Abstract
Objectives
Multiple feline diseases involving the gastrointestinal tract, pancreas, liver and biliary tract are known to cause abnormal serum cobalamin and folate concentrations. Measuring the serum concentration of these vitamins can therefore be a helpful diagnostic tool. However, factors other than disease, in particular age, have also been suggested to have an effect on the serum concentration of cobalamin and folate. In previous studies, the dietary intake was not standardised, or even known, despite diet being the prinicpal source of both vitamins. Therefore, we evaluated the effect of age, sex and body weight on the serum concentration of folate and cobalamin in cats fed the same diet.
Methods
The serum cobalamin and folate concentrations were measured in 65 apparently healthy cats in a nutrition colony that had been fed an identical diet. A linear model was used to test the relationship between the serum concentration of cobalamin and folate with the variables age, sex and body weight.
Results
There was a large variation in the serum concentration of both folate and cobalamin, despite identical intake. Serum cobalamin was inversely associated with age (P = 0.002), and males had higher concentrations than females (P = 0.039). Serum folate was positively associated with age (P = 0.01).
Conclusions and relevance
Independent of diet, serum cobalamin concentration decreases with age. Changes in gastrointestinal function, microflora or metabolism may be responsible. Older cats may be more susceptible to cobalamin deficiency secondary to inappetence or gastrointestinal disease.
Introduction
Several diseases of the gastrointestinal tract, pancreas and liver can alter serum cobalamin and folate concentrations in cats.1–7 Diseases that have been associated with hypocobalaminaemia in cats include inflammatory bowel disease, alimentary lymphoma, pancreatitis, cholangitis–cholangiohepatitis and exocrine pancreatic insufficiency (EPI).1–4 A similar spectrum of diseases has been associated with decreased folate serum concentrations, although in feline EPI, serum folate may be within or above normal range.1,7 In addition to typical disease associations, some authors have suggested that feline cardiomyopathy, arterial thromboembolism and hyperthyroidism are associated with decreased serum cobalamin concentrations;8,9 although a recent study found that the prevalence of hypocobalaminaemia in hyperthyroid cats was low, while folate was significantly lower in hyperthyroid cats compared with when the same cats were euthyroid post-radioiodine treatment. 10 Lastly, congenital abnormalities impeding cobalamin absorption have been reported, albeit rarely, in cats.11,12
Factors other than disease have been found to have an effect on cobalamin and folate serum concentrations. Older cats have repeatedly been shown to have lower serum concentrations of cobalamin, while folate has been found to have a weaker inverse relationship with age.13–16 Breed differences have also been reported, with pedigree cats being more likely to have higher cobalamin concentrations than mixed-breed cats. 17 In one study, Birman and Maine Coon cats had significantly higher serum cobalamin concentrations than domestic short- or longhair cats, although the Maine Coon population in that study was significantly younger than the domestic breed cats, which may have confounded the results. 13
Independent of the underlying disease process, hypocobalaminaemia can have harmful effects on multiple organ systems. Cobalamin is a cofactor for enzymes involved in fatty acid and amino acid metabolism, and the synthesis of the nucleic acid thymidine. In cats, hypocobalaminaemia can lead to alterations in the concentrations of the sulfur-containing amino acids, methionine, cysteine and cystothionine, and cause significant increases in methylmalonic acid.11,15 Owing to its role in DNA production, hypocobalaminaemia affects the rapidly dividing cells, in particular cells of the gastrointestinal and haematopoietic systems. In humans, but not cats, defective DNA synthesis secondary to hypocobalaminaemia results in megaloblastic anaemia.18,19 In dogs, gastrointestinal tract histopathology changes associated with hypocobalaminaemia include moderate diffuse atrophy of the gastric mucosa, mild-to-moderate diffuse atrophy of the duodenal and proximal jejunal mucosa, and mild-to-moderate mucosal oedema and lymphangectasia of the entire intestinal tract. 