Abstract
Objectives
The goals of this retrospective study were to evaluate the use of mechlorethamine, vincristine, melphalan and prednisolone (MOMP) chemotherapy for rescue of feline lymphoma, to describe the protocol’s toxicity and to determine prognostic indicators for progression-free survival.
Methods
The medical records of 12 cats treated with MOMP chemotherapy at the University of Tennessee Veterinary Medical Center between 2007 and 2017 were evaluated. Parameters assessed included lymphoma cell size, anatomical location, number of previous chemotherapy drugs and number of previous rescue protocols received. Chemotherapy-related toxicity was also described.
Results
Seven of 12 cats responded to this rescue protocol. Three cats experienced complete response and four cats achieved partial response for a median duration of 39 days (range 14–345 days). Cats that achieved complete response had a significantly longer median progression-free survival than cats that did not respond to treatment. Five of 12 cats developed hematologic toxicity (neutropenia) and one cat developed gastrointestinal toxicity. Toxicity was mild in most cases; no cats needed to be hospitalized. Neutropenia was associated with increased progression-free survival.
Conclusions and relevance
MOMP is a safe and effective rescue chemotherapy protocol for cats with relapsing and refractory lymphoma.
Introduction
Lymphoma accounts for approximately one-third of all feline cancers. 1 According to the largest compilation of feline lymphoma cases, the majority fit intermediate (35%) to high-grade (50%) histologic classification. 2 Several chemotherapeutic agents have been used to treat this disease, with mixed success. Response rates range from approximately 22–90% with an overall survival of 2–12 months.3–10 One of the major therapeutic challenges remains re-induction of remission in an attempt to increase survival after failure of frontline therapy. Only a few rescue chemotherapy protocols for cats have been evaluated in the literature.11,12 In one report, the median progression-free interval in cats treated with lomustine for all histologic subtypes of lymphoma was 39 days. 11 In another report, doxorubicin-based rescue therapy resulted in a 22% response rate. 12
Mechlorethamine-based chemotherapy is frequently utilized to treat relapsed lymphoma in dogs. The protocols consist of mechlorethamine, vincristine, prednisone and either procarbazine or melphalan (MOPP or MOMP, respectively).13,14 Both produce an objective response in >50% of dogs for a median of 47–63 days.13,14 To date there is almost no information in the literature about the use of mechlorethamine-based rescue chemotherapy in cats. In a study of 16 cats with gastric lymphoma, eight cats were treated with rescue chemotherapy, four of which received a mechlorethamine-based protocol. The number of cats that responded to it was not reported. 15
The goals of this retrospective study were to evaluate the use of MOMP chemotherapy for rescue of feline lymphoma, to describe the protocol’s toxicity and to determine prognostic indicators for progression-free survival (PFS). This is the first study to describe the use of MOMP protocol in cats.
Materials and methods
The medical records of cats treated with MOMP chemotherapy at the University of Tennessee Veterinary Medical Center between 1 January 2007 and 1 March 2017 were evaluated. Cats were included in the study if they had either a histologically or cytologically confirmed lymphoma, documentation of relapse after treatment with frontline chemotherapy (cyclophosphamide, doxorubicin, vincristine and prednisolone [CHOP] with or without L-asparaginase), had received at least one dose of MOMP and had follow-up information at least 1 week after MOMP administration. Data obtained from the records included age, sex, weight, breed, feline leukemia virus (FeLV)/feline immunodeficiency virus (FIV) status, anatomic location of lymphoma, number of chemotherapy drugs received before MOMP and number of rescue protocols received before MOMP. Lymphoma cell size was obtained from the pathology reports. Immunophenotype was recorded when it was available. Anatomical location was classified as intra-abdominal (gastrointestinal, splenic, hepatic and abdominal lymph node involvement), mediastinal, renal, multicentric (mediastinal and intra-abdominal involvement) and other.
The MOMP protocol is described in Table 1. The dose of vincristine was determined based on the history of previous side effects during CHOP treatment and clinician preference. Melphalan was administered either as one 0.5 mg capsule four consecutive days a week or one 2 mg capsule once a week, based on availability.
Table 1.
