Abstract
Post-traumatic stress disorder (PTSD) is a protracted and debilitating consequence of traumatic events. Identifying early predictors of PTSD can inform the disorder’s risk stratification and prevention. We used advanced computational models to evaluate the contribution of early neurocognitive performance measures to the accuracy of predicting chronic PTSD from demographics and early clinical features. We consecutively enrolled adult trauma survivors seen in a general hospital emergency department (ED) to a 14-month long prospective panel study. Extreme Gradient Boosting algorithm evaluated the incremental contribution to 14 months PTSD risk of demographic variables, 1-month clinical variables, and concurrent neurocognitive performance. The main outcome variable was PTSD diagnosis, 14 months after ED admission, obtained by trained clinicians using the Clinician-Administered PTSD Scale (CAPS). N = 138 trauma survivors (mean age = 34.25 ± 11.73, range = 18–64; n = 73 [53%] women) were evaluated 1 month after ED admission and followed for 14 months, at which time n = 33 (24%) met PTSD diagnosis. Demographics and clinical variables yielded a discriminatory accuracy of AUC = 0.68 in classifying PTSD diagnostic status. Adding neurocognitive functioning improved the discriminatory accuracy (AUC = 0.88); the largest contribution emanating from poorer cognitive flexibility, processing speed, motor coordination, controlled and sustained attention, emotional bias, and higher response inhibition, and recall memory. Impaired cognitive functioning 1-month after trauma exposure is a significant and independent risk factor for PTSD. Evaluating cognitive performance could improve early screening and prevention.
INTRODUCTION
About 20% of patients who are admitted to the emergency department (ED) after potentially traumatic event develop non-remitting, long-lasting, burdensome post-traumatic stress disorder (PTSD) [1, 2]. The early aftermath of trauma exposure might offer a window of opportunity for preventive interventions [3], which optimally should target survivors at significant risk [3]. Evaluating PTSD risk, however, is compounded by the disorder’s complexity, heterogeneous course [4, 5] and uncertainty regarding underlying mechanisms [6]. Published predictive models to-date encompassed demographics (e.g., gender, education, lifetime mental disorders) [6] and early clinical manifestations (such as PTSD, depression or anxiety symptoms’ severity) [1, 7-10]. Studies have additionally shown that neurocognitive dysfunction, shortly after trauma exposure contributes to PTSD risk [6, 11-13]. Dysfunctions associated with PTSD risk included verbal learning, short-term and declarative memory, working memory, information processing speed [14, 15], attention, executive functioning [16, 17], altered response inhibition, attentional switching, and cognitive flexibility [18-22]. It is unclear, however, if the contribution of neurocognitive factors to PTSD risk is redundant with that of demographics (e.g., education level) and early symptoms severity, or else if they, or some of which, contribute independently, properly increasing PTSD likelihood, and thus potentially informing its pathogenesis.
Examining the contribution of multiple heterogeneous predictors and determining the unique non-redundant contribution of each requires statistical methods that accommodate predictors’ eventual multicollinearity, and account for non-linear associations among them [23]. Advanced computational approaches in statistical learning [23], such as extreme gradient boosting (XGB) algorithm [24], can effectively address these challenges [23], and thereby reveal and validate the independent contribution of single- or sets of candidate features. Evaluating the predictive value of diverse predictors additionally requires labor-intensive and methodologically challenging longitudinal studies of sufficient length (from acute responses to chronic PTSD), sufficient sample size, limited attrition, documented retention-bias, and reliable, clinically informative metrics (e.g., structured clinical interviews rather than self-report questionnaires) [25-27]. To date, these requirements have rarely been met.
The present study addresses these computational- and study-design limitations by following 138 intensely documented trauma survivors for 14 months, carefully accounting for selection and attrition biases, and using previously validated, clinically meaningful metrics. Specifically, it evaluates the extent to which a panel of neurocognitive performance indicators uniquely contributes to the discriminatory accuracy of classifying PTSD status by demographics and clinical predictors. We also evaluated which neurocognitive domains specifically contribute to PTSD risk, and whether such unique contribution remains significant in a subgroup of individuals who meet 1-month PTSD symptoms criteria—a group previously shown at high risk for chronic PTSD and likely beneficial of early interventions [28, 29].
METHODS AND MATERIALS
Data informing this report was collected as part of the NIMH-funded Neurobehavioral Moderators of Post-traumatic Disease Trajectories study (MH103287). The data was collected between January 2015 and March 2020. The study’s design and methodologies have been previously published [27] and those informing this work are summarized below.
Participants
Inclusion per ED records.
Subject participants in this study were 18 to 65 years old adult civilians consecutively admitted to Tel Aviv Sourasky Medical Center (TASMC) ED between January 2015 and November 2019 after one of the following events: motor-vehicle accident, bicycle accident, physical assault, robbery, hostilities, electric shock, fire, drowning, work accident, terror attack or a large-scale disaster.
Participants were included in the study if they expressed distressing PTSD criteria 1 month after the traumatic event and provided informed consent. Participants were not included if they had an ED notation of severe head injury, coma upon ED admission, medical condition that interfered with their ability to provide informed consent or apprehend the study’s procedures, a diagnosis of PTSD prior to ED admission, current substance abuse disorder, current suicidal ideations, lifetime psychotic illness, conditions precluding MRI scanning (pacemaker, metal implants, known claustrophobia, permanent makeup or large tattoos) or medical/psychological conditions that constituted treatment priority. Participants provided an oral assent to the study’s screening telephone interview and written informed consent upon attending a subsequent diagnostic and eligibility ascertainment clinical interview.
Instruments
Diagnostics.
