) who advertised this study among the Italian breeders.
Footnotes
Measurement of symmetric dimethylarginine was kindly offered by IDEXX Laboratories Italia. Dr Eleonora Piseddu is an employee of IDEXX Laboratories Italia Srl.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Accepted: 6 September 2017
References
- 1. Reynolds BS, Concordet D, Germain CA, et al. Breed dependency of reference intervals for plasma biochemical values in cats. J Vet Intern Med 2010; 24: 809–818. [DOI] [PubMed] [Google Scholar]
- 2. Paltrinieri S, Ibba F, Rossi G. Hematological and biochemical reference intervals of four feline breeds. J Feline Med Surg 2014; 16: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reynolds BS, Lefebvre HP. Feline CKD: pathophysiology and risk factors – what do we know? J Feline Med Surg 2013; (Suppl 1): 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Renal Interest Society. IRIS 2015 staging of CKD. http://www.iris-kidney.com (accessed September 12, 2016).
- 5. Pesteanu-Somogyi LD, Radzai C, Pressler BM. Prevalence of feline infectious peritonitis in specific cat breeds. J Feline Med Surg 2006; 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedersen NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet J 2014; 201: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barthez PY, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg 2003; 5: 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jepson RE, Syme HM, Vallance C, et al. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008; 22: 317–324. [DOI] [PubMed] [Google Scholar]
- 9. Braff J, Obare E, Yerramilli M, et al. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med 2014; 28: 1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014; 28: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleck C, Schweitzer F, Karge E, et al. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in patients with chronic kidney diseases. Clin Chim Acta 2003; 336: 1–12. [DOI] [PubMed] [Google Scholar]
- 12. Braun JP, Lefebvre HP, Watson AD. Creatinine in the dog: a review. Vet Clin Pathol 2003; 32: 162–179. [DOI] [PubMed] [Google Scholar]
- 13. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012; 41: 441–453 [DOI] [PubMed] [Google Scholar]
- 14. Geffré A, Concordet D, Braun JP, et al. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 2011; 40: 107–112. [DOI] [PubMed] [Google Scholar]
- 15. National Committee for Clinical Laboratory Standards. Defining, establishing and verifying reference intervals in the clinical laboratory. Approved guideline. 3rd ed. CLSI document C28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI), 2010. [Google Scholar]
- 16. Bourges-Abella N, Geffré A, Concordet D, et al. Reference value advisor (V2) now performs regression-based reference intervals with continuous covariables. Vet Clin Pathol 2012; 41: E16. [Google Scholar]
- 17. Ricós C, Alvarez V, Cava F, et al. Current databases on biological variation: pros, cons and progress. J Clin Lab Invest 1999; 59: 491–500. [DOI] [PubMed] [Google Scholar]
- 18. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2016; 46: 941–960. [DOI] [PubMed] [Google Scholar]
- 19. Geffré A, Friedrichs K, Harr K, et al. Reference values: a review. Vet Clin Pathol 2009; 38: 288–298. [DOI] [PubMed] [Google Scholar]
- 20. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med 2015; 29: 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L-carnitine, and medium-chain triglycerides. Vet J 2014; 202: 588–596. [DOI] [PubMed] [Google Scholar]
- 22. Hall JA, Yerramilli M, Obare M, et al. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med 2015; 29: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaldívar-López S, Marin LM, Iazbik MC, et al. Clinical pathology of greyhounds and other sighthounds. Vet Clin Pathol 2011; 40: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]


