Abstract
Objectives
The aim of this study was to evaluate changes in circulating insulin-like growth factor type 1 (IGF-1) concentrations in hyperthyroid cats, before and after thiamazole treatment.
Methods
Thirty-four hyperthyroid cats were retrospectively included. Plasma free thyroxine (fT4) and IGF-1 concentrations were measured at diagnosis and 3 months after initiating antithyroid drug therapy.
Results
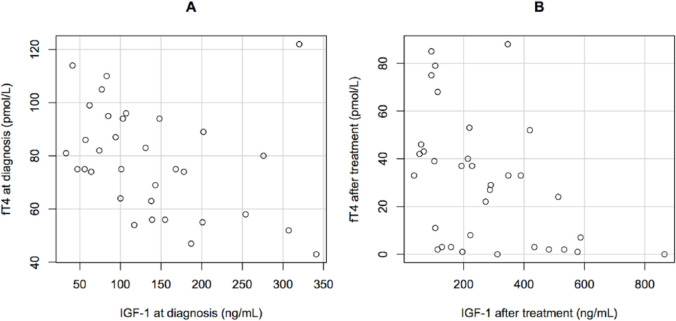
Median fT4 significantly decreased (P <0.001) after treatment (from 78 pmol/l [range 43–122 pmol/l] to 31 pmol/l [range below assay limit of detection to 88 pmol/l]), whereas IGF-1 values significantly increased (P <0.001) after treatment (from 117 ng/ml [33–341 ng/ml] to 221 ng/ml [36–865 ng/ml]). fT4 and IGF-1 concentrations were significantly negatively correlated both at diagnosis (r = −0.43, P = 0.01) and after treatment (r = −0.51, P = 0.002).
Conclusions and relevance
In cats, IGF-1 concentrations appear to be inversely proportional to the severity of hyperthyroidism and significantly increase after treatment with thiamazole.
Introduction
Normal secretion and action of the thyroid hormones and growth hormone (GH)/insulin-like growth factor type 1 (IGF-1) are interdependent.1–3 Somatostatin inhibits hypothalamic thyrotropin-releasing hormone (TRH) and pituitary thyroid-stimulating hormone (TSH) secretion. 4 Conversely, thyroid cells express IGF-1 receptors allowing the pro-mitogenic effect of IGF-1 and enhancing thyroglobulin secretion.5–8 Hyperplastic thyroid cells also synthesise both IGF-1 and insulin-like growth factor binding protein 3.9,10
The relationship between thyroid hormone dysfunction and miscellaneous endocrine and metabolic disturbances has been studied in some species. 3 For example, a previous study showed that hyperthyroid cats had higher IGF-1 concentrations than healthy animals, although the difference was not statistically significant and the results varied according to the assay methods. 11 Furthermore, IGF-1 is increased in dogs with primary hypothyroidism when compared with healthy animals. 12 In addition, a previous study showed that thiamazole (Felimazole; Dechra Veterinary Products) treatment of hyperthyroid cats was associated with a significant rise in leptin concentrations. 13 As there is increasing evidence that leptin and IGF-1 signaling cooperate to promote cellular proliferation, altered metabolic/hormonal state and increased body weight (BW) through common intracellular pathways and bidirectional cross-talks,14,15 it was hypothesised that hyperthyroid cats had higher serum IGF-1 concentrations after antithyroid treatment, with a negative correlation between IGF-1 and free thyroxine (fT4), in contrast to what is observed in the hypothyroid dog. The aim of the present work was to measure plasma IGF-1 concentrations in hyperthyroid cats before and after antithyroid treatment with thiamazole.
Materials and methods
Study design
Thirty-four cats were retrospectively included in the study, based on a diagnosis of spontaneous hyperthyroidism (compatible physical signs, ie, weight loss, polyphagia, polyuria/polydipsia, gastrointestinal signs or tachycardia associated with palpable thyroid nodule[s]together with a plasma fT4 above 40 pmol/l)16,17 leading to thiamazole treatment and subsequent follow-up 3 months after diagnosis. Cats were considered as well treated as soon as fT4 was <30 pmol/l. Animals with concurrent diseases and/or receiving other treatments at the time of diagnosis were excluded. IGF-1 was measured at the time of diagnosis and 3 months after the beginning of the antithyroid treatment in all cats, using surplus plasma samples. Informed owner consent was obtained in all cases.