The Clinician Administered PTSD Scale (CAPS) provided PTSD diagnosis and symptom severity estimates. To maintain continuity with decades of DSM-IV-based PTSD research, and following evidence of non-overlapping PTSD-diagnosed groups selected DSM IV or DSM 5 criteria, and a consequent recommendation to use “broader” cross-templates PTSD definitions for empirical research [30-32] we administered the CAPS-IV and CAPS 5 simultaneously, using a combined clinical interview schedule, and scored both DSM-IV and DSM 5 CAPS’ items (CAPS-IV and CAPS-5 [33, 34]). A positive diagnosis of PTSD was inferred when a participant met either DSM-IV or DSM-5 PTSD diagnostic criteria or, in line with previous recommendations Weathers, Ruscio [35] endorsed CAPS-IV symptom severity of ≥40.
The structured clinical interview for DSM IV (SCID-IV) [36] was used to identify current and lifetime Axis I disorders [37, 38], of which current major depression and any current anxiety disorder were considered for this study. All diagnostic interviews were administered by trained and certified clinicians and corroborated, when required, by bi-weekly consensus meeting with the study PI (AS).
Psychometrics.
The PTSD checklist IV for civilians (PCL) [39] evaluated concurrent PTSD symptoms severity during clinical assessment.
The beck depression inventory (BDI-II) [40] evaluated current depressive symptoms’ severity.
The beck anxiety inventory (BAI) [41] evaluated current anxiety symptoms.
The clinical global impression instrument (CGI-P) [42] evaluated patients’ perception of their symptom severity on a 1 to 7 scale (respectively, “normal feeling” to 7 “the worst feeling there is”).
Neurocognitive assessment.
The WebNeuro, a previously validated web-based cognitive functioning testing battery, evaluated participants’ performance in 11 cognitive domains: Motor Coordination, Processing Speed, Sustained Attention, Controlled Attention, Cognitive Flexibility, Response Inhibition, Working Memory, Recall Memory, Executive Function, Emotion Identification, and Emotional Bias [43] (detailed in [27]). WebNeuro tests were administered in the laboratory. Cognitive functioning performance were calculated by WebNeuro’s dedicated software, standardized for age and years of education and expressed as z-scores (see table 1 in ref. [27]).
Procedure
The hospital’s ED electronic records were available to the study personnel within 24 h of ED admission. Within these records, an ED “trauma” notation generated initial screening contacts starting 3 days after the ED.
Telephone screening.
Eligible ED participants were contacted by telephone within 10–14 days of ED admission. Oral assent was obtained prior to starting the interview, else the interviewer thanked the subject and terminated the interview. The interviewers firstly ascertaining the occurrence of a psychologically traumatic event, associated distress, availability for the study and salient exclusion criteria specified above (alias a 5–10 min “short interview”). Individuals with ascertained traumatic event, continued to 20–30 min “long interview.” The long interviews evaluated PTSD symptom severity using the PCL, study availability and exclusion criteria as specified above. Participants were invited for the clinical assessments if they endorsed distressing early PTSD symptoms in the telephone screening interview.
Clinical interviews.
The clinical interviews took place 23.9 ± 8.20 after ED admission by trained clinicians. PTSD symptoms severity and status were identified using the Clinician Administered PTSD Scale. Participants were invited to participate in the study if they had CAPS-based full or subthreshold (3 out of 4 symptom criteria) PTSD in the clinical assessments.
Current and lifetime disorders were identified using the SCID as above. Additionally, the interviewers, re-assessed, in-person, the presence of previously unnoticed study exclusion criteria. Eligible participants were invited to continue the study (i.e., neurocognitive assessment and functional magnetic resonance imaging (fMRI) session (scheduled a week later).
Statistical analysis
Outcome measure.
The study’s primary outcome is the PTSD diagnostic status 14 months after ED admission per diagnostic criteria specified above.
Predictor variables.
Predictor variables include demographics, clinical and neurocognitive measures collected 1-month after trauma exposure, evaluated stepwise as specified below: The first step included demographic information (age, gender, marital status, years of education, and trauma type) and clinical data (self-report measures of PCL, CGI-P, BDI-II, BAI; present or past major depression or any anxiety disorder). In a second step, we added patients’ cognitive performance scores in the eleven neurocognitive domains specified above. The stepwise approach aims to facilitate comparisons between distinct sets of candidate predictors such as sociodemographic-clinical vs. cognitive functioning and to examine the added discriminatory accuracy of neurocognitive functioning in addition to sociodemographic-clinical information alone.
Data pre-processing.
Categorical variables were transformed into numeric values and transformed into one-hot encoded numeric arrays using the OneHotEncoder function scikit-learn [44]. Continuous variables were standardized using the function StandardScaler in scikit-learn and missing values were imputed using k-nearest neighbor estimation (KNNImputer in scikit-learn) with k equals 5.
Model development.
We used XGB algorithm [24] in scikit-learn package 0.23.2 [44] using python 3.7.9. We randomly split the data into a model discovery dataset including 75% of the cases (“training set”) and a separate dataset including the remaining 25% of the cases for model validation (“holdout test set”). The random split was stratified for the outcome variable. We used 10-fold cross-validation on the training set to assess the bias-variance trade-off and to gauge the extent of potential “overfitting” of the model [45].
Model evaluation.
The predictive performance was assessed in terms of discriminatory accuracy and calibration. Discrimination is reported in terms of precision, recall, f1, confusion matrix, and the Area under the ROC Curve (AUC). Discriminatory accuracy was further evaluated using calibration plots and Hosmer-Lemeshow statistic [46]. In the stepwise approach, we first compared the predictions based on demographic and clinical data (alias “clinical model”) with the predictions of a second prediction model (alias: “full model”) adding neurocognitive performance in the eleven domains as candidate predictors in addition to the demographic and clinical data.