20 Thus, cobalamin deficiency exacerbates intestinal malabsorption, which, in turn, can exacerbate the deficient state. Cobalamin’s role in central nervous system function is multifactorial and not completely understood, but glial cells, the interstitium and myelin production can be negatively affected by cobalamin deficiency. 21 Clinically apparent neurological dysfunction occurs in both people and cats with hypocobalaminaemia, with encephalopathy and myelopathy being described in cases of severe feline hypocobalaminaemia.11,12,22–24 Anecdotally, supplemental cobalamin is required in conjunction with primary therapies for clinical remission of some gastrointestinal diseases, emphasising the importance of recognising the deficient state.25–27
Similarly, folate is a cofactor for nucleic acid, amino acid and vitamin synthesis, and is also involved in DNA replication, repair and methylation. Kittens that were fed diets deficient in folic acid were found to have reduced appetite and growth, macrocytic anaemia, leukopenia and bone marrow changes (megaloblastic erythrocyte precursors).28–30 To our knowledge there have been no reports of clinical disease responding to folate supplementation; however, folate supplementation has been reported to resolve clinical signs associated with experimentally induced folate deficiency. 28
In none of the previously mentioned studies that reported an apparent relationship between either cobalamin or folate and age or breed, were the cobalamin and folate content of the diet being consumed defined. Thus, it is possible that previously identified associations were, in fact, due to differences in intake alone. To be able to determine if factors other than disease have an effect on serum cobalamin and folate concentrations in cats, diet must be removed as a variable. Therefore, the aim of this study was to determine if age, sex and body weight have an effect on serum cobalamin and folate concentrations in a population of apparently healthy cats with a known and consistent cobalamin and folate intake. The null hypothesis was that age, sex and body weight would not affect the serum concentration of cobalamin and folate.
Materials and methods
Apparently healthy cats were obtained from and housed in the Massey University Feline Nutrition Unit, a closed breeding unit. Cats were defined as being healthy on the basis of there being no history of unexplained weight loss, vomiting, regurgitation or diarrhoea in the 12 months prior to and 12 months after the time of the blood draw. Cats on any medication were excluded. All cats had been vaccinated against feline herpesvirus-1, calicivirus and panleukopenia virus using a modified live vaccine (Felocell CVR; Norden Laboratories). The cats were exposed to natural light cycles and provided with ad libitum access to water and food, except on the day of blood collection.
The diet that the cats consumed was an American Association of Feed Control Officials (AAFCO) growth and maintenance commercial moist diet. The percentage distribution of metabolisable energy from protein, fat and carbohydrate was 36%, 58% and 5%, respectively. The folic acid content of the diet was 1060 µg/kg, and cobalamin content was 350 µg/kg on a dry matter basis. The AAFCO recommended allowance for feline adult maintenance based on dry matter for folic acid is 800 µ/kg and 20 µg /kg for cobalamin, assuming an energy density of 4 kcal/g dry matter. 31
Following an overnight fast, jugular venous samples were taken from the cats that met the inclusion criteria. Each sample was left at room temperature for 1 h to allow for clotting. The samples were then centrifuged for 6 mins at 3000 g to obtain serum, which was stored at −80ºC for 12 months before folate and cobalamin were assayed. All serum samples were thawed at room temperature and cobalamin and folate were measured once at a commercial veterinary laboratory (New Zealand Veterinary Pathology, Massey University, Palmerston North, New Zealand) using an electrochemiluminescent assay (Roche E170; Roche Diagnostics NZ). All samples were run on the same day. Maximum and minimum detection limits for folate were 1.45–45.4 nmol/l and 22–1476 pmol/l for cobalamin. The study protocol was approved by the Massey University animal ethics committee.