Feline mechlorethamine, vincristine, melphalan and prednisolone (MOMP)* rescue chemotherapy protocol
| Drug | Dosage | Route | Days |
|---|---|---|---|
| Mechlorethamine | 3 mg/m2 | IV | 0, 7 |
| Vincristine | 0.5–0.75 mg/m2 | IV | 0, 7 |
| Melphalan | 2 mg weekly | PO | 0–13 |
| Prednisolone | 5 mg q12h | PO | 0–28 |
*MOMP is a 28 day cycle protocol. The second cycle starts on day 28
IV = intravenously; PO = per os
Response to treatment during follow-up visits was determined based on physical examination, measurement of palpable lesions, laboratory tests (complete blood count [CBC] and serum chemistry panels, when applicable), clinical signs reported by owners and imaging (thoracic, and/or abdominal) if performed. Imaging was performed if it was recommended by the attending clinician and agreed to by the owners. Complete response (CR) was defined as resolution of all measurable disease and clinical signs, partial response (PR) as >50% but <100% decrease in measurable disease and no response (NR) as <50% decrease or increase in measurable disease and/or worsening clinical signs. MOMP toxicity evaluation was conducted according to the Veterinary Comparative Oncology Group-Common Terminology Criteria for Adverse Events version 1.1. 16
PFS was defined as time from initiation of MOMP chemotherapy to disease progression or death. The Kaplan–Meier product limit method was used to estimate PFS, which was the end point of this study. Log-rank test was used to determine whether anatomic location of lymphoma, response to MOMP treatment, type of response, hematologic toxicity and number of rescue protocols used before MOMP significantly affected PFS. Statistical analyses were carried out using IBM SPSS, version 24. Results were considered significant if P <0.05.
Results
Twelve cats met the inclusion criteria for this study. A summary of patient demographics can be found in Table 2.
Table 2.
Demographics of 12 cats treated with the mechlorethamine, vincristine, melphalan and prednisolone (MOMP) chemotherapy protocol
| Characteristic | Category | Number of cats |
|---|---|---|
| Sex | Female spayed | 3 |
| Male castrated | 9 | |
| Breed | Domestic shorthair | 8 |
| Domestic longhair | 1 | |
| Siamese | 1 | |
| Persian | 1 | |
| Maine Coon | 1 | |
| Anatomic location | Intra-abdominal | 4 |
| Mediastinal | 1 | |
| Multicentric | 2 | |
| Renal | 3 | |
| Other | 2 |
The median age at the time of diagnosis was 9 years (range 3–13 years). The median weight was 3.96 kg (range 2.65–5.58 kg). Four of the 12 cats had intra-abdominal lymphoma: one had a gastric mass, two had intestinal masses with or without mesenteric lymphadenopathy, and one had hepatic and splenic involvement. Three cats had renal lymphoma, one cat had mediastinal lymphoma, and two had mediastinal and intra-abdominal lymphoma with splenic involvement in one cat, and hepatic and nodal involvement in the other. These two cats were classified as having multicentric lymphoma. Of the two remaining cats that were classified as other, one had lymphoma in the retrobulbar space with extension into the nasal cavity and subcutis; the other had multifocal cutaneous nodules and a retrobulbar mass. The first cat was FeLV positive and the second one FIV positive. All other cats in this study were negative for FeLV and FIV. The majority of cats (11/12) had large-cell lymphoma. Intermediate-cell lymphoma was present in one cat. The diagnosis of lymphoma was made from biopsy in three cats; the remaining nine cats had a cytologic diagnosis. Immunophenotyping was performed in 3/12 cats; flow cytometry was used in one cat and immunohistochemistry in the other two cats. Two cats had B-cell lymphoma (one renal and one intra-abdominal), and one cat with a mediastinal tumor location had a T-cell rich B-cell lymphoma.
Prior to initiation of the MOMP rescue, cats received a median of three different chemotherapy drugs (range 2–5). These drugs included cyclophosphamide, vincristine, doxorubicin, lomustine and L-asparaginase. All cats received prednisolone as a part of their first protocol. Most cats (10/12) in this study received MOMP as their first rescue protocol; the remaining two cats were given one rescue protocol before being switched to MOMP: lomustine/L-asparaginase/prednisolone (n = 1) and vincristine/cyclophosphamide/prednisolone (n = 1). The median duration of treatment before starting the MOMP protocol was 76 days (range 11–400 days).