Hormone assays
All the assay procedures were performed in accordance with the manufacturer’s instructions. Plasma fT4 was measured by radioimmunoassay (RIA kit 1363; Beckman Coulter). The kit was validated for linearity beforehand by the manufacturer (between 11.0 and 100.0 pmol/l) and analytical sensitivity (2.4 pmol/l). These data were then checked in situ at three levels (15, 35 and 60 pmol/l) with 20 replicates per level. Maximal intra- and interassay coefficients of variation (CVs) were 2.3% and 14.3%, respectively, for the high level (which best approximated the level of clinical decision of hyperthyroidism).
After dissociation from the insulin-like growth factor binding proteins by an acidic buffer, total IGF-1 concentrations were measured using a non-specific immunoradiometric commercial kit based on two region-restricted, affinity-purified polyclonal antibodies (Mediagnost IGF1 RI-CT R22; Diasorin). The manufacturer’s validation data (linearity between 25.0 and 900.0 ng/ml) and analytical sensitivity (2.6 ng/ml) for this kit were checked in situ at two levels (120 and 190 ng/ml) with 20 replicates per level. Maximal intra- and interassay CVs were then 6.7% and 8.9% for the low level and 6.5% and 11.3% for the high level, respectively.
Statistical analysis
Normality of the data was assessed by Shapiro–Wilk test, using Statview software. None of the results were normally distributed and are therefore presented as median and range. The effects of age, sex and BW on IGF-1 concentrations were determined by the Kruskal–Wallis test. The IGF-1 values before and after treatment with thiamazole were compared using the Wilcoxon signed-rank test. Correlations between variables were determined by Spearman’s test. P <0.05 was considered significant.
Results
The hyperthyroid cats included 31 domestic shorthairs and three Siamese; 17 were females (14 spayed) and 17 were castrated males, ranging in age from 10 to 17 years (median 14 years). Clinical signs of hyperthyroidism had developed 1–24 months (median 5 months) before presentation. The physical examination revealed weight loss (n = 24; 70.6%), polyphagia (n = 15; 44.1%), tachycardia (n = 11; 32.3%), polyuria/polydipsia (n = 10; 29.4%), vomiting (n = 8; 23.5%), hyperactivity (n = 4; 11.8%) and diarrhoea (n=3; 8.8%).
Reported thiamazole dose varied according to the fT4 concentration and clinical signs at diagnosis (mean dose 7.7 ± 2.7 mg/cat q24h, range 5–10 mg/cat q24h).
Median fT4 concentrations significantly decreased after 3 months of thiamazole treatment (from 78 pmol/l [range 43–122 pmol/l] to 31 pmol/l [range below assay limit of detection to 88 pmol/l]), (P <0.001) with fT4 decreasing below 30 pmol/l in 17 cats. No significant change in BW after 3 months of treatment was observed. There was no effect of sex, castration status, breed, BW or age on IGF-1 concentrations. Hyperthyroid cats exhibited a significant increase in IGF-1 values after treatment (from 117 ng/ml [range 33–341 ng/ml] to 221 ng/ml [range 36–865 ng/ml]) (P <0.001) (Figure 1). A significant negative correlation between fT4 and IGF-1 was observed at diagnosis (r = −0.43; P = 0.01) and after treatment (r = −0.51; P = 0.002) (Figure 2). When cats were split according to their biological response to thiamazole, IGF-1 concentrations at diagnosis did not differ significantly between well-treated (median 117 ng/ml [range 41–341 ng/ml]) and insufficiently treated (median 119 ng/ml [range 33–320 ng/ml]) cats (P = 0.87). IGF-1 concentrations significantly increased after treatment both for well-treated (P <0.001) and insufficiently treated (P = 0.02) cats. However, it seems that the increase in IGF-1 concentrations was significantly higher (P = 0.005) in well-treated cats (median 290 ng/ml [range 106–865 ng/ml]) compared with the others (median 114 ng/ml [range 36–419 ng/ml]).
