Abstract
Objectives
The primary study objective was to assess two injectable anesthetic protocols, given to facilitate castration surgery in cats, for equivalence in terms of postoperative analgesia. A secondary objective was to evaluate postoperative eating behavior.
Methods
Male cats presented to a local clinic were randomly assigned to receive either intramuscular ketamine (5 mg/kg, n = 26; KetHD) or alfaxalone (2 mg/kg, n = 24; AlfHD) in combination with dexmedetomidine (25 μg/kg) and hydromorphone (0.05 mg/kg). All cats received meloxicam (0.3 mg/kg SC) and intratesticular lidocaine (2 mg/kg). Species-specific pain and sedation scales were applied at baseline, 1, 2 and 4 h postoperatively. Time taken to achieve sternal recumbency and begin eating were also recorded postoperatively.
Results
Pain scale scores were low and showed equivalence between the treatment groups at all time points (1 h, P = 0.38, 95% confidence interval [CI] of the difference between group scores 0–0; 2 h, P = 0.71, 95% CI 0–0; 4 h, P = 0.97, 95% CI 0–0). Four cats crossed the threshold for rescue analgesia (KetHD, n = 1; AlfHD, n = 3). At 1 h, more cats in the KetHD (65%) group than in the AlfHD (42%) group were sedated, but statistical significance was not detected (P = 0.15, 95% CI −1 to 0). Most AlfHD cats (88%) began eating by 1 h vs 65% of KetHD cats (P = 0.039). Time to recover sternal recumbency did not differ between groups (P = 0.86, 95% CI −14.1 to 11.8).
Conclusions and relevance
These results show that AlfHD and KetHD provide equivalent analgesia as part of a multimodal injectable anesthetic protocol. Alfaxalone is associated with an earlier return to eating.
Introduction
The use of intramuscular (IM) drug combinations in cats to produce general anesthesia for short procedures, such as castration, is popular for its simplicity and consistency.1–7 Drug combinations and doses vary, although it is common to combine an anesthetic, opioid and alpha2-adrenergic agonist. Ketamine is a common choice for the anesthetic component as it produces a predictable effect, supports cardiovascular function, depresses respiratory function less than other anesthetic agents, has analgesic properties and typical doses (5–10 mg/kg) have a low volume of injection.8,9 However, ketamine is associated with prolonged recoveries, during which cats display a range of emergence phenomena, including increased sensitivity to touch and noise (tactile and auditory hyperesthesia), ataxia and increased motor activity, which may last several hours after injection.10–13 These phenomena slow the return to normal function and interfere with pain assessment.14,15
A rapid and smooth return to normal function with appropriate pain management is central to the concept of enhanced recovery after surgery (ERAS), whereby perioperative care is optimized through a multidisciplinary approach by evaluating each process in the care pathway.16,17 By focusing on analgesia, fluid therapy, surgical technique and nutrition, ERAS has been successfully applied to numerous human patient populations and its implementation is supported by high-quality evidence.18–20 The development of ERAS in veterinary medicine is in the early stages, but promising results have been achieved in efforts to improve postoperative pain assessment and recovery.14,15,21
As a refinement to IM ketamine (in combination with an opioid and dexmedetomidine), substitution with the neurosteroid anesthetic alfaxalone has been explored.14,22 IM administration of alfaxalone is currently licensed in Australia, New Zealand and South Africa, with a restriction to intravenous use in other countries. However, extralabel IM use is gaining popularity for the ability to provide a rapid, smooth induction of profound sedation or general anesthesia accompanied by cardiorespiratory stability and complete recovery.1,14,22,23 Some reports of IM alfaxalone use describe hyperesthesia during recovery, though this appears to be related to higher doses (exceeding 2–2.5 mg/kg) or when alfaxalone is administered alone.24–26 In contrast to ketamine, it is unclear if alfaxalone has analgesic properties.27,28
The goal of this study was to evaluate IM ketamine or alfaxalone, combined with hydromorphone and dexmedetomidine, as injectable anesthetic protocols for castration. The primary objective was to assess if the treatment groups provided equivalent postoperative analgesia. The recent publication of feline-specific composite measures pain assessment scales, tested for validity and reliability in a range of experimental and clinical settings, allows the quantitative assessment of analgesia and facilitates comparisons between studies.14,21,29,30 Such scales are crucial to generating evidence for developing perioperative care protocols and ERAS. A secondary objective was to document postoperative eating behavior as we suspected that the relatively long recovery period associated with ketamine would interfere with a return to normal behavior.
Our hypothesis was that an alfaxalone-based IM anesthetic protocol would result in an early return to eating postoperatively without compromising analgesia.
Methods
Animals
The study protocol for this prospective, blinded, randomized clinical trial was provided by the University of Calgary Veterinary Sciences Animal Care Committee in accordance with the Canadian Council on Animal Care guidelines (study ID: AC13-0146). Cats were recruited from a local clinic (City of Calgary Animal Services Centre Clinic), either through the shelter service or were client-owned. Informed consent for shelter animals was provided by the clinic manager or veterinarian and by the owner for pet cats. All recruited cats underwent a physical examination at admission by a veterinarian, to assess health status. Exclusion criteria were a baseline pain assessment score exceeding the intervention threshold of the UNESP-Botucatu multidimensional pain expression subscale (U-B MCPSpainex)29; aggression, an American Society of Anesthesiologists physical classification status >2, requirement for additional procedures during the same anesthetic or body mass <1.5 kg. Perioperatively, cats were excluded if they became hyperthermic (>40°C) or hypothermic (<36°C), or there was incomplete injection of treatment drugs. During pre- and postoperative time periods, cats were housed individually in stainless steel kennels, each with a litter tray, plastic igloo shelter and a towel. Dogs were excluded from this area. Room temperature was maintained at approximately 21°C. Cats had food and water withheld for approximately 12 and 2 h before surgery, respectively.