Statistical analysis
The results are expressed as means ± SEM. Data were tested for normality using the Shapiro–Wilk test and, where appropriate, non-parametric methods were used. Reference intervals (RIs) were calculated using the ReferenceIntervals package from R statistical software (R v 3.1.0; R Development Core Team, 2012). Outliers were recognised visually by inspection of scatter plot and histogram, confirmed by a Cook’s distance of >1, and were removed from the analysis. A simple linear model was used to test the relationship between the concentration of cobalamin or folate and the following variables: age, sex and body weight. Non-significant variables were excluded from the model. An alpha value of P = 0.05 was used to define statistical significance.
Results
A total of 65 cats were included in the study. The median age of the population was 5.4 years (range 2.8–9.06 years). Age was not normally distributed (P <0.01; W = 0.89) and, as such, was grouped into three categories for further analysis: 2.80–3.86 years (category one, 22 cats), 3.86–6.75 years (category two, 24 cats) and 6.75–9.06 years (category three, 19 cats). Of the 65 cats, 31 were male neutered, 18 were female neutered, 15 were female entire and one cat was male entire. Body weight was not normally distributed (P = 0.03100, W = 0.9592) and the median body weight was 4.05 kg (range 2.81–5.72 kg).
The serum cobalamin concentrations were normally distributed (P = 0.4162, W = 0.981) and RIs and confidence intervals were calculated parametrically. The mean serum cobalamin concentration was 737.1 pmol/l (range 162–1427 pmol/l). The calculated RI was 202.4–1271.8 pmol/l. Four outliers were identified following a plot of Cook’s distances. With the outliers removed, the data remained normally distributed (P = 0.2607 [Shapiro-Wilk]) with a mean serum cobalamin concentration of 715.7 pmol/l (range 315–1248 pmol/l). The recalculated RI was 261.7–1169.7 pmol/l.
The serum folate concentrations were not normally distributed (P = 0.0204, W = 0.9556) and non-parametric analysis was performed. There were eight cats with serum folate concentrations greater than the detection limit of 45 nmol/l. For statistical analysis these cats were recorded as having serum folate concentrations of 45 nmol/l. The median serum folate concentration was found to be 38 nmol/l (range 25.2–45 nmol/l) with a RI calculated as 26–45 nmol/l. Using Cook’s method, three outliers were identified and following removal of the outliers the data was not normally distributed (P = 0.0334). The median folate serum concentration was 38.45 nmol/l (range 27.8–45 nmol/l) and the RI 28.2–45 nmol/l.
Multiple linear regression analysis determined that serum cobalamin concentrations was significantly associated with sex and age (P = 0.006). The model had an adjusted R2 value of 14.19%, and the estimates and associated errors are presented in Table 1. The relationship between serum cobalamin and age was an inverse relationship, with increasing age being significantly associated with decreasing serum cobalamin concentration (Figure 1, P = 0.002). Male cats had significantly higher concentrations of serum cobalamin than female cats (P = 0.039).
Table 1.
Effect of age category and sex on serum vitamin B12 concentration in 65 cats consuming the same diet
| Variable | Serum vitamin B12 (pmol/l) |
||
|---|---|---|---|
| Estimated value | SEM | P value | |
| Age | |||
| Category 1 (2.80–3.86 years) | 796.18 | 58.67 | <0.01 |
| Category 2 (3.86–6.75 years) | 662.74 | 75.9 | 0.08 |
| Category 3 (6.75–9.06 years) | 535.33 | 79.83 | 0.00178 |
| Sex | |||
| Female | 796.18 | 58.67 | <0.01 |
| Male | 931.05 | 63.86 | 0.0388 |
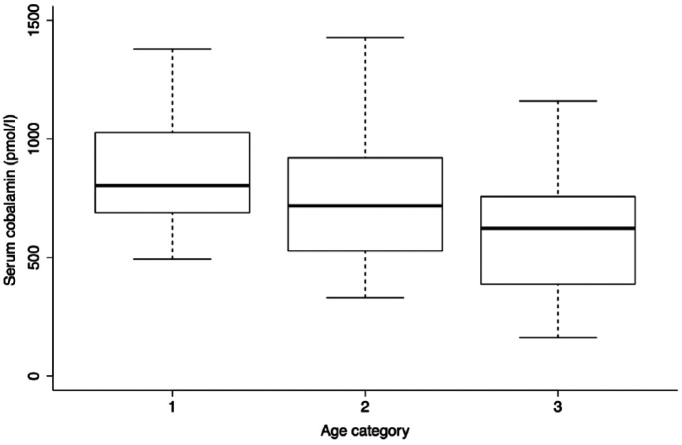
Figure 1.
