Abstract
Leptin acts as an anorexigenic hormone in the brain, where the long form of the leptin receptor (LRb) is widely expressed in hypothalamic and extra-hypothalamic sites that are known to participate in diverse feeding circuits. The important role of leptin in energy homeostasis is demonstrated by the profound hyperphagia and morbid obesity in humans and rodents null for leptin or LRb. However, common forms of obesity are associated with high leptin levels and a failure to respond effectively to exogenous leptin; indicating a state of leptin resistance. Leptin resistance is thought to be an important component in the development of obesity. Several defects may contribute to the leptin resistant state, including a defective leptin transport across the blood-brain barrier, which reduces the availability of leptin at its receptor. Furthermore, defects in LRb signal transduction involving reduced LRb expression or the induction of feedback inhibitors have been found in leptin resistance; these defects are commonly termed cellular leptin resistance,. Finally, reduced leptin action can result in the disruption of proper neuronal interactions, by altering neuronal wiring. Interestingly, some leptin functions remain intact in the leptin-resistant state, such as cardiovascular leptin effects. The appearance of selective leptin resistance is mirrored by the observation that cellular leptin resistance has been found only in some subpopulations of hypothalamic LRb neurons. Current efforts to dissect leptin function in specific populations of LRb neurons will increase our understanding of these complexities of leptin physiology.
Leptin Action in the Central Nervous System
Leptin Receptor
The adipocyte-derived hormone leptin executes its anorexigenic actions via leptin receptors (LR), which belong to the category of class I cytokine receptors. Six LR variants (LRa-f in the mouse) exist, all derived from alternative splicing of the lepr gene. LR variants are subcategorized as short form (LRa, c, d, f, g), soluble form (LRe) and long form (LRb). LRa and LRb consist of identical extracellular domains, transmembrane domains and the initial 29 amino acids of the intracellular domain, but only LRb possesses a long intracellular signaling domain [1, 2]. Even though the function of short and soluble LR is still unclear, LRb appears to be the critical receptor for leptin action, as the dramatic obese and hyperphagic phenotype of leprdb mice is due to a mutation that prevents the protein expression of LRb, whereas all other LR variants are still functional [2]. Also, leprdb mice are phenotypically identical to leptin-deficient lepob mice as well as to mice with deletion of all LR isoforms [3].
LRb is predominantly expressed in the CNS, and central LRb expression has been found sufficient to normalize the phenotype of leprdb mice [3]. LRb expression is particularly high in the hypothalamus, where diverse sensory information is integrated and processed to adjust homeostatic function, as discussed in several chapter in this volume[4, 5]. Apart from its obvious effects on body weight and food intake, leptin has also been found to control thyroid axis, reproductive axis, glucose homeostasis, immune function, growth and autonomic nervous system and other physiological functions [6].
Leptin Action in AgRP and POMC Neurons in the Arcuate Nucleus
The hypothalamic arcuate nucleus (Arc), which stretches along the base of the hypothalamus, is so far the best-characterized site of leptin action. At least two distinct neuronal populations in the Arc express LRb: the orexigenic agouti-related peptide (AgRP) neurons and the anorexigenic pro-opiomelanocortin (POMC)-expressing neurons [7]. Leptin stimulates the expression and release of the POMC-derived peptide α-melanocyte-stimulating hormone (α-MSH), which in turn activate CNS melanocortin receptors (MC3R and MC4R) on target neurons. In contrast, leptin reduces AgRP expression, which inhibits of MC3R and MC4R signaling [8, 9]. POMC- and AgRP- expressing neurons densely innervate the paraventricular hypothalamic nucleus (PVN), the dorsomedial hypothalamus, and the lateral hypothalamic area and other sites [10, 11] ultimately leading to neuroendocrine, autonomic and behavioral outputs [12]. Deletion of MC4R leads to morbid obesity [8]. Thus, it has been hypothesized that leptin action on Arc melanocortin neurons is the key mechanism for leptin-regulated energy homeostasis.
