Abstract
When left untreated, Hepatitis B virus (HBV) and Hepatitis C virus (HCV) infections may cause severe illnesses. Since these infections remain asymptomatic for many years, routine screening of populations at risk is critical for therapy initiation. Current standard of care mandates a screening antibody test for HCV, followed with confirmatory laboratory-based molecular test and treatment. Multiple visits to the clinic are inconvenient and many patients fail to follow up. To address this challenge, we have developed sensitive, two-stage, isothermal molecular (Penn-RAMP) point of care tests to enable test and treat strategy. Penn-RAMP’s first stage is comprised of recombinase polymerase amplification (RPA) while its second stage is loop mediated isothermal amplification (LAMP). Penn-RAMP is more sensitive than LAMP or RPA alone. We designed a custom pre-LAMP buffer to maximize the volume of RPA products that can be added to the LAMP reaction mix without inhibition and forward and backward primers. Penn-RAMP was implemented in a single pot comprised of two compartments separated with a thermally removable barrier. RAMP’s first stage is carried out above the barrier at the RPA incubation temperature. When the pot is heated to the LAMP incubation temperature, the barrier melts away, and the RPA reaction volume mixes with the pre-LAMP buffer, facilitating second-stage amplification. This entire process can be carried out with minimal instrumentation. Our HBV and HCV tests detect, respectively, as few as 10 and 25 virions within 30 minutes by threshold time and the viral load can be estimated based on signal.
Keywords: Hepatitis B virus, Hepatitis C virus, Isothermal nucleic acid amplification, Point-of-care, Molecular diagnostics
Graphical Abstract
Blood-borne viruses infect millions of people worldwide and, in the absence of early diagnostics and treatment, cause chronic diseases, morbidity, and mortality, and endanger the blood supply. Hepatitis B Virus (HBV), Hepatitis C virus (HCV), and Human immunodeficiency virus (HIV) are among the greatest public health threats 1, 2. According to the World Health Organization, over 250 million people are currently infected with HBV, more than 70 million with HCV, and many are co-infected with both.
HCV and HBV can be acquired by exposure to blood through injection drug use, sexual contact, unsafe health care, and transfusion of unscreened blood and blood products 3. HCV and HBV can cause both acute and chronic hepatitis, ranging in severity from a mild illness lasting a few weeks to a serious, lifelong chronic illness. A significant fraction of those who are chronically infected develop cirrhosis or liver cancer 4. Often new infections are asymptomatic, leaving patients unaware of their condition. While effective treatments are available 5, many patients remain untreated.
The current standard of care for HCV requires two tests: an antibody test followed up with a confirmatory PCR test. This procedure requires multiple visits to the clinic, resulting in many patients lost to follow up. A point of care test capable of distinguishing between active state of infection and past infection would enable a direct linkage to care, keep patients engaged, increase the number of patients tested, and provide means to monitor therapy’s efficacy. Such a test is likely to be impactful, particularly in resource poor settings and in populations that do not have equitable access to health care 2, 6, 7.
Molecular tests rely on nucleic acid amplification and are typically carried out with polymerase chain reaction (PCR) 8 that requires stringent sample preparation, complex instruments,, and skilled personnel 9. To overcome these challenges, various isothermal amplification methods have been developed in recent years as alternatives to PCR. These include recombinase polymerase amplification (RPA) 7, 10, helicase dependent amplification (HDA) 11, rolling-circle amplification (RCA) 12, and loop-mediated isothermal amplification (LAMP) 5, 13, 14. Isothermal amplification methods provide many advantages over PCR for point of care applications and for resource poor settings. Since isothermal amplification does not require temperature cycling, it can be processed with simpler equipment or even equipment-free. Typically, isothermal amplification schemes are more tolerant of contaminants than PCR, simplifying sample preparation 15. Furthermore, isothermal schemes produce much greater abundance of amplicons than PCR, simplifying amplicon detection. For example, LAMP products can be detected with colorimetric dyes without a need for a fluorescent reader. Isothermal amplification methods such as LAMP and RPA, originally developed for use in resource poor countries have gained a broad acceptability during the recent COVID-19 pandemic.
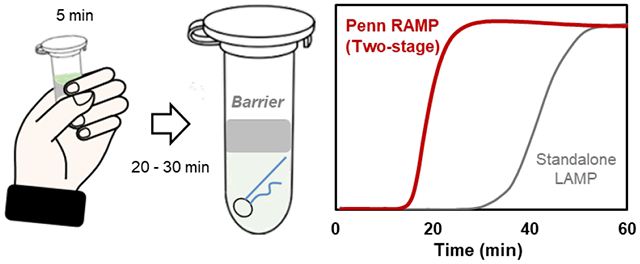
To enable high level multiplexing, a two-stage isothermal amplification method dubbed Penn-RAMP 16 was developed. The first stage of RAMP is RPA, and the second stage is LAMP. RAMP has shown to have a better sensitivity than standalone LAMP and RPA 16. Here, we adapt RAMP for single plex detection of the hepatitis viruses B and C in a closed pot. To enable spontaneous transfer of first stage RPA products into the second stage LAMP, we devised a novel tube that is separated with a thermally removable barrier into two compartments. The RPA is carried out in the upper compartment above the barrier and the LAMP in the lower compartment beneath the barrier. The barrier disintegrates when the tube is heated from the RPA incubation temperature to the LAMP incubation temperature, allowing the RPA products to undergo the LAMP reaction. The RPA and LAMP buffers are, however, incompatible. To maximize the volume of RPA products that can be transferred to the LAMP process, we formulated a new pre-LAMP buffer that is deficient in certain components and is made whole with the addition of the RPA products. Our pre-LAMP buffer allows us to operate with a 1:4 RPA:LAMP volumes, without adversely affecting the LAMP process. Furthermore, to optimize performance, we have designed custom LAMP B3/F3 primers. Overall, our RAMP assay achieves ten-fold better sensitivity than standalone LAMP. The first step RPA can be incubated with body heat by simply holding the tube in one’s fist for a few minutes. The second stage can be incubated with a thermal block, heat bath, or instrumentation-free with chemical heating (exothermic reaction of the type used in meals ready to eat) and phase change material for temperature control 17.