Box plot of serum cobalamin in 65 cats according to age. Serum cobalmin decreased significantly with age (P = 0.002). Age category 1: 2.80–3.86 years (22 cats); age category 2: 3.86–6.75 years (24 cats); age category 3: 6.75–9.06 years (19 cats)
With serum folate concentration as the outcome, a model including age category, sex, body weight and serum cobalamin concentration identified no significant relationship (P = 0.4492). However, when age was treated as a numeric variable, it was found to be positively associated with folate (P = 0.01), although the adjusted R2 value was only 0.09.
The regression parameter estimate for folate was 33.5073 nmol/l (± 1.7426 SEM), with 0.816 nmol/l (± 0.3075 SEM) for each year of life (P = 0.0101, adjusted R2 = 0.09).
Discussion
The principal aim of this study was to determine if age, sex and body weight had an effect on serum cobalamin and folate concentrations in a population of apparently healthy cats consuming the same dietary concentration of cobalamin and folate. The null hypothesis was that age, sex and body weight would not have an effect.
In this population of cats, the age of the cats had an effect on serum concentrations of cobalamin and folate independent of dietary content. The effect of age was most pronounced for serum cobalamin, which had an inverse relationship with age, while the relationship between age and serum folate concentration was positive. The effect of age on serum cobalamin concentration in cats has been identified previously, but this is the first time diet has been excluded as a confounding factor.13–16 To our knowledge, a positive relationship between age and serum folate concentrations has not been demonstrated in cats. We also identified that male cats have higher concentrations of cobalamin than female cats, which has not been reported previously. Body weight was not associated with the serum concentrations of either folate or cobalamin.
In elderly people low serum cobalamin concentration is frequently identified, with the most common cause being food cobalamin malabsorption (FCM).32,33 FCM is a term that includes non-specific gastritis, type A atrophic gastritis, partial gastrectomy, gastric bypass, vagotomy, gastric bacterial overgrowth, alcohol abuse and ingestion of acid-suppressing drugs. 34 It is possible that gastric disease could develop in cats with age, but clinical signs of gastric dysfunction such as vomiting or a history of acid-suppressing drugs were not present in this population.