Of the 12 cats, five experienced complete remission (CR), four experienced partial remission (PR), one had stable disease (SD) and two had progressive disease (PD) after the initial treatment. Response to the initial chemotherapy did not correlate with response to the MOMP rescue protocol. Out of the five cats that were in complete remission after the initial treatment two responded to MOMP (one CR, one PR), whereas three did not (one SD, two PD). Of the four cats that were in partial remission after the initial treatment one subsequently had a CR and two a PR to MOMP, whereas the third cat’s disease progressed. Two of the three cats that did not respond to the initial chemotherapy were in remission after the MOMP protocol (one CR, one PR); one cat had stable disease. Lymphoma progression was suspected based on clinical signs reported by the owners in all 12 cats. It was documented on physical examination in eight cats. Three of these cats had circulating neoplastic cells on CBC. Four of 12 cats were restaged with imaging. Abdominal ultrasound was performed in two cats with intra-abdominal lymphoma, one cat with multicentric and one with renal lymphoma. Thoracic radiographs were performed in one cat with multicentric lymphoma.
Sixteen 28 day MOMP cycles were administered to the cats in this study. The median number of cycles was 1 (range 0.5–3.5). The starting dose of vincristine (0.5–0.75 mg/m2) was determined by the attending clinician based on patient history of toxicity with previous chemotherapy and clinician preference. Vincristine was dosed at 0.5 mg/m2 (n = 9) and 0.75 mg/m2 (n = 3). The MOMP protocol was altered in one cat to every other week instead of the 28 day cycle, owing to neutropenia on day 7.
The median PFS of all cats treated with the MOMP chemotherapy protocol was 22 days (range 0–345 days). All cats had follow-up information available and all had died or were euthanized because of lymphoma. Seven of 12 cats responded to MOMP. Three cats experienced a CR and four cats achieved a PR. The duration of CR ranged from 39–345 days (median 62 days). The duration of PR ranged from 14–53 days (median 22 days). Response to treatment was significantly associated with PFS (P = 0.018). Cats that responded had a median PFS of 39 days, whereas cats that did not had a median PFS of 0 days. Cats that achieved CR had significantly longer median PFS than cats that had NR (P = 0.010) (Figure 1).
Figure 1.
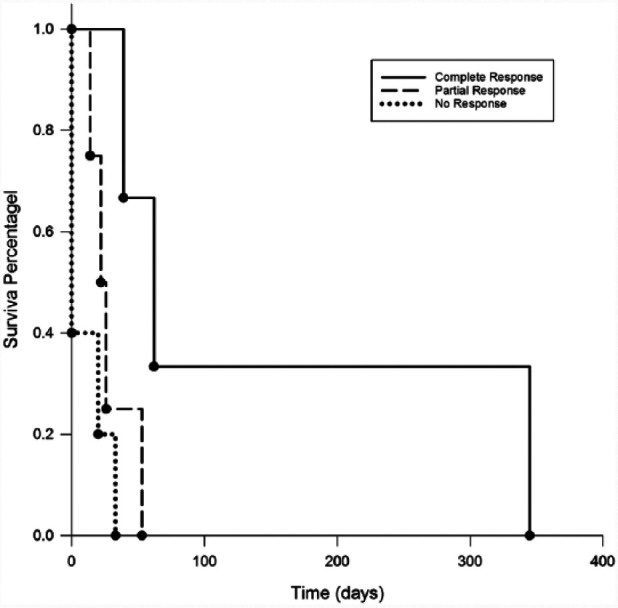
Progression-free survival (PFS) comparison in cats by type of response. Cats that experienced complete response had a significantly longer median PFS (62 days) than cats that did not respond to treatment (0 days) (P = 0.019). Cats that experienced partial response did not have a significantly longer median PFS (22 days) than cats that had no response (P = 0.242)
There was no significant difference in PFS between cats that received other rescue chemotherapy before MOMP and those that did not. Anatomic location of lymphoma did not have a significant impact on PFS.