Leptin Action Outside of the Arc
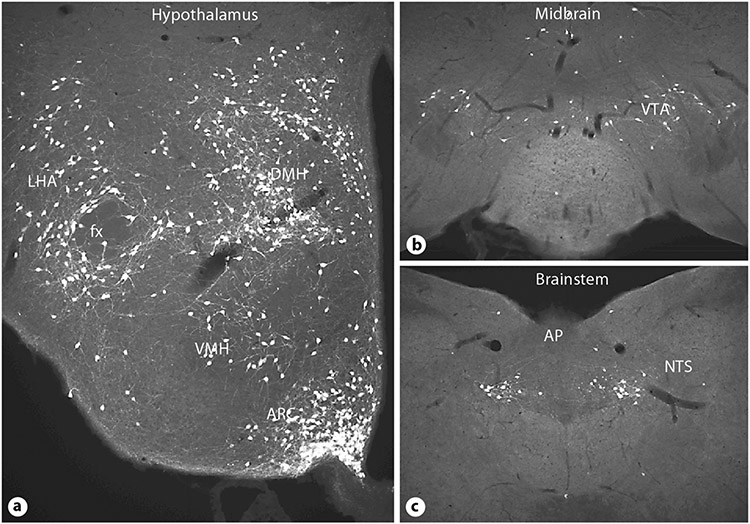
Several sites in the CNS express LRb, e.g. the hypothalamus, midbrain and brainstem (fig. 1) [13]. Most of these LRb-expressing sites are known to regulate energy homeostasis, suggesting that anorexigenic leptin actions involve several components. Nevertheless, the functions of LRb neurons outside the Arc are not yet fully understood. While LRb-expressing Arc neurons are important for the response to leptin, it has become clear from recent literature that they may not account for all or even the majority of anorexigenic leptin action in vivo. For example, LRb neurons in the ventromedial hypothalamus (VMH) [14], and the ventral tegmental area (VTA) [15, 16] have been shown to participate in the regulation of eating and energy balance, whereas LRb neurons in the premammillary nucleus do not participate in eating-related circuits, but are involved in reproductive function [17]. In contrast, reactivation of LRb specifically in the Arc of LRb-deficient mice surprisingly reduced body weight only modestly, but normalized glucose homeostasis and locomotor activity [18]. Furthermore, deletion of the leptin receptor gene (Lepr) from AgRP and/or POMC neurons results in modest obesity compared to that of leprdb animals [19, 20]. Hence, while deletion of LRb from specific neuronal populations in the ARC, VMH or the VTA clearly demonstrate that leptin has important actions in these sites, the dramatic obesity and hyperphagia found in leptin-signaling deficient lepob or leprdb mice could not be recapitulated. Therefore, despite our increased understanding of central leptin action, many details still await discovery and further efforts are on the way to define specific subpopulation of LRb neurons that contribute to anorexigenic leptin actions.
Fig. 1.
Immunohistochemical visualization of green fluorescent protein expressing LRb neurons in the hypothalamus (a), midbrain (b) and brainstem (c). LRb neurons are found in central sites that contribute importantly to the control of eating. LHA = Lateral hypothalamic area; fx = fornix; DMH = dorsomedial hypothalamus; VMH = ventromedial hypothalamus; Arc = arcuate nucleus; VTA = ventral tegmental area; AP = area postrema; NTS = nucleus of the solitary tract.
Leptin Signaling Pathways
LRb is a typical class I cytokine receptor without intrinsic kinase activity to initiate the phosphorylation of tyrosin residues (which are common stimulatory events to induce signaling pathways). Therefore, LRb binds janus kinase 2 (JAK2) for the induction of signaling pathways. Leptin binding to LRb results in conformational changes of LRb that enables binding and autophosphorylation of JAK2. JAK2 promotes the phosphorylation of three conserved tyrosine residues (Y) in the LRb signaling domain (Y985, Y1077, Y1138). Phosphorylated tyrosine residues (PY) are common docking stations for adaptor molecules that interact with PY via so called SH-2 domains. In cell culture experiments these three tyrosine residues on LRb have been found to interact with distinct adaptor proteins from discrete signaling pathways [21]. Thus, together with tyrosine phosphorylated JAK2, there are 4 PY sites that induce specific downstream LRb signaling events (fig. 2).
Fig. 2.