Given the importance of predicting from acute PTSD (i.e., the ones likely to raise early clinical concern), we repeated this analysis for a subgroup of participants who met PTSD diagnostic criteria specified above at the first (1-month) assessment session.
To formally test whether the full model is significantly better than the clinical model, we used a bootstrap resampling-based significance test [47, 48] to compare the discriminatory performance of the two models.
Variable importance.
To further describe which features of each information set (demographic, clinical data, and neurocognitive data) are most influential in predicting 14 months’ PTSD, we calculate a variable importance metric using SHapley Additive exPlanation (SHAP) values [49]. This approach ranks all variables in order of their contribution to prediction and thereby illustrates how distinct features can inform individual predictions.
RESULTS
Screening, enrollment, and attrition
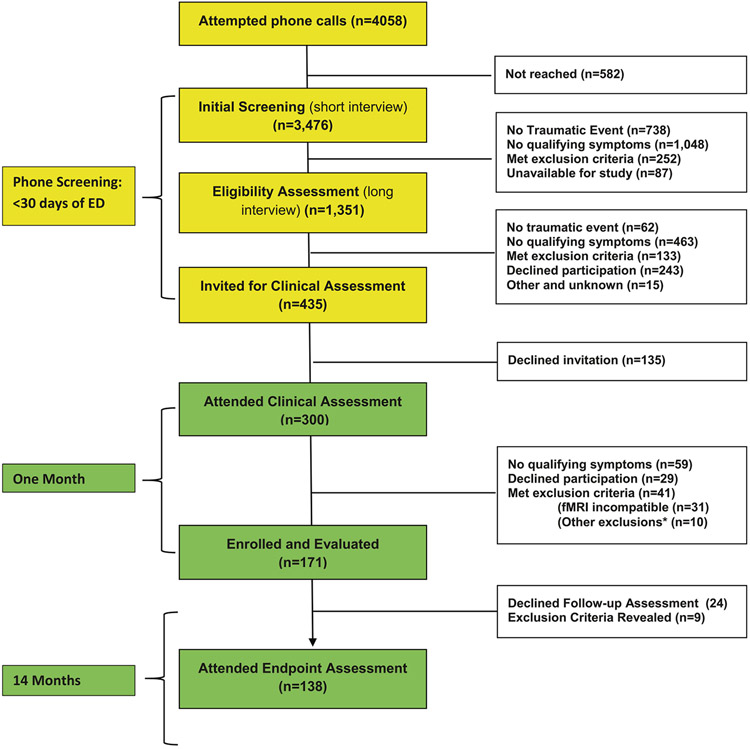
Figure 1 summarized the study’s participants identification, screening, and successive evaluations. Informed by the ED electronic records, we attempted 4058 telephone calls within 14 days of ED admission, reached and obtained informed assent from 3476 participants, confirmed the occurrence of psychologically traumatic event, participants availability for the study and absence of exclusion criteria in n = 1351, and invited 435 participants to attend clinical interviews (see Fig. 1 for details). Of the latter, 300 attended the interviews and 135 declined. Of those 300 individuals, n = 171 attended the first fMRI assessment and were formally enrolled, and n = 138 attended the 14 months’ clinical and fMRI assessment and are included in the current analysis (alias’ study completers’).
Fig. 1. Consort diagram.
Flow chart depicting the inclusion and exclusion of patients.
*Other exclusions (n=10) include: Serious medical/surgical condition requiring clinical attention (n=5). Chronic PTSD before current event (n=2), Substance use disorder - current (n=l). Head injury (n=l) and No traumatic event *n=l)
Evaluating sampling bias
The study sample’s demographic and clinical characteristics are presented in Table 1. By design, and towards obtaining large-enough number of PTSD patients at 14 months, participants with more severe PTSD symptoms were elected for inclusion in early screening and clinical assessment. To evaluate, however, eventual sampling bias as to age, gender, trauma type, we performed a series of comparisons between individuals included and those not included at each step on variable known to contribute to PTSD risk. Among participants reached for telephone assessments (n = 3476), those included in the long telephone interview (n = 1351) did not differ from those screened and not included (n = 2707) in age (35.51 ± 11.91 vs. 35.59 ± 11.88 years), gender distribution (55.3% females vs. 56.2%), and Trauma Type (92.8% MVA’s vs. 92.6%; all p-values > 0.05).
Table 1.
Participants’ demographic and clinical characteristics.
| Demographics (at study onset) | ||||
|---|---|---|---|---|
| Measure | M | SD | ||
| Age at onset | 34.25 | 11.73 | ||
| Gender (F:M) | 73 (52.9%):65 (47.1%) | |||
| Marital status (S:M:D) | 89 (69.0%):27 (20.9%):13 (10.1%) | |||
| Education (years) | 14.35 | 2.67 | ||
| Trauma type (%) | MVA: n = 122 (88.4%) Assault n = 9 (6.5%) 7 Other events n = 7 (5.1%) |
|||
| Clinical variables | 1-month post-trauma | 14-month post-trauma |
||
| Measure | M | SD | M | SD |
| PCL total score | 45.55 | 14.10 | 30.23 | 13.19 |
| BDI-II total score | 16.16 | 8.63 | 9.73 | 8.61 |
| BAI total score | 19.69 | 11.84 | 10.55 | 11.17 |
| CGI-P total score | 3.44 | 1.43 | 2.47 | 1.61 |
| CAPS-IV total score | 50.85 | 22.61 | 21.89 | 20.8 |
| CAPS-5 total score | 24.35 | 11.70 | 10.78 | 10.38 |
| Met PTSD diagnosis | 103 (74.6%) | 33 (23.9%) | ||
N = 9 participants did not provide information about their marital status. MVA motor vehicle accidents, PCL PTSD checklist, BDI beck depression inventory, BAI beck anxiety inventory, CGI clinical global impression, CAPS Clinician Administered PTSD Scale, IV for DSM IV and 5 for DSM 5.