Experimental Section
Materials.
Docosane, KCl, MgSO4, (NH4)2SO4, betaine, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tris-HCl was purchased from Boston Bioproducts (Ashland, MA, USA). dNTPs were purchased from Denville Scientific (South Plainfield, NJ, USA). Warmstart Bst 2.0 polymerase was purchased from New England Biolab (Ipswich, MA, USA). HBV and HCV plasma samples from real patients were purchased from Seracare life science (Milford, MA, USA). TwistAmp RPA Basic kit was purchased from TwistDx (Cambridge, UK).
LAMP primer sets.
LAMP Primer sets sequences (Table S1) for HBV and HCV were designed with Primer Explorer V5 software (Eiken) or adapted from literature 18 as stated; synthesized (Integrated DNA Technologies, IDT, Coral-ville, IA, USA); dissolved in molecular grade water (Invitrogen, Carlsbad, CA, USA) to 100 μM concentrations; and added to LAMP reaction mixes to final concentrations of 1.6 μM FIP and BIP, 0.8 μM of LF and LB, and 0.2 μM F3 and B3. To improve the efficiency of the first stage amplification (RPA), we designed 30 – 35 nt long F3/B3 primers that are longer than common for LAMP primers (~20nt).
Reaction buffers.
The RPA reaction mix (Table 1a) was prepared following manufacturer’s protocol (TwistDx RPA basic kit). The enzyme pellet from the RPA kit was dissolved in RPA rehydration buffer and supplemented with 500 nM of F3 and B3 primers, 14 mM of Mg(OAc)2, and template. A 1 μL of target was added to 10 μL of RPA reaction mix, and then aliquoted to four RAMP reaction volumes. The LAMP reaction mix for standalone LAMP was prepared following manufacturer’s recommendations (Table 1b). The LAMP reaction was carried out in a 10 μL volume comprised of 1 μL sample, 4 U of Bst polymerase 2.0 (NEB), 2.5 U of AMV reverse transcriptase (Promega), 2 μM of SYTO-9 (Invitrogen), 1 μL of primer mixture (resulting in final concentrations of 1.6 μM of FIP and BIP, 0.8 μM of LF and LB, and 0.2 μM of F3 and B3), and 5 μL of LAMP buffer (Table 1b). Two-stage, pre-LAMP buffer (Table 1c) was formulated to form standard LAMP buffer after mixing with RPA buffer in the volume ratio 1:4.
Table 1.
Chemical compositions of (a) RPA buffer (TwistDx) 19; (b) standalone LAMP buffer 13, 20; and (c) custom made pre-LAMP buffer for two-stage amplification prior and after blending with RPA products at 1:4 volume ratio.
| a | RPA Buffer (Partial) | ||
|---|---|---|---|
| Chemical | Concentration | ||
| [K+] | KCl | - | |
| CH3CO2K | 100 mM | ||
| [Mg2+] | MgSO4 | - | |
| Mg(CH3CO2)2 | 14 mM | ||
| Tris-HCl (pH 7.9) | 50 mM | ||
| dNTPs | 0.2 mM | ||
| DTT | 2 mM | ||
| ATP | 3 mM | ||
| (b) | LAMP Buffer | ||
|---|---|---|---|
| Chemical | Concentration | ||
| [K+] | KCl | 10 mM | |
| CH3CO2K | - | ||
| [Mg2+] | MgSO4 | 8 mM | |
| Mg(CH3CO2)2 | - | ||
| (NH4)2SO4 | 10 mM | ||
| Tris-HCl (pH 8.8) | 20 mM | ||
| dNTPs | 1.4mM each | ||
| Betaine | 0.8 M | ||
| Tween 20 | 0.1 % | ||
| (c) | LAMP reconstitution (for VRPA : VLAMP = 1:4 mix) | |||
|---|---|---|---|---|
| Chemical | Concentration | |||
| Pre-LAMP | Reconstituted LAMP | |||
| [K+] | KCI | - | - | |
| CH3CO2K | - | 20 mM | ||
| [Mg2+] | MgSO4 | - | - | |
| Mg(CH3CO2)2 | 7 mM | 9.8 mM | ||
| (NH4)2SO4 | 12.5 mM | 10 mM | ||
| Tris-HCl (pH 8.0) | 10 mM | 20 mM | ||
| dNTPs | 1.8mM each | 1.4mM each | ||
| Betaine | 1.0 M | 0.8 M | ||
| Tween 20 | 0.125 % | 0.1 % | ||
Sample processing.
HBV and HCV samples were stored at −20 °C prior to use. HBV dsDNA were extracted with Qiamp DNA extraction kit (Qiagen). HCV RNA was extracted with Qiamp viral RNA extraction kit (Qiagen). The concentrations of the purified nucleic acids were estimated with Qubit DNA assay kit, NA HS assay kit, and fluorimeter (molecular probe life science, Waltham, MA, USA). Copy numbers of each sample were calculated based on molecular masses (NCBI), and then mixed with molecular water to form dilution series. Our estimated viral loads favorably agreed with vendor’s (Seracare) data after converting international units (IU) to copy numbers 21.
Thermally removable barrier.
To enable two-stage assay in single-pot, we use a thermally removable barrier above the LAMP reaction volume (Figure 1b). To prove the concept, we inserted 10 μL pre-LAMP solution in the tube with 20 μL of docosane. The tube was then heated to 50°C (above the docosane’s melting temperature of 45 °C). The molten docosane floated to the surface of the pre-LAMP buffer. The tube then was cooled to room temperature and the docosane solidified to form a an impermeable barrier above the pre-LAMP volume. Later, in the discussion section, we describe an alternative that is compatible with long shelf life.