Gastrointestinal and hematological toxicity occurred in 5/12 cats. One cat had gastrointestinal toxicity; five cats experienced hematologic toxicity. The one cat with gastrointestinal toxicity had grade I anorexia and vomiting; this same cat had also experienced hematological toxicity (grade I neutropenia). This cat received 0.75 mg/m2 vincristine. Follow-up CBCs were performed on days 7, 14 and 21 during the first MOMP cycle and days 7 and 14 during subsequent cycles. Hematological toxicity occurred at a median of 7 days (range 7–14 days) in the 28 day MOMP cycle. Four cats developed grade I neutropenia; one cat had grade III neutropenia. Three of the four cats with grade I neutropenia received 0.75 mg/m2 vincristine. The cat with grade III neutropenia received 0.5 mg/m2 vincristine. None of the remaining cats that received 0.5 mg/m2 of vincristine developed neutropenia. Thrombocytopenia was not observed in any of the cats treated with MOMP. No cats required hospitalization during MOMP treatment.
The MOMP protocol was modified in three cats that experienced toxicity in order to be able to continue treatment. The dose of vincristine was reduced to 0.5 mg/m2 in the cat that had both gastrointestinal and hematological toxicity. The dose of mechlorethamine was reduced to 2.7 mg/m2 in the cat with grade III neutropenia. In one cat that developed grade I neutropenia MOMP was administered every other week instead of the 28 day cycle. Cats that developed neutropenia during MOMP treatment had a significantly longer PFS (P = 0.013) (Figure 2).
Figure 2.
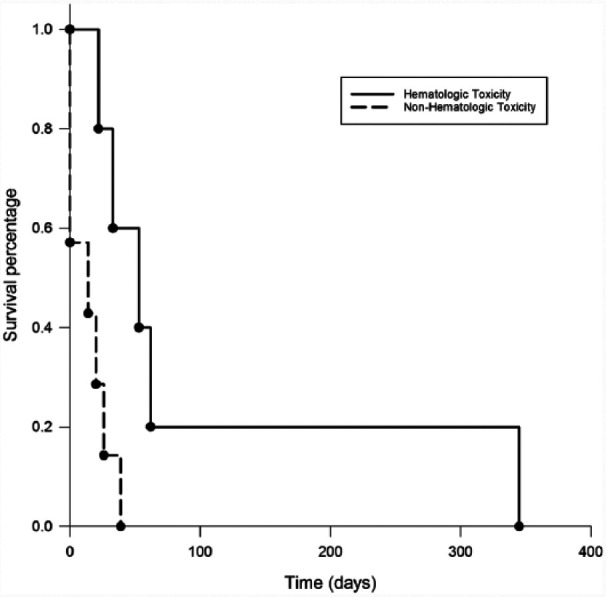
Progression-free survival (PFS) comparison between cats that experienced neutropenia and cats that did not. The median PFS for cats that developed neutropenia on MOMP treatment was 53 days vs 14 days for cats that did not develop neutropenia (P = 0.013)
Median PFS for cats that developed neutropenia was 53 days vs 14 days in cats that had no hematologic toxicity from MOMP chemotherapy.
Discussion
To date, very little information on outcomes of chemotherapy for rescue of feline lymphoma is available in the literature. In a study by Dutelle et al, 11 lomustine was evaluated for rescue of resistant lymphoma in cats. This study included 39 cats with small-, intermediate- and large-cell lymphoma. The median progression-free interval was 39 days for all histologies. The overall response rate to lomustine was not reported. Cats with small- and intermediate-cell lymphoma and cats with gastrointestinal location had a longer PFS. A study by Oberthaler et al looked at 23 cats with relapsing and refractory lymphoma treated with doxorubicin-based rescue chemotherapy. 12 This study reported that 22% of cats, all with non-large-cell lymphoma, responded to treatment. More than half of the responding cats received other concurrent chemotherapy. The study concluded that doxorubicin-based chemotherapy was not effective as rescue for feline lymphoma.