Similarly, individuals invited for a first clinical interview (n = 435) did not differ from those who were not invited (n = 916) in age (34.11 ± 11.16 vs. 34.82 ± 12.01 years), gender distribution (50.9% vs. 51.5% females) and trauma type (89.3% vs. 90.1% MVA’s; all p-values > 0.05).
Finally, among 171 participants enrolled at 1 month, study completers (n = 138; i.e., those who were seen again 14 months after ED admission), did not differ from those lost to follow-up (n = 33) in age (33.97 ± 10.48 years vs. 34.25 ± 11.73), trauma type (87.9% MVA’s vs. 88.4% MVA’s), gender distribution (53%; (73 of 138) vs. 39% (13 of 33) females), education (14.14 ± 2.57 vs. 14.35 ± 2.67 years of schooling;all p-values > 0.05), and 1-month PTSD symptom severity (CAPS-IV total score = 56.52 ± 20.48 and 50.85 ± 22.62 (t (168) = 1.31, p = 0.191).
Comparing clinical-demographic model with the full model
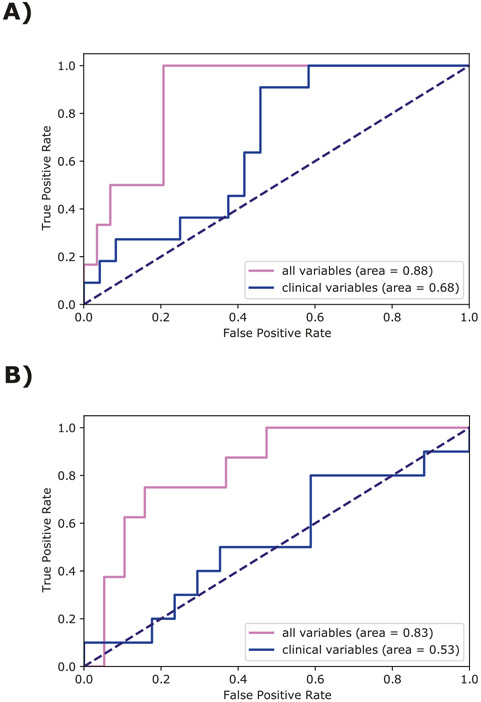
In the hold-out set (n = 35) of n = 138 completers, the XGB algorithm applied to demographic and clinical variables at 1-month achieved a discriminatory accuracy for classifying PTSD diagnosis 14 months after ED admission with an AUC of 0.68 (weighted average precision = 0.62, weighted average recall = 0.63, weighted average f1-score = 0.62) (Fig. 2A). Using the full model (demographics, clinical and neurocognitive performance) the discriminatory accuracy improved to an AUC of 0.88 (weighted average precision = 0.83, weighted average recall = 0.77, weighted average f1-score = 0.79). Based on 10,000 bootstrapped resamples, the bootstrap resampling significance test was D = 1.84, p = 0.066. The model predictions were assessed for calibration within 10 bins of probability deciles to gauge the agreement between observed PTSD diagnosis at 14 months follow-up and the predictions. The non-significant Hosmer–Lemeshow test (X2 = 10.76, df = 8, p = 0.22, g = 10) shows that there is no evidence of poor fit.
Fig. 2. Discriminatory accuracy for classifying PTSD diagnosis.
A ROC curve on the holdout test set for predicting PTSD diagnosis at 14 months by using all variables and only demographic and clinical variables (alias “clinical variables” in Fig. 2). B ROC curve for predicting PTSD diagnosis at 14 months by using all variables vs. only demographic and clinical variables in the subset of participants who met PTSD diagnostic criteria at 1-month post-trauma in the holdout test set.
Examining a subset of participants who met 1-month PTSD symptom criteria (N = 103), the predictive accuracy of demographics and clinical variable at 1-month for classifying PTSD diagnosis at 14 months yielded an AUC of 0.53 (weighted average precision = 0.55, weighted average recall = 0.52, weighted average f1-score = 0.53) and, upon including neurocognitive functioning had an AUC of 0.83 (weighted average precision = 0.78, weighted average recall = 0.78, weighted average f1-score = 0.78). The difference between these two models (demographic and clinical data with versus without neurocognitive data; see Fig. 2B) was significant (D = 2.11, p = 0.035, based on 10,000 bootstrapped resamples). A non-significant Hosmer-Lemeshow test (X2 = 3.29 df = 8, p = 0.91, g = 10) has shown no evidence of poor fit.
The 10-fold cross-validation yielded an AUC of 0.78 for the full model among all participants, and an AUC of 0.72 for the full model among the subset of participants who met the diagnostic criteria for PTSD 1 month after ED admission.