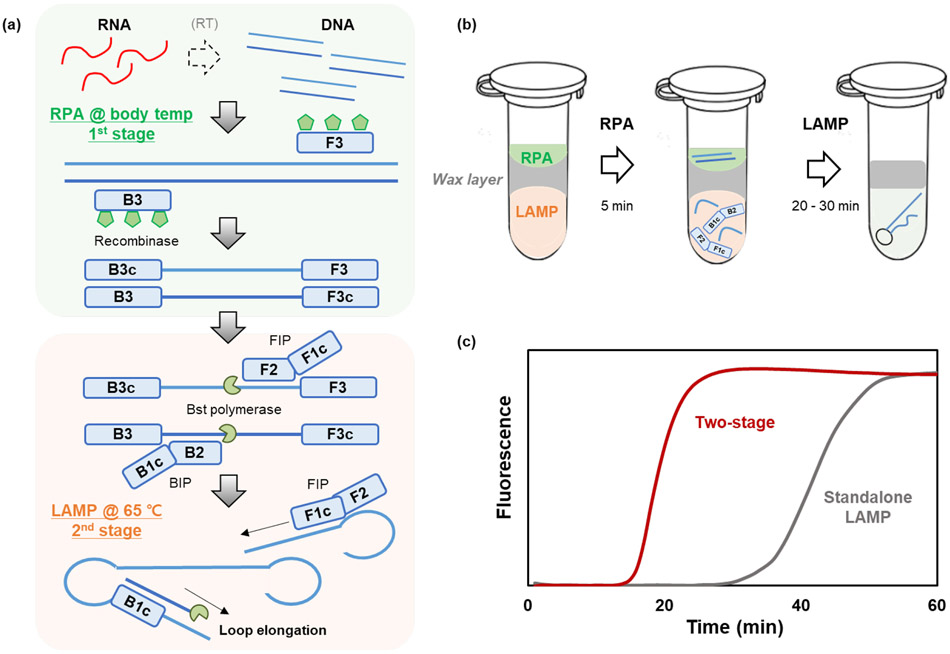
Figure 1:
(a) The working principle of the two-stage Penn RAMP assay. The first stage consists of RPA at 36°C and the second stage of LAMP at 65°C. (b) The reaction tube comprises of two compartments separated with a thermally removable barrier. The lower compartment houses the pre-LAMP buffer and the upper compartment the RPA reaction mix. Upon heating from the RPA incubation temperature to the LAMP incubation temperature, the barrier melts and float to the top, allowing the RPA products to mix with the pre-LAMP buffer, (c) Representative real-time amplification curves of the second stage amplification process (after 5 min first stage incubation) contrasted with standalone LAMP.
Two-stage amplification.
A 2.5 μL of RPA solution with templates was inserted above the solid docosane barrier, and the tube tube’s lid was closed. The reaction tube was incubated with body heat (by holding the tube in palm of one’s hand) for 5 min. Then, the second stage amplification was carried out at 65 °C for 40 min, with CFX-96 real-time thermal cycler (Bio-Rad, Hercules, CA, USA) operating at a fixed temperature. Threshold time in the system software was measured in three times, then average and standard deviation were calculated in the main data for viral detection. The LAMP amplification process was monitored in real time with a fluorescent intercalating dye (SYTO-9).
Results and discussion
Simple, rapid two-stage RAMP amplification in a single tube.
The Penn RAMP assay consists of two stages (Figure 1a). RAMP’s first stage is RPA, enable to target analyze quantitatively 22, with incubation temperature ranging from 35°C to 42°C. The amplicons of the 1st stage are then subjected to LAMP with incubation temperature ranging from 63° to 65°C. RAMP provides higher sensitivity than either standalone LAMP or standalone RPA 16. Previously, single plex RAMP was implemented in a closed pot by incubating the first (RPA) stage in the tube’s lid and the second (LAMP) stage in the tube’s body. Such an approach limits the volume of the RPA reaction mix and requires one to mechanically manipulate the tube to mix first stage products with the second stage buffer. Here, we devised a novel method that removes restrictions on the first stage reaction volume and eliminates the need for mechanical manipulation of the tube.
To this end, we separate the tube into two compartments with a thermally removable barrier (Figure 1b) that has a melting temperature (e.g., 45°C) between the first stage and the second stage incubation temperatures. The compartment above the barrier houses the RPA reaction mix and the templates. The chamber beneath the barrier contains the pre-LAMP buffer and LAMP primers. After a few minutes (typically, 5 min) of first stage incubation, the tube is heated to the second stage incubation temperature (65°C). During this process, the barrier melts, floats to the top, providing a barrier against evaporation, and the RPA products mix with the pre-LAMP buffer.
Custom-designed pre-LAMP buffer.
Since the RPA and LAMP buffers are incompatible, the volume of the RPA reaction buffer (VRPA) that can be added to the LAMP reaction mix (VLAMP) must be restricted to avoid significant inhibition of LAMP. With manufacturers’ recommended RPA and LAMP buffer compositions (Table 1a and 1b), the RAMP assay performs optimally when the ratio between the RPA reaction mix volume and the LAMP buffer volume VRPA:VLAMP = 1:15 23. Although RAMP with VRPA:VLAMP = 1:9 significantly outperforms standalone LAMP and standalone RPA, we wish to further increase the volume ratio VRPA:VLAMP to maintain high sensitivity. We hypothesize that by depleting the LAMP assay from selected compounds, we can form a pre-LAMP assay such that upon the addition of the RPA volume VRPA, we end up with the standard LAMP composition.
To quantify the effects of various RPA reaction mix components on LAMP, we define the metric ,where Tt is the LAMP threshold time (min), and Tmin is the smallest threshold time in the same comparison group. Threshold times greater than 60 min, were assigned the value of 60 min. We examined η as a function of the concentrations of various LAMP reaction mix components (Figure S1). The LAMP buffer includes 10 mM KCl whereas the RPA buffer includes 100 mM potassium acetate. Among other things, K+ may bind to the DNA phosphate backbone; anions may bind to proteins and interfere with polymerase; and the salt concentration affects ionic strength (and to the Debye screening length). LAMP performs optimally with 10 mM KCl or 20 mM CH3CO2K (Figure S1a). Divalent cations (Mg2+) are essential for enzyme activity and affect amplification efficiency and specificity. The standard RPA reaction mix contains 14 mM of magnesium acetate while the standard LAMP buffer contains 8 mM of magnesium sulfate. LAMP performs optimally with either 8 mM magnesium sulfate or 10 mM magnesium acetate (Figure S1b). Tris-HCl is used as pH buffer. LAMP was only mildly sensitive to Tris-HCl concentration in the range from PH=7.4 to 8.8 (Figure S1c) and performed optimally with 20 mM Tris-HCl. LAMP is insensitive to primer concentration in the range from 0.5X (2.6 μM) to 2X (10.4 μM total primer); and Bst polymerase concentration in the range from 0.5X (0.16 U/μL) to 2X (0.64 U/μL) (Figure S1d).