The intracellular LRb signaling cascade involves the binding, phosphorylation and activation of JAK2 (janus kinase 2) as well as phosphorylation of conserved tyrosine residues on LRb. These initiate distinct signaling pathways to mediate the diverse functions of leptin, including the transcriptional regulation of SOCS-3 (suppressor-of-cytokine-signaling-3) and PTP1B (phosphotyrosine phosphatase 1B) which are negative regulator or LRb signaling and are involved in the development of leptin resistance in the obese state.
PY985 interacts with tyrosine phosphatase SHP-2, which leads to the activation of the ERK signaling pathway. PY1077 binds the nuclear transcription factor signal-transducer-and-activator-5 (STAT5), which is then phosphorylated by JAK2, dimerized, and translocated into the nucleus, where STAT5 acts as a transcription factor. Similarly, PY1138 binds specifically to STAT3, which is also phosphorylated, dimerized, and transported into the nucleus for transcriptional control [21]. The majority of LRb/STAT induced transcripts are unknown, although leptin-induced transcription of the neuropeptide POMC as well as the suppressor-of-cytokine-signaling-3 (SOCS3) has been demonstrated to require LRb/STAT3 signaling [22, 23]. SOCS-3 acts as a negative feedback signal on LRb and has been shown to inhibit JAK2 phosphorylation by interacting with PY985 as well as directly via JAK2 [24].
Most of these interactions of LRb with specific signaling pathways are based on cell-culture models, but some physiological roles for discrete LRb signaling pathways have been dissected. For example, the majority of leptin’s anorexigenic action can be attributed to PY1138, because mice with targeted mutation of Y1138, which leads to a loss of LRb/STAT3 binding without affecting other LRb signaling pathways, are severely obese and hyperphagic, comparable to leprdb mice [25]. To the contrary, targeted mutation of Y938, which leads to a selective loss of LRb/SHP-2 binding, results in lean mice with increased leptin sensitivity, suggesting that an inhibitory component of the LRb signal has been lost in these mice [26]. Therefore, the results from Y938-mutated mice support an important role of this phosphorylation site for the binding and function of the negative-feedback mediator SOCS-3, as suggested by earlier cell-culture experiments [24].
Leptin also has been found to regulate several other pathways involved in the regulation of energy homeostasis, including the mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), and the IRS/PI3K pathway [27-29]. However, the exact molecular mechanisms resulting in their interaction with the leptin signaling pathway, and whether leptin indeed directly regulates these pathways remains unknown. Furthermore, leptin regulates cellular function on multiple levels involving – aside from signaling pathways and transcriptional events – the stimulation or inhibition of neuronal activity (leading to neuropeptide and transmitter release), neuronal plasticity via axonal outgrowth, and reorganization as well as neurogenesis [9, 10, 30-32].
Leptin Resistance
Despite the dramatic effects of leptin deficiency on body weight and food intake in leptin-deficient mice, the use of leptin as an anorexigenic drug to treat human obesity was disappointing [33]. Because obesity is generally associated with elevated leptin levels [34] it was suggested that obesity causes a decrease in leptin efficiency. The reduced potency of leptin to inhibit eating has been termed leptin resistance.
Leptin resistance can be dramatically demonstrated in rodents in which obesity is induced by feeding a palatable, high-fat diet (this condition has been termed diet-induced obesity, DIO). Such mice become progressively less sensitive to leptin administration [35]. Leptin resistance has been also described in aged individuals [36], seasonal obese rodents [37] and DIO prone (compared to DIO resistant) rodent strains [38], which all share a state of increased adiposity and elevated leptin levels. Whereas the concept of leptin resistance is well accepted, its mechanisms remain incompletely understood. Leptin transport defects [39], defects within the LRb signaling cascade (cellular leptin resistance) [35, 40], and defective neuronal wiring and function of eating circuits [41, 42] all may contribute.
Defective Leptin Transport across the Blood-Brain Barrier
Leptin is transported across the blood-brain barrier into the brain by a saturable, regulated transporter [43, 44]. In DIO rats the ratio of cerebrospinal fluid (CSF) leptin versus serum leptin is decreased. Thus, even though CSF leptin levels in DIO rats is still elevated compared to lean control rats, the central leptin availability at LRb might not be sufficient to translate into appropriate LRb signaling action [39]. Consistent with this model is the fact that DIO-induced leptin resistance can be improved (but not completely recovered) by central leptin injections, which circumvent any defective transport mechanisms [35]. Thus, defective leptin transport represents one component in the leptin resistant state.