Experimental procedure
Cats were block randomized (sealedenvelope.com) to one of two treatment groups. Group KetHD were administered ketamine hydrochloride (5 mg/kg, 100 mg/ml [Vetalar; Bioniche Animal Health Canada]), hydromorphone hydrochloride (0.05 mg/kg, 2 mg/ml, [Hydromorphone HCl; Sandoz Canada]) and dexmedetomidine hydrochloride (25 μg/kg, 0.5 mg/ml [Dexdomitor; Zoetis]). Group AlfHD were administered alfaxalone (2 mg/kg, 10 mg/ml [Jurox; Alfaxan]), hydromorphone hydrochloride (0.05 mg/kg) and dexmedetomidine hydrochloride (25 μg/kg). Drugs were drawn up separately and combined in a single syringe immediately before IM injection (lumbar epaxial muscles).
On the morning of surgery baseline data were collected for demeanor, pain and sedation. These assessments were repeated at 1, 2 and 4 h postoperatively in all cats, and at 24 h in cats from the shelter population.
A published demeanor scale was modified for use, as two items (litter tray use and appetite 24 h prior to surgery) could not be assessed. 31 Scale range is 0–21, with higher scores indicating withdrawn behavior or aggression. Two pain scales were used: the UNESP-Botucatu multidimensional pain scale (U-B MCPS) and the revised Composite Measures Pain Scale-Feline (rCMPS-F). 30 The ‘pain expression’ (analgesic intervention threshold >2, range 0–12) and ‘psychomotor’ (range 0–12) subscales were used from the U-B MCPS scale and the decision to provide rescue analgesia was determined by the pain expression subscale as the psychomotor subscale may be influenced by ketamine. 14 The rCMPS-F scale has a score range of 0–16, with scores above a threshold of ⩾4 associated with an increased likelihood of pain. The sedation scale ranged from 0 to 4, with a higher score indicating an increased level of sedation (0 = no sedation, 1 = can stand but is wobbly, 2 = in sternal recumbency, 3 = can lift its head, 4 = no response to clicker sound). 32 Sedation was additionally assessed 10 mins after treatment injection (time PM10). All assessments were performed by one of two trained veterinary students (MCW, TA) blinded to the treatment group. Inter-rater reliability was good, as assessed with an intra-class correlation coefficient using data collected from five cats (20 time points) at the start of the study (U-B MCPS: 0.79 [95% CI 0.75–0.82]; rCMPS-F: 0.62 [95% CI 0.54–0.68]; demeanor scale: 0.72 [95% CI 0.67–0.77]).
Immediately following treatment injection, cats were returned to their kennels (igloo and litter tray removed). After 10 mins, sedation was assessed and cats carried to the adjacent surgery area and placed in lateral recumbency. Oxygen (1 l/min) was provided via face mask and anesthesia monitoring started during surgical preparation (fur overlying scrotum clipped and skin cleaned with chlorhexidine). The following variables were monitored during anesthesia (SurgiVet Veterinary Anesthesia and Monitoring Equipment; Smiths Medical): saturation of arterial hemoglobin with oxygen (SpO2, pulse oximeter probe placed on digit pad or ear), heart rate (from pulse oximeter and thoracic auscultation), systemic arterial blood pressure (indirectly with the oscillometric technique) and respiratory rate (observation of thoracic excursions).
An intratesticular block was performed with lidocaine hydrochloride (2 mg/kg, divided equally between two testes [Lidocaine Neat 2%; Pfizer Canada]) at least 1 min before surgery. Castration was undertaken by an experienced veterinarian using a figure-of-eight method with pedicle ties. A scalpel was used to make scrotal incisions, and mosquito hemostats used to ligate the spermatic cord and vessels. If movement occurred in response to surgical stimulation, isoflurane was delivered via the face mask (beginning at 0.5–1%). Surgery duration was defined as the time of initial incision until return of the second spermatic cord in to the scrotal sac. At the end of surgery, the following actions were immediately performed: isoflurane was turned off (if administered), a rectal temperature measured with a digital thermometer and meloxicam (0.3 mg/kg subcutaneously, 5 mg/ml [Metacam; Boehringer Ingelheim]) and atipamezole (125 μg/kg IM, 5 mg/ml [Antisedan; Zoetis]) given. Anesthesia duration was defined as time from the treatment injection until the end of surgery.
Completion of surgery was defined as time 0 for all postoperative time points. All cats were placed in lateral recumbency for recovery and monitored every 15 mins, to determine return of sternal recumbency, defined as all four paws positioned under the body.14,15,21 Once sternal, the igloo, litter tray and food were placed in the kennel (approximately 20 g each of Purina Veterinary Diets Essential Care Adult Formula for Cats and Purina Veterinary Diets Essential Care Senior Formula for Cats). Time to begin eating was recorded, with cats observed every 60 mins from completion of surgery. Rectal temperature measurement was repeated 60 mins postoperatively.
An assessment to determine need for rescue analgesia (buprenorphine 0.01 mg/kg IM, 0.3 mg/ml [Vetergesic Multidose; Champion Alstoe Animal Health]) was performed by the veterinarian if the analgesic intervention threshold for the U-B MCPSpainex was crossed.
Statistical methods
Continuous data were assessed for normality with a D’Agostino Pearson omnibus normality test. Normally distributed data were analyzed with an unpaired t-test (time to sternal recumbency) and non-normally distributed data were analyzed with a Mann–Whitney test (age, weight, surgery duration, time from premedication to surgery, anesthesia duration). To ensure similar baseline demeanor between groups, baseline demeanor scores were compared with a Mann–Whitney test.