The most frequently cited explanation for the inverse relationship between serum cobalamin concentration and age in cats is underlying gastrointestinal tract disease in elderly cats.14–16 The possibility of age-related changes impairing cellular metabolism and causing changes to cobalamin-related metabolites has also been proposed. 15 It has been found that with age, the function of the feline digestive system is altered. As an example, fat digestibility is significantly lower in senior cats compared with young cats.35,36 In the healthy adult cat, fat digestibility of a typical commercial diet is 90–95%, which decreases to <80% in aged cats. Ten to 15% of cats between 7 and 12 years of age show decreased fat digestibility, which increases to 33% in cats over 12 years of age. 36 It has been suggested that this could be due to decreased secretion and activity of pancreatic enzymes and/or decreased production, transport and secretion of bile acids.37,38 Dietary protein digestibility also decreases with increasing age and affects 20% of cats after the age of 14 years. 36 Given the effect of age on the digestibility of macronutrients, it is possible that there may be an age-related decrease in the digestibility of micronutrients such as cobalamin. Alternatively, an age-related decline in focal ileal function might be responsible for decreasing cobalamin absorption
Similar to nutrient digestibility, the faecal microflora is also altered with age. A study of 155 cats found that the numbers of bifidobacteria and lactobacilli were lower in cats >9 years compared with younger cats, while faecal Clostridium perfringens was 0.9 log units higher in cats >9 years of age compared with other age groups. 39 Given that changes to microbiota can alter cobalamin metabolism, it might be that changes in gastrointestinal microbiota that occur with age are responsible for the effect on cobalamin. 40
None of the older cats in this study had clinical signs suggestive of EPI at the time of blood sampling or during the following 12 months. However, 90% of the pancreas is lost before clinical signs of EPI develop in people. 41 Therefore, it might be possible that a loss of less than 90% of pancreatic function may decrease serum cobalamin concentration in cats without the classical signs of EPI developing. Subclinical age-related reduction in pancreatic function could explain the relationship between age and serum cobalamin concentration. Although folate is usually reported to remain unchanged or decreased in EPI, it has been found to increase in some patients.1,3 Therefore, age-associated pancreatic dysfunction may also explain the positive association between age and serum folate concentration seen in this study. Feline trypsin-like immunoreactivity (fTLI) was not measured in this study; therefore, it is not known if EPI was present or not in the cats studied.
A previous study found a weak inverse relationship between age and serum folate concentration in cats, which is the opposite to the finding of this study. 14 In people, serum folate concentration can decrease with age, but not to the same degree as serum cobalamin. 42 In the diet folate is in a polyglutamate form, and to be absorbed in the jejunum all but one of the glutamate residues must be removed by the brush-boarded enzyme folate conjugase, which could be decreased in older age. Some intestinal bacteria such as strains of bifidobacteria are able to produce folate. 43 Although bifidobacteria decrease with age in cats, other bacteria that produce folate may increase with age. The changes to the intestinal microbiota that occurs with age may explain the tendency for serum folate concentrations to increase with age in cats.
It is unknown why males had higher serum cobalamin concentration than females in this study. However, as this is the first time this has been documented in a population of cats, repeat studies to determine if it is consistent finding are warranted. A similar effect has not been documented in other species.
As the adjusted R2 value for the serum cobalamin concentration was 0.1419, age and male sex only explain 14.19% of variation in this parameter. Age only explained 8.6% of the variation in serum folate concentration. Explanations for the remaining variation in these parameters could to be owing to differences in intestinal transit time among the cats. As the cats in the study were fed ad libitum, the size and timing of the last meal relative to the start of the 12 h fast could have created the variation seen. Gastric emptying times have been shown to be variable and prolonged in cats, and as diet is the sole source of cobalamin in cats and also provides folate, timing of the blood draw relative to a cat’s last meal should be taken into consideration when interpreting serum cobalamin and folate concentrations. 44 Underlying disease among our population could have also accounted for some variability.
With serum cobalamin concentration decreasing with age in cats, older cats are likely to be at an increased risk of cobalamin deficiency in the face of underlying gastrointestinal, hepatobiliary or pancreatic disease and/or reduced dietary intake. Therefore, as cats age, they may be more susceptible to cobalamin deficiency secondary to inappetence or gastrointestinal, pancreatic and hepatic disease.
Excluding the outliers, our RIs for serum cobalamin and folate concentrations were 261.7–1169.7 pmol/l (354.6–1585.0 pg/l) and 28.2–45 nmol/l (12.4–19.9 μg/l), respectively. Although there are a number of RIs for serum cobalamin and folate cited in the literature, those most commonly referred to are 214–1107 pmol/l (290–1500 pg/l) and 21.98–48.96 nmol/l (9.7–21.6 μg/l). 45 Both of these RIs are similar to those determined in the present study.