Cellular Leptin Resistance and Negative Feedback Signals
Cellular leptin resistance refers to any decrease in intracellular LRb signaling events. Indeed, DIO can be also characterized by decreased leptin-induced STAT3 activation [35]. Furthermore, negative-feedback mechanisms have been described for LRb in vivo via SOCS-3 and protein tyrosine phosphatase PTP1B.
SOCS-3 is upregulated in response to leptin within hypothalamic areas known to express LRb [22]. Thus, increased circulating leptin, as found in most obese individuals, may chronically induce SOCS-3 levels and therefore attenuate leptin signaling via LRb. Recent data demonstrated that targeted deletion of the SOCS-3 gene as well as conditional, neuron-specific SOCS-3 deletion resulted in reduced weight gain on a high fat diet and increased leptin sensitivity [45], thus supporting that SOCS-3 indeed interacts with LRb signaling in vivo and importantly mediates leptin resistance.
PTB1B is a cytoplasmic protein that, like SOCS-3, has been found to decrease JAK2 phosphorylation and also decreases LRb signaling [46]. Similar to SOCS-3 the complete deletion of PTP1B or conditional PTB1B deletion in neurons resulted in decreased body weight gain on a high-fat diet and a lean phenotype [47]. Leptin seems to increase PTB1B via unknown mechanisms [47, 48]. Thus, an important open question is whether PTB1B and SOCS-3 are independently regulated by different mechanisms.
It is also possible that other leptin-independent factors may be involved in the regulation of PTB1B or SOCS-3. For example, both have been shown to be upregulated by high-fat diet feeding [40, 48] and SOCS-3 is also regulated by several cytokines that are known to be induced in obese subjects [49]. Thus, SOCS-3 and PTB1B seem to have important roles in the development of leptin resistance and, therefore, are potential targets for the development of anti-obesity drugs.
Leptin-Induced Leptin Resistance
Leptin resistance is commonly associated with hyperleptinemia, and leptin can induce SOCS-3 as well as PTB1B, suggesting that leptin causes its own resistance. This concept was further supported in rats receiving prolonged central leptin infusion: Even though these animals initially lose weight, their body weights normalize over time despite ongoing leptin treatment. In this state even additional acute leptin injections failed to induce any anorexigenic effect [50-52]. Indeed, when put on a high-fat diet, animals with acquired leptin resistance gained more weight than control animals, further indicating that leptin resistance contributes significantly to DIO.
In contrast, low leptin levels (e.g. in mice after prolonged fasting) as well as lack of leptin (lepob mice) increases leptin sensitivity, consistent with the idea that low leptin levels result in decreased SOCS-3 levels. Therefore, leptin induced STAT3 activation (stimulating SOCS-3 expression) and SOCS-3 induction (inhibiting leptin induced STAT3 activation) might normally be in a steady state for any given level of circulating leptin [6]. The evolutionary adaptiveness of a leptin-driven feedback mechanism in which low body weight (low leptin levels) produces the most potent anorexigenic effect seems questionable. A potential explanation for this is that leptin action may be less important in states of plenty, but be critical to coordinate reproductive behavior with information about body fat stores in states of scarcity.
Site-Specific Leptin Resistance
Although leptin’s anorexigenic action is reduced in leptin resistant states, other leptin-regulated processes have been shown to be preserved. For example, leptin’s effect to increase sympathetic tone in the kidney, resulting in increased blood pressure, is preserved in leptin-resistant mice [53]. This selective leptin resistance cannot be easily explained by a defect in leptin transport or general cellular signaling mechanisms. Rather, this suggests that only subpopulations of LRb neurons are involved in either central eating-control circuits or cardiovascular circuits and that these LRb circuits are selectively affected during leptin resistance.
Indeed, in DIO mice, cellular leptin resistance with reduced leptin-induced STAT3 phophorylation and increased SOCS-3 was found to be restricted to the Arc, with no such resistance evident in other hypothalamic and extrahypothalamic sites [40]. Similarly, SOCS-3 levels were selectively elevated in the Arc in a seasonal mammal during the period of increased adiposity and hyperphagia [37], further supporting the existence of site-specific leptin resistance in the brain. Many LRb neurons in the Arc are neuroendocrine cells, with processes exposed to the portal vascular system. These cells have been shown to respond more sensitively and faster to changes in circulating leptin levels, suggesting that leptin could directly stimulate these neurons in addition to a leptin transport across the blood-brain barrier [47]. Therefore, increased leptin access to arcuate LRb neurons would make them more prone to the development of increased SOCS-3 levels and cellular leptin resistance [54].