A planned interim analysis was performed with statistical software (PASS 14.0.7; NCSS) to determine the final sample size required to test for equivalence in levels of analgesia between treatment groups. With a power of 0.8 and alpha of 0.0125, after adjusting for multiple comparisons (baseline, postoperative hours 1, 2 and 4), approximately 12 animals were needed in each treatment group to detect a difference in U-B MCPSpainex score of 1.5 at each time point (the 24 h time point was not included as a reduced number of available animals was predicted). A predetermined margin of equivalence was set as a median difference U-B MCPSpainex score ranging from −1.5 to 1.5 between groups, with equivalence claimed if the 95% confidence interval (CI) of the median difference fell within this range. The target sample size was set at approximately 30 animals to increase power for the secondary objective (appetite: power of 0.8, α = 0.05, for a 40% difference between groups in the proportion of animals eating at 1 h postoperatively). Postoperative temperature was analyzed with a one-way ANOVA and Bonferroni multiple comparison test to compare immediate and 60 min postoperative temperatures between groups. Owing to unequal group sizes, pain and sedation scale scores were analyzed with a Mann–Whitney test with P values corrected for the number of comparisons: comparisons between groups were made at postoperative hours 1, 2 and 4 for sedation (significance set at P <0.017) and at baseline; postoperative hours 1, 2 and 4 for pain (significance set at P <0.0125).
The number of cats eating at 1 h postoperatively and number of cats requiring isoflurane were analyzed with an Exact Unconditional Independence test. 33
Where a U-B MCPSpainex score exceeding threshold was recorded, that score was carried forward for remaining time points and included in the analyses. This treatment of pain score data avoids underestimating pain and differences between treatments resulting from either excluding animals after administering rescue analgesia or by using pain scores following rescue analgesia. For the other pain assessment scales (U-B MCPSpsych and rCMPS-F), scores recorded at each time point were included in analysis. Data are presented as mean ± SD and median (range) with 95% CI of the difference (KetHD–AlfHD), where available. Unless otherwise specified, data were analyzed with commercial software (Prism version 7.0a; GraphPad Software).
Results
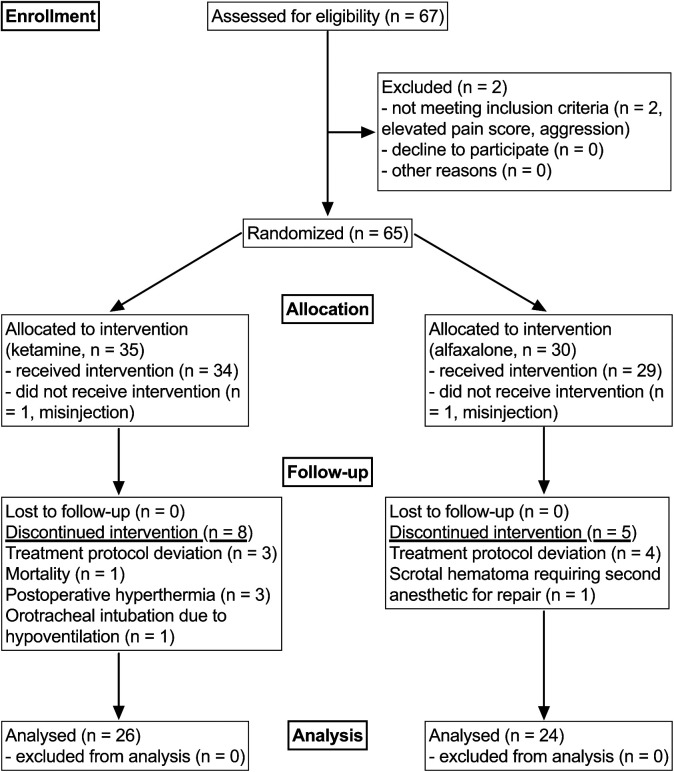
Sixty-seven cats were eligible for the study. Seventeen cats were excluded at various stages before data analysis, including a mortality during recovery (Figure 1). The final study population consisted of 26 cats in the KetHD group and 24 cats in the AlfHD group. There were 33 domestic shorthair and 17 domestic longhair cats.
Figure 1.
A Consolidated Standards of Reporting Trials Statement (CONSORT) flow diagram showing the flow of animals through the study. 34 Treatment groups are identified as ketamine or alfaxalone, combined with dexmedetomidine and hydromorphone
Age, weight, surgery duration and time to attain sternal recumbency after surgery were not significantly different between groups (Table 1).
Table 1.
Demographic and perioperative data for 50 cats anesthetized for orchiectomy with intramuscular ketamine (KetHD) or alfaxalone (AlfHD) in combination with dexmedetomidine and hydromorphone and supplemented with oxygen
| Variable | KetHD (n = 26) | AlfHD (n = 24) | P value (95% CI) |
|---|---|---|---|
| Age (years) | 0.6 (0.2–8.0) | 0.7 (0.2–4.0) | 0.99 (–0.4 to 0.3) |
| Weight (kg) | 4.0 (1.5–6.1) | 3.7 (1.5–5.9) | 0.94 (–0.8 to 0.8) |
| Demeanor (baseline) | 4 (0–6) | 3 (0–8) | 0.15 (0–2) |
| Surgery duration (mins) | 2 (1–4) | 1 (1–4) | 0.59 (0–1) |
| Premedication to surgery time (mins) | 17.5 (13.0–29.0) | 20.0 (17.0–29.0) | 0.004 (−5 to −1) |
| Anesthetic duration (mins) | 19.0 (15.0–31.0) | 22.0 (18.0–33.0) | 0.011 (−4 to −1) |
| Postoperative temperature (°C) | 38.1 ± 0.7 | 37.7 ± 0.5 | 0.15 (–0.1 to 0.8) |
| Temperature 60 mins after surgery (°C) | 38.2 ± 0.8 | 37.6 ± 0.8 | 0.008 (0.1–1.0) |
| Time to achieve sternal recumbency (mins) | 43.9 ± 19.9 | 45.0 ± 25.4 | 0.86 (–14.1 to 11.8) |
CI = confidence interval
Pain scores
Pain scores from the U-B MCPSpainex were equivalent between treatment groups, with no significant differences between time points and the 95% CI lying within the predetermined margin of equivalence (±1.5): baseline, P >0.99 (95% CI 0–0); 1 h, P = 0.38 (95% CI 0–0); 2 h, P = 0.71 (95% CI 0–0); 4 h, P = 0.97 (95% CI 0–0) (Figure 2). Four cats crossed the analgesic intervention threshold (KetHD, n = 1; AlfHD, n = 3) and two of these cats were given buprenorphine (KetHD, n = 1; AlfHD, n = 1).