Figure 1.
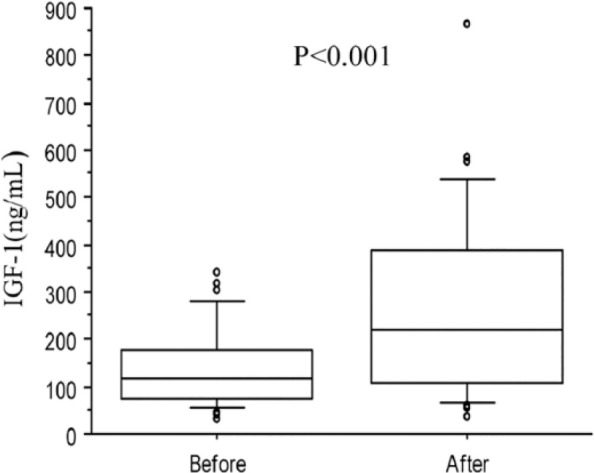
Box plots of insulin-like growth factor type 1 (IGF-1) concentration in 34 hyperthyroid cats at diagnosis and after thiamazole treatment. The horizontal line in each box represents the median value. The boxes represent the interquartile range (25th and 75th percentiles). Whiskers represent the 5th and 95th percentiles. Outliers are plotted separately as dots
Figure 2.
Correlation between free thyroxine (fT4; pmol/l) and insulin-like growth factor type 1 (IGF-1; ng/ml) concentrations in 34 hyperthyroid cats (a) at diagnosis and (b) after treatment with thiamazole. fT4 and IGF-1 were significantly negatively correlated both before (r = −0.43; P = 0.01) and after treatment (r = −0.51; P = 0.002)
Discussion
The results of the present study indicate that IGF-1 concentrations appear to be inversely proportional to the severity of hyperthyroidism as assessed by measurement of fT4 concentrations, and to increase significantly after thiamazole treatment in the absence of significant BW gain. The rise in IGF-1 concentrations was of particular significance in well-treated cats, which exhibited significantly higher IGF-1 values compared with the insufficiently treated hyperthyroid cats. The lower IGF-1 concentrations in untreated hyperthyroid cats is in contrast to the increased GH and IGF-1 concentrations previously demonstrated in dogs with primary hypothyroidism.12,18–20
Numerous hypotheses for these lower IGF-1 values in hyperthyroidism are possible. First, the lower IGF-1 values could be due to reduced GH secretion. Thyroid hormones directly stimulate somatostatin secretion in order to slow down hypothalamic TRH and pituitary TSH secretions. Consequently, GH (and IGF-1) release is inhibited.2,5 Second, hyperthyroidism as a chronic illness is associated with a chronic stress response. The subsequent corticotropin-releasing hormone, adrenocorticotropic hormone and glucocorticoid secretions result in decreased GH release and inhibition of IGF-1 action. 4 Third, feline hyperthyroidism has been previously associated with decreased leptin concentrations that significantly increase after thiamazole treatment. 13 Leptin is known to stimulate indirectly GH secretion by inhibiting hypothalamic somatostatin and neuropeptide Y, and by stimulating growth hormone releasing hormone.5,21 The liver is the main organ producing IGF-1, 5 and various hepatic diseases induce decreased IGF-1 concentration in humans and dogs.3,22–24 Although liver enzyme activities are increased in hyperthyroid cats, 25 liver function is preserved and therefore an unlikely cause of decreased IGF-1 concentrations. 26 Finally, in humans, it has been demonstrated that excess thyroid hormones decrease peripheral vascular resistance by dilating the peripheral arterioles. 27 Consequently, the effective volume decreases and stimulating the renin–angiotensin–aldosterone system (RAAS) in order to re-establish euvolaemia through increased renal sodium retention.27,28 Although interactions between RAAS and the IGF system are complex and poorly understood, some studies have shown that these two systems are negatively correlated.28–31 The pathophysiological mechanisms responsible for the changes in IGF-1 concentrations in hyperthyroid cats remain unclear and additional investigations, including other components of the somatotropic system and evaluation of the RAAS, would be necessary for a better understanding. A longer study would also be interesting to explore the correlation between BW gain and changes in IGF-1 concentrations.