Variable importance
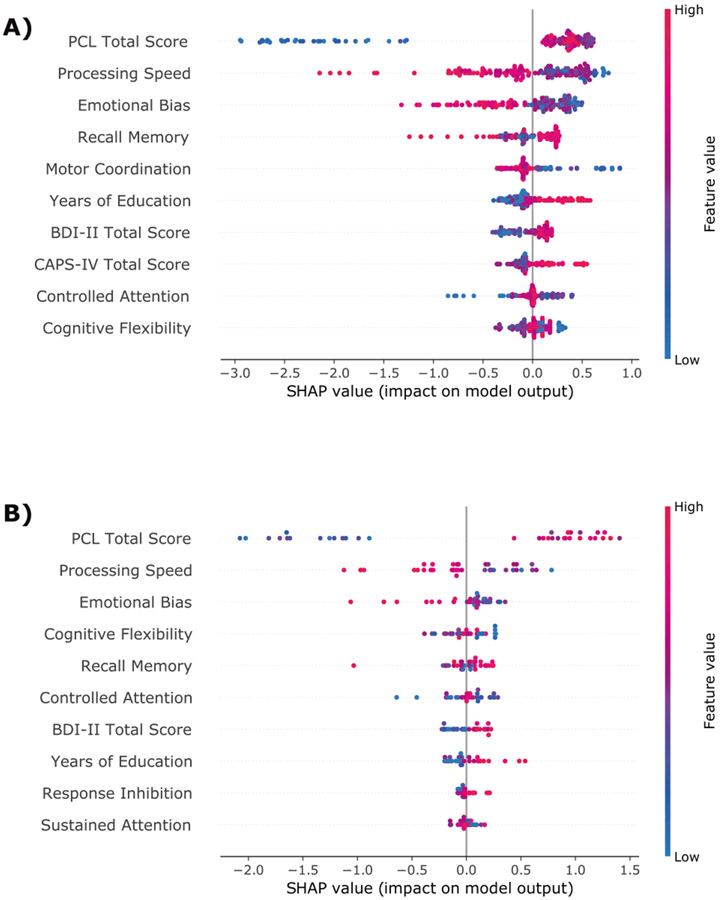
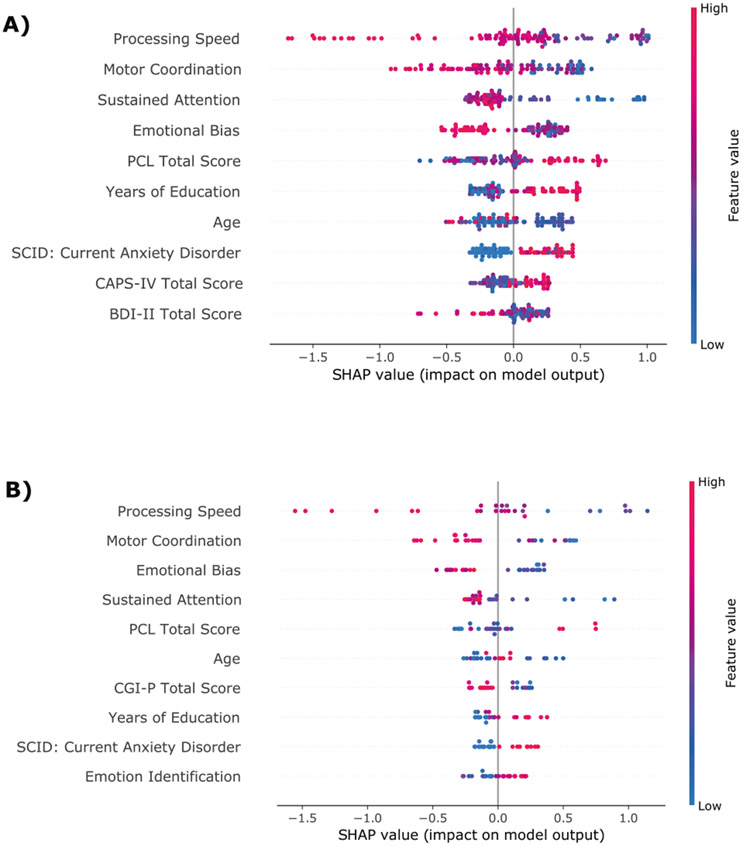
The variable importance is presented in Figs. 3 and 4. In both study completers and study completers with PTSD, the same neurocognitive domains were consistently the most relevant for identifying patients with high risk for chronic PTSD. Poorer cognitive flexibility, processing speed, motor coordination, controlled and sustained attention, emotional bias, higher response inhibition, and higher recall memory at 1-month post-trauma were associated with a higher likelihood for PTSD 14-month post-trauma.
Fig. 3. Variable importance.
SHAP summary dot plot of the XGB model based on sociodemographic, clinical, and neurocognitive predictor variables for the training set (A) and hold-out test set (B). The higher the SHAP value of a feature, the higher the log odds of the PTSD diagnosis. On the y-axis, the features are sorted by their general feature importance. For each participant and variable, the dots represent how the given value of a variable impacts the classification of the patient to one of the two outcome classes. Dots that are far on the left side shift the classification of a given patient to the class “no PTSD”, whereas dots on the far-right side of the x-axis shift the classification of participants to the class “PTSD”. The color represents the range of the feature values from low (blue) to high (red).
Fig. 4. Variable importance for the subset of participants who met 1-month PTSD symptom criteria.
SHAP summary dot plot of the XGB model based on sociodemographic, clinical, and neurocognitive predictor variables for the training set (A) and hold-out set (B) for the subset of participants who met PTSD diagnostic criteria at 1-month post-trauma. The higher the SHAP value of a feature, the higher the log odds of the PTSD diagnosis. On the y-axis, the features are sorted by their general feature importance. For each participant and variable, the dots represent how the given value of a variable impacts the classification of the patient to one of the two outcome classes. Dots that are far on the left side shift the classification of a given patient to the class “no PTSD”, whereas dots on the far-right side of the x-axis shift the classification of participants to the class “PTSD”. The color represents the range of the feature values from low (blue) to high (red).
DISCUSSION
Our results show that 1 month after trauma exposure, measures of neurocognitive functioning improve the performance of demographic information and clinical symptoms in predicting 14 months PTSD status. Impaired neurocognitive functioning in eight specific domains contributed to the difference. The added contribution of neurocognitive functioning impairment was particularly striking among highly symptomatic trauma-survivors.
These results are in line with previous research findings of an association between early lower neurocognitive performance and higher risk for PTSD development [13]. For example, several studies suggested that deficits in processing speed, controlled- and sustained attention (as found here) are closely linked to PTSD progression [16, 50, 51]. Furthermore, lower cognitive flexibility shortly after trauma exposure predicted more severe PTSD symptoms a year later, and intervention-induced improvement in this domain were associated with greater subsequent clinical improvement [21]. While this work cannot infer on the mechanistic meaning of these neurocognitive predictors, we encourage future research examining their potential in preventive interventions for PTSD.