The main limitation of the present study was how apparently healthy cats were defined, as cats with intermittent hyporexia might be able to maintain their body weight. The absence of diagnostic tests to ensure the absence of gastrointestinal, hepatic and pancreatic disease, for example serum fTLI, faecal parasitology, serum biochemistry, haematology, abdominal ultrasound and intestinal biopsies may have resulted in cats with subclinical disease being included in the study. However, serum folate and cobalamin were measured a year after sampling and only cats that were healthy at the time of the blood draw and maintained their health and body weight, within seasonal variation, during the 12 months were included. Therefore, despite the lack of extensive diagnostics, it is argued that cats with underlying disease were not included as it would be expected that they would have at least lost weight or would have developed clinical signs of gastrointestinal disease, for example vomiting, diarrhoea and regurgitation, during the ensuing 12 month interval. A second limitation of this study was that 8/65 cats had a serum folate concentration >45 nmol/l. As one of the aims of this study was to determine a folate serum RI, the cats should have had their folate concentration determined by serial dilution.
Conclusions
This finding suggests that older cats, independent of diet, are at an increased risk of cobalamin deficiency. The study also identified that male cats have higher serum concentrations of cobalamin compared with female cats. We do not argue that the decrease in cobalamin justifies a lower RI for older cats, but rather that it is indicative of an increased risk of deficiency with age. Further work is needed to determine the mechanisms for the age-associated decline, the clinical significance and if supplementation will confer any benefit.
Supplemental Material
A spreadsheet of the age, sex, neuter status, body weight, serum folate concentration and serum cobalamin concentration of each cat in this study
Acknowledgments
The authors would like to acknowledge Janis Bridges for her assistance with the statistical analysis.
Footnotes
Accepted: 21 February 2017
Supplementary material: A spreadsheet of the age, sex, neuter status, body weight, serum folate concentration and serum cobalamin concentration of each cat in this study.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was internally funded.
References
- 1. Thompson KA, Parnell NK, Hohenhaus AE, et al. Feline exocrine pancreatic insufficiency: 16 cases (1992–2007). J Feline Med Surg 2009; 11: 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maunder CL, Day MJ, Hibbert A, et al. Serum cobalamin concentrations in cats with gastrointestinal signs: correlation with histopathological findings and duration of clinical signs. J Feline Med Surg 2012; 14: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steiner JM, Williams DA. Serum feline trypsin-like immunoreactivity in cats with exocrine pancreatic insufficiency. J Vet Intern Med 2000; 14: 627–629. [DOI] [PubMed] [Google Scholar]
- 4. Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (Vitamin B-12) in cats with gastrointestinal disease. J Vet Intern Med 2001; 15: 26–32. [DOI] [PubMed] [Google Scholar]
- 5. Berghoff N, Steiner JM. Laboratory tests for the diagnosis and management of chronic canine and feline enteropathies. Vet Clin North Am Small Anim Pract 2011; 41: 311–328. [DOI] [PubMed] [Google Scholar]
- 6. Nicola R, Gunn-Moore D, Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg 2007; 9: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dossin O. Laboratory tests for diagnosis of gastrointestinal and pancreatic diseases. Top Comp Anim Med 2011; 26: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMichael MA, Freeman LM, Selhub J, et al. Plasma homocysteine, B vitamins, and amino acid concentrations in cats with cardiomyopathy and arterial thromboembolism. J Vet Intern Med 2000; 14: 507–512. [DOI] [PubMed] [Google Scholar]