Site-specific cellular leptin resistance has been also demonstrated in pregnant, hyperphagic rats, although in this model leptin resistance was confined to the VMH [55]. Many VMH neurons co-express LRb and estrogen receptors [56], so that the substantial changes in levels of estrogens during the ovarian cycle and pregnancy might interact with LRb signaling in the VMH. Thus, the leptin resistant state might be a highly regulated response to diverse metabolic situations, suggesting that we are only beginning to understand the many facets that contribute to the development of leptin resistance and obesity.
Conclusion
The research initiated by the discovery of leptin and its receptor, together with advances in novel genetic and histological methodologies, has tremendously increased our knowledge about central circuits that control energy homeostasis. Future research aimed at dissecting the functional effects of LRb signaling in discrete populations of LRb neurons and the neuronal circuits that they affect will certainly improve our understanding of the LRb function in the control of eating and regulation of body weight.
References
- 1.Chua SC Jr, Koutras IK, Han L, Liu SM, Kay J, Young SJ, Chung WK, Leibel RL: Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics 1997;45:264–270. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia LA: The leptin receptor. J Biol Chem 1997;272:6093–6096. [DOI] [PubMed] [Google Scholar]
- 3.Kowalski TJ, Liu SM, Leibel RL, Chua SC Jr: Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 2001;50:425–435. [DOI] [PubMed] [Google Scholar]
- 4.Blevins JE, Baskin DG: Hypothalamic-brainstem circuits controlling eating and energy homeostasis; in Geary N, Langhans W (eds): Frontiers in Eating and Weight Regulation. Forum Nutr. Basel, Karger, 2010, vol 63, pp 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langhans W, Geary N: Overview of the physiological control of eating; in Langhans W, Geary N (eds): Frontiers in Eating and Weight Regulation. Forum Nutr. Basel, Karger, 2010, vol 63, pp 9–53. [DOI] [PubMed] [Google Scholar]
- 6.Myers MG, Cowley MA, Munzberg H: Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008;70:537–556. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG: Central nervous system control of food intake. Nature 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 8.Butler AA, Cone RD: The melanocortin receptors: lessons from knockout models. Neuropeptides 2002;36:77–84. [DOI] [PubMed] [Google Scholar]
- 9.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ: Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411:480–484. [DOI] [PubMed] [Google Scholar]
- 10.Bouret SG, Draper SJ, Simerly RB: Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004;304:108–110. [DOI] [PubMed] [Google Scholar]
- 11.Jacobowitz DM, O’Donohue TL: Alpha-melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA 1978;75:6300–6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD: Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 1994;8:1298–1308. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB: Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 1998;395:535–547. [PubMed] [Google Scholar]
- 14.Dhillon H, Zigman JM, Ye C, et al. : Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006;49:191–203. [DOI] [PubMed] [Google Scholar]
- 15.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS: Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006;51:811–822. [DOI] [PubMed] [Google Scholar]
- 16.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ: Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006;51:801–810. [DOI] [PubMed] [Google Scholar]
- 17.Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG Jr: Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 2009;29:3138–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppari R, Ichinose M, Lee CE, et al. : The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 2005;1:63–72. [DOI] [PubMed] [Google Scholar]
- 19.Balthasar N, Coppari R, McMinn J, et al. : Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004;42:983–991. [DOI] [PubMed] [Google Scholar]
- 20.van de Wall E, Leshan R, Xu AW, et al. : Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 2008;149:1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers MG Jr: Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 2004;59:287–304. [DOI] [PubMed] [Google Scholar]
- 22.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS: Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1998;1:619–625. [DOI] [PubMed] [Google Scholar]
- 23.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C: Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 2003;144:2121–2131. [DOI] [PubMed] [Google Scholar]
- 24.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG Jr: SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 2000;275:40649–40657. [DOI] [PubMed] [Google Scholar]
- 25.Bates SH, Stearns WH, Dundon TA, et al. : STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003;421:856–859. [DOI] [PubMed] [Google Scholar]