From a risk prediction perspective, our findings highlight the independent prognostic relevance of early neurocognitive functioning, a finding that emphasizes the relevance of assessing individuals’ cognitive functioning in addition to clinical and demographic variables and as a way to improve the early screening and identification of ED trauma survivors at risk. Importantly, a contribution of neurocognitive evaluation to PTSD prediction particularly concerned survivors with salient 1-month PTSD symptoms, who are at higher current distress and long-term PTSD risk.
Additionally, the association of early neurocognitive deficits with 14 months PTSD implies a mechanistic contribution to PTSD emergence and persistence. Such contribution is in line with previous findings of early symptoms persistence among survivors with mild traumatic brain injury (MTBI) and with the replicated link between education levels and PTSD risk [52, 53]. Moreover, such deficits likely implicate brain circuits and functions that extend beyond those mediating fear learning and extinction (i.e., the amygdala and vmPFC) in PTSD development;in line with published models of PTSD pathogenesis that include the hippocampus, dorsolateral and prefrontal cortical regions others [6, 54, 55].
While this work cannot separate pre-trauma neurocognitive traits from trauma-induced, or amplified, neurocognitive difficulties, it nonetheless shows a significant contribution, worthy of early detection and informing potential therapy or palliation. Either way, our finding suggests that screening for trauma type, gender, and early clinical symptoms does not exhaust the potential of early information to predict PTSD, and thus the addition of neurocognitive functioning yields clinically important information for the prognosis of the symptom development of trauma-survivors.
While prior studies have discussed neurocognitive deficits as predictors of poorer outcome of psychotherapy [56-58], only a few interventions exist that directly target cognitive functioning in PTSD patients [13, 59]. Notwithstanding, studies have shown that it is, in principle, possible to modulate neurocognitive performance [21, 60] and that neurocognitive deficits are ameliorated after successful trauma-focused therapy [61]. Our results raise the possibility that improving early neurocognitive functioning could mitigate PTSD risk. They thereby identify hypothetical mechanistic targets for preventive interventions for PTSD.
A major barrier to conducting early neurocognitive assessment is its eventual cost and the dearth of qualified personnel. As this work has shown, web-based neurocognitive assessment batteries efficiently captured an incremental PTSD risk. This is in line with new developments of web-based digital biomarkers and digital phenotyping tools [62] that require limited resources and personnel qualification and time. These new computational advancements may facilitate the implementation of such neurocognitive assessments shortly after trauma exposure. Such methods should be further validated, towards offering a promising opportunity for larger scale implementation.
Strength and limitations
This work is limited by addressing a sample of ED-admitted symptomatic civilians enrolled in a single medical center after single-impact short traumatic events. Additionally, participants with head trauma and severe lifetime and mental disorders were not included. Finally, the use of web-based neurocognitive instrument not-especially built to measure traumatic stress consequences is another limitation.
Within these limitations, however, sampling and retention biases have been reasonably good and thus participants included in this work fairly well represent our ED trauma admissions’ age, gender and trauma type, and those lost to follow up (n = 33) do not differ from those who remained in the study (n = 138). Participants were screened for early PTSD symptoms to enrich the sample and increase the likelihood of endpoint PTSD. Further, whilst the diagnosis of PTSD varies upon DSM taxonomic classification and is frequently criticized [63, 64], we attempted to optimize participants’ characterization by combining two “gold standard” structured clinical instruments capturing both DSM IV and 5 PTSD. We additionally followed subjects for 14 months—a time period indicative of a chronic course [65, 66]. We therefore believe that our results can be reliably generalized to ED trauma among adults; a frequent modality of trauma exposure among civilians, and that they can safely guide further explorations.
CONCLUSION
This work shows an independent contribution of early neurocognitive functioning to chronic PTSD risk. Further studies are needed to better understand the origin of the observed neurocognitive deficits, i.e., the extent to which they represent pre-trauma, “vulnerability” attribute, a reaction to traumatic stress or a mixture of both.
ACKNOWLEDGEMENTS
We would like to thank the research team at Tel-Aviv Sourasky Medical Center—including Nili Green, Mor Halevi, Sheli Luvton, Yael Shavit, Olga Nevenchannaya, Iris Rashap, Efrat Routledge, and Ophir Leshets for their significant contributions to participants, screening, enrollment, assessments, and follow-up and to Naomi Fine and Michal Achituv for setting up the clinical aspect of the research. Last but not least, we extend our gratitude to all the participants of this study, who completed all the assessments at three different time-points after experiencing a traumatic event. The work was supported by award number R01-MH-103287 from the National Institute of Mental Health (NIMH) given to AYS (PI), IL, and TH (co-Investigators, subcontractors), and had undergone critical review by the NIMH Adult Psychopathology and Disorders of Aging study section.
Footnotes
COMPETING INTERESTS
TH is the chief medical officer of GrayMatters Health Co Haifa Israel. All other authors have no potential conflicts of interest to declare.