Figure 2.
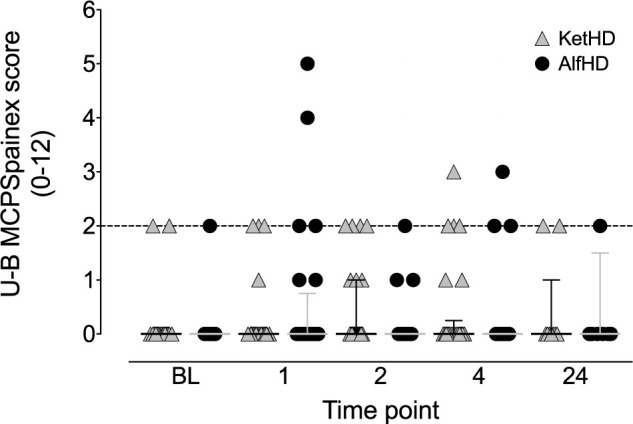
Plot of pain scale scores for the UNESP-Botucatu multidimensional pain expression subscale (U-B MCPSpainex). The analgesia intervention threshold (>2/12) is indicated by the broken horizontal line. Symbols represent individual data points: ketamine–dexmedetomidine–hydromorphone (gray triangles, n = 26 cats) and alfaxalone–dexmedetomidine–hydromorphone (black circles, n = 24 cats). Data are median and interquartile range. BL = baseline
Median U-B MCPS psychomotor pain scores did not differ between groups at any time point: baseline (KetHD 0 [0–3], ALfHD 0 [0–3]; P = 0.23 [95% CI 0–0]), 1 h (KetHD 0 [0–3], ALfHD 0 [0–3]; P = 0.89 [95% CI 0–0]), 2 h (KetHD 0 [0–4], ALfHD 0 [0–3]; P = 0.12 [95% CI 0–0]) or 4 h (KetHD 0 [0–3], ALfHD 0 [0–1]; P >0.32 [95% CI 0–0]). The same pattern was observed with scores from the rCMPS-F scale, with no significant differences between groups at baseline (KetHD 0 [0–3], ALfHD 0 [0–5]; P = 0.30 [95% CI 0–0]), 1 h (KetHD 0 [0–3], ALfHD 0 [0–5]; P = 0.90 [95% CI 0–0]), 2 h (KetHD 0 [0–4], ALfHD 0 [0–6]; P = 0.63 [95% CI 0–0]) or 4 h (KetHD 0 [0–4], ALfHD 0 [0–3]; P >0.25 [95% CI 0–0]).
Though results from the U-B MCPSpainex and rCMPS-F were broadly similar, there were small differences in the cats that crossed the intervention threshold of each scale. Four cats crossed the U-B MCPSpainex intervention threshold and five cats crossed the rCMPS-F intervention threshold (Table 2). Two cats crossed the intervention threshold of both scales at the same time point.
Table 2.
Cat identification number and pain assessment scale score for cats that crossed the analgesia intervention threshold of either the UNESP-Botucatu multidimensional pain expression subscale (U-B MCPSpainex) or revised Composite Measures Pain Scale-Feline (rCMPS-F)
| Time point | U-B MCPSpainex | rCMPS-F |
|---|---|---|
| Baseline | A19:2 | A19:5 |
| 1 | A3:1, A19:4, A13:5 | A3:4, A19:5, A13:3 |
| 2 | K16:0 | K16:4 |
| 4 | K14:3, K16:2, K17:2, A8:3 | K14:4, K16:4, K17:4, A8:0 |
| 24 | – | – |
Where the intervention threshold was crossed for one scale, the corresponding score for the other scale is included. Bold data indicate cats that crossed intervention thresholds of both scales at the same time point. Intervention thresholds are >2/12 and ⩾4/16 for the U-B MCPSpainex and rCMPS-F, respectively
A = alfaxalone treatment group; K = ketamine treatment group
Eating
There was a significant difference in the proportion of cats eating by 1 h postoperatively (P = 0.039; Figure 3). At 1 h 65% (n = 17) of KetHD cats had begun eating vs 88% (n = 21) of AlfHD cats. By 4 h 81% (n = 21) of KetHD cats had begun eating vs 92% (n = 22) of the AlfHD cats (Figure 3).
Figure 3.
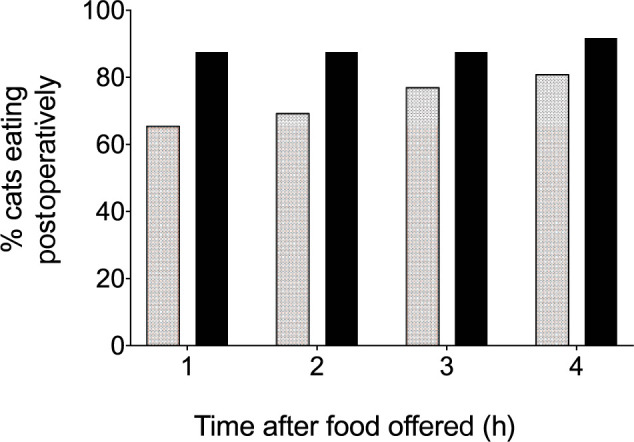
Percentage of cats eating at 1–4 h postoperatively. Stippled bars represent the ketamine-dexmedetomidine-hydromorphone group (n = 26 cats) and black bars represent the alfaxalone-dexmedetomidine-hydromorphone group (n = 24 cats)
Sedation
All cats had a sedation score of 0 at baseline, 4 and 24 h postoperatively. At PM10 all but one cat in each group received the maximum sedation score of 4. More cats received a sedation score of 1 (‘wobbly’) at 1 h postoperatively in the KetHD (n = 17/26; 65%) than in the AlfHD group (n = 10/24; 42%). By 2 h, three cats in the KetHD group were assigned a score of 1, while all cats in the AlfHD group were assigned scores of 0. However, no significant differences were identified at individual time points (1 h, P = 0.15 [95% CI −1 to 0]; 2 h, P >0.24 [95% CI 0–0]; 4 h, P >0.99 [95% CI 0–0]).