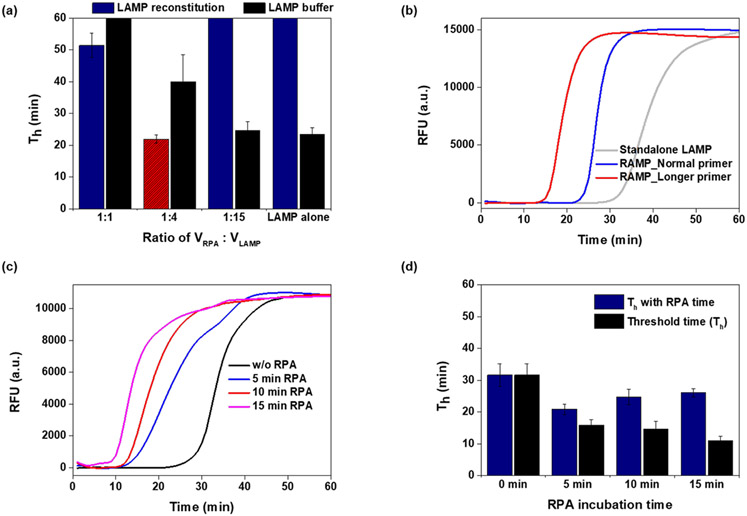
We kept the RPA buffer composition unaltered (Table 1a). For the two-stage reaction, guided by Figure S1, we replace the standalone LAMP composition with a depleted LAMP buffer (dubbed pre-LAMP, Table 1c) designed so that pre-LAMP combined with RPA buffer results in a reaction mix equivalent to standalone LAMP. To keep the monovalent salt concentration under 20 mM and prevent LAMP inhibition, our pre-LAMP buffer must be free of monovalent salt. This suggests VRPA:VLAMP = 1:4. Pre-LAMP buffer mixed with RPA products in the ratio VRPA:VLAMP = 1:4 provides the smallest threshold time among all the volume ratios tested (Figure 2a).
Figure 2:
Optimization of two-stage assay conditions, using 100 copies of HBV viral DNA. (a) Threshold time as a function of the volume ratio VRPA:VLAMP. Th values longer than 60 min are displayed as 60 min. (N=3 and average with standard deviation was displayed) (b) Real-time amplification curves of RAMP with long (30-35 nt) F3/B3 primers, RAMP with short (17-20 nt) F3/B3 primers; and standalone LAMP with short (17-20 nt) F3/B3 primers, (c) RAMP amplification curves following various RPA incubation times, (d) RAMP and second stage LAMP threshold times as functions of RPA incubation time (N=3).
RAMP operates optimally with long F3/B3 primers.
RAMP’s first stage (RPA) employs F3 and B3 LAMP primers to amplify templates. LAMP F3 and B3 primers are typically 17-20 nt long 24 while optimal RPA primers range in length from 30 to 35 nt25. When we use short F3/B3 primers, RPA operates sub optimally while LAMP operates optimally. On the other hand, if we use long F3/B3 primers, RPA will be optimal while LAMP will be less so. What is better? To answer this question, we amplified 100 copies of HBV DNA with RAMP, and recorded the threshold time when operating with long (32 nt) and short (20 nt) F3/B3 primers (Figure 2b). RAMP with long F3/B3 primers has the shortest threshold time. In all the experiments that follow, we used RAMP with long F3/B3 primers (Table S1).
Five minutes first stage incubation time minimizes overall reaction time.
RAMP requires two incubation temperatures. The first stage incubation (RPA) is carried out in the temperature range from 35 °C to 42 °C for the time interval TRPA while the second stage (LAMP) is carried out in the temperature range from 60 °C to 65 °C for up to 60 min. As the first stage incubation time TRPA increases, the number of templates available to the second stage reaction increases, and the second stage threshold time Tt decreases (Figure 2c). RAMP’s accumulative threshold time is, however, TRPA +Tt. The total time TRPA +Tt initially decreases as TRPA increases, attains a minimum at TRPA ~ 5 min, and then increases again (Figure 2d). The optimal TRPA likely depends on the number of templates in the sample. In all the experiments reported here, we use TRPA =5 min with good results.
A closed pot with thermally removable partition for two stage amplification.
The two-stage amplification process requires one to transfer first stage amplicons to the second stage. It is desirable to accomplish this task without opening the reaction tube and exposing the amplicon rich first stage products to the ambient, risking the contamination of the workspace. To achieve single pot RAMP, we divide the tube into two compartments with a liquid-impermeable paraffin barrier that has a melting temperature between the RPA and LAMP incubation temperatures (Figure 1b). The lower chamber houses the pre-LAMP buffer (including the LAMP primer set). The RPA reaction is carried out in the space above the barrier. The tube is first incubated at the RPA incubation temperature (35 - 42°C) for about 5 min - a temperature at which the barrier remains solid, and then the temperature is ramped up to the LAMP incubation temperature (60 - 65°C), which is above the barrier melting temperature. When transitioning from the first stage incubation temperature to the second stage incubation temperature, the docosane barrier melts and floats to the surface and the RPA products mix with the pre-LAMP buffer. The docosane then forms an evaporation barrier above the reaction volume keeping the tube’s lid free of condensation to provide unobstructed imaging of fluorescence emission or color change.
We experimented with various barrier compounds for their compatibility with polymerase and selected docosane (C22H46). Docosane is insoluble (8 × 10−10 g/L at 25°C) in water; is lighter (density 0.8 g cm3) than water; and has a melting temperature of 44°C 26 Although alternative phase change materials such as PureTemp 42 and PureTemp 63 (PureTemp, Minneapolis, MN, USA) are attractive because their phase change temperature can be customized, they inhibit amplification (Figure S2b).