- 9. Cook AK, Suchodolski JS, Steiner JM, et al. The prevalence of hypocobalaminaemia in cats with spontaneous hyperthyroidism. J Small Anim Pract 2011; 52: 101–106. [DOI] [PubMed] [Google Scholar]
- 10. Geesaman BM, Whitehouse WH, Viviano KR. Serum cobalamin and methylmalonic acid concentrations in hyperthyroid cats before and after radioiodine treatment. J Vet Intern Med 2016; 30: 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaden SL, Wood PA, Ledley FD, et al. Cobalamin deficiency associated with methylmalonic acidemia in a cat. J Am Vet Med Assoc 1992; 200: 1101–1103. [PubMed] [Google Scholar]
- 12. Kelmer E, Shelton GD, Williams DA, et al. Organic acidemia in a young cat associated with cobalamin deficiency. J Vet Emerg Crit Care 2007; 17: 299–304. [Google Scholar]
- 13. Ibarrola P, Blackwood L, Graham PA, et al. Hypocobalaminaemia is uncommon in cats in the United Kingdom. J Feline Med Surg 2005; 7: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parnell NK, Moore GE, Suchodolski JS, et al. Influence of age on serum cobalamin and folate concentrations in healthy cats [abstract]. J Vet Intern Med 2008; 22: 809. [Google Scholar]
- 15. Ruaux CG, Steiner JM, Williams DA. Metabolism of amino acids in cats with severe cobalamin deficiency. Am J Vet Res 2001; 62: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 16. Anna SM, Manuelian CL, Gargante M, et al. Relation between B12 deficiency in cats and age, gastrointestinal inflammation and pancreas dysfunction [abstract]. The Waltham International Sciences Symposium 2013: from pet food to pet care – bridging the gap; 2013 Oct 1–4; Portland, OR, USA. MARS Incorporated, p 62. [Google Scholar]
- 17. Trehy MR, German AJ, Silvestrini P, et al. Hypercobalaminaemia is associated with hepatic and neoplastic disease in cats: a cross sectional study. BMC Vet Res 2014; 10: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients 2013; 5: 4521–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn JK, Hirsch VM, Searcy GP. Serum folate and vitamin-B12 levels in anemic cats. J Am Anim Hosp Assoc 1984; 20: 999–1002. [Google Scholar]
- 20. Fyfe JC, Giger U, Hall CA, et al. Inherited selective intestinal cobalamin malabsorption and cobalamin deficiency in dogs. Pediatr Res 1991; 29: 24–31. [DOI] [PubMed] [Google Scholar]
- 21. Scalabrino G. The multi-faceted basis of vitamin B-12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Prog Neurobiol 2009; 88: 203–220. [DOI] [PubMed] [Google Scholar]
- 22. Salvadori C, Cantile C, De Ambrogi G, et al. Degenerative myelopathy associated with cobalamin deficiency in a cat. J Vet Med Ser A Physiol Pathol Clin Med 2003; 50: 292–296. [DOI] [PubMed] [Google Scholar]
- 23. Simpson K, Battersby I, Lowrie M. Suspected acquired hypocobalaminaemic encephalopathy in a cat: resolution of encephalopathic signs and MRI lesions subsequent to cobalamin supplementation. J Feline Med Surg 2012; 14: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Packer RA, Cohn LA, Wohlstadter DR, et al. D-lactic acidosis secondary to exocrine pancreatic insufficiency in a cat. J Vet Intern Med 2005; 19: 106–110. [DOI] [PubMed] [Google Scholar]
- 25. Ruaux CG. Cobalamin in companion animals: diagnostic marker, deficiency states and therapeutic implications. Vet J 2013; 196: 145–152. [DOI] [PubMed] [Google Scholar]
- 26. Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med 2005; 19: 155–160. [DOI] [PubMed] [Google Scholar]
- 27. Steiner JM, Williams DA. Feline exocrine pancreatic disorders. Vet Clin North Am Small Anim Pract 1999; 29: 551–575. [PubMed] [Google Scholar]