There was no difference in immediate postoperative temperature between groups, though cats in the KetHD group had a slightly higher rectal temperature (0.6ºC difference) 1 h postoperatively (Table 1). There was no difference in the number of cats requiring supplementation with isoflurane between treatment groups (KetHD, n = 5; AlfHD, n = 4 [P = 1.0]). The median interval between premedication to the start of surgery was slightly longer in the AlfHD group (P = 0.004), with a consequent increase in anesthetic duration (P = 0.01; Table 1).
Mean arterial blood pressure (KetHD, 88 ± 5 mmHg; AlfHD, 88 ± 6 mmHg), heart rate (KetHD, 128 ± 19 beats per min [bpm]; AlfHD, 123 ± 26 bpm) and respiratory rate (KetHD, 33 ± 13 breaths/min; AlfHD, 43 ± 10 breaths/min) values were within acceptable limits. Recorded values for SpO2 were low (KetHD, 90% [70–97%; interquartile range 84–92%]; AlfHD 87% [78–99%; interquartile range 82–93%]).
One cat was found unresponsive in its cage approximately 15 mins after the end of surgery. After confirmation of cardiac arrest, closed-chest cardiopulmonary resuscitation was performed for 25 mins without success. The body was submitted for necropsy examination by a board-certified veterinary pathologist. Mild cardiomegaly and limited microscopic changes were suggestive of hypertrophic cardiomyopathy, though a definitive cause of death could not be confirmed. This cat was in the KetHD treatment group and surgery had been uneventful.
Discussion
These data show that both ketamine- and alfaxalone-based anesthesia protocols studied provide statistically equivalent levels of postoperative analgesia as part of a multimodal analgesia regimen. Furthermore, the alfaxalone-based protocol has the advantage of a potentially shorter delay to begin eating postoperatively.
The decision to compare alfaxalone and ketamine was based on previous work showing that alfaxalone, combined with dexmedetomidine and hydromorphone, resulted in minimal postanesthetic sedation. 14 This is an appealing outcome, fulfilling the goal of ERAS, to achieve an optimal recovery, which is smooth and pain-free.15,16 As the analgesic properties of alfaxalone are unclear, it was important to compare its performance against ketamine, which does provide analgesia.27,28,35 This was done through statistical testing for equivalence. 34 An equivalence trial differs from superiority testing, the more common form of statistical testing in clinical trials in which the aim is to assess if one treatment is superior to another, by instead assessing if one treatment is acceptably similar to another. In this study the question was: Is performance of a AlfHD acceptably similar to that of KetHD? The narrow 95% CI for the difference in U-B MCPSpainex scores between treatment groups fell within the predetermined margin of equivalence, showing that the different treatments provided equivalent analgesia. The margin of equivalence was set at ± 1.5 as it was felt that a difference between groups greater than this represented a clinically important finding. An alternative approach would have been to use the number of animals requiring rescue analgesia as an indicator of differences between groups. However, as supported by the results, the use of a multimodal analgesia protocol in both groups makes it unlikely that large differences in rescue analgesia requirement would be identified.
It is highly likely that any difference in analgesia provided by ketamine is outweighed by the benefits of multimodal analgesia, with the inclusion of hydromorphone, meloxicam and lidocaine. Furthermore, related work using a similar multimodal approach has shown that pharmacological antagonism of the sedative and analgesic properties of dexmedetomidine, with atipamezole, shortens recovery without compromising analgesia. 15
In contrast to previous results using a similar anesthetic protocol, 14 sedation levels did not differ statistically between treatment groups, though more cats in the KetHD group had measurable sedation at the 1 and 2 h time points. This lack of difference is likely to be multifactorial, including the absence of surgery and longer duration of anesthesia (40 mins, with isoflurane) in the experimental study and a lack of statistical power for this outcome in this study.
Comparing recovery speed and quality between studies in which an injectable anesthetic protocol has been employed is greatly hindered by variations in methodology including, but not limited to, pain assessment, use and dose of pharmacological antagonists, drug doses, duration of surgery and anesthesia and evaluation of recovery.
The pain expression subscale, rather than the psychomotor subscale, of the U-B MCPS was used as the basis for equivalence testing as psychomotor subscale scores have been shown to be confounded by ketamine in an experimental (non-surgical) anesthesia study. 14 Additionally, using the pain expression subscale and confirming the even distribution of demeanor scores between groups controlled for the possibility of demeanor to influence pain assessment scores. 21 The potential for ketamine and demeanor to interfere with behavioral responses underlines the importance of assessing scale performance in situations that may differ from those in which the scale was originally developed and tested. The recent introduction of feline-specific pain assessment scales that have undergone validity and reliability testing in clinically relevant situations facilitates the comparison of results between studies.29,30 However, recent work has shown that the generalizability of these scales to all clinical situations should not be assumed: drug choice and demeanor can inhibit scale performance.14,21 The continued use of pain-assessment scales that have not been tested for validity or reliability does not necessarily invalidate individual studies but limits generalizability of findings to different situations (animals, observers, drugs, procedures, environment).