REFERENCES
- 1.Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, et al. Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLoS ONE. 2013;8:e70084. 10.1371/journal.pone.0070084. Erratum in: PLoS ONE. 2013;8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe SR, Ratanatharathorn A, Lai BS, van der Mei W, Barbano AC, Bryant RA, et al. Posttraumatic stress disorder symptom trajectories within the first year following emergency department admissions: pooled results from the International Consortium to predict PTSD. Psychol Med. 2021;51:1129–39. 10.1017/S0033291719004008. Epub 2020 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultebraucks K, Chang BP. The opportunities and challenges of machine learning in the acute care setting for precision prevention of posttraumatic stress sequelae. Exp Neurol. 2021;336:113526. 10.1016/j.expneurol.2020.113526. Epub 2020 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heim C, Schultebraucks K, Marmar CR, Nemeroff CB. Neurobiological Pathways Involved in Fear, Stress, and PTSD. In: Nemeroff CB, Marmar C (editors) Post-traumatic stress disorder. Oxford University Press; 2018. pp. 331–351. [Google Scholar]
- 5.Galatzer-Levy IR, Huang SH, Bonanno GA. Trajectories of resilience and dysfunction following potential trauma: a review and statistical evaluation. Clin Psychol Rev. 2018;63:41–55. [DOI] [PubMed] [Google Scholar]
- 6.Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N Engl J Med. 2017;376:2459–69. [DOI] [PubMed] [Google Scholar]
- 7.Schultebraucks K, Shalev A, Michopoulos V, Grudzen C, Shin S, Stevens J, et al. A validated predictive algorithm of posttraumatic stress course following emergency department admission after a traumatic stressor. Nat Med. 2020;26:1084–8. [DOI] [PubMed] [Google Scholar]
- 8.Koren D, Arnon I, Klein E. Long term course of chronic posttraumatic stress disorder in traffic accident victims: a three-year prospective follow-up study. Behav Res Ther. 2001;39:1449–58. [DOI] [PubMed] [Google Scholar]
- 9.Perkonigg A, Pfister H, Stein MB, Höfler M, Lieb R, Maercker A, et al. Longitudinal course of posttraumatic stress disorder and posttraumatic stress disorder symptoms in a community sample of adolescents and young adults. Am J Psychiatry. 2005;162:1320–7. [DOI] [PubMed] [Google Scholar]
- 10.Stein DJ, Karam EG, Shahly V, Hill ED, King A, Petukhova M, et al. Post-traumatic stress disorder associated with life-threatening motor vehicle collisions in the WHO World Mental Health Surveys. BMC Psychiatry. 2016;16:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, et al. Predeployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Mol Psychiatry. 2020;26:5011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuelson KW, Newman J, Abu Amara D, Qian M, Li M, Schultebraucks K, et al. Predeployment neurocognitive functioning predicts postdeployment posttraumatic stress in Army personnel. Neuropsychology. 2020;34:276. [DOI] [PubMed] [Google Scholar]
- 13.Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bull. 2015;141:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsen GE, Asbjørnsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111:74–82. [DOI] [PubMed] [Google Scholar]
- 15.Samuelson KW. Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues Clin Neurosci. 2011;13:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141:11–21. [DOI] [PubMed] [Google Scholar]
- 18.Casada JH, Roache JD. Behavioral inhibition and activation in posttraumatic stress disorder. J Nerv Ment Dis. 2005;193:102–9. [DOI] [PubMed] [Google Scholar]
- 19.Hart RP, Bagrodia R, Rahman N, Bryant RA, Titcombe-Parekh R, Marmar CR, et al. Neuropsychological predictors of trauma centrality in OIF/OEF veterans. Front Psychol. 2017;8:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenen KC, Driver KL, Oscar-Berman M, Wolfe J, Folsom S, Huang MT, et al. Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain Cogn. 2001;45:64–78. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Zion Z, Fine NB, Keynan NJ, Admon R, Green N, Halevi M, et al. Cognitive flexibility predicts PTSD symptoms: observational and interventional studies. Front Psychiatry. 2018;9:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Zion Z, Zeevi Y, Keynan NJ, Admon R, Kozlovski T, Sharon H, et al. Multi-domain potential biomarkers for post-traumatic stress disorder (PTSD) severity in recent trauma survivors. Transl Psychiatry. 2020;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. New York: Springer Science & Business Media, 2009. [Google Scholar]
- 24.Chen T, Guestrin C. XGBoost: a scalable tree boosting system. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, California, USA: ACM; 2016, p. 785–94. [Google Scholar]
- 25.Shalev AY, Ankri YL, Peleg T, Israeli-Shalev Y, Freedman S. Barriers to receiving early care for PTSD: results from the Jerusalem trauma outreach and prevention study. Psychiatr Serv. 2011;62:765–73. [DOI] [PubMed] [Google Scholar]
- 26.Qi W, Ratanatharathorn A, Gevonden M, Bryant R, Delahanty D, Matsuoka Y, et al. Application of data pooling to longitudinal studies of early post-traumatic stress disorder (PTSD): the International Consortium to Predict PTSD (ICPP) project. Eur J Psychotraumatol. 2018;9:1476442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Zion Z, Fine NB, Keynan NJ, Admon R, Halpern P, Liberzon I, et al. Neurobehavioral moderators of post-traumatic stress disorder (PTSD) trajectories: study protocol of a prospective MRI study of recent trauma survivors. Eur J Psychotraumatol. 2019;10:1683941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem Trauma Outreach And Prevention study. Arch Gen Psychiatry. 2012;69:166–76. [DOI] [PubMed] [Google Scholar]
- 29.Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Systematic review and meta-analysis of multiple-session early interventions following traumatic events. Am J Psychiatry. 2009;166:293–301. [DOI] [PubMed] [Google Scholar]
- 30.Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1:269–77. [DOI] [PubMed] [Google Scholar]