Since docosane in its solid form is brittle and difficult to handle, we devised a simple procedure to cast the docosane insitu. As proof of concept, we filled the tube with 10 μL LAMP reaction mix and casted molten docosane on top of the aqueous solution (Figure S2c). The docosane wetted the polypropylene tube forming a disk-shaped layer with an upright paraboloid-like top surface, nearly flat bottom, and minimum thickness at the disk’s center. Alternatively, we can introduce a solid block of docosane in the liquid-filled lower chamber and heat the tube to above the docosane’s phase transition temperature. The molten docosane then spreads on top of the liquid’s surface. When the tube is cooled to room temperature, a solid barrier forms.
To determine the shelf life of the docosane barrier in the presence of liquid, we filled the bottom of the tube with water dyed with reddish food coloring; added 15 μL, 17.5 μL, and 20 μL docosane barrier, and topped with water dyed with green food coloring above the docosane barrier (Figure S2c). The tubes were then stored in a horizontal position at room temperature. The 15 μL and 17.5 μL docosane barriers were, respectively, compromised within 3 and 5 days while the 20 μL barrier maintained its integrity for a few weeks (Figure S2d). Since in applications, the barrier is needed just for a few minutes, a 20 μL barrier exceeds our needs. While the formation of the thermal barrier as described above is appropriate for proof of concept, it is not compatible with long term storage. We have, however, a solution for long shelf life that we will describe in this paper’s conclusion and outlook section.
The first stage can be incubated with body heat.
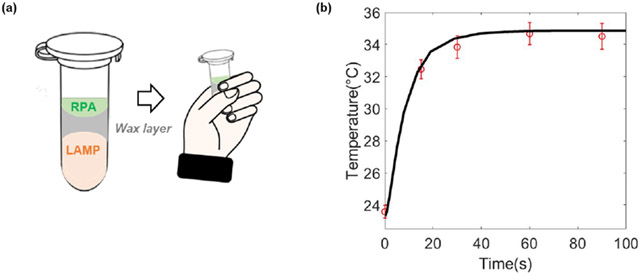
The two-stage process requires two incubation temperatures. The first stage incubation requires a temperature ranging from 35 °C to 42 °C. While it is possible to carry out this incubation with a thermal cycler or a heat block, this relatively low incubation temperature provides one with the opportunity to carry out the incubation instrument-free, increasing test affordability. Since the first stage incubation temperature matches human body temperature and requires short time, we carried out the first stage incubation with body heat as previously described 27 by holding the tube in our fist (Figure 3a). To assure that this method provides the required temperature, we inserted a thermo-couple (OMEGA, K type, # TFIR-24S-50) inside the tube, and both monitored and computed the tube’s temperature as functions of time at the tube’s center. Our computational model assumes a heating process dominated by conduction. The tube’s temperature reached nearly steady state of about 35°C in about 30 s and remained at that level for the duration of the first stage incubation, relying on one’s body ability to regulate temperature (Figure 3b). The reasonable agreement between the experimental observations and theory suggests that the heat transfer in the tube is dominated by conduction.
Figure 3:
(a) First stage incubation by body heat. (b) Measured (symbols, N=3) and computed (solid line) tube temperature as a function of time when the tube is held in a person’s fist. The computational model is based on a tube subjected to body heat around its surface and heat loss to the ambient at its top.
In the experiments described herein, we carried out the second stage incubation with a thermal cycler operating at a fixed temperature (65°C). Since no temperature cycling is needed, the second stage incubation can be carried out with a simple heat block or even electricity free, wherein heat is provided by an exothermic reaction and regulated with a phase change material 28, making the two-stage assay amenable for use in resource poor settings and at home, electricity-free.
HBV RAMP assay.
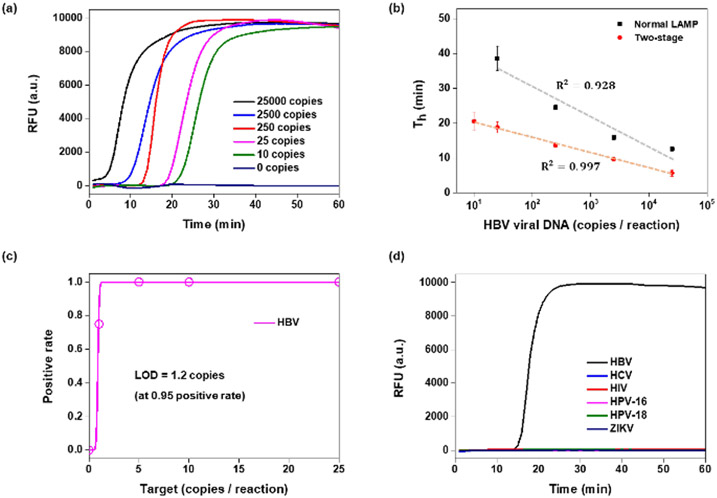
We designed a RAMP primer set with long F3/B3 primers (Table S1) targeting the S gene of HBV DNA and tested samples with various concentrations of HBV DNA isolated from plasma with both our RAMP assay (with long F3/B3 primers) and standalone LAMP (with short F3/B3 primers). The RAMP assays were incubated in tubes partitioned with our thermally removable barrier (Figure 1b) and with first stage body heat incubation. The second stage amplification and the standalone LAMP were incubated with a thermal cycler operating at a fixed temperature (65°C). Real-time amplification curves were obtained by monitoring fluorescent emission from an intercalating dye during the LAMP stage (Figure 4a). The threshold times of the RAMP (excluding the five minutes first stage incubation) and of the standalone LAMP correlated linearly with the log of the number of templates (Figure 4b). Threshold times of RAMP were shorter than those of standalone LAMP (Figure 4b). More importantly, RAMP provided better sensitivity and reproducibility than the standalone LAMP. RAMP HBV detected reproducibly (100% sensitivity) 5 or more copies per the 12.5 μL reaction volume and less reproducibly (75%) one copy per reaction volume (Figure 4c). The latter can be attributed to sampling error and is comparable with the probability of producing a sample with more than one molecule during dilution. Probit analysis suggests the limit of detection of 1.2 copies per reaction volume. RAMP exhibited an order of magnitude better sensitivity than our standalone LAMP and better sensitivity than other published assays (ESI Table S2). Within the 60 min monitoring, no false positive signals were observed in the absence of templates (no-template control) and with other common blood-borne viruses such as HCV, human immunodeficiency virus (HIV), human papilloma viruses HPV-16 and HPV-18, and zika virus (ZIKV) (Fig 4d).