In general, where antagonism of dexmedetomidine (or medetomidine) is not performed, recovery time (variably defined as time to extubation, displaying ‘awake’ behavior, time to sternal or standing) can be prolonged, exceeding 2 h.5,7,22 In the presence of reduced monitoring (frequency and intensity) during recovery, a period associated with an increased incidence of death (61% of anesthetic and sedation related deaths in cats), 36 this makes a strong case for antagonism of the α2-adrenergic agonist component of injectable protocols as a means of shortening recovery without compromising analgesia.2,15
There is no standard protocol for injectable anesthetic combinations using an anesthetic (ketamine or alfaxalone), opioid (eg, butorphanol or hydromorphone) and α2-adrenergic agonist (dexmedetomidine or medetomidine).4,7,14,15,21,22 Dose selection is typically based on veterinarian experience and planned procedure. A recent dose-finding study, performed in cats undergoing minor procedures (wound cleaning and dressing change, radiography, pin removal) suggested that 14 μg/kg dexmedetomidine, 2.5 mg/kg alfaxalone and 0.3 mg/kg butorphanol, delivered IM as a single injection, produced the optimal quality of sedation/anesthesia with minimal side effects (vomiting, hypersalivation, respiratory depression, increased muscle tone), a smooth recovery and an acceptable total volume for injection.1,37 The dose selected here reflected a desire to attain a depth of anesthesia suitable for the planned procedure with minimal requirement for supplemental inhalational anesthesia, and was consistent with previous work.3,4,7 It is possible that a lower dose of dexmedetomidine, in the region of 15 μg/kg, would provide suitable conditions for castration, in combination with multimodal analgesia. 22
A dichotomy in the literature exists between neuter studies conducted with student surgeons vs experienced veterinarians. This is readily apparent in the extremes of reported surgery durations (and, consequently, anesthesia) observed, ranging from approximately 12 to 145 mins for ovariohysterectomy and 3 to 30 mins for castration.4,5,15,22,38 These large differences preclude comparisons between studies as differences in tissue trauma and drug pharmacokinetics are likely to produce clinically relevant differences in pain and recovery.
When recovery is evaluated, a seemingly objective measure such as return of sternal recumbency is rarely defined in the literature, making comparisons between studies difficult. The definition applied here is the same as in previous studies by our group,14,15,21 but it is unknown if the stipulation for all four limbs to be tucked under the body is universal as most studies do not define this behavioral outcome.1,3–5,22,35,39 To take a single recent example, Khenissi et al describe short times to return to sternal recumbency (15–30 mins) following administration of either alfaxalone (3 mg/kg) or ketamine (5 mg/kg) with dexmedetomidine (10 μg/kg) and butorphanol (0.2 mg/kg) for feline castration surgery, despite not administering atipamezole. 22 However, once the long surgical and anesthesia durations (approximately 27 and 50 mins, respectively), involvement of student surgeons and stimulation to stand during recovery are taken into account it becomes clear that attempts to compare studies directly are difficult.
An unexpected and interesting benefit observed in the AlfHD group was the shorter time to begin eating postoperatively. It is unclear if this difference results from residual sedation in the KetHD group (coupled with associated behavioral changes), appetite suppression by ketamine, the interaction of the drugs given in combination or the possibility that alfaxalone stimulates appetite.13,40 To our knowledge, the effect of ketamine and alfaxalone on subsequent eating behavior during postoperative recovery has not been investigated in cats. Several anesthetic and adjunctive agents have been shown to promote appetite stimulation in animals, including propofol, maropitant and the benzodiazepines.41–43 Chen et al showed that several neurosteroids, including alfaxalone, induced a dose-dependent increase in eating in adult male rats. 40 This effect was reversible with picrotoxin, suggesting a gamma-aminobutyric acid A-mediated mechanism of action. Therefore, alfaxalone may directly stimulate appetite resulting in the early return of eating postoperatively. The implications of these findings to other surgeries and species in their role in ERAS requires further study.
The low number of cats that required rescue analgesia is similar to that reported elsewhere, using similar drug combinations.4,7,22 This contrasts with the high requirement for rescue analgesia when unimodal analgesia with butorphanol, a mu-opioid receptor antagonist kappa-opioid receptor agonist, was used in feline ovariohysterectomy. 44 The use of buprenorphine for rescue analgesia was a compromise between its relatively slow onset of action but long duration of action compared with hydromorphone, and the likelihood of providing analgesia once a cat had returned home. The decision to provide rescue analgesia was based on a combination of the pain assessment scale and veterinarian judgment, as analgesia intervention thresholds are intended to guide clinical decision making.29,45,46
The SpO2 values were variable in both treatment groups and low values (<90%) were not uncommon. In principle, the presence of low SpO2 values indicates hypoxemia; however, the profound vasoconstriction resulting from dexmedetomidine may interfere with pulse oximeter performance. 47 None of the cats was observed to be cyanotic, though observation of cyanosis is unreliable. 48 Using a similar drug combination and the same pulse oximeter, but with a lower dose of dexmedetomidine (15 μg/kg), low values of SpO2 were not observed. 15 And a study using a higher dose of medetomidine (equivalent to 30 μg/kg dexmedetomidine) did not identify hypoxemia. 6 Simultaneous arterial blood gas sampling would be required to differentiate between hypoxemia and equipment inaccuracy.
The death of a study cat was a sobering reminder of the risks of anesthesia, even when the intensity and frequency of monitoring is increased as part of a clinical trial. We speculate that a fatal arrhythmia was precipitated in a predisposed abnormal myocardium. Of the drugs given, dexmedetomidine and ketamine have the greatest cardiovascular effects. Dexmedetomidine causes coronary vasoconstriction, reducing blood flow to the myocardium, and ketamine increases myocardial workload and oxygen demand through sympathetic system stimulation.8,49 These changes are tolerated in healthy animals but the consequences are difficult to predict in the presence of hypertrophic cardiomyopathy with the associated risk of myocardial hypoperfusion. Following a debrief and discussion, it was decided to institute continuous observation of all cats during recovery from anesthesia until return of gross purposeful movement.