In contrast to these reports, our study population consisted of cats with intermediate- and large-cell lymphoma, with the majority (11/12) diagnosed with large-cell lymphoma. The large-cell subtype of lymphoma has been reported as a negative prognostic indicator for response to treatment, progression-free and overall survival in cats with lymphoma.1,4,10–12,17 The MOMP rescue protocol was effective at inducing remission in this population of cats. Seven of 12 cats responded to this rescue protocol. Three cats experienced CR for a median of duration of 62 days, and four cats experienced PR for a median of 24 days. Cats that experienced CR had a significantly longer median PFS than cats that did not respond to treatment. This is consistent with previous reports of response to treatment being one of the most commonly found positive prognostic indicators in feline lymphoma studies.5,7,8,18,19
No statistical significance in PFS was identified between cats that received MOMP as the first rescue and cats that were treated with another rescue protocol before MOMP. However, only two cats in this study had been treated with another rescue protocol before receiving MOMP. Future prospective studies with larger numbers of patients in each subgroup should be performed in order to more accurately address the question of whether the number of previous chemotherapy protocols affects PFS of cats treated with MOMP. Anatomic location was not identified as a negative prognostic indicator in this study, unlike in several previous reports.3,8,10,11 A likely explanation for this is the low number of cases within each anatomic location group resulting in a low statistical power for analysis.
In this study we defined PFS as the time of MOMP initiation to the time of disease progression. While this may have led to a slight overestimation of the true remission duration, this method of estimating remission duration is frequently used in feline lymphoma studies because it can be difficult to pinpoint the exact time remission is achieved in some anatomic locations of this disease.7,12 We did not look at the overall survival time in this study, and instead chose to focus on PFS because many of the cats went on to have one or more rescue protocols after MOMP. The overall survival can be impacted by the owner’s willingness to pursue additional treatments, perception of their cat’s quality of life and willingness to consider euthanasia.
The MOMP rescue protocol was well tolerated by the majority of cats in this study. Gastrointestinal toxicity was identified in only 1/12 cats. This form of toxicity may have been under-recognized because three other cats (one with a gastric mass and two with intestinal masses) in this study already had clinical signs associated with gastrointestinal upset due to lymphoma. These cats did not experience worsening of their clinical signs during MOMP treatment; therefore, they were not described as having chemotherapy-related gastrointestinal upsets. Five of 12 cats experienced hematologic toxicity. Four cats had grade I neutropenia, which did not affect their quality of life, and one had grade III neutropenia. This cat was afebrile and was treated with oral broad-spectrum antibiotics for 7 days. Because this cat was originally treated with 0.5 mg/m2 vincristine, the mechlorethamine dose was reduced by 10%. Most of the neutropenic episodes were associated with a 0.75 mg/m2 dose of vincristine, although one cat developed grade I and one grade III neutropenia at 0.5 mg/m2. Neutropenia was associated with a significantly longer PFS in this study. However, the number of neutropenic cats was small and no conclusions can be drawn from this finding. Neutropenia resulted in treatment delay in all affected cats. In one cat vincristine was subsequently dose-reduced to 0.5 mg/m2 in order to be able to continue the 28 day MOMP cycle; in another cat the MOMP schedule was changed to every other week.