Figure 4:
HBV RAMP in a single pot. (a) Amplification curves from various concentrations of HBV viral DNA. (b) RAMP and standalone LAMP threshold times as functions of the number of templates in the reaction volume. (c) Probit plot for positive detection rate of each 16 tests in 0, 1, 5, 10, 25 copies of HBV DNA. (d) Specificity of two-stage assay: there is no signal in the absence of HBV and in the presence of 1000 copies of other, common blood borne viruses.
HCV RAMP.
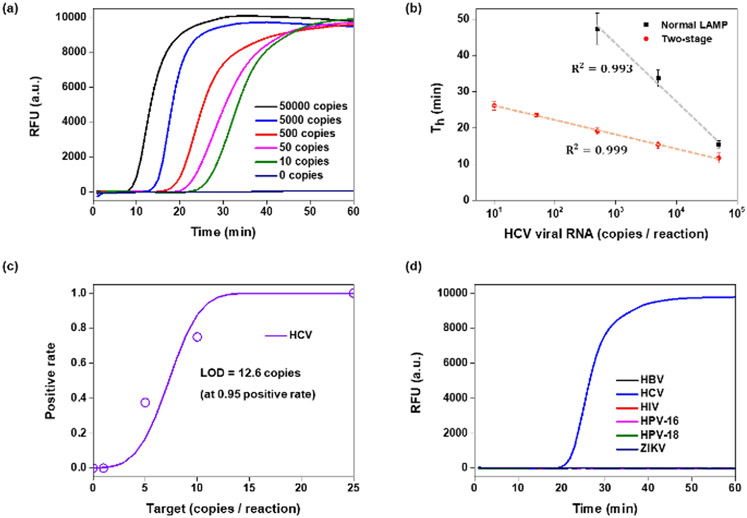
We designed a RAMP primer set with long F3/B3 primers (Table S1) targeting the 5’UTR gene of HCV RNA and tested various concentrations of HCV RNA samples isolated from plasma with both our RAMP assay (with long F3/B3 primers) and standalone LAMP (with short F3/B3 primers). The HCV RAMP assays were incubated in tubes partitioned with the thermally removable barrier (Figure 1b) and with first stage body heat incubation. The second stage amplification and the standalone LAMP were incubated with a thermal cycler operating at a fixed temperature (65°C). Real-time amplification curves were obtained by monitoring fluorescent emission form an intercalating dye during the LAMP stage (Figure 5a). The HCV RAMP successfully detected RNA concentrations ranging from 10 to 5×105 per reaction (Figure 5a). The threshold times of the HCV RAMP (excluding the five minutes first stage incubation) and of the LAMP correlated linearly with the log of the number of templates. The threshold times of RAMP were shorter than those of standalone LAMP (Figure 5b). More importantly, RAMP provided better sensitivity and reproducibility than the standalone LAMP. RAMP HCV detected reproducibly (100% sensitivity) 25 or more copies per the 12.5 μL reaction volume (Figure 5c). RAMP HCV detected less reproducibly (75%) 10 copies per reaction volume. Probit analysis suggests a limit of detection of 12.6 copies per reaction volume. RAMP exhibited an order of magnitude better sensitivity than our standalone LAMP and better sensitivity than other assays operating with similar samples (ESI Table S3). Within the 60 min monitoring time, no false positive signals were observed in the absence of templates (no-template control) and with other common blood-borne viruses such as HCV, human immunodeficiency virus (HIV), human papilloma viruses HPV-16 and HPV-18, and zika virus (ZIKV) (Fig 5d).
Figure 5:
HCV RAMP in a single pot. (a) Amplification curves from various concentrations of HCV viral RNA. (b) RAMP and standalone LAMP threshold times as functions of the number of templates in the reaction volume. (c) Probit plot for positive detection rate of each 16 tests in 0, 1, 5, 10, 25 copies of HCV RNA. (d) Specificity of two-stage assay: there is no signal in the absence of HCV and in the presence of 1000 copies of other, common blood borne viruses.
Conclusions
This study describes a two stage, isothermal molecular assay dubbed RAMP for HBV and HCV. The first stage of RAMP is comprised of RPA and the second stage of LAMP. Although RAMP was originally designed for high level of multiplexing, we found it to be useful for single plex assays as it provides a better sensitivity than standalone LAMP. RAMP requires two different incubation temperatures, but the first incubation process does not require any specialized equipment, and able to do in our body temperature.
Since RPA and LAMP buffers are incompatible, we formulated a pre-LAMP buffer that is deficient in certain components. When RPA products are added to the pre-LAMP buffer in the volume ratio of 1 to 4, standard standalone LAMP buffer is reconstituted. HBV RAMP and HCV RAMP exhibit ten-fold higher sensitivity than our standalone HBV LAMP and standalone HCV LAMP, enabling the detection of fewer than 10 copies of HBV DNA and 25 copies of HCV RNA per reaction volume within 30 min. Both HBV RAMP and HCV RAMP are semi-quantitative exhibiting linear relationship between threshold time and the log of template concentration. In our experiments, we have not observed any false positives even when incubating the LAMP reaction for over sixty minutes. Hence, we can use generic intercalating dye to monitor the amplification process. In the event of false positives, one can replace the intercalating dye with molecular beacons.
We have devised a new method that allows us to carry out RAMP in a single pot without a need to open the tube and transfer first stage reaction products into the second stage. We achieve this by dividing the tube into two chambers with a thermally removable partition. The first stage RPA reaction mix and the templates are placed above the barrier to carry out the RPA reaction. During this process, the barrier remains impermeable separating the RPA reaction mix from the pre-LAMP buffer. When the tube’s temperature is increased from the first stage incubation temperature to the second stage incubation temperature, the barrier melts and float to the surface, allowing the first stage products to mix with the pre-LAMP buffer. The second stage reaction is then incubated. The amplification process can be monitored in real time with intercalating dye or molecular beacons. Alternatively, test results can be detected with colorimetric dyes that change color either in the presence of amplicons or reaction byproducts 29.