- 26.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG Jr: Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 2007;117:1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ: Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930. [DOI] [PubMed] [Google Scholar]
- 28.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB: Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002;415:339–343. [DOI] [PubMed] [Google Scholar]
- 29.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW: Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 2001;413:794–795. [DOI] [PubMed] [Google Scholar]
- 30.Bouret SG: Development of hypothalamic neural networks for controlling appetite; in Langhans W, Geary N (eds): Frontiers in Eating and Weight Regulation. Forum Nutr. Basel, Karger, 2010, vol 63, pp 84–93. [DOI] [PubMed] [Google Scholar]
- 31.Kokoeva MV, Yin H, Flier JS: 2005. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683. [DOI] [PubMed] [Google Scholar]
- 32.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL: Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004;304:110–115. [DOI] [PubMed] [Google Scholar]
- 33.Heymsfield SB, Greenberg AS, et al. : Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999;282:1568–1575. [DOI] [PubMed] [Google Scholar]
- 34.Considine RV, Sinha MK, Heiman ML, et al. : Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;34:292–295. [DOI] [PubMed] [Google Scholar]
- 35.El Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS: Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 2000;105:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarpace PJ, Matheny M, Moore RL, Tumer N: Impaired leptin responsiveness in aged rats. Diabetes 2000;49:431–435. [DOI] [PubMed] [Google Scholar]
- 37.Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, Klingenspor M: Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology 2004;145:1185–1193. [DOI] [PubMed] [Google Scholar]
- 38.Levin BE, Dunn-Meynell AA, Banks WA: Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 2004;286:R143–R150. [DOI] [PubMed] [Google Scholar]
- 39.Banks WA, DiPalma CR, Farrell CL: Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 1999;20:1341–1345. [DOI] [PubMed] [Google Scholar]
- 40.Munzberg H, Flier JS, Bjorbaek C: Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004;145:4880–4889. [DOI] [PubMed] [Google Scholar]
- 41.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB: Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 2008;7:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enriori PJ, Evans AE, Sinnayah P, et al. : Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007;5:181–194. [DOI] [PubMed] [Google Scholar]
- 43.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM: Leptin enters the brain by a saturable system independent of insulin. Peptides 1996;17:305–311. [DOI] [PubMed] [Google Scholar]
- 44.Banks WA: Blood-brain barrier as a regulatory interface; in Langhans W, Geary N (eds): Frontiers in Eating and Weight Regulation. Forum Nutr. Basel, Karger, 2010, vol 63, pp 102–110. [DOI] [PubMed] [Google Scholar]
- 45.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS: Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 2006;4:123–132. [DOI] [PubMed] [Google Scholar]
- 46.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. : PTP1B regulates leptin signal transduction in vivo. Dev Cell 2002;2:489–495. [DOI] [PubMed] [Google Scholar]
- 47.Picardi PK, Calegari VC, Prada PO, Moraes JC, Araujo E, Marcondes MC, Ueno M, Carvalheira JB, Velloso LA, Saad MJ: Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 2008;149:3870–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD: HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab 2009;296:E291–E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wellen KE, Hotamisligil GS: Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003;112:1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogus S, Ke Y, Qiu J, Wang B, Chehab FF: Hyperleptinemia precipitates diet-induced obesity in transgenic mice overexpressing leptin. Endocrinology 2003;144:2865–2869. [DOI] [PubMed] [Google Scholar]
- 51.Sahu A: Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol 2002;14:796–804. [DOI] [PubMed] [Google Scholar]
- 52.Scarpace PJ, Tumer N: Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav 2001;74:721–727. [DOI] [PubMed] [Google Scholar]
- 53.Rahmouni K, Haynes WG, Morgan DA, Mark AL: Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension 2002;39:486–490. [DOI] [PubMed] [Google Scholar]
- 54.Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H: Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 2007;148:5414–5423. [DOI] [PubMed] [Google Scholar]
- 55.Ladyman SR, Grattan DR: Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 2004;145:3704–3711. [DOI] [PubMed] [Google Scholar]
- 56.Diano S, Kalra SP, Sakamoto H, Horvath TL: Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res 1998;812:256–259. [DOI] [PubMed] [Google Scholar]