- 31.Hoge CW, Yehuda R, Castro CA, McFarlane AC, Vermetten E, Jetly R, et al. Unintended consequences of changing the definition of posttraumatic stress disorder in DSM-5: critique and call for action. JAMA Psychiatry. 2016;73:750–2. [DOI] [PubMed] [Google Scholar]
- 32.Stein DJ, McLaughlin KA, Koenen KC, Atwoli L, Friedman MJ, Hill ED, et al. DSM-5 and ICD-11 definitions of posttraumatic stress disorder: investigating “narrow” and “broad” approaches. Depress Anxiety. 2014;31:494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. [DOI] [PubMed] [Google Scholar]
- 34.Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the clinician-administered posttraumatic stress disorder scale. Psychol Assess. 1999;11:124–33. [Google Scholar]
- 36.First M, Spitzer R, Gibbon M, Williams J. Biometrics research. New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. Clin Trials Version (SCID-CT). 1995;9:92–104. [Google Scholar]
- 37.Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE Jr, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873–5. [DOI] [PubMed] [Google Scholar]
- 38.Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. Am J Psychiatry. 1997;154:1593–8. [DOI] [PubMed] [Google Scholar]
- 39.Weathers FW, Huska JA, Keane TM. PCL-C for DSM-IV. Boston: National Center for PTSD-Behavioral Science Division; 1991. [Google Scholar]
- 40.Beck A, Steer R, Brown G. Beck depression inventory-II. San Antonio. 1996;78:490–8. [Google Scholar]
- 41.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consulting Clin Psychol. 1988;56:893. [DOI] [PubMed] [Google Scholar]
- 42.Guy W Clinical Global Impressions, ECDEU Assessment Manual for Psychopharmacology, revised (DHEW Publ. No. ADM 76-338). National Institute of Mental Health, Rockville; 1976. pp. 218–222. [Google Scholar]
- 43.Silverstein SM, Berten S, Olson P, Paul R, Williams LM, Cooper N, et al. Development and validation of a World-Wide-Web-based neurocognitive assessment battery: WebNeuro. Behav Res Methods. 2007;39:940–9. [DOI] [PubMed] [Google Scholar]
- 44.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30. [Google Scholar]
- 45.Cawley GC, Talbot NL. On over-fitting in model selection and subsequent selection bias in performance evaluation. J Mach Learn Res. 2010;11:2079–107. [Google Scholar]
- 46.Hosmer DW Jr., Lemeshow S, Sturdivant RX. Applied logistic regression, vol. 398. New York: John Wiley & Sons; 2013. [Google Scholar]
- 47.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–64. [DOI] [PubMed] [Google Scholar]
- 48.Pepe MS, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009;9:1–16. [PMC free article] [PubMed] [Google Scholar]
- 49.Lundberg SM, Lee SI. A unified approach to interpreting model predictions. In Proceedings of the 31st international conference on neural information processing systems. 2017; pp. 4768–4777. [Google Scholar]
- 50.Qureshi SU, Long ME, Bradshaw MR, Pyne JM, Magruder KM, Kimbrell T, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23:16–28. [DOI] [PubMed] [Google Scholar]
- 51.Dutra SJ, Marx BP, McGlinchey R, DeGutis J, Esterman M. Reward ameliorates posttraumatic stress disorder-related impairment in sustained attention. Chronic Stress. 2018;2:2470547018812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roitman P, Gilad M, Ankri YL, Shalev AY. Head injury and loss of consciousness raise the likelihood of developing and maintaining PTSD symptoms. J Trauma Stress. 2013;26:727–34. [DOI] [PubMed] [Google Scholar]
- 53.Bryant RA, Creamer M, O’Donnell M, Silove D, Clark CR, McFarlane AC. Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J Int Neuropsychological Soc. 2009;15:862–7. [DOI] [PubMed] [Google Scholar]
- 54.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Zion Z, Artzi M, Niry D, Keynan NJ, Zeevi Y, Admon R, et al. Neuroanatomical risk factors for posttraumatic stress disorder in recent trauma survivors. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2020;5:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falconer E, Allen A, Felmingham KL, Williams LM, Bryant RA. Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. J Clin Psychiatry. 2013;74:895–901. [DOI] [PubMed] [Google Scholar]
- 57.Nijdam MJ, de Vries G-J, Gersons BP, Olff M. Response to psychotherapy for posttraumatic stress disorder: The role of pretreatment verbal memory performance. J Clin Psychiatry. 2015;76:1023–8. [DOI] [PubMed] [Google Scholar]
- 58.Wild J, Gur RC. Verbal memory and treatment response in post-traumatic stress disorder. Br J Psychiatry. 2008;193:254–5. [DOI] [PubMed] [Google Scholar]
- 59.Fine NB, Achituv M, Etkin A, Merin O, Shalev AY. Evaluating web-based cognitive-affective remediation in recent trauma survivors: study rationale and protocol. Eur J Psychotraumatol. 2018;9:1442602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fonzo GA, Fine NB, Wright RN, Achituv M, Zaiko YV, Merin O, et al. Internet-delivered computerized cognitive & affective remediation training for the treatment of acute and chronic posttraumatic stress disorder: two randomized clinical trials. J Psychiatr Res. 2019;115:82–9. [DOI] [PubMed] [Google Scholar]
- 61.Nijdam MJ, Martens IJ, Reitsma JB, Gersons BP, Olff M. Neurocognitive functioning over the course of trauma-focused psychotherapy for PTSD: Changes in verbal memory and executive functioning. Br J Clin Psychol. 2018;57:436–52. [DOI] [PubMed] [Google Scholar]
- 62.Schultebraucks K, Yadav V, Galatzer-Levy IR. Utilization of machine learning-based computer vision and voice analysis to derive digital biomarkers of cognitive functioning in trauma survivors. Digital Biomark. 2021;5:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoellner LA, Bedard-Gilligan MA, Jun JJ, Marks LH, Garcia NM. The evolving construct of posttraumatic stress disorder (PTSD): DSM-5 criteria changes and legal implications. Psychological Inj Law. 2013;6:277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychological Sci. 2013;8:651–62. [DOI] [PubMed] [Google Scholar]
- 65.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. [DOI] [PubMed] [Google Scholar]
- 66.Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry. 2005;162:1188–91. [DOI] [PubMed] [Google Scholar]