Conclusions
It appears that the more severe hyperthyroidism is in cats, as assessed by fT4 estimation, the lower the IGF-1 concentration. Following treatment of hyperthyroidism, IGF-1 concentrations significantly increase. A longer study would be necessary to assess whether IGF-1 measurement provides any prognostic information in hyperthyroid cats at diagnosis or undergoing treatment. Indeed, because IGF-1 has major roles in cell proliferation, tissue growth and immunity, its increase could be involved in the general health status improvement during the course of treatment of feline hyperthyroidism, as it does in many human diseases. 32
Footnotes
Accepted: 9 January 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Näntö-Salonen K, Muller HL, Hoffman AR, et al. Mechanisms of thyroid hormone action on the insulin-like growth factor system: all thyroid hormone effects are not growth hormone mediated. Endocrinology 1993; 132: 781–788. [DOI] [PubMed] [Google Scholar]
- 2. Giustina A, Wehrenberg WB. Influence of thyroid hormones on the regulation of growth hormone secretion. Eur J Endocrinol 1995; 133: 646–653. [DOI] [PubMed] [Google Scholar]
- 3. Völzke H, Friedrich N, Schipf S, et al. Association between serum insulin-like growth factor-i levels and thyroid disorders in a population-based study. J Clin Endocrinol Metab 2007; 92: 4039–4045. [DOI] [PubMed] [Google Scholar]
- 4. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67: 259–284. [DOI] [PubMed] [Google Scholar]
- 5. Butler AA, Le Roith D. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol 2001; 63: 141–164. [DOI] [PubMed] [Google Scholar]
- 6. Feldt-Rasmussen U. Interactions between growth hormone and the thyroid gland – with special reference to biochemical diagnosis. Curr Med Chem 2007; 14: 2783–2788. [DOI] [PubMed] [Google Scholar]
- 7. Malaguarnera R, Frasca F, Garozzo A, et al. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J Clin Endocrinol Metab 2011; 96: 766–774. [DOI] [PubMed] [Google Scholar]
- 8. Morgan SJ, Neumann S, Marcus-Samuels B, et al. Thyrotropin and insulin-like growth factor 1 receptor crosstalk upregulates sodium-iodide symporter expression in primary cultures of human thyrocytes. Thyroid 2016; 26: 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewiński A, Marcinkowska M, Brzeziańska E, et al. Expression of insulin-like growth factor I (IGF-I) gene and of genes for IGF-binding proteins 1, 2, 3, 4 (IGFBP-1-IGFBP-4) in non-neoplastic human thyroid cells and in certain human thyroid cancers. Effect of exogenous IGF-I on this expression. Endocr Res 2004; 30: 47–59. [DOI] [PubMed] [Google Scholar]
- 10. Kursunluoglu R, Turgut S, Akin F, et al. Insulin-like growth factor-i gene and insulin-like growth factor binding protein-3 polymorphism in patients with thyroid dysfunction. Arch Med Res 2009; 40: 42–47. [DOI] [PubMed] [Google Scholar]
- 11. Tschuor F, Zini E, Schellenberg S, et al. Evaluation of four methods used to measure plasma insulin-like growth factor 1 concentrations in healthy cats and cats with diabetes mellitus or other diseases. Am J Vet Res 2012; 73: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 12. Jaillardon L, Martin L, Nguyen P, et al. Serum insulin-like growth factor type 1 concentrations in healthy dogs and dogs with spontaneous primary hypothyroidism. Vet J 2011; 190: e95–99. [DOI] [PubMed] [Google Scholar]
- 13. Jaillardon L, Burger M, Siliart B. Leptin levels in hyperthyroid cats before and after treatment. Vet Rec 2012; 170: 155. [DOI] [PubMed] [Google Scholar]