Conclusions
Alfaxalone or ketamine, in combination with dexmedetomidine and hydromorphone, provide equivalent and appropriate analgesia for feline castration. The systematic administration of atipamezole to speed recovery and early return to eating observed in the alfaxalone group promotes the aims of ERAS in veterinary medicine.
Acknowledgments
Dr Sarah Engbers and technical staff (Arlene Johnson, Doris Porter, Jackie Walters, Karen Brick, Lee Head, Tiffany Ramsden, Sonja Gavora, Wendy Weed) of the City of Calgary Animal Services Centre Clinic. Dr Grace PS Kwong (University of Calgary) for statistical support.
Footnotes
Accepted: 14 January 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by Zoetis and the University of Calgary Faculty of Veterinary Medicine Clinical Research Fund. TA received a scholarship from the Department of Veterinary Clinical and Diagnostic Sciences and Zoetis. Dexmedetomidine and atipamezole were supplied by Zoetis. Alfaxalone was supplied by Jurox. MCW received a scholarship from the Faculty of Veterinary Medicine Summer Undergraduate Research Experience Programme.
References
- 1. Adami C, Imboden T, Giovannini AE, et al. Combinations of dexmedetomidine and alfaxalone with butorphanol in cats: application of an innovative stepwise optimisation method to identify optimal clinical doses for intramuscular anaesthesia. J Feline Med Surg 2016; 18: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffin B, Bushby PA, McCobb E, et al. The Association of Shelter Veterinarians’ 2016 veterinary medical care guidelines for spay-neuter programs. J Am Vet Med Assoc 2016; 249: 165–188. [DOI] [PubMed] [Google Scholar]
- 3. Harrison KA, Robertson SA, Levy JK, et al. Evaluation of medetomidine, ketamine and buprenorphine for neutering feral cats. J Feline Med Surg 2011; 13: 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko JC, Austin BR, Barletta M, et al. Evaluation of dexmedetomidine and ketamine in combination with various opioids as injectable anesthetic combinations for castration in cats. J Am Vet Med Assoc 2011; 239: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 5. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiese AJ, Muir WW. Anaesthetic and cardiopulmonary effects of intramuscular morphine, medetomidine and ketamine administered to telemetered cats. J Feline Med Surg 2007; 9: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeiler GE, Dzikiti BT, Fosgate GT, et al. Anaesthetic, analgesic and cardiorespiratory effects of intramuscular medetomidine-ketamine combination alone or with morphine or tramadol for orchiectomy in cats. Vet Anaesth Analg 2014; 41: 411–420. [DOI] [PubMed] [Google Scholar]
- 8. Kastner SBR. Intravenous anaesthetics. In: Duke-Novakovski T, de Vries M, Seymour C. (eds). BSAVA manual of canine and feline anaesthesia and analgesia. 3rd ed. Quedgeley: British Small Animal Veterinary Association, 2016, pp 133–149. [Google Scholar]
- 9. Selmi AL, Mendes GM, Lins BT, et al. Evaluation of the sedative and cardiorespiratory effects of dexmedetomidine, dexmedetomidine-butorphanol, and dexmedetomidine-ketamine in cats. J Am Vet Med Assoc 2003; 222: 37–41. [DOI] [PubMed] [Google Scholar]
- 10. Gieseg M, Hon H, Bridges J, et al. A comparison of anaesthetic recoveries in cats following induction with either alfaxalone or ketamine and diazepam. N Z Vet J 2014; 62: 103–109. [DOI] [PubMed] [Google Scholar]
- 11. Ilkiw JE, Suter C, McNeal D, et al. The optimal intravenous dose of midazolam after intravenous ketamine in healthy awake cats. J Vet Pharmacol Ther 1998; 21: 54–61. [DOI] [PubMed] [Google Scholar]
- 12. Larenza MP, Althaus H, Conrot A, et al. Anaesthesia recovery quality after racemic ketamine or S-ketamine administration to male cats undergoing neutering surgery. Schweiz Arch Tierheilkd 2008; 150: 599–607. [DOI] [PubMed] [Google Scholar]
- 13. Wright M. Pharmacologic effects of ketamine and its use in veterinary medicine. J Am Vet Med Assoc 1982; 180: 1462–1471. [PubMed] [Google Scholar]
- 14. Buisman M, Wagner MC, Hasiuk MM, et al. Effects of ketamine and alfaxalone on application of a feline pain assessment scale. J Feline Med Surg 2016; 18: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasiuk MM, Brown D, Cooney C, et al. Application of fast-track surgery principles to evaluate effects of atipamezole on recovery and analgesia following ovariohysterectomy in cats anesthetized with dexmedetomidine-ketamine-hydromorphone. J Am Vet Med Assoc 2015; 246: 645–653. [DOI] [PubMed] [Google Scholar]
- 16. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997; 78: 606–617. [DOI] [PubMed] [Google Scholar]
- 17. Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008; 248: 189–198. [DOI] [PubMed] [Google Scholar]
- 18. Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011; CD007635. [DOI] [PubMed] [Google Scholar]
- 19. Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010; 29: 434–440. [DOI] [PubMed] [Google Scholar]
- 20. Zhu F, Lee A, Chee YE. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev 2012; 10: CD003587. [DOI] [PubMed] [Google Scholar]
- 21. Buisman M, Hasiuk MMM, Gunn M, et al. The influence of demeanor on scores from two validated feline pain assessment scales during the perioperative period. Vet Anaesth Analg 2017; 44: 646–655. [DOI] [PubMed] [Google Scholar]