Here, we have demonstrated proof of concept of carrying the two-stage process in a closed pot with a thermally removable barrier. To enable the use of this method in practice, it would be necessary to pre-store the reagents in the tube. This can be accomplished, for example by equipping the tube with vertical vias that allow access to the compartment beneath the thermally removable barrier (Figure S3). We envision storing dry pre-LAMP reaction mix in the lower compartment and dry RPA reaction mix in the upper compartment above the barrier. Prior to the test, the user fills the lower compartment with molecular water, add the sample to the upper compartment and incubate the reaction.
While the two stage RAMP process can be incubated with a thermal cycler, a heat block, or a water bath, it can also be processed instrumentation free. Although we used a thermal cycler in this work, simple heating and fluorescence measurements are the only required functions. Since the first stage of RAMP requires a relatively low temperature (35-42°C), we incubated this reaction with body heat, simply by holding the tube in our fist for five minutes. Although RAMP’s second stage requires higher temperature (60-65°C), it can also be incubated without an instrument or electricity. We can produce heat with a chemical heater (exothermic reaction) like the one used in meals ready to eat and regulate the temperature with a phase change material 17.
Given their simplicity, our HCV RAMP and HBV RAMP can be processed during a visit to the clinic or to the doctor’s office, reducing the number of visits that currently are needed for diagnostics (e.g., antibody test followed with confirmatory molecular test), enabling detection of active stage of disease and prompt/direct linkage to therapy. Our molecular tests can also be used to monitor disease progression and therapy’s efficacy. Our system is particularly suitable for resource poor settings, where sophisticated laboratory facilities are in short supply, and many patients fail to return to the doctor’s office for follow up care. The RAMP assay was originally developed to accommodate a multiplexed assay in the microfluidic chip 16. In the future, we plan to combine detection of HBV, HCV, and other viruses into a single test using the RAMP platform.
Supplementary Material
ACKNOWLEDGMENT
This work has been supported, in part, by NIH grant R61 AI140484-01 to the University of Pennsylvania.
Footnotes
Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.”
LAMP efficiency as a function of the buffer composition, Reaction tube with docosane partition for two-stage RAMP amplification, A schematic description of a tube with a removable partition and vertical vias providing access to the LAMP chamber, Standalone LAMP and two-stage RAMP primer sequences, Comparison of HBV RAMP with various HBV assays, Comparison of HCV-RAMP with various HCV assays, Positive rate in low concentration range for 16 tests
REFERENCES
- (1).Global H. AIDS statistics—2018 fact sheet. Geneva: UNAIDS; 2019. [Google Scholar]; Beyrer C; Pozniak A HIV drug resistance—an emerging threat to epidemic control. New England Journal of Medicine 2017, 377 (17), 1605–1607. [DOI] [PubMed] [Google Scholar]; Chen HW; Belinskaya T; Zhang Z; Ching WM Simple Detection of Hepatitis B Virus in Using Loop-Mediated Isothermal Amplification Method. Mil Med 2019, 184 (7-8), e275–e280. DOI: 10.1093/milmed/usy421. [DOI] [PubMed] [Google Scholar]; Shin DJ; Trick AY; Hsieh YH; Thomas DL; Wang TH Sample-to-Answer Droplet Magnetofluidic platform for Point-of-Care Hepatitis C Viral Load Quantitation. Sci Rep 2018, 8 (1), 9793. DOI: 10.1038/s41598-018-28124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kim MJ; Park Q; Min HK; Kim HO Residual risk of transfusion-transmitted infection with human immunodeficiency virus, hepatitis C virus, and hepatitis B virus in Korea from 2000 through 2010. BMC infectious diseases 2012, 12 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hughes E; Bassi S; Gilbody S; Bland M; Martin F Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. The Lancet Psychiatry 2016, 3 (1), 40–48. DOI: 10.1016/s2215-0366(15)00357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chang FM; Wang YP; Lang HC; Tsai CF; Hou MC; Lee FY; Lu CL Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology 2017, 66 (3), 896–907. DOI: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]; Perz JF; Armstrong GL; Farrington LA; Hutin YJ; Bell BP The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006, 45 (4), 529–538. DOI: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- (5).Nyan DC; Swinson KL A method for rapid detection and genotype identification of hepatitis C virus 1-6 by one-step reverse transcription loop-mediated isothermal amplification. Int J Infect Dis 2016, 43, 30–36. DOI: 10.1016/j.ijid.2015.12.002. [DOI] [PubMed] [Google Scholar]
- (6).Sun W; Du Y; Li X; Du B Rapid and Sensitive Detection of Hepatitis C Virus in Clinical Blood Samples Using Reverse Transcriptase Polymerase Spiral Reaction. J Microbiol Biotechnol 2020, 30 (3), 459–468. DOI: 10.4014/jmb.1910.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Shen XX; Qiu FZ; Shen LP; Yan TF; Zhao MC; Qi JJ; Chen C; Zhao L; Wang L; Feng ZS; et al. A rapid and sensitive recombinase aided amplification assay to detect hepatitis B virus without DNA extraction. BMC Infect Dis 2019, 19 (1), 229. DOI: 10.1186/s12879-019-3814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Waggoner JJ; Gresh L; Mohamed-Hadley A; Ballesteros G; Davila MJ; Tellez Y; Sahoo MK; Balmaseda A; Harris E; Pinsky BA Single-Reaction Multiplex Reverse Transcription PCR for Detection of Zika, Chikungunya, and Dengue Viruses. Emerg Infect Dis 2016, 22 (7), 1295–1297. DOI: 10.3201/eid2207.160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Batule BS; Seok Y; Kim MG Paper-based nucleic acid testing system for simple and early diagnosis of mosquito-borne RNA viruses from human serum. Biosens Bioelectron 2020, 151, 111998. DOI: 10.1016/j.bios.2019.111998. [DOI] [PubMed] [Google Scholar]