- 28. Dasilva AC, Deangelis RC, Pontes MA, et al. The domestic cat as a laboratory animal for experimental nutrition studies. IV folic acid deficiency. J Nutr 1955; 56: 199–213. [DOI] [PubMed] [Google Scholar]
- 29. Yu S, Morris JG. Folate requirement of growing kittens to prevent elevated formiminoglutamic acid excretion following histidine loading. J Nutr 1998; 128: 2606–2608. [DOI] [PubMed] [Google Scholar]
- 30. Thenen SW, Rasmussen KM. Megaloblastic erythropoiesis and tissue depletion of folic-acid in cat. Am J Vet Res 1978; 39: 1205–1207. [PubMed] [Google Scholar]
- 31. Dzanis DA. The association of American feed control officials dog and cat food nutrient profiles: substantiation of nutritional adequacy of complete and balanced pet foods in the United States. J Nutr 1994; 124: 2535–2539. [DOI] [PubMed] [Google Scholar]
- 32. Andres E, Vidal-Alaball J, Federici L, et al. Clinical aspects of cobalamin deficiency in elderly patients. Epidemiology, causes, clinical manifestations, and treatment with special focus on oral cobalamin therapy. Eur J Intern Med 2007; 18: 456–462. [DOI] [PubMed] [Google Scholar]
- 33. Matthews JH. Cobalamin and folate deficiency in the elderly. Baillieres Clin Haematol 1995; 8: 679–697. [DOI] [PubMed] [Google Scholar]
- 34. Carmel R. Malabsorption of food cobalamin. Baillieres Clin Haematol 1995; 8: 639–655. [DOI] [PubMed] [Google Scholar]
- 35. Peachey SE, Dawson JM, Harper EJ. The effect of ageing on nutrient digestibility by cats fed beef tallow, sunflower oil or olive oil enriched diets. Growth Develop Aging 1999; 63: 61–70. [PubMed] [Google Scholar]
- 36. Perez-Camargo G. Cat nutrition: what is new in the old. Nestlé Purina Nutrition Forum Proceeding; 2003 Sept 25–28; St Louis, MO, USA. Compen Contin Educ Pract Vet 2004; 26: 5–10. [Google Scholar]
- 37. Harper EJ. Changing perspectives on aging and energy requirements: aging and digestive function in humans, dogs and cats. J Nutr 1998; 128: 2632–2635. [DOI] [PubMed] [Google Scholar]
- 38. Burkholder WJ. Age-related changes to nutritional requirements and digestive function in adult dogs and cats. J Am Vet Med Assoc 1999; 215: 625–629. [PubMed] [Google Scholar]
- 39. Patil AR, Rayner L, Carrión PA. Effect of age on fecal microflora of cats. Nestlé Purina Nutrition Forum Proceeding; 2003 Sept 25–28; St Louis, MO, USA. Compend Contin Educ Pract Vet 2004; 26: 60. [Google Scholar]
- 40. Barker SJ. Contribution of the microflora of the small intestine to the vitamin B12 nutriture of man. Nutr Rev 1980; 38: 274–275. [DOI] [PubMed] [Google Scholar]
- 41. Dimagno EP, Go VLW, Summerskill WHJ. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med 1973; 288: 813–815. [DOI] [PubMed] [Google Scholar]
- 42. Wong CW, Ip CY, Leung CP, et al. Vitamin B-12 deficiency in the institutionalized elderly: a regional study. Exp Gerontol 2015; 69: 221–225. [DOI] [PubMed] [Google Scholar]
- 43. Pompei A, Cordisco L, Amaretti A, et al. Folate production by bifidobacteria as a potential probiotic property. Appl Environ Microbiol 2007; 73: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coradini M, Rand JS, Filippich LJ, et al. Associations between meal size, gastric emptying and post-prandial plasma glucose, insulin and lactate concentrations in meal-fed cats. J Anim Physiol Anim Nutr 2015; 99: 757–766. [DOI] [PubMed] [Google Scholar]
- 45. Steiner J. Serum cobalamin (vitamin B12) and folate. http://vetmed.tamu.edu/gilab/service/assays/b12folate (2012, accessed August 6, 2016)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A spreadsheet of the age, sex, neuter status, body weight, serum folate concentration and serum cobalamin concentration of each cat in this study