- 22. Khenissi L, Nikolayenkova-Topie O, Broussard S, et al. Comparison of intramuscular alfaxalone and ketamine combined with dexmedetomidine and butorphanol for castration in cats. J Feline Med Surg. Epub ahead of print 11 July 2016. DOI: 10.1177/1098612X16657951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribas T, Bublot I, Junot S, et al. Effects of intramuscular sedation with alfaxalone and butorphanol on echocardiographic measurements in healthy cats. J Feline Med Surg 2015; 17: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grubb TL, Greene SA, Perez TE. Cardiovascular and respiratory effects, and quality of anesthesia produced by alfaxalone administered intramuscularly to cats sedated with dexmedetomidine and hydromorphone. J Feline Med Surg 2013; 15: 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodrigo-Mocholi D, Belda E, Bosmans T, et al. Clinical efficacy and cardiorespiratory effects of intramuscular administration of alfaxalone alone or in combination with dexmedetomidine in cats. Vet Anaesth Analg 2016; 43: 291–300. [DOI] [PubMed] [Google Scholar]
- 26. Tamura J, Ishizuka T, Fukui S, et al. Sedative effects of intramuscular alfaxalone administered to cats. J Vet Med Sci 2015; 77: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svensson E, Persson J, Fitzsimmons B, et al. Intrathecal neurosteroids and a neurosteroid antagonist: effects on inflammation-evoked thermal hyperalgesia and tactile allodynia. Neurosci Lett 2013; 548: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winter L, Nadeson R, Tucker AP, et al. Antinociceptive properties of neurosteroids: a comparison of alphadolone and alphaxalone in potentiation of opioid antinociception. Anesth Analg 2003; 97: 798–805. [DOI] [PubMed] [Google Scholar]
- 29. Brondani JT, Mama KR, Luna SP, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 2013; 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calvo G, Holden E, Reid J, et al. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract 2014; 55: 622–629. [DOI] [PubMed] [Google Scholar]
- 31. Zeiler GE, Fosgate GT, van Vollenhoven E, et al. Assessment of behavioural changes in domestic cats during short-term hospitalisation. J Feline Med Surg 2014; 16: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slingsby LS, Lane EC, Mears ER, et al. Postoperative pain after ovariohysterectomy in the cat: a comparison of two anaesthetic regimens. Vet Rec 1998; 143: 589–590. [DOI] [PubMed] [Google Scholar]
- 33. Berger RL. Exact unconditional homogeneity/independence tests for 2x2 tables. http://www4.stat.ncsu.edu/~boos/exact/ (2005, accessed October 10, 2016).
- 34. Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012; 308: 2594–2604. [DOI] [PubMed] [Google Scholar]
- 35. Kalchofner Guerrero KS, Reichler IM, Schwarz A, et al. Alfaxalone or ketamine-medetomidine in cats undergoing ovariohysterectomy: a comparison of intra-operative parameters and postoperative pain. Vet Anaesth Analg 2014; 41: 644–653. [DOI] [PubMed] [Google Scholar]
- 36. Brodbelt DC, Blissitt KJ, Hammond RA, et al. The risk of death: the confidential enquiry into perioperative small animal fatalities. Vet Anaesth Analg 2008; 35: 365–373. [DOI] [PubMed] [Google Scholar]
- 37. Biermann K, Hungerbuhler S, Mischke R, et al. Sedative, cardiovascular, haematologic and biochemical effects of four different drug combinations administered intramuscularly in cats. Vet Anaesth Analg 2012; 39: 137–150. [DOI] [PubMed] [Google Scholar]
- 38. Kennedy KC, Tamburello KR, Hardie RJ. Peri-operative morbidity associated with ovariohysterectomy performed as part of a third-year veterinary surgical-training program. J Vet Med Educ 2011; 38: 408–413. [DOI] [PubMed] [Google Scholar]
- 39. Mathis A, Pinelas R, Brodbelt DC, et al. Comparison of quality of recovery from anaesthesia in cats induced with propofol or alfaxalone. Vet Anaesth Analg 2012; 39: 282–290. [DOI] [PubMed] [Google Scholar]
- 40. Chen SW, Rodriguez L, Davies MF, et al. The hyperphagic effect of 3 alpha-hydroxylated pregnane steroids in male rats. Pharmacol Biochem Behav 1996; 53: 777–782. [DOI] [PubMed] [Google Scholar]
- 41. Long JP, Greco SC. The effect of propofol administered intravenously on appetite stimulation in dogs. Contemp Top Lab Anim Sci 2000; 39: 43–46. [PubMed] [Google Scholar]
- 42. Marquez M, Boscan P, Weir H, et al. Comparison of NK-1 receptor antagonist (maropitant) to morphine as a pre-anaesthetic agent for canine ovariohysterectomy. PLOS ONE 2015; 10: e0140734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mereu GP, Fratta W, Chessa P, et al. Voraciousness induced in cats by benzodiazepines. Psychopharmacology (Berl) 1976; 47: 101–103. [DOI] [PubMed] [Google Scholar]
- 44. Warne LN, Beths T, Holm M, et al. Evaluation of the perioperative analgesic efficacy of buprenorphine, compared with butorphanol, in cats. J Am Vet Med Assoc 2014; 245: 195–202. [DOI] [PubMed] [Google Scholar]
- 45. Reid J, Nolan AM, Hughes JML, et al. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim Welfare 2007; 16: 97–104. [Google Scholar]
- 46. Oliver V, De Rantere D, Ritchie R, et al. Psychometric assessment of the Rat Grimace Scale and development of an analgesic intervention score. PLOS ONE 2014; 9: e97882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ibanez J, Velasco J, Raurich JM. The accuracy of the Biox 3700 pulse oximeter in patients receiving vasoactive therapy. Intensive Care Med 1991; 17: 484–486. [DOI] [PubMed] [Google Scholar]
- 48. Comroe JHJ, Botelho S. The unreliability of cyanosis in the recognition of arterial anoxemia. Am J Med Sci 1947; 124: 1–6. [DOI] [PubMed] [Google Scholar]
- 49. Murrell JC, Hellebrekers LJ. Medetomidine and dexmedetomidine: a review of cardiovascular effects and antinociceptive properties in the dog. Vet Anaesth Analg 2005; 32: 117–127. [DOI] [PubMed] [Google Scholar]