- (10).Yi TT; Zhang HY; Liang H; Gong GZ; Cai Y Betaine-assisted recombinase polymerase assay for rapid hepatitis B virus detection. Biotechnol Appl Biochem 2020. DOI: 10.1002/bab.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Natoli ME; Rohrman BA; De Santiago C; van Zyl GU; Richards-Kortum RR Paper-based detection of HIV-1 drug resistance using isothermal amplification and an oligonucleotide ligation assay. Anal Biochem 2018, 544, 64–71. DOI: 10.1016/j.ab.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Soares RRG; Varela JC; Neogi U; Ciftci S; Ashokkumar M; Pinto IF; Nilsson M; Madaboosi N; Russom A Sub-at-tomole detection of HIV-1 using padlock probes and rolling circle amplification combined with microfluidic affinity chromatography. Biosens Bioelectron 2020, 166, 112442. DOI: 10.1016/j.bios.2020.112442. [DOI] [PubMed] [Google Scholar]
- (13).Seok Y; Batule BS; Kim M-G Lab-on-paper for all-in-one molecular diagnostics (LAMDA) of zika, dengue, and chikungunya virus from human serum. Biosensors and Bioelectronics 2020, 165, 112400. [DOI] [PubMed] [Google Scholar]
- (14).Yu M; Chen X; Qu H; Ma L; Xu L; Lv W; Wang H; Ismagilov RF; Li M; Shen F Multistep SlipChip for the Generation of Serial Dilution Nanoliter Arrays and Hepatitis B Viral Load Quantification by Digital Loop Mediated Isothermal Amplification. Anal Chem 2019, 91 (14), 8751–8755. DOI: 10.1021/acs.anal-chem.9b01270. [DOI] [PubMed] [Google Scholar]
- (15).Walker FM; Hsieh K Advances in Directly Amplifying Nucleic Acids from Complex Samples. Biosensors (Basel) 2019, 9 (4). DOI: 10.3390/bios9040117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yi C; Luo Z; Lu Y; Belwal T; Pan X; Lin X Nanoporous hydrogel for direct digital nucleic acid amplification in untreated complex matrices for single bacteria counting. Biosens Bioelectron 2021, 184, 113199. DOI: 10.1016/j.bios.2021.113199. [DOI] [PubMed] [Google Scholar]
- (16).Song J; Liu C; Mauk MG; Rankin SC; Lok JB; Green-berg RM; Bau HH Two-Stage Isothermal Enzymatic Amplification for Concurrent Multiplex Molecular Detection. Clin Chem 2017, 63 (3), 714–722. DOI: 10.1373/clinchem.2016.263665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Li RJ; Mauk MG; Seok Y; Bau HH Electricity-free chemical heater for isothermal nucleic acid amplification with applications in COVID-19 home testing. Analyst 2021. DOI: 10.1039/d1an00309g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).V L. Application of Real Time Loop Mediated Isothermal Amplification Assay on Dried Blood Spots in the Detection of HCV RNA among High Risk Patients. Journal of Emerging Diseases and Virology 2016, 2 (1). DOI: 10.16966/2473-1846.111. [DOI] [Google Scholar]
- (19).Piepenburg O; Williams CH; Stemple DL; Armes NA DNA detection using recombination proteins. PLoS Biol 2006, 4 (7), e204. DOI: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Tomita N; Mori Y; Kanda H; Notomi T Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 2008, 3 (5), 877–882. DOI: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- (21).Saldanha J; Gerlich W; Lelie N; Dawson P; Heermann K; Heath A; Group, T. W. C. S. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sanguinis 2001, 80 (1), 63–71. DOI: 10.1046/j.1423-0410.2001.00003.x. [DOI] [PubMed] [Google Scholar]; Saldanha J; Heath A; Aberham C; Albrecht J; Gentili G; Gessner M; Pisani G World Health Organization collaborative study to establish a replacement WHO international standard for hepatitis C virus RNA nucleic acid amplification technology assays. Vox Sanguinis 2005, 88 (3), 202–204. DOI: 10.1111/j.1423-0410.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- (22).Crannell ZA; Rohrman B; Richards-Kortum R Development of a quantitative recombinase polymerase amplification assay with an internal positive control. Journal of visualized experiments: JoVE 2015, (97). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).El-Tholoth M; Anis E; Bau HH Two stage, nested isothermal amplification in a single tube. Analyst 2021, 146 (4), 1311–1319. DOI: 10.1039/d0an01835j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Notomi T; Mori Y; Tomita N; Kanda H Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. Journal of microbiology 2015, 53 (1), 1–5. [DOI] [PubMed] [Google Scholar]
- (25).Li J; Macdonald J; von Stetten F Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2018, 144 (1), 31–67. DOI: 10.1039/c8an01621f. [DOI] [PubMed] [Google Scholar]
- (26).<Docosane _ C22H46 - PubChem.pdf>.
- (27).Crannell ZA; Rohrman B; Richards-Kortum R Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PLoS One 2014, 9 (11), e112146. DOI: 10.1371/journal.pone.0112146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Goertz JP; White IM Phase-Change Partitions for Thermal Automation of Multistep Reactions. Anal Chem 2018, 90 (6), 3708–3713. DOI: 10.1021/acs.analchem.7b05400. [DOI] [PubMed] [Google Scholar]
- (29).Song J; Mauk MG; Hackett BA; Cherry S; Bau HH; Liu C Instrument-Free Point-of-Care Molecular Detection of Zika Virus. Anal Chem 2016, 88 (14), 7289–7294. DOI: 10.1021/acs.analchem.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seok Y; Joung HA; Byun JY; Jeon HS; Shin SJ; Kim S; Shin YB; Han HS; Kim MG A Paper-Based Device for Performing Loop-Mediated Isothermal Amplification with Real-Time Simultaneous Detection of Multiple DNA Targets. Theranostics 2017, 7 (8), 2220–2230. DOI: 10.7150/thno.18675. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miyamoto S; Sano S; Takahashi K; Jikihara T Method for colorimetric detection of double-stranded nucleic acid using leuco triphenylmethane dyes. Anal Biochem 2015, 473, 28–33. DOI: 10.1016/j.ab.2014.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.