The limitations of this study include its retrospective nature, and a relatively small sample size. Because of the small number of cats, it was not possible to draw meaningful conclusions from some of the findings, such as lack of statistically significant difference in PFS between cats with CR and PR, and lack of impact of anatomic location on PFS. Collecting a large number of feline lymphoma cases for this type of study remains a challenge owing to the fact that non-small-cell lymphoma in cats generally carries a poor prognosis, and a limited number of owners choose to pursue rescue chemotherapy. Ideally, prospective multi-institutional studies should be performed to better assess rescue chemotherapy options for feline lymphoma. Although all cats in this study were fully staged with a minimum database, thoracic radiographs and abdominal ultrasound on initial presentation, not all of them were fully restaged at relapse. In some cases, a cat’s performance status and imaging of the previously affected body cavity only were used to determine response or lack of thereof. While this method can affect accurate interpretation of response, it has been frequently used in previous feline lymphoma studies for practical purposes.12,20,21
Conclusions
The results of this study suggest that the MOMP protocol is safe and effective as a rescue option for feline lymphoma. It justifies future prospective studies of MOMP in cats with relapsed lymphoma in various anatomic locations.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 14 September 2017
References
- 1. Vail DM. Hematopoietic tumors. In: Withrow SJ, Vail DM, Page RL. (eds). Small animal clinical oncology. 5th ed. St Louis, MO: Elsevier Saunders, 2013, pp 638–641. [Google Scholar]
- 2. Valli VE, Jacobs RM, Norris A, et al. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest 2000; 12: 295–306. [DOI] [PubMed] [Google Scholar]
- 3. Simon D, Eberle N, Laacke-Singer L, et al. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 4. Mooney SC, Hayes AA, MacEwen EG, et al. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981). J Am Vet Med Assoc 1989; 194: 696–702. [PubMed] [Google Scholar]
- 5. Zwahlen CH, Lucroy MD, Kraegel SA, et al. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997). J Am Vet Med Assoc 1998; 213: 1144–1149. [PubMed] [Google Scholar]
- 6. Kristal O, Lana SE, Ogilvie GK, et al. Single agent chemotherapy with doxorubicin for feline lymphoma: a retrospective study of 19 cases (1994–1997). J Vet Intern Med 2001; 15: 125–130. [DOI] [PubMed] [Google Scholar]
- 7. Teske E, Van Straten G, Van Noort R, et al. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med 2002; 16: 179–186. [DOI] [PubMed] [Google Scholar]
- 8. Waite AH, Jackson K, Gregor TP, et al. Lymphoma in cats treated with a weekly cyclophosphamide-, vincristine-, and prednisone-based protocol: 114 cases (1998–2008). J Am Vet Med Assoc 2013; 242: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 9. Krick EL, Cohen RB, Gregor TP, et al. Prospective clinical trial to compare vincristine and vinblastine in a COP-based protocol for lymphoma in cats. J Vet Intern Med 2013; 27: 134–140. [DOI] [PubMed] [Google Scholar]
- 10. Collette SA, Allstadt SD, Chon EM, et al. Treatment of feline intermediate- to high-grade lymphoma with a modified university of Wisconsin-Madison protocol: 119 cases (2004–2012). Vet Comp Oncol 2015; 14: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dutelle AL, Bulman-Fleming JC, Lewis CA, et al. Evaluation of lomustine as a rescue agent for cats with resistant lymphoma. J Feline Med Surg 2012; 14: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oberthaler KT, Mauldin E, McManus PM, et al. Rescue therapy with doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma: a retrospective study of 23 cases. J Feline Med Surg 2009; 11: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rassnick KM, Mauldin GE, Al-Sarraf R, et al. MOPP chemotherapy for treatment of resistant lymphoma in dogs: a retrospective study of 117 cases (1989–2000). J Vet Intern Med 2002; 16: 576–580. [DOI] [PubMed] [Google Scholar]
- 14. Back AR, Schleis SE, Smrkovski OA, et al. Mechlorethamine, vincristine, melphalan and prednisone (MOMP) for the treatment of relapsed lymphoma in dogs. Vet Comp Oncol 2015; 13: 398–408. [DOI] [PubMed] [Google Scholar]
- 15. Gustafson TL, Villamil A, Taylor BE, et al. A retrospective study of feline gastric lymphoma in 16 chemotherapy-treated cats. J Am Anim Hosp Assoc 2014; 50: 46–52. [DOI] [PubMed] [Google Scholar]
- 16. Veterinary cooperative oncology group-common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016: 14; 417–446. [DOI] [PubMed] [Google Scholar]
- 17. Pohlman LM, Higginbotham ML, Welles EG, et al. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Path 2009; 46: 259–268. [DOI] [PubMed] [Google Scholar]
- 18. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 19. Milner RJ, Peyton J, Cooke K, et al. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin–Madison chemotherapy protocol: 38 cases (1996–2003). J Am Vet Med Assoc 2005; 227: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 20. Peaston AE, Maddison JE. Efficacy of doxorubicin as an induction agent for cats with lymphosarcoma. Austral Vet J 1999; 77: 442–444. [DOI] [PubMed] [Google Scholar]
- 21. Fondacaro JV, Richter KP, Carpenter JL, et al. Feline gastrointestinal lymphoma: 67 cases (1988–1996). Eur J Comp Gastroenterol 1999; 4: 5–11. [Google Scholar]