- 14. Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J 2006; 393: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saxena NK, Taliaferro-Smith L, Knight BB, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res 2008; 68: 9712–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broussard JD, Peterson ME, Fox PR. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. J Am Vet Med Assoc 1995; 206: 302–305. [PubMed] [Google Scholar]
- 17. Scott-Moncrieff JC. Thyroid disorders in the geriatric veterinary patient. Vet Clin North Am Small Anim Pract 2012; 42: 707–725. [DOI] [PubMed] [Google Scholar]
- 18. Diaz-Espiñeira MM, Mol JA, van den Ingh TSGAM, et al. Functional and morphological changes in the adenohypophysis of dogs with induced primary hypothyroidism: loss of TSH hypersecretion, hypersomatotropism, hypoprolactinemia, and pituitary enlargement with transdifferentiation. Domest Anim Endocrinol 2008; 35: 98–111. [DOI] [PubMed] [Google Scholar]
- 19. Diaz-Espiñeira MM, Galac S, Mol JA, et al. Thyrotropin-releasing hormone-induced growth hormone secretion in dogs with primary hypothyroidism. Domest Anim Endocrinol 2008; 34: 176–181. [DOI] [PubMed] [Google Scholar]
- 20. Lee WM, Diaz-Espineira M, Mol JA, et al. Primary hypothyroidism in dogs is associated with elevated GH release. J Endocrinol 2001; 168: 59–66. [DOI] [PubMed] [Google Scholar]
- 21. Ahima RS, Flier JS. Leptin. Annu Rev Physiol 2000; 62: 413–437. [DOI] [PubMed] [Google Scholar]
- 22. Conchillo M, Prieto J, Quiroga J. Insulin-like growth factor I (IGF-I) and liver cirrhosis. Rev Esp Enferm Dig 2007; 99: 156–164. [DOI] [PubMed] [Google Scholar]
- 23. Neumann S, Welling H, Thuere S. Insulin-like growth factor I concentration in dogs with inflammatory and neoplastic liver diseases. J Vet Med Ser A 2007; 54: 612–617. [DOI] [PubMed] [Google Scholar]
- 24. Wu Y-L, Ye J, Zhang S, et al. Clinical significance of serum IGF-I, IGF-II and IGFBP-3 in liver cirrhosis. World J Gastroenterol 2004; 10: 2740–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foster DJ, Thoday KL. Tissue sources of serum alkaline phosphatase in 34 hyperthyroid cats: a qualitative and quantitative study. Res Vet Sci 2000; 68: 89–94. [DOI] [PubMed] [Google Scholar]
- 26. Berent AC, Drobatz KJ, Ziemer L, et al. Liver function in cats with hyperthyroidism before and after 131I therapy. J Vet Intern Med 2007; 21: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 27. Van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol 2009; 160: 205–215. [DOI] [PubMed] [Google Scholar]
- 28. Vargas F. Vascular and renal function in experimental thyroid disorders. Eur J Endocrinol 2006; 154: 197–212. [DOI] [PubMed] [Google Scholar]
- 29. Cooper SA, Whaley-Connell A, Habibi J, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. AJP Hear Circ Physiol 2007; 293: H2009–H2023. [DOI] [PubMed] [Google Scholar]
- 30. Perticone F, Sciacqua A, Perticone M, et al. Low-plasma insulin-like growth factor-I levels are associated with impaired endothelium-dependent vasodilatation in a cohort of untreated, hypertensive Caucasian subjects. J Clin Endocrinol Metab 2008; 93: 2806–2810. [DOI] [PubMed] [Google Scholar]
- 31. Zandbergen AAM. Short-term administration of an angiotensin-receptor antagonist in patients with impaired fasting glucose improves insulin sensitivity and increases free IGF-I. Eur J Endocrinol 2006; 155: 293–296. [DOI] [PubMed] [Google Scholar]
- 32. Franceschi C, Olivieri F, Marchegiani F, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev 2005; 126: 351–361. [DOI] [PubMed] [Google Scholar]